The Role of Curcumin in Prostate Cancer Cells and Derived Spheroids
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Cell Cultures
2.3. Cell Survival Analysis
2.4. Western Blotting Analysis
2.5. Miniaturized 3D Cultures in ECM
2.6. Immunofluorescence (IF) Analysis of Organoids
2.7. Assessment of Organoids and Cell Viability via MTT Reduction
2.8. Statistical Analyses
3. Results
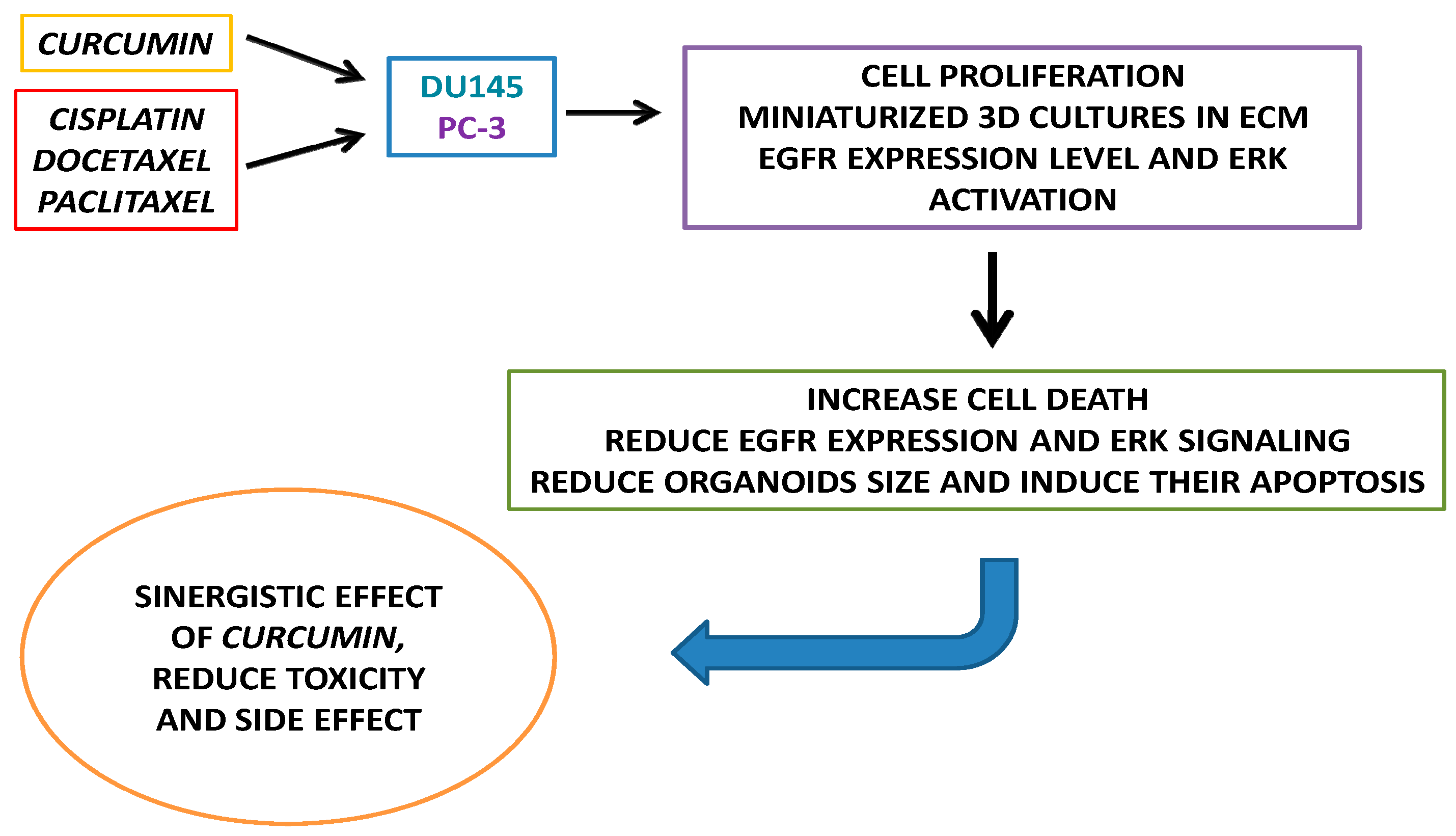
3.1. Drugs Treatment Effect on PC-3 and DU145 Cells Viability
3.2. Effect of Curcumin Alone or in Combination with Drugs on PC-3 and DU145 Cell Viability
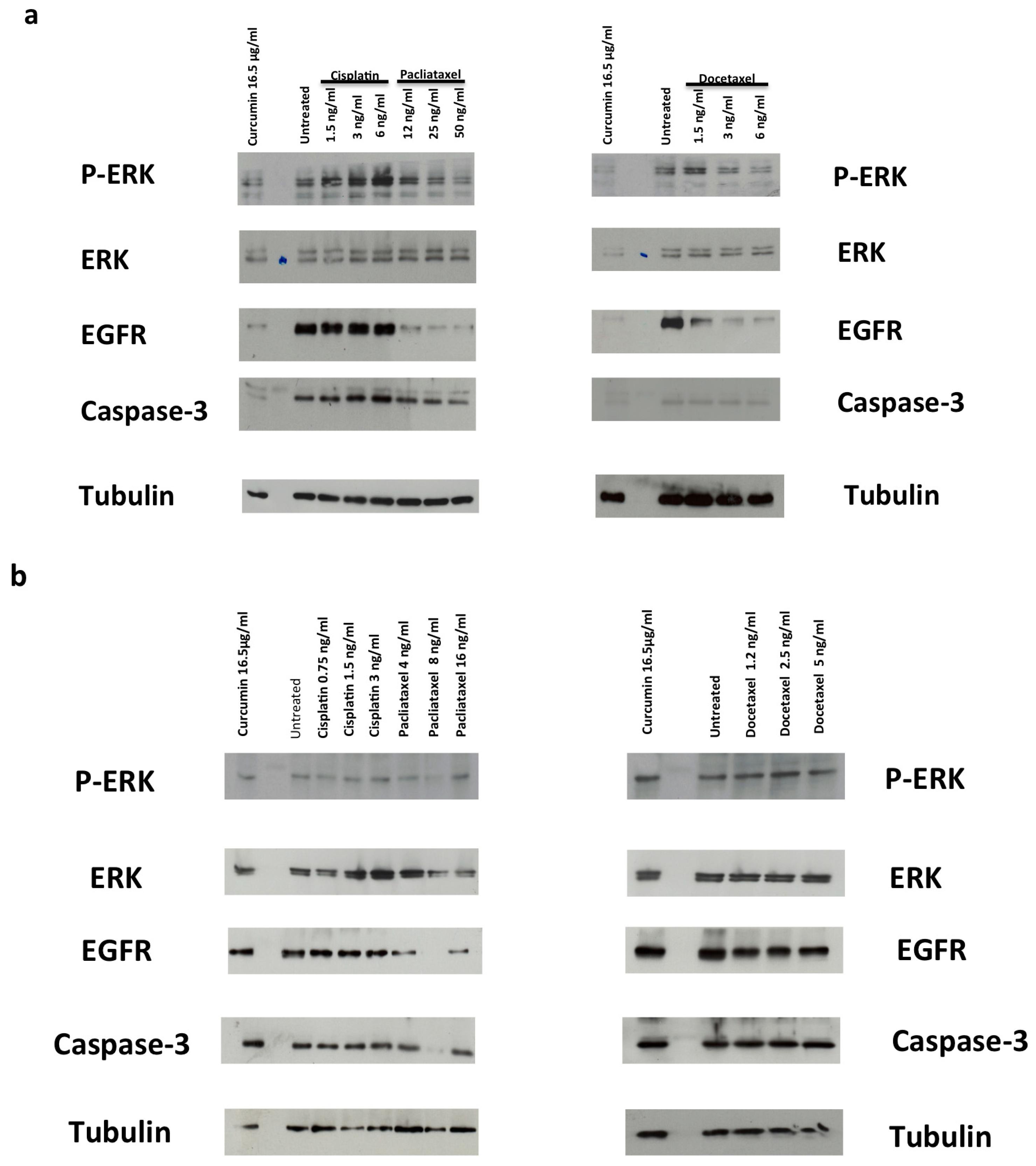
3.3. Drugs Treatment Effects on EGFR Expression Level and ERK Activation in PC-3 and DU145 Cells
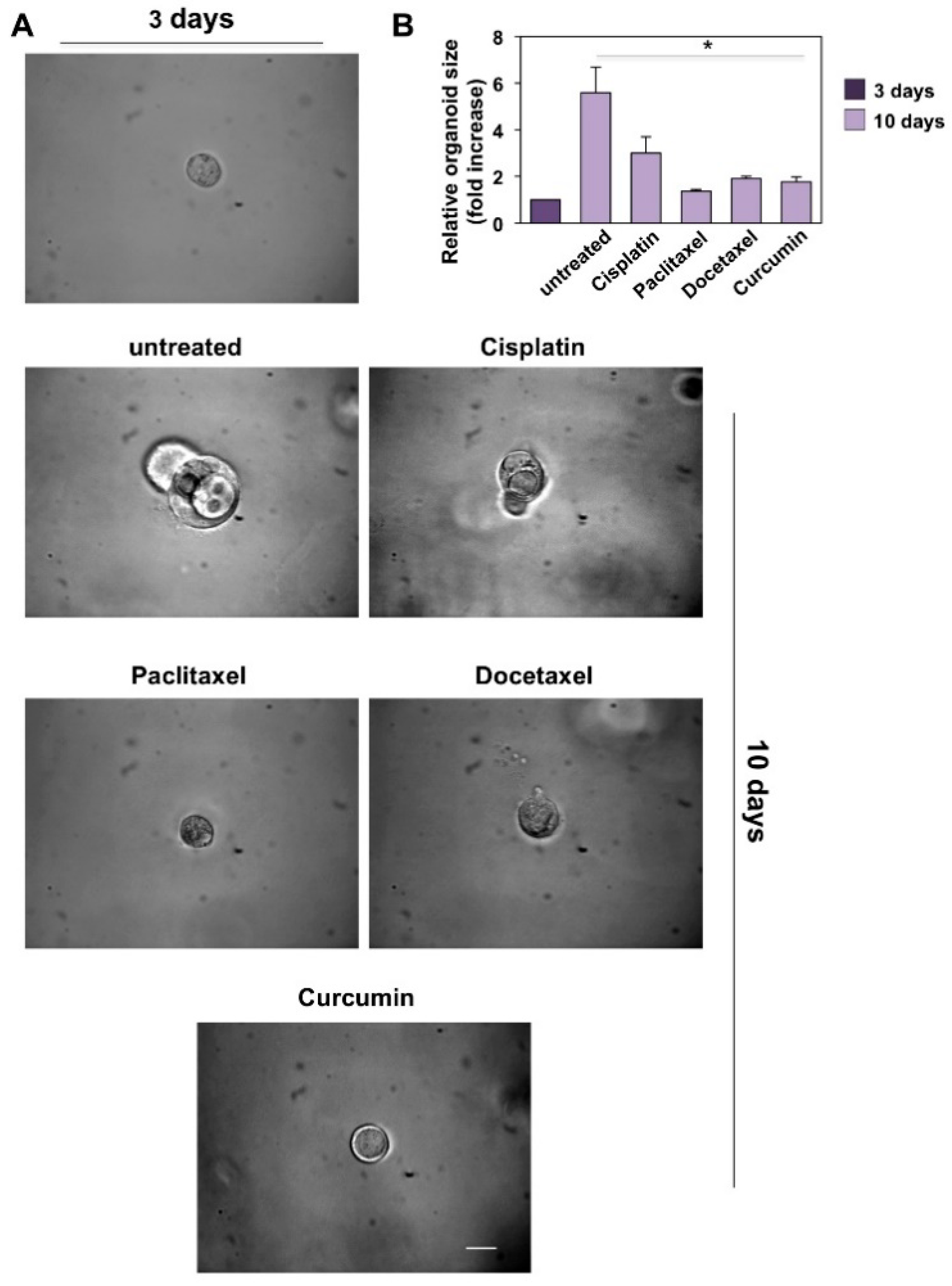
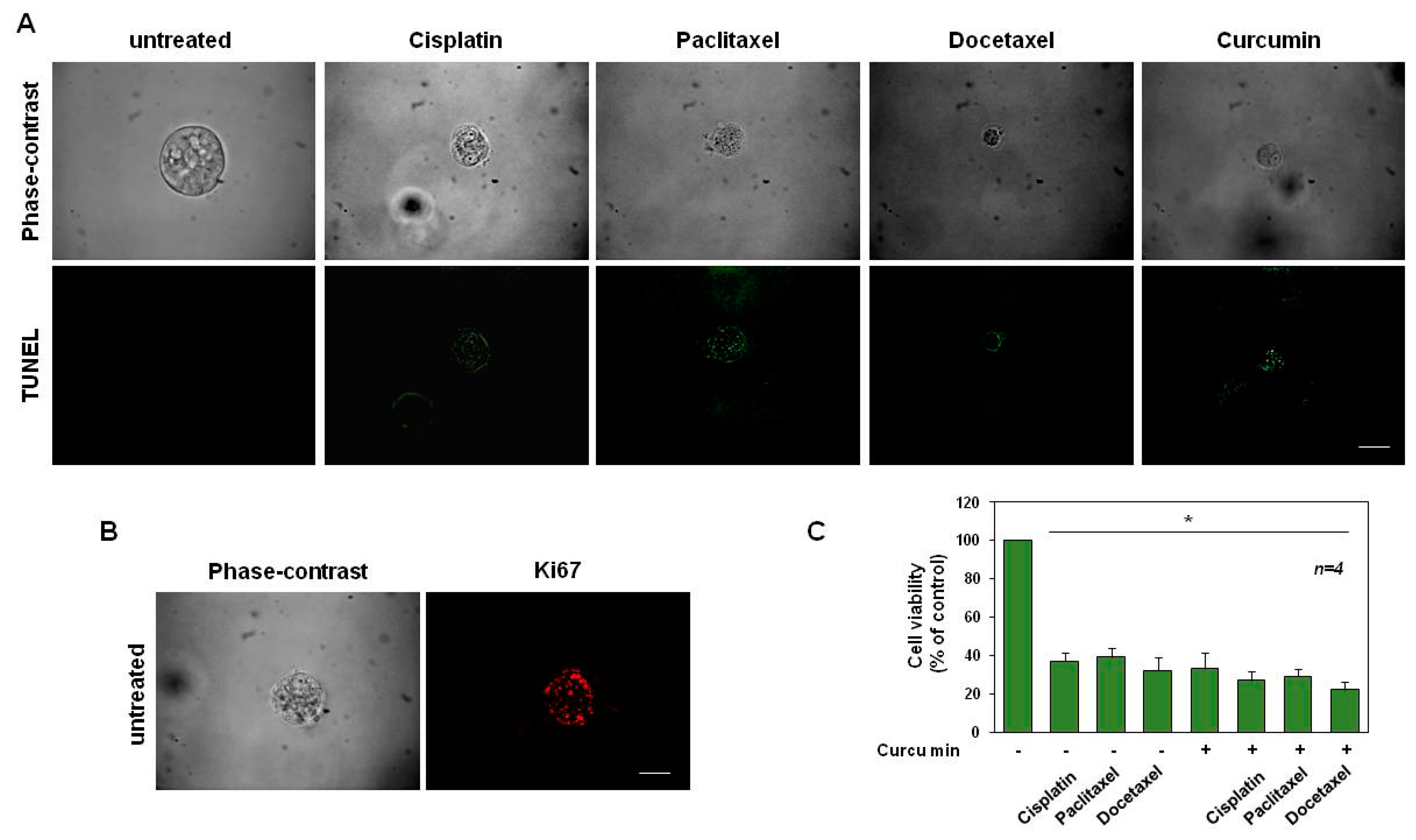
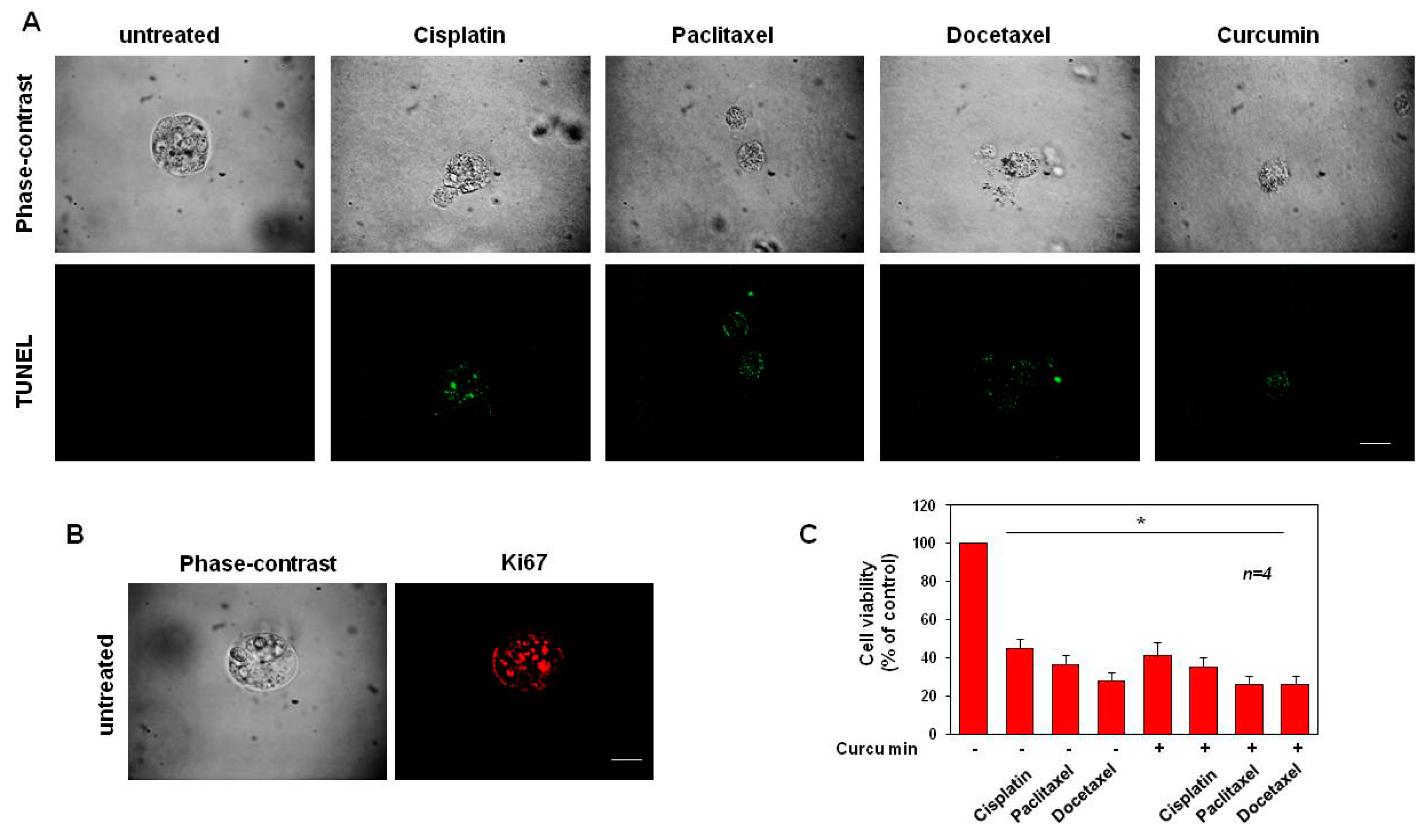
3.4. CRPC 3D Organoid Size Reduction
3.5. Cisplatin, Paclitaxel, Docetaxel and Curcumin Induce Organoid Disruption and Apoptosis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McCullough, A.R. Sexual dysfunction after radical prostatectomy. Rev. Urol. 2005, 7 (Suppl. 2), S3–S10. [Google Scholar] [PubMed]
- Grimaldi, A.; Santini, D.; Zappavigna, S.; Lombardi, A.; Misso, G.; Boccellino, M.; Desiderio, V.; Vitiello, P.P.; Di Lorenzo, G.; Zoccoli, A.; et al. Antagonistic effects of chloroquine on autophagy occurrence potentiate the anticancer effects of everolimus on renal cancer cells. Cancer Biol. Ther. 2015, 16, 567–579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spugnini, E.P.; Melillo, A.; Quagliuolo, L.; Boccellino, M.; Vincenzi, B.; Pasquali, P.; Baldi, A. Definition of novel electrochemotherapy parameters and validation of their in vitro and in vivo effectiveness. J. Cell. Physiol. 2014, 229, 1177–1181. [Google Scholar] [CrossRef] [PubMed]
- Varriale, E.; Ferrante, A.; Crocetto, F.; Cianciulli, M.; Palermo, E.; Vitale, A.; Benincasa, G. Assessment of glutathione-S-trasnferase (GSTP1) methylation status in a reliable molecular biomarker for early diagnosis of prostatic intraepithelial neoplasia. World Cancer Res. J. 2019, 6, e1384. [Google Scholar] [CrossRef]
- Crocetto, F.; Barone, B.; De Luca, L.; Creta, M. Granulomatous prostatitis: A challenging differential diagnosis to take into consideration. Future Oncol. 2020, 16, 805–806. [Google Scholar] [CrossRef]
- Rapisarda, S.; Bada, M.; Crocetto, F.; Barone, B.; Arcaniolo, D.; Polara, A.; Imbimbo, C.; Grosso, G. The role of multiparametric resonance and biopsy in prostate cancer detection: Comparison with definitive histological report after laparoscopic/robotic radical prostatectomy. Abdom. Radiol. 2020, 45, 4178–4184. [Google Scholar] [CrossRef]
- Di Zazzo, E.; Galasso, G.; Giovannelli, P.; Di Donato, M.; Di Santi, A.; Cernera, G.; Rossi, V.; Abbondanza, C.; Moncharmont, B.; Sinisi, A.A.; et al. Prostate cancer stem cells: The role of androgen and estrogen receptors. Oncotarget 2016, 7, 193–208. [Google Scholar] [CrossRef] [Green Version]
- Vanacore, D.; Boccellino, M.; Rossetti, S.; Cavaliere, C.; D’Aniello, C.; Di Franco, R.; Romano, F.J.; Montanari, M.; La Mantia, E.; Piscitelli, R.; et al. Micrornas in prostate cancer: An overview. Oncotarget 2017, 8, 50240–50251. [Google Scholar] [CrossRef] [Green Version]
- Castoria, G.; Lombardi, M.; Barone, M.V.; Bilancio, A.; Di Domenico, M.; De Falco, A.; Varricchio, L.; Bottero, D.; Nanayakkara, M.; Migliaccio, A.; et al. Rapid signalling pathway activation by androgens in epithelial and stromal cells. Steroids 2004, 69, 517–522. [Google Scholar] [CrossRef]
- Migliaccio, A.; Castoria, G.; Di Domenico, M.; de Falco, A.; Bilancio, A.; Lombardi, M.; Barone, M.V.; Ametrano, D.; Zannini, M.S.; Abbondanza, C.; et al. Steroid-induced androgen receptor-oestradiol receptor beta-Src complex triggers prostate cancer cell proliferation. EMBO J. 2000, 19, 5406–5417. [Google Scholar] [CrossRef] [Green Version]
- Di Zazzo, E.; Galasso, G.; Giovannelli, P.; Di Donato, M.; Castoria, G. Estrogens and their receptors in prostate cancer: Therapeutic implications. Front. Oncol. 2018, 8, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nardone, V.; Botta, C.; Caraglia, M.; Martino, E.C.; Ambrosio, M.R.; Carfagno, T.; Tini, P.; Semeraro, L.; Misso, G.; Grimaldi, A.; et al. Tumor infiltrating T lymphocytes expressing FoxP3, CCR7 or PD-1 predict the outcome of prostate cancer patients subjected to salvage radiotherapy after biochemical relapse. Cancer Biol. Ther. 2016, 17, 1213–1220. [Google Scholar] [CrossRef] [PubMed]
- Buonerba, C.; Ferro, M.; Dolce, P.; Crocetto, F.; Verde, A.; Lucarelli, G.; Scafuri, L.; Facchini, S.; Vaia, A.; Marinelli, A.; et al. Predictors of efficacy of androgen-receptor-axis-targeted therapies in patients with metastatic castration-sensitive prostate cancer: A systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 2020, 151, 102992. [Google Scholar] [CrossRef] [PubMed]
- Ferro, M.; Lucarelli, G.; Crocetto, F.; Dolce, P.; Verde, A.; La Civita, E.; Zappavigna, S.; de Cobelli, O.; Di Lorenzo, G.; Facchini, B.A.; et al. First-line systemic therapy for metastatic castration-sensitive prostate cancer: An updated systematic review with novel findings. Crit. Rev. Oncol. Hematol. 2021, 157, 103198. [Google Scholar] [CrossRef]
- Migliaccio, A.; Pagano, M.; De Goeij, C.C.; Di Domenico, M.; Castoria, G.; Sluyser, M.; Auricchio, F. Phosphorylation and estradiol binding of estrogen receptor in hormone-dependent and hormone-independent GR mouse mammary tumors. Int. J. Cancer 1992, 51, 733–739. [Google Scholar] [CrossRef]
- Castoria, G.; Migliaccio, A.; Bilancio, A.; Di Domenico, M.; de Falco, A.; Lombardi, M.; Fiorentino, R.; Varricchio, L.; Barone, M.V.; Auricchio, F. PI3-kinase in concert with Src promotes the S-phase entry of oestradiol-stimulated MCF-7 cells. EMBO J. 2001, 20, 6050–6059. [Google Scholar] [CrossRef] [Green Version]
- Mulholland, D.J.; Tran, L.M.; Li, Y.; Cai, H.; Morim, A.; Wang, S.; Plaisier, S.; Garraway, I.P.; Huang, J.; Graeber, T.G.; et al. Cell autonomous role of PTEN in regulating castration-resistant prostate cancer growth. Cancer Cell 2011, 19, 792–804. [Google Scholar] [CrossRef] [Green Version]
- Feola, A.; Ricci, S.; Kouidhi, S.; Rizzo, A.; Penon, A.; Formisano, P.; Giordano, A.; Di Carlo, A.; Di Domenico, M. Multifaceted breast cancer: The molecular connection with obesity. J. Cell. Physiol. 2017, 232, 69–77. [Google Scholar] [CrossRef]
- Di Zazzo, E.; Feola, A.; Zuchegna, C.; Romano, A.; Donini, C.F.; Bartollino, S.; Costagliola, C.; Frunzio, R.; Laccetti, P.; Di Domenico, M.; et al. The p85 regulatory subunit of PI3K mediates cAMP-PKA and insulin biological effects on MCF-7 cell growth and motility. Sci. World J. 2014, 2014, 565839. [Google Scholar] [CrossRef] [Green Version]
- Donini, C.F.; Di Zazzo, E.; Zuchegna, C.; Di Domenico, M.; D’Inzeo, S.; Nicolussi, A.; Avvedimento, E.V.; Coppa, A.; Porcellini, A. The p85α regulatory subunit of PI3K mediates cAMP-PKA and retinoic acid biological effects on MCF7 cell growth and migration. Int. J. Oncol. 2012, 40, 1627–1635. [Google Scholar] [CrossRef]
- Cosentino, C.; Di Domenico, M.; Porcellini, A.; Cuozzo, C.; De Gregorio, G.; Santillo, M.R.; Agnese, S.; Di Stasio, R.; Feliciello, A.; Migliaccio, A.; et al. p85 regulatory subunit of PI3K mediates cAMP-PKA and estrogens biological effects on growth and survival. Oncogene 2007, 26, 2095–2103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ballaré, C.; Uhrig, M.; Bechtold, T.; Sancho, E.; Di Domenico, M.; Migliaccio, A.; Auricchio, F.; Beato, M. Two domains of the progesterone receptor interact with the estrogen receptor and are required for progesterone activation of the c-Src/Erk pathway in mammalian cells. Mol. Cell. Biol. 2003, 23, 1994–2008. [Google Scholar] [CrossRef] [Green Version]
- Buommino, E.; Boccellino, M.; De Filippis, A.; Petrazzuolo, M.; Cozza, V.; Nicoletti, R.; Ciavatta, M.L.; Quagliuolo, L.; Tufano, M.A. 3-O-methylfunicone produced by penicillium pinophilum affects cell motility of breast cancer cells, downregulating alphavbeta5 integrin and inhibiting metalloproteinase-9 secretion. Mol. Carcinog. 2007, 46, 930–940. [Google Scholar] [CrossRef]
- Boccellino, M.; Camussi, G.; Giovane, A.; Ferro, L.; Calderaro, V.; Balestrieri, C.; Quagliuolo, L. Platelet-activating factor regulates cadherin-catenin adhesion system expression and beta-catenin phosphorylation during Kaposi’s sarcoma cell motility. Am. J. Pathol. 2005, 166, 1515–1522. [Google Scholar] [CrossRef]
- Fiorelli, A.; Vitiello, F.; Morgillo, F.; Santagata, M.; Spuntarelli, C.; Di Domenico, M.; Santini, M.; Bianco, A. Pembrolizumab monotherapy in advanced NSCLC patients with low PD-L1 expression: Is there real evidence? Transl. Cancer Res. 2019, 8, S618–S620. [Google Scholar] [CrossRef] [PubMed]
- Baldi, A.; Piccolo, M.T.; Boccellino, M.R.; Donizetti, A.; Cardillo, I.; La Porta, R.; Quagliuolo, L.; Spugnini, E.P.; Cordero, F.; Citro, G.; et al. Apoptosis induced by piroxicam plus cisplatin combined treatment is triggered by p21 in mesothelioma. PLoS ONE 2011, 6, e23569. [Google Scholar] [CrossRef] [Green Version]
- Castoria, G.; Migliaccio, A.; D’Amato, L.; Di Stasio, R.; Ciociola, A.; Lombardi, M.; Bilancio, A.; Di Domenico, M.; de Falco, A.; Auricchio, F. Integrating signals between cAMP and MAPK pathways in breast cancer. Front. Biosci. 2008, 13, 1318–1327. [Google Scholar] [CrossRef] [Green Version]
- Shin, D.H.; Choi, Y.J.; Park, J.W. SIRT1 and AMPK mediate hypoxia-induced resistance of non-small cell lung cancers to cisplatin and doxorubicin. Cancer Res. 2014, 74, 298–308. [Google Scholar] [CrossRef] [Green Version]
- Franco, R.; Nicoletti, G.; Lombardi, A.; Di Domenico, M.; Botti, G.; Zito Marino, F.; Caraglia, M. Current treatment of cutaneous squamous cancer and molecular strategies for its sensitization to new target-based drugs. Expert Opin. Biol. Ther. 2013, 13, 51–66. [Google Scholar] [CrossRef]
- Basourakos, S.P.; Li, L.; Aparicio, A.M.; Corn, P.G.; Kim, J.; Thompson, T.C. Combination platinum-based and DNA damage response-targeting cancer therapy: Evolution and future directions. Curr. Med. Chem. 2017, 24, 1586–1606. [Google Scholar] [CrossRef]
- Chien, J.; Ota, T.; Aletti, G.; Shridhar, R.; Boccellino, M.; Quagliuolo, L.; Baldi, A.; Shridhar, V. Serine protease HtrA1 associates with microtubules and inhibits cell migration. Mol. Cell. Biol. 2009, 29, 4177–4187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Domenico, M.; Ricciardi, C.; Fusco, A.; Pierantoni, G.M. Anti-VEGF therapy in breast and lung mouse models of cancers. J. Biomed. Biotechnol. 2011, 2011, 947928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Bono, J.S.; Oudard, S.; Ozguroglu, M.; Hansen, S.; Machiels, J.P.; Kocak, I.; Gravis, G.; Bodrogi, I.; Mackenzie, M.J.; Shen, L.; et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: A randomised open-label trial. Lancet 2010, 376, 1147–1154. [Google Scholar] [CrossRef]
- Alaia, C.; Boccellino, M.; Zappavigna, S.; Amler, E.; Quagliuolo, L.; Rossetti, S.; Facchini, G.; Caraglia, M. Ipilimumab for the treatment of metastatic prostate cancer. Expert Opin. Biol. Ther. 2018, 18, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Cardillo, I.; Spugnini, E.P.; Galluzzo, P.; Contestabile, M.; Dell’Anna, M.L.; Picardo, M.; Crispi, S.; Calogero, R.A.; Piccolo, M.T.; Arigoni, M.; et al. Functional and pharmacodynamic evaluation of metronomic cyclophosphamide and docetaxel regimen in castration-resistant prostate cancer. Future Oncol. 2013, 9, 1375–1388. [Google Scholar] [CrossRef]
- Beer, T.M.; Armstrong, A.J.; Rathkopf, D.E.; Loriot, Y.; Sternberg, C.N.; Higano, C.S.; Iversen, P.; Bhattacharya, S.; Carles, J.; Chowdhury, S.; et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N. Engl. J. Med. 2014, 371, 424–433. [Google Scholar] [CrossRef] [Green Version]
- Loriot, Y.; Fizazi, K.; De Bono, J.S.; Forer, D.; Hirmand, M.; Scher, H.I. Outcomes in patients with liver or lung metastatic castration-resistant prostate cancer (mCRPC) treated with the androgen receptor inhibitor enzalutamide: Results from the phase III AFFIRM trial. J. Clin. Oncol. 2013, 31, 5065. [Google Scholar] [CrossRef]
- Li, K.; Zhan, W.; Chen, Y.; Jha, R.K.; Chen, X. Docetaxel and doxorubicin codelivery by nanocarriers for synergistic treatment of prostate cancer. Front. Pharmacol. 2019, 10, 1436. [Google Scholar] [CrossRef] [Green Version]
- Di Domenico, M.; Pozzi, D.; Palchetti, S.; Digiacomo, L.; Iorio, R.; Astarita, C.; Fiorelli, A.; Pierdiluca, M.; Santini, M.; Barbarino, M.; et al. Nanoparticle-biomolecular corona: A new approach for the early detection of non-small-cell lung cancer. J. Clin. Oncol. 2019, 234, 9378–9386. [Google Scholar] [CrossRef]
- Papi, M.; Palmieri, V.; Palchetti, S.; Pozzi, D.; Digiacomo, L.; Guadagno, E.; Del Basso De Caro, M.; Di Domenico, M.; Ricci, S.; Pani, R.; et al. Exploitation of nanoparticle-protein interactions for early disease detection. Appl. Phys. Lett. 2019, 114, 163702. [Google Scholar] [CrossRef] [Green Version]
- Borghese, C.; Casagrande, N.; Pivetta, E.; Colombatti, A.; Boccellino, M.; Amler, E.; Normanno, N.; Caraglia, M.; De Rosa, G.; Aldinucci, D. Self-assembling nanoparticles encapsulating zoledronic acid inhibit mesenchymal stromal cells differentiation, migration and secretion of proangiogenic factors and their interactions with prostate cancer cells. Oncotarget 2017, 8, 42926–42938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boccellino, M.; Pedata, P.; Castiglia, L.; La Porta, R.; Pieri, M.; Quagliuolo, L.; Acampora, A.; Sannolo, N.; Miraglia, N. Doxorubicin can penetrate nitrile gloves and induces apoptosis in keratinocytes cell lines. Toxicol. Lett. 2010, 197, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Boccellino, M.; Alaia, C.; Misso, G.; Cossu, A.M.; Facchini, G.; Piscitelli, R.; Quagliuolo, L.; Caraglia, M. Gene interference strategies as a new tool for the treatment of prostate cancer. Endocrine 2015, 49, 588–605. [Google Scholar] [CrossRef] [PubMed]
- De Bono, J.S.; Logothetis, C.J.; Molina, A.; Fizazi, K.; North, S.; Chu, L.; Chi, K.N.; Jones, R.J.; Goodman, O.B., Jr.; Saad, F.; et al. Abiraterone and increased survival in metastatic prostate cancer. N. Engl. J. Med. 2011, 364, 1995–2005. [Google Scholar] [CrossRef]
- Crocetto, F.; Boccellino, M.; Barone, B.; Di Zazzo, E.; Sciarra, A.; Galasso, G.; Settembre, G.; Quagliuolo, L.; Imbimbo, C.; Boffo, S.; et al. The crosstalk between prostate cancer and microbiota inflammation: Nutraceutical products are useful to balance this interplay? Nutrients 2020, 12, 2648. [Google Scholar] [CrossRef]
- Capece, M.; Creta, M.; Calogero, A.; La Rocca, R.; Napolitano, L.; Barone, B.; Sica, A.; Fusco, F.; Santangelo, M.; Dodaro, C.; et al. Does physical activity regulate prostate carcinogenesis and prostate cancer outcomes? A narrative review. Int. J. Environ. Res. Public Health 2020, 17, 1441. [Google Scholar] [CrossRef] [Green Version]
- Moyad, M.A.; Newton, R.U.; Tunn, U.W.; Gruca, D. Integrating diet and exercise into care of prostate cancer patients on androgen deprivation therapy. Res. Rep. Urol. 2016, 8, 133–143. [Google Scholar] [CrossRef] [Green Version]
- Di Domenico, M.; Pinto, F.; Quagliuolo, L.; Contaldo, M.; Settembre, G.; Romano, A.; Coppola, M.; Ferati, K.; Bexheti-Ferati, A.; Sciarra, A.; et al. The role of oxidative stress and hormones in controlling obesity. Front. Endocrinol. 2019, 10, 540. [Google Scholar] [CrossRef] [Green Version]
- Giudice, A.; Montella, M.; Boccellino, M.; Crispo, A.; D’Arena, G.; Bimonte, S.; Facchini, G.; Ciliberto, G.; Botti, G.; Quagliuolo, L.; et al. Epigenetic changes induced by green tea catechins are associated with prostate cancer. Curr. Mol. Med. 2017, 17, 405–420. [Google Scholar] [CrossRef]
- Schmidt, K.T.; Figg, W.D. The potential role of curcumin in prostate cancer: The importance of optimizing pharmacokinetics in clinical studies. Transl. Cancer Res. 2016, 5, S1107–S1110. [Google Scholar] [CrossRef]
- Saberi-Karimian, M.; Katsiki, N.; Caraglia, M.; Boccellino, M.; Majeed, M.; Sahebkar, A. Vascular endothelial growth factor: An important molecular target of curcumin. Crit. Rev. Food. Sci. Nutr. 2019, 59, 299–312. [Google Scholar] [CrossRef] [PubMed]
- Jordan, B.C.; Mock, C.D.; Thilagavathi, R.; Selvam, C. Molecular mechanisms of curcumin and its semisynthetic analogues in prostate cancer prevention and treatment. Life Sci. 2016, 152, 135–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, Y.; Long, Q.; Zhang, L.; Shi, Y.; Liu, X.; Li, X.; Guan, B.; Tian, Y.; Wang, X.; Li, L.; et al. Curcumin inhibits cancer-associated fibroblast-driven prostate cancer invasion through MAOA/mTOR/HIF-1α signaling. Int. J. Oncol. 2015, 47, 2064–2072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, A.L.; Hsu, C.H.; Lin, J.K.; Hsu, M.M.; Ho, Y.F.; Shen, T.S.; Ko, J.Y.; Lin, J.T.; Lin, B.R.; Ming-Shiang, W.; et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001, 21, 2895–2900. [Google Scholar] [PubMed]
- Chua, C.W.; Shibata, M.; Lei, M.; Toivanen, R.; Barlow, L.J.; Bergren, S.K.; Badani, K.K.; McKiernan, J.M.; Benson, M.C.; Hibshoosh, H.; et al. Single luminal epithelial progenitors can generate prostate organoids in culture. Nat. Cell Biol. 2014, 16, 951–961. [Google Scholar] [CrossRef] [Green Version]
- Di Donato, M.; Cernera, G.; Migliaccio, A.; Castoria, G. Nerve growth factor induces proliferation and aggressiveness in prostate cancer cells. Cancers 2019, 11, 784. [Google Scholar] [CrossRef] [Green Version]
- Boccellino, M.; Di Domenico, M.; Donniacuo, M.; Bitti, G.; Gritti, G.; Ambrosio, P.; Quagliuolo, L.; Rinaldi, B. AT1-receptor blockade: Protective effects of irbesartan in cardiomyocytes under hypoxic stress. PLoS ONE 2018, 13, e0202297. [Google Scholar] [CrossRef] [Green Version]
- Boccellino, M.; Quagliuolo, L.; Verde, A.; La Porta, R.; Crispi, S.; Piccolo, M.T.; Vitiello, A.; Baldi, A.; Signorile, P.G. In vitro model of stromal and epithelial immortalized endometriotic cells. J. Cell. Biochem. 2012, 113, 1292–1301. [Google Scholar] [CrossRef]
- Pieri, M.; Quagliuolo, L.; La Porta, R.; Silvestre, A.; Miraglia, N.; Pedata, P.; Acampora, A.; Castiglia, L.; Sannolo, N.; Boccellino, M. Epirubicin permeation of personal protective equipment can induce apoptosis in keratinocytes. J. Expo. Sci. Environ. Epidemiol. 2013, 23, 428–434. [Google Scholar] [CrossRef] [Green Version]
- Spugnini, E.P.; Cardillo, I.; Fanciulli, M.; Crispi, S.; Vincenzi, B.; Boccellino, M.; Quagliuolo, L.; Baldi, A. Electroporation as a strategy to promote HtrA1 gene uptake and chemotherapy efficacy in a mouse model of mesothelioma. Front. Biosci. 2013, 5, 974–981. [Google Scholar] [CrossRef]
- Di Donato, M.; Zamagni, A.; Galasso, G.; Di Zazzo, E.; Giovannelli, P.; Barone, M.V.; Zanoni, M.; Gunelli, R.; Costantini, M.; Auricchio, F.; et al. The androgen receptor/filamin A complex as a target in prostate cancer microenvironment. Cell Death. Dis. 2021, 12, 127. [Google Scholar] [CrossRef] [PubMed]
- Grant, S.; Qiao, L.; Dent, P. Roles of ERBB family receptor tyrosine kinases, and downstream signaling pathways, in the control of cell growth and survival. Front. Biosci. 2002, 7, d376–d389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grandal, M.V.; Madshus, I.H. Epidermal growth factor receptor and cancer: Control of oncogenic signalling by endocytosis. J. Cell. Mol. Med. 2008, 12, 1527–1534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Härmä, V.; Virtanen, J.; Mäkelä, R.; Happonen, A.; Mpindi, J.P.; Knuuttila, M.; Kohonen, P.; Lötjönen, J.; Kallioniemi, O.; Nees, M. A comprehensive panel of three-dimensional models for studies of prostate cancer growth, invasion and drug responses. PLoS ONE 2010, 5, e10431. [Google Scholar] [CrossRef]
- Grabinger, T.; Luks, L.; Kostadinova, F.; Zimberlin, C.; Medema, J.P.; Leist, M.; Brunner, T. Ex vivo culture of intestinal crypt organoids as a model system for assessing cell death induction in intestinal epithelial cells and enteropathy. Cell Death Dis. 2014, 5, e1228. [Google Scholar] [CrossRef] [Green Version]
- Di Domenico, M.; Giordano, A. Signal transduction growth factors: The effective governance of transcription and cellular adhesion in cancer invasion. Oncotarget 2017, 8, 36869–36884. [Google Scholar] [CrossRef] [Green Version]
- Castoria, G.; Lombardi, M.; Barone, M.V.; Bilancio, A.; Di Domenico, M.; Bottero, D.; Vitale, F.; Migliaccio, A.; Auricchio, F. Androgen-stimulated DNA synthesis and cytoskeletal changes in fibroblasts by a nontranscriptional receptor action. J. Cell Biol. 2003, 161, 547–556. [Google Scholar] [CrossRef]
- Feldman, B.J.; Feldman, D. The development of androgen-independent prostate cancer. Nat. Rev. Cancer 2001, 1, 34–45. [Google Scholar] [CrossRef]
- Teiten, M.H.; Gaascht, F.; Eifes, S.; Dicato, M.; Diederich, M. Chemopreventive potential of curcumin in prostate cancer. Genes Nutr. 2010, 5, 61–74. [Google Scholar] [CrossRef] [Green Version]
- Zhou, D.Y.; Zhao, S.Q.; Du, Z.Y.; Zheng, X.I.; Zhang, K. Pyridine analogues of curcumin exhibit high activity for inhibiting CWR-22Rv1 human prostate cancer cell growth and androgen receptor activation. Oncol. Lett. 2016, 11, 4160–4166. [Google Scholar] [CrossRef] [Green Version]
- Hong, J.H.; Lee, G.; Choi, H.Y. Effect of curcumin on the interaction between androgen receptor and Wnt/β-catenin in LNCaP xenografts. Korean J. Urol. 2015, 56, 656–665. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| PC-3 | DU145 | |
|---|---|---|
| Drug | Concentration | |
| DOCETAXEL | 0.3–50 µg/mL 0.37–61.9 µM | 1.5–200 ng/mL 1.86–247.56 nM |
| PACLITAXEL | 3.25–500 µg/mL 3.81–585.55 µM | 1.5–200 ng/mL 1.76–234.22 nM |
| CISPLATIN | 0.3–50 µg/mL 0.996–166.06 nM | 1.5–200 ng/mL 4.99–664.23 nM |
| CURCUMIN | 1.72–55.2 µg/mL 4.67–149.85 µM | 1.72–55.2 µg/mL 4.67–149.85 µM |
| PC-3 | DU145 | |||
|---|---|---|---|---|
| IC50 * | 48 h | 72 h | 48 h | 72 h |
| CISPLATIN | 2.35 µg/mL 7.80 µM | 1.32 µg/mL 4.38 µM | 3.26 ng/mL 10.83 µM | 2.17 ng/mL 7.21 µM |
| PACLITAXEL | 13.1 µg/mL 15.34 µM | 10.07 µg/mL 11.79 µM | N.D | 4.42 ng/mL 5.18 nM |
| DOCETAXEL | 9.14 µg/mL 11.3 µM | 6.5 µg/mL 8.05 µM | 12.5 ng/mL 15.47 nM | 3.76 ng/mL 4.65 nM |
| CURCUMIN | 8.64 µg/mL 23.45 µM | 6.52 µg/mL 17.70 µM | 16.5 µg/mL 44.79 µM | 8.62 µg/mL 23.40 µM |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boccellino, M.; Ambrosio, P.; Ballini, A.; De Vito, D.; Scacco, S.; Cantore, S.; Feola, A.; Di Donato, M.; Quagliuolo, L.; Sciarra, A.; et al. The Role of Curcumin in Prostate Cancer Cells and Derived Spheroids. Cancers 2022, 14, 3348. https://doi.org/10.3390/cancers14143348
Boccellino M, Ambrosio P, Ballini A, De Vito D, Scacco S, Cantore S, Feola A, Di Donato M, Quagliuolo L, Sciarra A, et al. The Role of Curcumin in Prostate Cancer Cells and Derived Spheroids. Cancers. 2022; 14(14):3348. https://doi.org/10.3390/cancers14143348
Chicago/Turabian StyleBoccellino, Mariarosaria, Pasqualina Ambrosio, Andrea Ballini, Danila De Vito, Salvatore Scacco, Stefania Cantore, Antonia Feola, Marzia Di Donato, Lucio Quagliuolo, Antonella Sciarra, and et al. 2022. "The Role of Curcumin in Prostate Cancer Cells and Derived Spheroids" Cancers 14, no. 14: 3348. https://doi.org/10.3390/cancers14143348
APA StyleBoccellino, M., Ambrosio, P., Ballini, A., De Vito, D., Scacco, S., Cantore, S., Feola, A., Di Donato, M., Quagliuolo, L., Sciarra, A., Galasso, G., Crocetto, F., Imbimbo, C., Boffo, S., Di Zazzo, E., & Di Domenico, M. (2022). The Role of Curcumin in Prostate Cancer Cells and Derived Spheroids. Cancers, 14(14), 3348. https://doi.org/10.3390/cancers14143348