Artificial Intelligence for Thyroid Nodule Characterization: Where Are We Standing?
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Radiomics
3.2. Deep Learning and Machine Learning and TIRADS Systems
3.3. Computer-Assisted Diagnosis (CAD)
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Curado, M.P.; Edwards, B.; Shin, H.R.; Storm, H.; Ferlay, J.; Heanue, M.; Boyle, P. Cancer Incidence in Five Continents; Iarc Scientific Publications: Lyon, France, 2014; Volume 10. [Google Scholar]
- Grani, G.; Zatelli, M.C.; Alfò, M.; Montesano, T.; Torlontano, M.; Morelli, S.; Deandrea, M.; Antonelli, A.; Francese, C.; Ceresini, G.; et al. Real-World Performance of the American Thyroid Association Risk Estimates in Predicting 1-Year Differentiated Thyroid Cancer Outcomes: A Prospective Multicenter Study of 2000 Patients. Thyroid 2021, 31, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Celletti, I.; Fresilli, D.; De Vito, C.; Bononi, M.; Cardaccio, S.; Cozzolino, A.; Durante, C.; Grani, G.; Grimaldi, G.; Isidori, A.M.; et al. TIRADS, SRE and SWE in INDETERMINATE thyroid nodule characterization: Which has better diagnostic performance? Radiol. Med. 2021, 126, 1189–1200. [Google Scholar] [CrossRef] [PubMed]
- Fresilli, D.; Grani, G.; De Pascali, M.L.; Alagna, G.; Tassone, E.; Ramundo, V.; Ascoli, V.; Bosco, D.; Biffoni, M.; Bononi, M.; et al. Computer-aided diagnostic system for thyroid nodule sonographic evaluation outperforms the specificity of less experienced examiners. J. Ultrasound 2020, 23, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Gabillard, J.C.; Ulisse, S.; Baldini, E.; Sorrenti, S.; Cremet, J.Y.; Coccaro, C.; Prigent, C.; D’Armiento, M.; Arlot-Bonnemains, Y. Aurora-C interacts with and phosphorylates the transforming acidic coiled-coil 1 protein. Biochem. Biophys. Res. Commun. 2011, 408, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Baldini, E.; Tuccilli, C.; Prinzi, N.; Sorrenti, S.; Falvo, L.; De Vito, C.; Catania, A.; Tartaglia, F.; Mocini, R.; Coccaro, C.; et al. Deregulated expression of Aurora kinases is not a prognostic biomarker in papillary thyroid cancer patients. PLoS ONE 2015, 10, e0121514. [Google Scholar] [CrossRef]
- Beam, A.L.; Kohane, I.S. Big Data and Machine Learning in Health Care. JAMA 2018, 319, 1317–1318. [Google Scholar] [CrossRef]
- Gillies, R.J.; Kinahan, P.E.; Hricak, H. Radiomics: Images Are More than Pictures, They Are Data. Radiology 2016, 278, 563–577. [Google Scholar] [CrossRef] [Green Version]
- Bishop, C.M. Pattern Recognition and Machine Learning; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Cantisani, V.; Grani, G.; Tovoli, F.; Piscaglia, F.; Catalano, C. Artificial Intelligence: What Is It and How Can It Expand the Ultrasound Potential in the Future? Ultraschall Med. 2020, 41, 356–360. [Google Scholar] [CrossRef]
- Thomas, J.; Ledger, G.A.; Mamillapalli, C.K. Use of artificial intelligence and machine learning for estimating malignancy risk of thyroid nodules. Curr. Opin. Endocrinol. Diabetes Obes. 2020, 27, 345–350. [Google Scholar] [CrossRef]
- Bini, F.; Pica, A.; Azzimonti, L.; Giusti, A.; Ruinelli, L.; Marinozzi, F.; Trimboli, P. Artificial Intelligence in Thyroid Field—A Comprehensive Review. Cancers 2021, 13, 4740. [Google Scholar] [CrossRef]
- Sorrenti, S.; Dolcetti, V.; Fresilli, D.; Del Gaudio, G.; Pacini, P.; Huang, P.; Camponovo, C.; Leoncini, A.; D’Andrea, V.; Pironi, D.; et al. The Role of CEUS in the Evaluation of Thyroid Cancer: From Diagnosis to Local Staging. J. Clin. Med. 2021, 10, 4559. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.C.; Jin, P.F.; Bao, J.; Jiang, Q.; Wang, X. Thyroid ultrasound image classification using a convolutional neural network. Ann. Transl Med. 2021, 9, 1526. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Liu, Y.; Lv, W.; Liu, L.; Zhou, Q.; Yang, H.; Ren, J.; Liu, G.; Wang, X.; Zhang, X.; et al. Deep learning-based artificial intelligence model to assist thyroid nodule diagnosis and management: A multicentre diagnostic study. Lancet Digit. Health 2021, 3, e250–e259. [Google Scholar] [CrossRef]
- Bai, Z.; Chang, L.; Yu, R.; Li, X.; Wei, X.; Yu, M.; Liu, Z.; Gao, J.; Zhu, J.; Zhang, Y.; et al. Thyroid nodules risk stratification through deep learning based on ultrasound images. Med. Phys. 2020, 47, 6355–6365. [Google Scholar] [CrossRef]
- Yoon, J.; Lee, E.; Kang, S.W.; Han, K.; Park, V.Y.; Kwak, J.Y. Implications of US radiomics signature for predicting malignancy in thyroid nodules with indeterminate cytology. Eur. Radiol. 2021, 31, 5059–5067. [Google Scholar] [CrossRef]
- Liu, Z.; Zhong, S.; Liu, Q.; Xie, C.; Dai, Y.; Peng, C.; Chen, X.; Zou, R. Thyroid nodule recognition using a joint convolutional neural network with information fusion of ultrasound images and radiofrequency data. Eur. Radiol. 2021, 31, 5001–5011. [Google Scholar] [CrossRef] [PubMed]
- Gomes Ataide, E.J.; Ponugoti, N.; Illanes, A.; Schenke, S.; Kreissl, M.; Friebe, M. Thyroid Nodule Classification for Physician Decision Support Using Machine Learning-Evaluated Geometric and Morphological Features. Sensors 2020, 20, 6110. [Google Scholar] [CrossRef]
- Ye, H.; Hang, J.; Chen, X.; Di Xu Chen, J.; Ye, X.; Zhang, D. An intelligent platform for ultrasound diagnosis of thyroid nodules. Sci. Rep. 2020, 10, 13223. [Google Scholar] [CrossRef]
- Wei, X.; Gao, M.; Yu, R.; Liu, Z.; Gu, Q.; Liu, X.; Zheng, Z.; Zheng, X.; Zhu, J.; Zhang, S. Ensemble Deep Learning Model for Multicenter Classification of Thyroid Nodules on Ultrasound Images. Med. Sci. Monit. 2020, 26, e926096. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, Y.; Chang, Q.; Liu, T.; Zhang, S.; Wang, X.; Guo, Q.; Yao, J.; Sun, W.; Niu, L. Evaluation of a deep learning-based computer-aided diagnosis system for distinguishing benign from malignant thyroid nodules in ultrasound images. Med. Phys. 2020, 47, 3952–3960. [Google Scholar] [CrossRef]
- Zhou, H.; Jin, Y.; Dai, L.; Zhang, M.; Qiu, Y.; Wang, K.; Tian, J.; Zheng, J. Differential Diagnosis of Benign and Malignant Thyroid Nodules Using Deep Learning Radiomics of Thyroid Ultrasound Images. Eur. J. Radiol. 2020, 127, 108992. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.T.; Kang, J.K.; Pham, T.D.; Batchuluun, G.; Park, K.R. Ultrasound Image-Based Diagnosis of Malignant Thyroid Nodule Using Artificial Intelligence. Sensors 2020, 20, 1822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Zhang, L.; Zhu, M.; Qi, X.; Yi, Z. Automatic diagnosis for thyroid nodules in ultrasound images by deep neural networks. Med. Image Anal. 2020, 61, 101665. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.; Haertling, T. AIBx, Artificial Intelligence Model to Risk Stratify Thyroid Nodules. Thyroid 2020, 30, 878–884. [Google Scholar] [CrossRef] [PubMed]
- Galimzianova, A.; Siebert, S.M.; Kamaya, A.; Rubin, D.L.; Desser, T.S. Quantitative Framework for Risk Stratification of Thyroid Nodules With Ultrasound: A Step Toward Automated Triage of Thyroid Cancer. AJR Am. J. Roentgenol. 2020, 214, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.T.; Pham, T.D.; Batchuluun, G.; Yoon, H.S.; Park, K.R. Artificial Intelligence-Based Thyroid Nodule Classification Using Information from Spatial and Frequency Domains. J. Clin. Med. 2019, 8, 1976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buda, M.; Wildman-Tobriner, B.; Hoang, J.K.; Thayer, D.; Tessler, F.N.; Middleton, W.D.; Mazurowski, M.A. Management of Thyroid Nodules Seen on US Images: Deep Learning May Match Performance of Radiologists. Radiology 2019, 292, 695–701. [Google Scholar] [CrossRef]
- Koh, J.; Lee, E.; Han, K.; Kim, E.K.; Son, E.J.; Sohn, Y.M.; Seo, M.; Kwon, M.R.; Yoon, J.H.; Lee, J.H.; et al. Diagnosis of thyroid nodules on ultrasonography by a deep convolutional neural network. Sci. Rep. 2020, 10, 15245. [Google Scholar] [CrossRef]
- Wang, L.; Yang, S.; Yang, S.; Zhao, C.; Tian, G.; Gao, Y.; Chen, Y.; Lu, Y. Automatic thyroid nodule recognition and diagnosis in ultrasound imaging with the YOLOv2 neural network. World J. Surg. Oncol. 2019, 17, 12. [Google Scholar] [CrossRef] [Green Version]
- Han, M.; Ha, E.J.; Park, J.H. Computer-Aided Diagnostic System for Thyroid Nodules on Ultrasonography: Diagnostic Performance Based on the Thyroid Imaging Reporting and Data System Classification and Dichotomous Outcomes. AJNR Am. J. Neuroradiol. 2021, 42, 559–565. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, Q.; Chen, Y.; Wang, Y. A Clinical Assessment of an Ultrasound Computer-Aided Diagnosis System in Differentiating Thyroid Nodules With Radiologists of Different Diagnostic Experience. Front. Oncol. 2020, 10, 557169. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Zhu, Y.; Zhang, S.; Xie, F.; Zhang, M.; Zhang, Y.; Tian, X.; Zhang, J.; Luo, Y.; Cao, J. Ultrasound Computer-Aided Diagnosis (CAD) Based on the Thyroid Imaging Reporting and Data System (TI-RADS) to Distinguish Benign from Malignant Thyroid Nodules and the Diagnostic Performance of Radiologists with Different Diagnostic Experience. Med. Sci. Monit. 2020, 26, e918452. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Yao, J.; Zhou, W.; Dong, Y.; Xu, S.; Zhou, J.; Zhan, W. A computer-aided diagnosing system in the evaluation of thyroid nodules-experience in a specialized thyroid center. World J. Surg. Oncol. 2019, 17, 210. [Google Scholar] [CrossRef] [PubMed]
- Jin, A.; Li, Y.; Shen, J.; Zhang, Y.; Wang, Y. Clinical Value of a Computer-Aided Diagnosis System in Thyroid Nodules: Analysis of a Reading Map Competition. Ultrasound Med. Biol. 2019, 45, 2666–2671. [Google Scholar] [CrossRef]
- Kim, H.L.; Ha, E.J.; Han, M. Real-World Performance of Computer-Aided Diagnosis System for Thyroid Nodules Using Ultrasonography. Ultrasound Med. Biol. 2019, 45, 2672–2678. [Google Scholar] [CrossRef]
- Chi, J.; Walia, E.; Babyn, P.; Wang, J.; Groot, G.; Eramian, M. Thyroid Nodule Classification in Ultrasound Images by Fine-Tuning Deep Convolutional Neural Network. J. Digit. Imaging 2017, 30, 477–486. [Google Scholar] [CrossRef]
- Zhao, W.J.; Fu, L.R.; Huang, Z.M.; Zhu, J.Q.; Ma, B.Y. Effectiveness evaluation of computer-aided diagnosis system for the diagnosis of thyroid nodules on ultrasound: A systematic review and meta-analysis. Medicine 2019, 98, e16379. [Google Scholar] [CrossRef]
- Watkins, L.; O’Neill, G.; Young, D.; McArthur, C. Comparison of British Thyroid Association, American College of Radiology TIRADS and Artificial Intelligence TIRADS with histological correlation: Diagnostic performance for predicting thyroid malignancy and unnecessary fine needle aspiration rate. Br. J. Radiol. 2021, 94, 20201444. [Google Scholar] [CrossRef]
- Wang, S.; Xu, J.; Tahmasebi, A.; Daniels, K.; Liu, J.B.; Curry, J.; Cottrill, E.; Lyshchik, A.; Eisenbrey, J.R. Incorporation of a Machine Learning Algorithm With Object Detection Within the Thyroid Imaging Reporting and Data System Improves the Diagnosis of Genetic Risk. Front. Oncol. 2020, 10, 591846. [Google Scholar] [CrossRef]
- Wildman-Tobriner, B.; Buda, M.; Hoang, J.K.; Middleton, W.D.; Thayer, D.; Short, R.G.; Tessler, F.N.; Mazurowski, M.A. Using Artificial Intelligence to Revise ACR TI-RADS Risk Stratification of Thyroid Nodules: Diagnostic Accuracy and Utility. Radiology 2019, 292, 112–119. [Google Scholar] [CrossRef]
- Lambin, P.; Rios-Velazquez, E.; Leijenaar, R.; Carvalho, S.; van Stiphout, R.G.; Granton, P.; Zegers, C.M.; Gillies, R.; Boellard, R.; Dekker, A.; et al. Radiomics: Extracting more information from medical images using advanced feature analysis. Eur. J. Cancer 2012, 48, 441–446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sollini, M.; Cozzi, L.; Chiti, A.; Kirienko, M. Texture analysis and machine learning to characterize suspected thyroid nodules and differentiated thyroid cancer: Where do we stand? Eur. J. Radiol. 2018, 99, 1–8. [Google Scholar] [CrossRef]
- Han, Z.; Feng, N.; Lu, Y.; Li, M.; Wei, P.; Yao, J.; Zhu, Q.; Lei, Z.; Xu, D. A Control Study on the Value of the Ultrasound Grayscale Ratio for the Differential Diagnosis of Thyroid Micropapillary Carcinoma and Micronodular Goiter in Two Medical Centers. Front. Oncol. 2021, 10, 625238. [Google Scholar] [CrossRef]
- Grani, G.; D’Alessandri, M.; Carbotta, G.; Nesca, A.; Del Sordo, M.; Alessandrini, S.; Coccaro, C.; Rendina, R.; Bianchini, M.; Prinzi, N.; et al. Grey-Scale Analysis Improves the Ultrasonographic Evaluation of Thyroid Nodules. Medicine 2015, 94, e1129. [Google Scholar] [CrossRef]
- Lei, Z.K.; Li, M.K.; Luo, D.C.; Han, Z.J. The clinical significance of ultrasound grayscale ratio in differentiating markedly hypoechoic and anechoic minimal thyroid nodules. J. Cancer Res. Ther. 2018, 14, 1567–1571. [Google Scholar] [PubMed]
- Chen, X.; Gao, M.; Hu, L.; Zhu, J.; Zhang, S.; Wei, X. The diagnostic value of the ultrasound gray scale ratio for different sizes of thyroid nodules. Cancer Med. 2019, 8, 7644–7649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, Z.; Lei, Z.; Li, M.; Luo, D.; Ding, J. Differential diagnosis value of the ultrasound gray scale ratio for papillary thyroid microcarcinomas and micronodular goiters. Quant. Imaging Med. Surg. 2018, 8, 507–513. [Google Scholar] [CrossRef]
- Liang, J.; Huang, X.; Hu, H.; Liu, Y.; Zhou, Q.; Cao, Q.; Wang, W.; Liu, B.; Zheng, Y.; Li, X.; et al. Predicting Malignancy in Thyroid Nodules: Radiomics Score Versus 2017 American College of Radiology Thyroid Imaging, Reporting and Data System. Thyroid 2018, 28, 1024–1033. [Google Scholar] [CrossRef] [PubMed]
- Kwon, M.R.; Shin, J.H.; Park, H.; Cho, H.; Hahn, S.Y.; Park, K.W. Radiomics Study of Thyroid Ultrasound for Predicting BRAF Mutation in Papillary Thyroid Carcinoma: Preliminary Results. AJNR Am. J. Neuroradiol. 2020, 41, 700–705. [Google Scholar] [CrossRef] [Green Version]
- Park, V.Y.; Han, K.; Kim, H.J.; Lee, E.; Youk, J.H.; Kim, E.K.; Moon, H.J.; Yoon, J.H.; Kwak, J.Y. Radiomics signature for prediction of lateral lymph node metastasis in conventional papillary thyroid carcinoma. PLoS ONE 2020, 15, e0227315. [Google Scholar] [CrossRef]
- Akkus, Z.; Cai, J.; Boonrod, A.; Zeinoddini, A.; Weston, A.D.; Philbrick, K.A.; Erickson, B.J. A Survey of Deep-Learning Applications in Ultrasound: Artificial Intelligence-Powered Ultrasound for Improving Clinical Workflow. J. Am. Coll. Radiol. 2019, 16, 1318–1328. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.; Lee, E.; Koo, J.S.; Yoon, J.H.; Nam, K.H.; Lee, J.; Jo, Y.S.; Moon, H.J.; Park, V.Y.; Kwak, J.Y. Artificial intelligence to predict the BRAFV600E mutation in patients with thyroid cancer. PLoS ONE 2020, 15, e0242806. [Google Scholar] [CrossRef] [PubMed]
- Ulisse, S.; Baldini, E.; Lauro, A.; Pironi, D.; Tripodi, D.; Lori, E.; Ferent, I.C.; Amabile, M.I.; Catania, A.; Di Matteo, F.M.; et al. Papillary Thyroid Cancer Prognosis: An Evolving Field. Cancers 2021, 13, 5567. [Google Scholar] [CrossRef] [PubMed]
- Baldini, E.; Tuccilli, C.; Pironi, D.; Catania, A.; Tartaglia, F.; Di Matteo, F.M.; Palumbo, P.; Arcieri, S.; Mascagni, D.; Palazzini, G.; et al. Expression and Clinical Utility of Transcription Factors Involved in Epithelial-Mesenchymal Transition during Thyroid Cancer Progression. J. Clin. Med. 2021, 10, 4076. [Google Scholar] [CrossRef] [PubMed]
- Daniels, K.; Gummadi, S.; Zhu, Z.; Wang, S.; Patel, J.; Swendseid, B.; Lyshchik, A.; Curry, J.; Cottrill, E.; Eisenbrey, J. Machine Learning by Ultrasonography for Genetic Risk Stratification of Thyroid Nodules. JAMA Otolaryngol. Head Neck Surg. 2020, 146, 36–41. [Google Scholar] [CrossRef]
- Prieditis, P.; Radzina, M.; Mikijanska, M.; Liepa, M.; Stepanovs, K.; Grani, G.; Durante, C.; Lamartina, L.; Trimboli, P.; Cantisani, V. Non-Marked Hypoechogenic Nodules: Multicenter Study on the Thyroid Malignancy Risk Stratification and Accuracy Based on TIRADS Systems Comparison. Medicina 2022, 58, 257. [Google Scholar] [CrossRef]
- Gild, M.L.; Chan, M.; Gajera, J.; Lurie, B.; Gandomkar, Z.; Clifton-Bligh, R.J. Risk stratification of indeterminate thyroid nodules using ultrasound and machine learning algorithms. Clin. Endocrinol. 2022, 96, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Gao, Z.; He, Y.; Mai, W.; Li, J.; Zhou, M.; Li, S.; Yi, W.; Wu, S.; Bai, T.; et al. An Artificial Intelligence Model Based on ACR TI-RADS Characteristics for US Diagnosis of Thyroid Nodules. Radiology 2022, 22, 211455. [Google Scholar] [CrossRef]
- Xu, L.; Gao, J.; Wang, Q.; Yin, J.; Yu, P.; Bai, B.; Pei, R.; Chen, D.; Yang, G.; Wang, S.; et al. Computer-Aided Diagnosis Systems in Diagnosing Malignant Thyroid Nodules on Ultrasonography: A Systematic Review and Meta-Analysis. Eur. Thyroid J. 2020, 9, 186–193. [Google Scholar] [CrossRef]
- Elliott Range, D.D.; Dov, D.; Kovalsky, S.Z.; Henao, R.; Carin, L.; Cohen, J. Application of a machine learning algorithm to predict malignancy in thyroid cytopathology. Cancer Cytopathol. 2020, 128, 287–295. [Google Scholar] [CrossRef]
- Fresilli, D.; David, E.; Pacini, P.; Del Gaudio, G.; Dolcetti, V.; Lucarelli, G.T.; Di Leo, N.; Bellini, M.I.; D’Andrea, V.; Sorrenti, S.; et al. Thyroid Nodule Characterization: How to Assess the Malignancy Risk. Update of the Literature. Diagnostics 2021, 11, 1374. [Google Scholar] [CrossRef]
- Friedrich-Rust, M.; Meyer, G.; Dauth, N.; Berner, C.; Bogdanou, D.; Herrmann, E.; Zeuzem, S.; Bojunga, J. Interobserver agreement of Thyroid Imaging Reporting and Data System (TIRADS) and strain elastography for the assessment of thyroid nodules. PLoS ONE 2013, 8, e77927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- LeCun, Y.; Bengio, Y.; Hinton, G. Deep learning. Nature 2015, 521, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Kezlarian, B.; Lin, O. Artificial Intelligence in Thyroid Fine Needle Aspiration Biopsies. Acta Cytol. 2021, 65, 324–329. [Google Scholar] [CrossRef]
- Bellini, M.I.; Biffoni, M.; Patrone, R.; Borcea, M.C.; Costanzo, M.L.; Garritano, T.; Melcarne, R.; Menditto, R.; Metere, A.; Scorziello, C.; et al. Poorly Differentiated Thyroid Carcinoma: Single Centre Experience and Review of the Literature. J. Clin. Med. 2021, 10, 5258. [Google Scholar] [CrossRef]
- Ippolito, A.M.; De Laurentiis, M.; La Rosa, G.L.; Eleuteri, A.; Tagliaferri, R.; De Placido, S.; Vigneri, R.; Belfiore, A. Neural network analysis for evaluating cancer risk in thyroid nodules with an indeterminate diagnosis at aspiration cytology: Identification of a low-risk subgroup. Thyroid 2004, 14, 1065–1071. [Google Scholar] [CrossRef]
- Wildman-Tobriner, B.; Taghi-Zadeh, E.; Mazurowski, M.A. Artificial Intelligence (AI) Tools for Thyroid Nodules on Ultrasound, From the AJR Special Series on AI Applications. AJR Am. J. Roentgenol. 2022, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Cheng, S.; Qian, K.; Yue, K.; Liu, H. Automatic Recognition and Classification System of Thyroid Nodules in CT Images Based on CNN. Comput. Intell. Neurosci. 2021, 2021, 5540186. [Google Scholar] [CrossRef]
- Zhao, H.B.; Liu, C.; Ye, J.; Chang, L.F.; Xu, Q.; Shi, B.W.; Liu, L.L.; Yin, Y.L.; Shi, B.B. A comparison between deep learning convolutional neural networks and radiologists in the differentiation of benign and malignant thyroid nodules on CT images. Endokrynol. Pol. 2021, 72, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Song, B.; Ye, N.; Ren, J.; Sun, X.; Dai, Z.; Zhang, Y.; Chen, B.T. Machine learning-based multiparametric MRI radiomics for predicting the aggressiveness of papillary thyroid carcinoma. Eur. J. Radiol. 2020, 122, 108755. [Google Scholar] [CrossRef] [Green Version]
- Gu, J.; Zhu, J.; Qiu, Q.; Wang, Y.; Bai, T.; Yin, Y. Prediction of Immunohistochemistry of Suspected Thyroid Nodules by Use of Machine Learning-Based Radiomics. AJR Am. J. Roentgenol. 2019, 213, 1348–1357. [Google Scholar] [CrossRef] [PubMed]


| Reference | Approach | Source Data | Method Details | Performance |
|---|---|---|---|---|
| Zhu, et al., 2021 [14] | Brief Efficient Thyroid Network (BETNET; a CSS model) | gray-scale US images of 592 patients with 600 TNs (internal dataset) 187 patients with 200 TNs (external validation dataset) | CNN approach with 24 layers: 13 convolution layers, 5 pooling layers, 3 fully connected layers with dropouts in between | AUC 0.970, 95% CI: 0.958–0.980 in the independent validation cohort; similar to two highly skilled radiologists (0.940 and 0.953) |
| Peng, et al. 2021 [15] | Deep-learning AI model (ThyNet) | 18,049 US images of 8339 patients (training set) 4305 images of 2775 patients (total test set) | combined architecture of three networks: ResNet, ResNeXt, and DenseNet | ThyNet AUC (0.922; 95% CI 0.910–0.934] higher than that of the radiologists (0.839; CI 0.834–0.844]; p < 0.0001) |
| Bai, et al., 2021 [16] | RS-Net evaluation AI model | 13,984 thyroid US images | CNN approach in which GoogLeNet is used as the backbone network. | Accuracy, sensitivity, specificity, PPV, and NPV were 88.0%, 98.1%, 79.1%, 80.5%, and 97.9%, comparable to that of a senior radiologist |
| Yoon, et al., 2021 [17] | Texture analysis; least absolute shrinkage and selection operator (LASSO) logistic regression model including clinical variables | 155 US images of indeterminate thyroid nodules in 154 patients. | Texture extraction using MATLAB 2019b.; the LASSO model was used to choose the most useful predictive features. Univariable and multivariable logistic regression analyses were performed to build malignancy prediction models. | Integrated model AUC 0.839 vs. 0.583 (clinical variables only). |
| Liu, et al., 2021 [18] | information fusion-based joint convolutional neural network (IF-JCNN) | 163 pairs of US images and raw radiofrequency signals of thyroid nodules | IF-JCNN contains two branched CNNs for deep feature extraction: one for US images (14 convolutional layers and 3 fully connected layers) and the other one for RF signals (12 convolutional layers and 3 fully connected layers) | The information carried by raw radiofrequency signals and ultrasound images for thyroid nodules is complementary IF-JCNN (both images and RF signals): AUC 0.956 (95% CI 0.926–0.987) |
| Gomes Ataide, et al., 2020 [19] | Feature extraction and Random Forest classifier | 99 original US images | Feature extraction using MATLAB 2018b; Random Forest classifier (400 Decision Trees; Criterion: Entropy, with Bootstrap) | RFC accuracy 99.3%, sensitivity 99.4%, specificity 99.2% |
| Ye, et al., 2020 [20] | Deep convolution neural network (VGG-16) | US images of 1601 nodules (training set) and test data including 209 nodules (test set) | CNN approach based on VGG-19 (16 layers with learnable weights, 13 convolutions and 3 fully connected layers) | AUC 0.9157, comparable to the experienced radiologist (0.8879; p > 0.1) |
| Wei, et al., 2020 [21] | Ensemble deep learning model (EDLC-TN) | 25,509 thyroid US images | CNN model based on DenseNet and adopted as a multistep cascade pathway for an ensemble learning model with voting system. | AUC 0.941 (0.936–0.946) |
| Zhou, et al., 2020 [23] | CNN-based transfer learning method named DLRT (deep-learning radiomics of thyroid) | US images of 1750 thyroid nodules (from 1734 patients) | CNN-based architecture with transfer learning strategy, with 4 hidden layers (3 transferred and a fine-tuned layer) and a fully connected layer | AUC in the external cohort 0.97 (0.95–0.99). Both a senior and a junior US radiologist had lower sensitivity and specificity than DLRT. |
| Nguyen, et al., 2020 [24] | Combination of multiple CNN models (ResNet-based and InceptionNet-based) | 450 US thyroid nodule images (from 298 patients) | Combination of ResNet50-based (50 layers) and Inception-based (4 layers) networks followed by global average pooling, batch normalization, dropout, and dense layer | Accuracy: 92.05% |
| Wang, et al., 2020 [25] | Three CNN networks (feature extraction network; attention-based feature aggregation network; classification network) | 7803 US thyroid nodule images from 1046 examinations | CNN approach based on Inception-Resnet-v2 (164 layers) | Method AUC 0.9006 Both the accuracy and sensitivity are significantly higher than sonographers. |
| Thomas, et al., 2020 [26] | AIBx, AI model to risk stratify thyroid nodules | 2025 US images of 482 thyroid nodules (internal dataset) and 103 nodules (external dataset) | CNN approach based on ResNet 34 (34 layers) | Negative predictive value (NPV), sensitivity, specificity, positive predictive value (PPV), and accuracy of the image similarity model were greater than other cancer risk stratification systems. |
| Galimzianova, et al., 2020 [27] | Feature extraction and regularized logistic regression model | 92 US images of 92 biopsy-confirmed thyroid nodules | Feature extraction (219 for each nodule) and elastic net regression analysis | Method AUC 0.828 (95% CI, 0.715–0.942), greater than or comparable to that of the expert classifiers |
| Nguyen, et al., 2019 [28] | AI-Based Thyroid Nodule Classification Using Information from Spatial and Frequency Domains | ultrasound thyroid images of 237 patients (training dataset) and 61 patients (test dataset). | CNN models (Resnet18, Resnet34, and Resnet50 were compared) | AI system with spatial domain based on deep learning, and frequency domain based on Fast Fourier transform (FFT) outperforms the state-of-the-art methods (especially CAD systems) |
| Buda, et al., 2019 [29] | CNN | 1377 US images of thyroid nodules in 1230 patients (training dataset) and 99 nodules (internal test dataset) | Custom CNN (six blocks with 3 × 3 convolutional filters, followed by Rectified Linear Unit activation function and max pooling layer with 2 × 2 kernels). | Method AUC: 0.87 [CI 0.76, 0.95] Three ACR-TIRADS readers 0.91 |
| Koh, et al., 2020 [30] | Two individual CNNs compared with experienced radiologist | 15,375 US images of thyroid nodules (training set), 634 (internal test), 1181 (external test set). | Four CNNs including two individual CNNs, ResNet50 (50 layers) and InceptionResNetV2 (164 layers), and two classification ensembles, AlexNet-GoogLeNet-SqueezeNet ensemble and AlexNet-GoogLeNetSqueezeNet-InceptionResNetv2 ensemble | CNNs AUC similar to experienced radiologist AUC (0.87) |
| Wang, et al., 2019 [31] | CNN compared with experienced radiologist | 351 US images with nodules and 213 images without nodules of 276 patients | CNN system in which the Resnet v2-50 (50 layers) network and YOLOv2 are integrated | CAD AUC 0.902 significantly higher than radiologist AUC 0.859 (p = 0.0434) |
| CAD systems | ||||
| Sun, et al., 2020 [22] | Fused features combing the CNN-based features (VGG F-based features) with hand-crafted features | 1037 US images of thyroid nodules (internal dataset) and 550 images (test dataset) | A support vector machine (SVM) is used for classification and fused features which combined the deep features extracted by a CNN with hand-crafted features, such as the histogram of oriented gradient (HOG), local binary patterns (LBP), and scale invariant feature transform (SIFT) | AUC of attending radiology lower than system (0.819 vs. 0.881, p = 0.0003) |
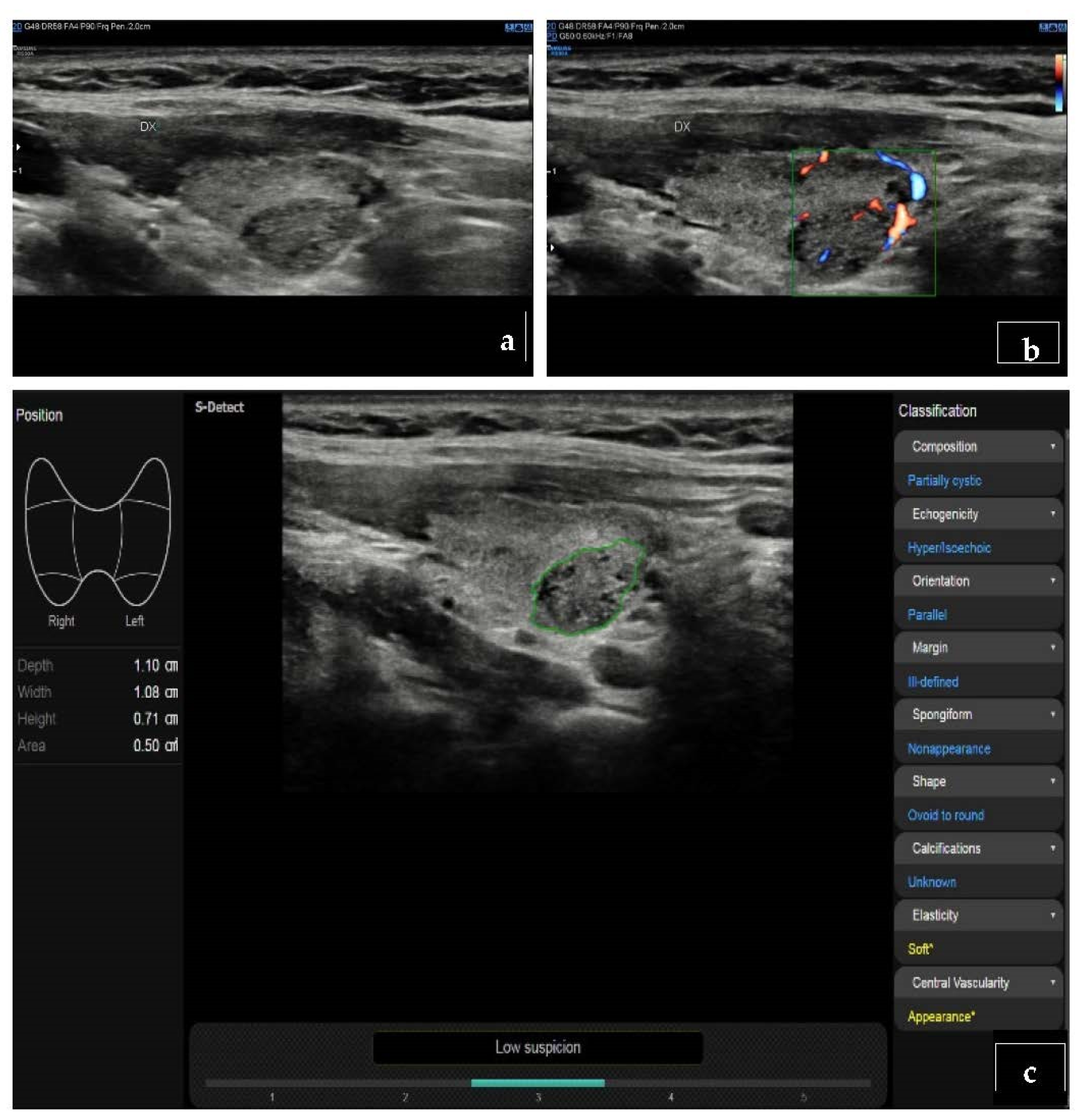
| Han, et al., 2021 [32] | S-Detect for Thyroid | US images of 454 thyroid nodules from 372 consecutive patients | S-Detect for Thyroid is an AI-based CAD software integrated in US equipment (Samsung Medison Co., Seoul, South Korea) | The sensitivities of the CAD system did not differ significantly from those of the radiologist (all p > 0.05); the specificities and accuracies were significantly lower than those of the radiologist (all p < 0.001). |
| Zhang, et al., 2020 [33] | AI-SONIC; Demetics Medical Technology Co., Zhejiang, China | US images of 365 thyroid nodules | AI-SONIC is a CAD based on deep learning (cascade CNN of two different CNN architectures (one with 15 convolutional layers/2 pooling layers for segmentation, and the other with 4 convolutional layers/4 pooling layers for detection), developed by Demetics Medical Technology Co., China | AUC CAD 0.788 vs. senior radiologist 0.906, p < 0.001). The use of CAD system improved the diagnostic sensitivities of both the senior and the junior radiologists |
| Fresilli, et al., 2020 [4] | S-Detect for Thyroid compared with an expert radiologist, a senior resident and a medical student evaluation | US images of 107 thyroid nodules | S-Detect for Thyroid is an AI-based CAD software integrated in US equipment (Samsung Medison Co., Seoul, South Korea) | The CAD system and the expert achieved similar values of a sensitivity and specificity (about 70%–87.5%). The specificity achieved by the student was significantly lower (76.25%). |
| Jin, et al., 2020 [34] | CAD system based on a modified, CNN-based TIRADS, evaluated by | US images of 789 thyroid nodules from 695 patients | CAD system based on the ACR TI-RADS automatic scoring using a CNN (no details provided). | AUC CAD 0.87 AUC Junior radiologist 0.73 (Junion + CAD): 0.83 AUC Senior radiologist 0.91 |
| Xia, et al., 2019 [35] | S-Detect for Thyroid | US images of 180 thyroid nodules in 171 consecutive patients | S-Detect for Thyroid is an AI-based CAD software integrated in US equipment (Samsung Medison Co., Seoul, South Korea) | AUC CADs 0.659 (0.577–0.740) AUC radiologist 0.823 (0.758–0.887) |
| Jin, et al., 2019 [36] | AmCad; AmCad BioMed, Taipei City, Taiwan | 33 images from 33 patients read by 81 radiologists | Commercial standalone CAD software: AmCad (version: Shanghai Sixth People’s Hospital; AmCad BioMed, Taipei City, Taiwan) | CAD AUC 0.985 (0.881–1.00) 177 contestants AUC 0.659 (0.645–0.673) (p < 0.01) |
| Kim, et al., 2019 [37] | S-Detect for Thyroid 1 and 2 | US images of 218 thyroid nodules from 106 consecutive patients | S-Detect for Thyroid is an AI-based CAD software integrated in US equipment (Samsung Medison Co., Seoul, South Korea) | AUC: radiologist 0.905 (95% CI, 0.859–0.941) S-Detect 1–assisted radiologist 0.865 (0.812–0.907) S-Detect 1 0.814 (0.756–0.863) S-Detect 2-assisted radiologist 0.802 (0.743–0.853) S-Detect 2 0.748 (0.685–0.804) |
| Chi, et al., 2017 [38] | CAD system for thyroid nodule | Database 1 includes 428 images in total while database 2 includes 164 images in total | CAD based on fine tuning of GoogLeNet CNN (22 convolutional layers including 9 inception modules) | CAD AUC 0.9920 Experienced radiologist AUC 0.9135 |
| Zhao, et al., 2019 [39] | CAD system for thyroid nodule systematic review and meta-analysis | Meta-analysis of 5 studies with 723 thyroid nodules from 536 patients | 4 studies with S-Detect; 1 study with internal CAD based on CNN. | CAD AUC 0.90 (95% CI 0.87–0.92) Experienced radiologist AUC 0.96 (95% CI 0.94–0.97) |
| AI-modified TIRADS | ||||
| Watkins, et al., 2021 [40] | AI-TIRADS | US images of 218 nodules from 212 patients | The AI-TIRADS is an optimization of ACR TIRADS generated by “genetic algorithms”, a subgroup of AI methods that focus on algorithms inspired by “natural selection”. | Sensitivity 93.44% Specificity 45.71% BTA, ACR-TIRADS, and AI-TIRADS have comparable diagnostic performance |
| Wang, et al., 2020 [41] | Google AutoML for automated nodule identification and risk stratification | US images of 252 nodules from 249 patients. | Google AutoML algorithm (AutoML Vision; Google LLC), with cloud computing and transfer learning | Accuracy of 68.7 ± 7.4% of AI-integrated TIRADS |
| Wildman-Tobriner, et al., 2010 [42] | AI-TIRADS | US images of 1425 biopsy-proven thyroid nodules from 1264 consecutive patients (training set); 100 nodules (test set) | The AI-TIRADS is an optimization of ACR TIRADS generated by “genetic algorithms”, a subgroup of AI methods that focus on algorithms inspired by “natural selection”. | ACR TI-RADS AUC 0.91 AI TI-RADS AUC 0.93 (with slight improvement of specificity and ease of use) |
| Main Advantages of AI | Main Disadvantages of AI |
|---|---|
| It is based on models, for the interpretation of thyroid nodules, that are able to match the performance characteristics of radiologists and pathologists | Too little experience at the moment; prospective multicenter trials on a wide population will be needed to improve the utility of artificial intelligence for the interpretation of thyroid nodules |
| Usable software for thyroid nodule risk stratification are already commercially available |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sorrenti, S.; Dolcetti, V.; Radzina, M.; Bellini, M.I.; Frezza, F.; Munir, K.; Grani, G.; Durante, C.; D’Andrea, V.; David, E.; et al. Artificial Intelligence for Thyroid Nodule Characterization: Where Are We Standing? Cancers 2022, 14, 3357. https://doi.org/10.3390/cancers14143357
Sorrenti S, Dolcetti V, Radzina M, Bellini MI, Frezza F, Munir K, Grani G, Durante C, D’Andrea V, David E, et al. Artificial Intelligence for Thyroid Nodule Characterization: Where Are We Standing? Cancers. 2022; 14(14):3357. https://doi.org/10.3390/cancers14143357
Chicago/Turabian StyleSorrenti, Salvatore, Vincenzo Dolcetti, Maija Radzina, Maria Irene Bellini, Fabrizio Frezza, Khushboo Munir, Giorgio Grani, Cosimo Durante, Vito D’Andrea, Emanuele David, and et al. 2022. "Artificial Intelligence for Thyroid Nodule Characterization: Where Are We Standing?" Cancers 14, no. 14: 3357. https://doi.org/10.3390/cancers14143357
APA StyleSorrenti, S., Dolcetti, V., Radzina, M., Bellini, M. I., Frezza, F., Munir, K., Grani, G., Durante, C., D’Andrea, V., David, E., Calò, P. G., Lori, E., & Cantisani, V. (2022). Artificial Intelligence for Thyroid Nodule Characterization: Where Are We Standing? Cancers, 14(14), 3357. https://doi.org/10.3390/cancers14143357











