Urine CA125 and HE4 for the Triage of Symptomatic Women with Suspected Endometrial Cancer
Abstract
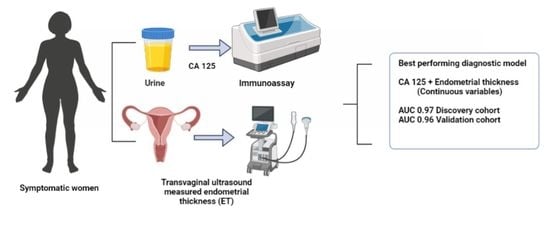
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Ethics, Approvals and Patient Involvement
2.2. Study Population
2.3. Bio Fluid Sample Collection and Processing
2.4. Statistical Analyses
3. Results
3.1. Clinical Characteristics of the Discovery Cohort Study Participants
3.2. Urine CA125 Levels in Discovery Cohort Samples
3.3. Urine HE4 Levels in Discovery Cohort Samples
3.4. Diagnostic Models for Endometrial Cancer Detection
3.5. Serum CA125 and HE4 for Endometrial Cancer Detection
3.6. Validation of Diagnostic Performance of Urine CA125 and HE4
3.6.1. Demographics of the Validation Cohort
3.6.2. Biomarker Performance in the Validation Cohort
3.6.3. Potential Clinical Utility of Urine CA125 in the Diagnostic Setting
4. Discussion
4.1. Main Findings
4.2. Strengths and Limitations
4.3. Interpretation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Crosbie, E.; Morrison, J. The emerging epidemic of endometrial cancer: Time to take action. Cochrane Database Syst. Rev. 2014, 12, ED000095. [Google Scholar] [CrossRef]
- CRUK. Uterine Cancer Incidence Statistics. 2020. Available online: www.cancerresearchuk.org (accessed on 1 June 2022).
- Njoku, K.; Ramchander, N.C.; Wan, Y.L.; Barr, C.E.; Crosbie, E.J. Pre-treatment inflammatory parameters predict survival from endometrial cancer: A prospective database analysis. Gynecol. Oncol. 2021, 164, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Jones, E.R.; O’Flynn, H.; Njoku, K.; Crosbie, E.J. Detecting endometrial cancer. Obstet. Gynaecol. 2021, 23, 103–112. [Google Scholar] [CrossRef]
- Crosbie, E.J.; Kitson, S.J.; McAlpine, J.N.; Mukhopadhyay, A.; Powell, M.E.; Singh, N. Endometrial cancer. Lancet 2022, 399, 1412–1428. [Google Scholar] [CrossRef] [PubMed]
- Funston, G.; O’Flynn, H.; Ryan, N.A.J.; Hamilton, W.; Crosbie, E.J. Recognizing gynecological cancer in primary care: Risk factors, red flags, and referrals. Adv. Ther. 2018, 35, 577–589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Njoku, K.; Chiasserini, D.; Jones, E.R.; Barr, C.E.; O’Flynn, H.; Whetton, A.D.; Crosbie, E.J. Urinary biomarkers and their potential for the non-invasive detection of endometrial cancer. Front. Oncol. 2020, 10, 559016. [Google Scholar] [CrossRef]
- Badrick, E.; Cresswell, K.; Ellis, P.; Detecting Cancer Early Priority Setting Partnership steering group; Renehan, A.G.; Crosbie, E.J. Top ten research priorities for detecting cancer early. Lancet Public Health 2019, 4, e551. [Google Scholar] [CrossRef] [Green Version]
- Paraskevaidi, M.; Morais, C.L.M.; Ashton, K.M.; Stringfellow, H.F.; McVey, R.J.; Ryan, N.A.J.; O’Flynn, H.; Sivalingam, V.N.; Kitson, S.J.; Mackintosh, M.L.; et al. Detecting Endometrial Cancer by Blood Spectroscopy: A Diagnostic Cross-Sectional Study. Cancers 2020, 12, 1256. [Google Scholar] [CrossRef]
- Behrouzi, R.; Barr, C.E.; Crosbie, E.J. HE4 as a Biomarker for Endometrial Cancer. Cancers 2021, 13, 4764. [Google Scholar] [CrossRef] [PubMed]
- Moss, E.L.; Hollingworth, J.; Reynolds, T.M. The role of CA125 in clinical practice. J. Clin. Pathol. 2005, 58, 308–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dochez, V.; Caillon, H.; Vaucel, E.; Dimet, J.; Winer, N.; Ducarme, G. Biomarkers and algorithms for diagnosis of ovarian cancer: CA125, HE4, RMI and ROMA, a review. J. Ovarian Res. 2019, 12, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charkhchi, P.; Cybulski, C.; Gronwald, J.; Wong, F.O.; Narod, S.A.; Akbari, M.R. CA125 and Ovarian Cancer: A Comprehensive Review. Cancers 2020, 12, 3730. [Google Scholar] [CrossRef] [PubMed]
- Aithal, A.; Rauth, S.; Kshirsagar, P.; Shah, A.; Lakshmanan, I.; Junker, W.; Jain, M.; Ponnusamy, M.P.; Batra, S.K. MUC16 as a novel target for cancer therapy. Expert Opin. Ther. Targets 2018, 22, 675–686. [Google Scholar] [CrossRef]
- Kanat-Pektas, M.; Yenicesu, O.; Gungor, T.; Bilge, Ü. Predictive power of sexual hormones and tumor markers in endometrial cancer. Arch. Gynecol. Obstet. 2010, 281, 709–715. [Google Scholar] [CrossRef]
- Kirchhoff, C.; Habben, I.; Ivell, R.; Krull, N. A major human epididymis-specific cdna encodes a protein with sequence homology to extracellular proteinase inhibitors1. Biol. Reprod. 1991, 45, 350–357. [Google Scholar] [CrossRef]
- Galgano, M.T.; Hampton, G.M.; Frierson, H.F., Jr. Comprehensive analysis of HE4 expression in normal and malignant human tissues. Mod. Pathol. 2006, 19, 847–853. [Google Scholar] [CrossRef] [Green Version]
- Zamani, N.; Gilani, M.M.; Mirmohammadkhani, M.; Sheikhhasani, S.; Mousavi, A.; Sharami, S.R.Y.; Akhavan, S.; Zamani, M.H.; Saffarieh, E. The utility of CA125 and HE4 in patients suffering from endometrial cancer. Int. J. Women’s Health Reprod. Sci. 2020, 8, 95–100. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Qu, W.; Wang, J.; Jiang, S.-W. Comparison of serum human epididymis protein 4 and CA125 on endometrial cancer detection: A meta-analysis. Clin. Chim. Acta 2019, 488, 215–220. [Google Scholar] [CrossRef]
- Knific, T.; Osredkar, J.; Smrkolj, Š.; Tonin, I.; Vouk, K.; Blejec, A.; Grazio, S.F.; Rižner, T.L. Novel algorithm including CA-125, HE4 and body mass index in the diagnosis of endometrial cancer. Gynecol. Oncol. 2017, 147, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.L.; Beverley-Stevenson, R.; Carlisle, D.; Clarke, S.; Edmondson, R.J.; Glover, S.; Holland, J.; Hughes, C.; Kitchener, H.C.; Kitson, S.; et al. Working together to shape the endometrial cancer research agenda: The top ten unanswered research questions. Gynecol. Oncol. 2016, 143, 287–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pepe, M.S.; Janes, H.; Li, C.I.; Bossuyt, P.M.; Feng, Z.; Hilden, J. Early-phase studies of biomarkers: What target sensitivity and specificity values might confer clinical utility? Clin. Chem. 2016, 62, 737–742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barr, C.E.; Funston, G.; Mounce, L.T.; Pemberton, P.W.; Howe, J.D.; Crosbie, E.J. Comparison of two immunoassays for the measurement of serum HE4 for ovarian cancer. Pract. Lab. Med. 2021, 26, e00235. [Google Scholar] [CrossRef] [PubMed]
- Njoku, K.; Chiasserini, D.; Whetton, A.D.; Crosbie, E.J. Proteomic Biomarkers for the Detection of Endometrial Cancer. Cancers 2019, 11, 1572. [Google Scholar] [CrossRef] [Green Version]
- Lycke, M.; Ulfenborg, B.; Lauesgaard, J.M.; Kristjansdottir, B.; Sundfeldt, K. Consideration should be given to smoking, endometriosis, renal function (eGFR) and age when interpreting CA125 and HE4 in ovarian tumor diagnostics. Clin. Chem. Lab. Med. 2021, 59, 1954–1962. [Google Scholar] [CrossRef]
- Ferraro, S.; Borille, S.; Carnevale, A.; Frusciante, E.; Bassani, N.; Panteghini, M. Verification of the harmonization of human epididymis protein 4 assays. Clin. Chem. Lab. Med. 2016, 54, 1635–1643. [Google Scholar] [CrossRef]
- Hellstrom, I.; Yip, Y.Y.; Darvas, M.; Swisher, E.; Hellstrom, K.E. Ovarian carcinomas express HE4 epitopes independently of each other. Cancer Treat. Res. Commun. 2019, 21, 100152. [Google Scholar] [CrossRef]
- Moore, R.G.; Brown, A.K.; Miller, M.C.; Skates, S.; Allard, W.J.; Verch, T.; Steinhoff, M.; Messerlian, G.; DiSilvestro, P.; Granai, C.; et al. The use of multiple novel tumor biomarkers for the detection of ovarian carcinoma in patients with a pelvic mass. Gynecol. Oncol. 2008, 108, 402–408. [Google Scholar] [CrossRef]
- Hellstrom, I.; Heagerty, P.J.; Swisher, E.M.; Liu, P.; Jaffar, J.; Agnew, K.; Hellstrom, K.E. Detection of the HE4 protein in urine as a biomarker for ovarian neoplasms. Cancer Lett. 2010, 296, 43–48. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Zhao, X.; Khimji, I.; Akbas, R.; Qiu, W.; Edwards, D.; Cramer, D.W.; Ye, B.; Demirci, U. Integration of cell phone imaging with microchip ELISA to detect ovarian cancer HE4 biomarker in urine at the point-of-care. Lab Chip 2011, 11, 3411–3418. [Google Scholar] [CrossRef] [PubMed]
- Macuks, R.; Baidekalna, I.; Donina, S. Urinary concentrations of human epidydimis secretory protein 4 (He4) in the diagnosis of ovarian cancer: A case-control study. Asian Pac. J. Cancer Prev. 2012, 13, 4695–4698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karakaya, B.K.; Baser, E.; Bildaci, B.; Cömert, E.; Bayraktar, N.; Dursun, P.; Kuscu, E.; Ayhan, A. Alternative tumor markers in the diagnosis of ovarian cancer. Ginekol. Polska 2016, 87, 565–769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandow, J.J.; Rainczuk, A.; Infusini, G.; Makanji, M.; Bilandzic, M.; Wilson, A.L.; Fairweather, N.; Stanton, P.; Garama, D.; Gough, D.; et al. Discovery and validation of novel protein biomarkers in ovarian cancer patient urine. Proteom. Clin. Appl. 2018, 12, e1700135. [Google Scholar] [CrossRef]
- Liao, J.B.; Yip, Y.Y.; Swisher, E.M.; Agnew, K.; Hellstrom, K.E.; Hellstrom, I. Detection of the HE4 protein in urine as a biomarker for ovarian neoplasms: Clinical correlates. Gynecol. Oncol. 2015, 137, 430–435. [Google Scholar] [CrossRef] [Green Version]
- Wang, A.; Jin, C.; Tian, X.; Wang, Y.; Li, H. Knockdown of HE4 suppresses aggressive cell growth and malignant progression of ovarian cancer by inhibiting the JAK/STAT3 pathway. Biol. Open 2019, 8, bio043570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, M.-M.; Deng, J.; Cheng, X.-L.; Yan, Z.; Li, Q.-C.; Xing, Y.-Y.; Fan, D.-M.; Tian, X.-Y. Diagnostic accuracy of urine HE4 in patients with ovarian cancer: A meta-analysis. Oncotarget 2017, 8, 9660–9671. [Google Scholar] [CrossRef] [Green Version]
- Long, B.; Clarke, M.A.; Morillo, A.D.M.; Wentzensen, N.; Bakkum-Gamez, J.N. Ultrasound detection of endometrial cancer in women with postmenopausal bleeding: Systematic review and meta-analysis. Gynecol. Oncol. 2020, 157, 624–633. [Google Scholar] [CrossRef]
- Njoku, K.; Barr, C.E.; Crosbie, E.J. Current and Emerging Prognostic Biomarkers in Endometrial Cancer. Front. Oncol. 2022, 12, 890908. [Google Scholar] [CrossRef]
- Ünsal, M.; Comert, G.K.; Karalok, A.; Basaran, D.; Turkmen, O.; Boyraz, G.; Tasci, T.; Koc, S.; Boran, N.; Tulunay, G.; et al. The preoperative serum CA125 can predict the lymph node metastasis in endometrioid-type endometrial cancer. Ginekol. Pol. 2018, 89, 599–606. [Google Scholar] [CrossRef]
- Hoon Chung, H.; Weon Kim, J.; Park, N.-H.; Song, Y.-S.; Kang, S.-B.; Lee, H.-P. Use of preoperative serum CA-125 levels for prediction of lymph node metastasis and prognosis in endometrial cancer. Acta Obstet. Gynecol. Scand. 2006, 85, 1501–1505. [Google Scholar] [CrossRef] [PubMed]
- Modarres-Gilani, M.; Vaezi, M.; Shariat, M.; Zamani, N.; Nourizadeh, R. The prognostic role of preoperative serum CA125 levels in patients with advanced endometrial carcinoma. Cancer Biomark. 2017, 20, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-L.; Huang, C.-Y.; Chien, T.-Y.; Huang, S.-H.; Wu, C.-J.; Ho, C.-M. Value of pre-operative serum CA125 level for prediction of prognosis in patients with endometrial cancer. Aust. N. Z. J. Obstet. Gynaecol. 2011, 51, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Behrouzi, R.; Ryan, N.A.J.; Barr, C.E.; Derbyshire, A.E.; Wan, Y.L.; Maskell, Z.; Stocking, K.; Pemberton, P.W.; Bolton, J.; McVey, R.J.; et al. Baseline serum HE4 but not tissue HE4 expression predicts response to the levonorgestrel-releasing intrauterine system in atypical hyperplasia and early stage endometrial cancer. Cancers 2020, 12, 276. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Gao, Y.; Tan, M.; Zhuang, H.; Gao, J.; Hu, Z.; Wang, H.; Zhu, L.; Liu, J.; Lin, B. Expression of HE4 in endometrial cancer and its clinical significance. BioMed Res. Int. 2015, 2015, 437468. [Google Scholar] [CrossRef] [Green Version]






| CA125 (IU/L) | HE4 (nm/L) | ||||
|---|---|---|---|---|---|
| Clinico-Pathological Characteristics | N (%) | Median (IQR) | p-Value | Median (IQR) | p-Value |
| Age | |||||
| <65 years | 44 (72.1%) | 3.2 (1.3–7.5) | <0.001 | 14.2 (7.8, 22.3) | 0.1203 |
| ≥65 years | 17 (27.9%) | 18.7 (13–51.6) | 19.3 (14.1, 21.6) | ||
| BMI (kg/m2) | |||||
| BMI < 30 | 35 (57.4%) | 3.5 (1.5–9.9) | 0.021 | 17.5 (8.0, 22.4) | 0.731 |
| BMI ≥30.0 | 26 (42.6%) | 16.8 (2.7–50.2) | 14.3 (9.2, 22.2) | ||
| Disease status | |||||
| Cancer | 30 (49.2%) | 18.7 (7.5, 77.8) | <0.001 | 19.3 (14.1, 26.3) | 0.011 |
| Control | 31 (50.8%) | 1.9 (0.9, 4.0) | 12.4 (2.6, 22.2) | ||
| Histology | |||||
| Type I EC | 20 (66.7%) | 18.7 (12.3, 72.4) | 0.611 | 19.3 (13.2, 23.3) | 0.610 |
| Type II EC | 10 (33.3%) | 11.9 (1.7, 271.9) | 20.0 (17.5, 27.5) | ||
| Stage | |||||
| FIGO I/II | 26 (86.7%) | 18.7 (9.9, 77.8) | 0.848 | 19.3 (12.4, 20.8) | 0.105 |
| FIGO III/IV | 4 (13.3%) | 29.0 (4.0, 161.7) | 24.6 (20.3, 51.7) | ||
| LVSI | |||||
| No | 17 (56.7%) | 18.7 (11.6, 77.8) | 0.812 | 20.3 (14.1, 28.0) | 0.703 |
| Yes | 13 (43.3%) | 18.7 (6.3, 51.6) | 19.1 (17.5, 21.6) | ||
| Myometrial invasion | |||||
| <50% | 17 (56.7%) | 18.7 (11.6, 77.8) | 0.628 | 19.3 (12.4, 20.8) | 0.502 |
| >50% | 13 (43.3%) | 18.7 (6.3, 51.6) | 19.3 (18.8, 27.5) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Njoku, K.; Barr, C.E.; Sutton, C.J.J.; Crosbie, E.J. Urine CA125 and HE4 for the Triage of Symptomatic Women with Suspected Endometrial Cancer. Cancers 2022, 14, 3306. https://doi.org/10.3390/cancers14143306
Njoku K, Barr CE, Sutton CJJ, Crosbie EJ. Urine CA125 and HE4 for the Triage of Symptomatic Women with Suspected Endometrial Cancer. Cancers. 2022; 14(14):3306. https://doi.org/10.3390/cancers14143306
Chicago/Turabian StyleNjoku, Kelechi, Chloe E. Barr, Caroline J. J. Sutton, and Emma J. Crosbie. 2022. "Urine CA125 and HE4 for the Triage of Symptomatic Women with Suspected Endometrial Cancer" Cancers 14, no. 14: 3306. https://doi.org/10.3390/cancers14143306
APA StyleNjoku, K., Barr, C. E., Sutton, C. J. J., & Crosbie, E. J. (2022). Urine CA125 and HE4 for the Triage of Symptomatic Women with Suspected Endometrial Cancer. Cancers, 14(14), 3306. https://doi.org/10.3390/cancers14143306