Targeted Therapeutic Options and Future Perspectives for HER2-Positive Breast Cancer
Abstract
:Simple Summary
Abstract
1. HER2-Targeted Therapy
Introduction
2. HER2-Positive Disease–Current Approaches
2.1. HER2-Positive Early Breast Cancer—Current Approaches
2.1.1. De-Escalation Approaches in the Early Setting
2.1.2. Escalation Approaches in HER2-Positive EBC
2.2. HER2-Positive Advanced Breast Cancer—Current Approaches
2.3. HER2 Heterogeneity as an Opportunity to Select the Best Therapy
3. The Role of the Estrogen Receptor in HER2-Positive Tumors
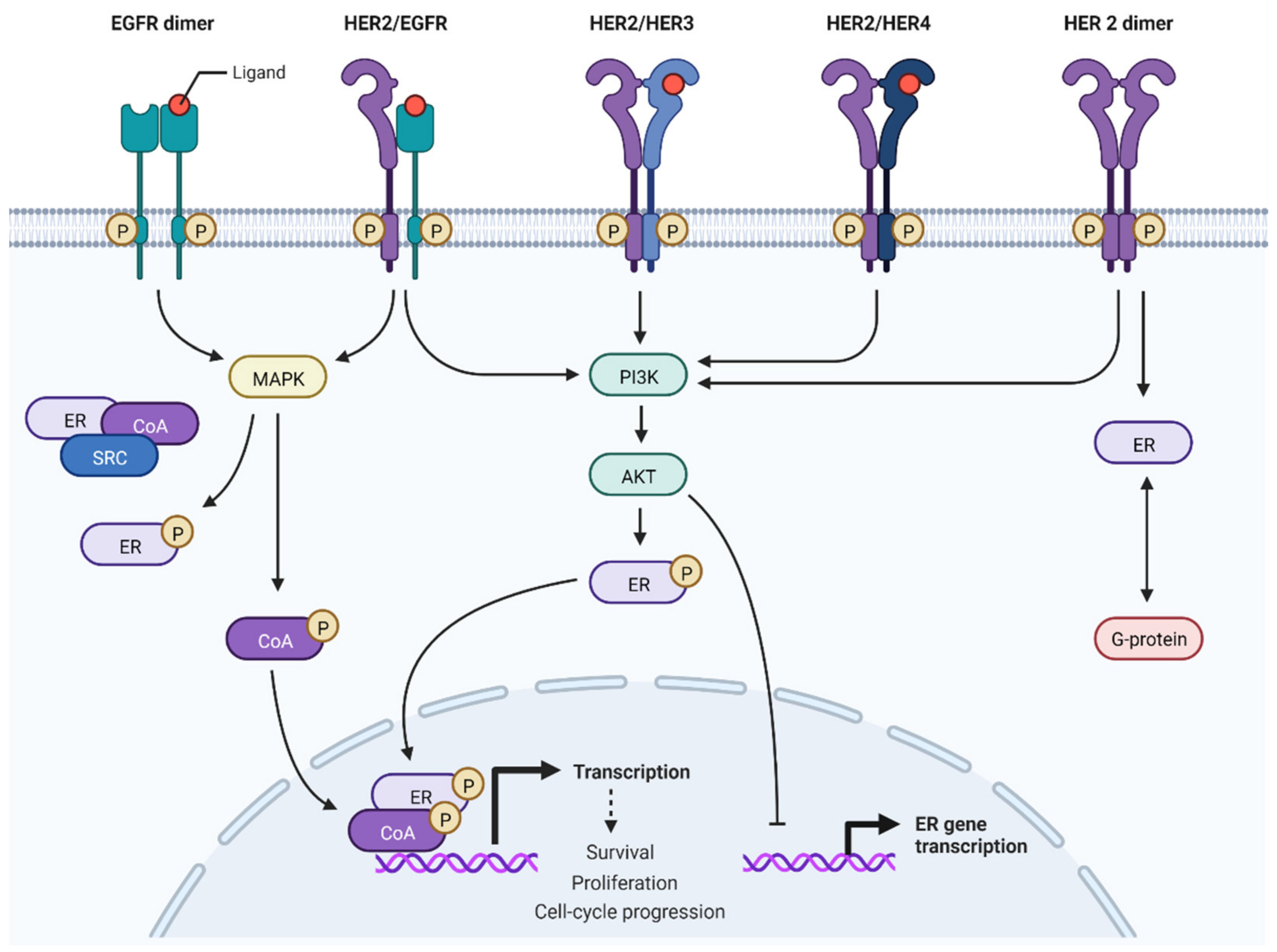
3.1. Crosslink between HER2 and Estrogen Receptors
3.2. Efficacy of Anti-HER2 and Endocrine Therapy in ER+/HER2+
3.3. Future Combination/Ongoing Studies to Improve the Efficacy of Endocrine Anti-HER2 in ER+/HER2+
4. Tumor Immunity and Immunotherapy in HER2-Positive Breast Cancer
4.1. Role of Tumor Immunity in HER2-Positive Breast Cancer
4.2. Trials Testing Combinations with Immune-Therapeutic Drugs in HER2-Positive Breast Cancer
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cronin, K.A.; Harlan, L.C.; Dodd, K.W.; Abrams, J.S.; Ballard-Barbash, R. Population-based Estimate of the Prevalence of HER-2 Positive Breast Cancer Tumors for Early Stage Patients in the US. Cancer Investig. 2010, 28, 963–968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slamon, D.J.; Clark, G.M.; Wong, S.G.; Levin, W.J.; Ullrich, A.; McGuire, W.L. Human breast cancer: Correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 1987, 235, 177–182. [Google Scholar] [CrossRef] [Green Version]
- Pernas, S.; Barroso-Sousa, R.; Tolaney, S.M. Optimal treatment of early stage HER2-positive breast cancer. Cancer 2018, 124, 4455–4466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moja, L.; Tagliabue, L.; Balduzzi, S.; Parmelli, E.; Pistotti, V.; Guarneri, V.; D’Amico, R. Trastuzumab containing regimens for early breast cancer. Cochrane Database Syst. Rev. 2012, 2021, CD006243. [Google Scholar] [CrossRef]
- Perez, E.A.; Romond, E.H.; Suman, V.J.; Jeong, J.; Sledge, G.; Geyer, C.E., Jr.; Martino, S.; Rastogi, P.; Gralow, J.; Swain, S.; et al. Trastuzumab Plus Adjuvant Chemotherapy for Human Epidermal Growth Factor Receptor 2–Positive Breast Cancer: Planned Joint Analysis of Overall Survival from NSABP B-31 and NCCTG N9831. J. Clin. Oncol. 2014, 32, 3744–3752. [Google Scholar] [CrossRef] [PubMed]
- Slamon, D.J.; Leyland-Jones, B.; Shak, S.; Fuchs, H.; Paton, V.; Bajamonde, A.; Fleming, T.; Eiermann, W.; Wolter, J.; Pegram, M.; et al. Use of Chemotherapy plus a Monoclonal Antibody against HER2 for Metastatic Breast Cancer That Overexpresses HER2. New Engl. J. Med. 2001, 344, 783–792. [Google Scholar] [CrossRef]
- Cameron, D.; Piccart-Gebhart, M.J.; Gelber, R.D.; Procter, M.; Goldhirsch, A.; de Azambuja, E.; Castro, G., Jr.; Untch, M.; Smith, I.; Gianni, L.; et al. 11 years’ follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive early breast cancer: Final analysis of the HERceptin Adjuvant (HERA) trial. Lancet 2017, 389, 1195–1205. [Google Scholar] [CrossRef] [Green Version]
- Slamon, D.; Eiermann, W.; Robert, N.; Giermek, J.; Martin, M.; Jasiowka, M.; Mackey, J.; Chan, A.; Liu, M.-C.; Pinter, T.; et al. Abstract S5-04: Ten year follow-up of BCIRG-006 comparing doxorubicin plus cyclophosphamide followed by docetaxel (AC→T) with doxorubicin plus cyclophosphamide followed by docetaxel and trastuzumab (AC→TH) with docetaxel, carboplatin and trastuzumab (TCH) in HER2+ early breast cancer. Cancer Res. 2016, 76, S5-04. [Google Scholar] [CrossRef]
- Carey, L.A.; Berry, D.A.; Cirrincione, C.T.; Barry, W.T.; Pitcher, B.N.; Harris, L.N.; Ollila, D.W.; Krop, I.E.; Henry, N.L.; Weckstein, D.J.; et al. Molecular Heterogeneity and Response to Neoadjuvant Human Epidermal Growth Factor Receptor 2 Targeting in CALGB 40601, a Randomized Phase III Trial of Paclitaxel Plus Trastuzumab With or Without Lapatinib. J. Clin. Oncol. 2016, 34, 542–549. [Google Scholar] [CrossRef] [Green Version]
- André, F.; Shahidi, J.; Lee, C.; Wang, K.; Krop, I. 197TiP—Phase III study of [fam-] trastuzumab deruxtecan vs. investigator’s choice in T-DM1-pretreated HER2+ breast cancer. Ann. Oncol. 2019, 30, iii63. [Google Scholar] [CrossRef]
- Murthy, R.K.; Loi, S.; Okines, A.; Paplomata, E.; Hamilton, E.; Hurvitz, S.A.; Lin, N.U.; Borges, V.; Abramson, V.; Anders, C.; et al. Tucatinib, Trastuzumab, and Capecitabine for HER2-Positive Metastatic Breast Cancer. N. Engl. J. Med. 2020, 382, 597–609. [Google Scholar] [CrossRef]
- Ciruelos, E.M.; Villagrasa, P.; Pascual, T.; Oliveira, M.; Pernas, S.; Paré, L.; Escrivá-De-Romaní, S.; Manso, L.; Adamo, B.; Martínez, E.; et al. Palbociclib and Trastuzumab in HER2-Positive Advanced Breast Cancer: Results from the Phase II SOLTI-1303 PATRICIA Trial. Clin. Cancer Res. 2020, 26, 5820–5829. [Google Scholar] [CrossRef]
- Loi, S.; Giobbie-Hurder, A.; Gombos, A.; Bachelot, T.; Hui, R.; Curigliano, G.; Campone, M.; Biganzoli, L.; Bonnefoi, H.; Jerusalem, G.; et al. Pembrolizumab plus trastuzumab in trastuzumab-resistant, advanced, HER2-positive breast cancer (PANACEA): A single-arm, multicentre, phase 1b–2 trial. Lancet Oncol. 2019, 20, 371–382. [Google Scholar] [CrossRef]
- Emens, L.A.; Esteva, F.J.; Beresford, M.; Saura, C.; De Laurentiis, M.; Kim, S.-B.; Im, S.-A.; Wang, Y.; Salgado, R.; Mani, A.; et al. Trastuzumab emtansine plus atezolizumab versus trastuzumab emtansine plus placebo in previously treated, HER2-positive advanced breast cancer (KATE2): A phase 2, multicentre, randomised, double-blind trial. Lancet Oncol. 2020, 21, 1283–1295. [Google Scholar] [CrossRef]
- Metzger, O.; Mandrekar, S.; Loibl, S.; Mundhenke, C.; Seiler, S.; Valagussa, P.; Lim, E.; Tripathy, D.; Winer, E.; Huang, C.; et al. Abstract OT3-02-07: PATINA: A ran-domized, open label, phase III trial to evaluate the efficacy and safety of palbociclib + anti-HER2 therapy + endocrine therapy (ET) vs. anti-HER2 therapy + ET after induction treatment for hormone receptor positive (HR+)/HER2-positive metastatic breast cancer (MBC). Cancer Res. 2019, 79 (Suppl. S4), OT3-02-07. [Google Scholar]
- Costa, R.L.B.; Czerniecki, B.J. Clinical development of immunotherapies for HER2+ breast cancer: A review of HER2-directed monoclonal antibodies and beyond. NPJ Breast Cancer 2020, 6, 10. [Google Scholar] [CrossRef] [Green Version]
- Swain, S.M.; Baselga, J.; Kim, S.-B.; Ro, J.; Semiglazov, V.; Campone, M.; Ciruelos, E.; Ferrero, J.-M.; Schneeweiss, A.; Heeson, S.; et al. Pertuzumab, Trastuzumab, and Docetaxel in HER2-Positive Metastatic Breast Cancer. New Engl. J. Med. 2015, 372, 724–734. [Google Scholar] [CrossRef] [Green Version]
- Bakhtiar, R. Antibody drug conjugates. Biotechnol. Lett. 2016, 38, 1655–1664. [Google Scholar] [CrossRef]
- Lewis Phillips, G.D.; Li, G.; Dugger, D.L.; Crocker, L.M.; Parsons, K.L.; Mai, E.; Blättler, W.A.; Lambert, J.M.; Chari, R.V.J.; Lutz, R.J.; et al. Targeting HER2-Positive Breast Cancer with Trastuzumab-DM1, an Antibody–Cytotoxic Drug Conjugate. Cancer Res. 2008, 68, 9280–9290. [Google Scholar] [CrossRef] [Green Version]
- Xia, W.; Mullin, R.J.; Keith, B.R.; Liu, L.-H.; Ma, H.; Rusnak, D.W.; Owens, G.; Alligood, K.J.; Spector, N.L. Anti-tumor activity of GW572016: A dual tyrosine kinase inhibitor blocks EGF activation of EGFR/erbB2 and downstream Erk1/2 and AKT pathways. Oncogene 2002, 21, 6255–6263. [Google Scholar] [CrossRef] [Green Version]
- Rabindran, S.K.; Discafani, C.M.; Rosfjord, E.C.; Baxter, M.; Floyd, M.B.; Golas, J.; Hallett, W.A.; Johnson, B.D.; Nilakantan, R.; Overbeek, E.; et al. Antitumor Activity of HKI-272, an Orally Active, Irreversible Inhibitor of the HER-2 Tyrosine Kinase. Cancer Res. 2004, 64, 3958–3965. [Google Scholar] [CrossRef] [Green Version]
- Kulukian, A.; Lee, P.; Taylor, J.; Rosler, R.; de Vries, P.; Watson, D.; Forero-Torres, A.; Peterson, S. Preclinical Activity of HER2-Selective Tyrosine Kinase Inhibitor Tucatinib as a Single Agent or in Combination with Trastuzumab or Docetaxel in Solid Tumor Models. Mol. Cancer Ther. 2020, 19, 976–987. [Google Scholar] [CrossRef] [Green Version]
- Saura, C.; Oliveira, M.; Feng, Y.-H.; Dai, M.-S.; Chen, S.-W.; Hurvitz, S.A.; Kim, S.-B.; Moy, B.; Delaloge, S.; Gradishar, W.; et al. Neratinib Plus Capecitabine Versus Lapatinib Plus Capecitabine in HER2-Positive Metastatic Breast Cancer Previously Treated With ≥ 2 HER2-Directed Regimens: Phase III NALA Trial. J. Clin. Oncol. 2020, 38, 3138–3149. [Google Scholar] [CrossRef]
- Pohlmann, P.R.; Mayer, I.A.; Mernaugh, R. Resistance to Trastuzumab in Breast Cancer. Clin. Cancer Res. 2009, 15, 7479–7491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goutsouliak, K.; Veeraraghavan, J.; Sethunath, V.; De Angelis, C.; Osborne, C.K.; Rimawi, M.F.; Schiff, R. Towards personalized treatment for early stage HER2-positive breast cancer. Nat. Rev. Clin. Oncol. 2019, 17, 233–250. [Google Scholar] [CrossRef] [PubMed]
- Schlam, I.; Swain, S.M. HER2-positive breast cancer and tyrosine kinase inhibitors: The time is now. NPJ Breast Cancer 2021, 7, 56. [Google Scholar] [CrossRef] [PubMed]
- Piccart-Gebhart, M.J.; Procter, M.; Leyland-Jones, B.; Goldhirsch, A.; Untch, M.; Smith, I.; Gianni, L.; Baselga, J.; Bell, R.H.; Jackisch, C.; et al. Trastuzumab after Adjuvant Chemotherapy in HER2-Positive Breast Cancer. N. Engl. J. Med. 2005, 353, 1659–1672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slamon, D.; Eiermann, W.; Robert, N.; Pienkowski, T.; Martin, M.; Press, M.; Mackey, J.; Glaspy, J.; Chan, A.; Pawlicki, M.; et al. Adjuvant Trastuzumab in HER2-Positive Breast Cancer. N. Engl. J. Med. 2011, 365, 1273–1283. [Google Scholar] [CrossRef] [Green Version]
- Conte, P.; Frassoldati, A.; Bisagni, G.; Brandes, A.; Donadio, M.; Garrone, O.; Piacentini, F.; Cavanna, L.; Giotta, F.; Aieta, M.; et al. Nine weeks versus 1 year adjuvant trastuzumab in combination with chemotherapy: Final results of the phase III randomized Short-HER study. Ann. Oncol. 2018, 29, 2328–2333. [Google Scholar] [CrossRef]
- Joensuu, H.; Fraser, J.; Wildiers, H.; Huovinen, R.; Auvinen, P.; Utriainen, M.; Nyandoto, P.; Villman, K.K.; Halonen, P.; Granstam-Bjrneklett, H.; et al. Effect of Adjuvant Trastuzumab for a Duration of 9 Weeks vs. 1 Year with Concomitant Chemotherapy for Early Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer: The SOLD Randomized Clinical Trial. JAMA Oncol. 2018, 4, 1199–1206. [Google Scholar] [CrossRef]
- Conte, P.F.; Bisagni, A.F.; Brandes, A.A.; Donadio, M.; Garrone, O.; Piacentini, F.; Cavanna, L.; Giotta, F.; Aieta, M.; Gebbia, V.; et al. Nine weeks vs. 1 year adjuvant trastuzumab: Long term outcomes of the ShortHER randomised trial. | OncologyPRO. Ann. Oncol. 2021, 32, S37–S47. [Google Scholar] [CrossRef]
- Pivot, X.; Romieu, G.; Debled, M.; Pierga, J.-Y.; Kerbrat, P.; Bachelot, T.; Lortholary, A.; Espié, M.; Fumoleau, P.; Serin, D.; et al. 6 months versus 12 months of adjuvant trastuzumab for patients with HER2-positive early breast cancer (PHARE): A randomised phase 3 trial. Lancet Oncol. 2013, 14, 741–748. [Google Scholar] [CrossRef]
- Earl, H.M.; Hiller, L.; Vallier, A.-L.; Loi, S.; McAdam, K.; Hughes-Davies, L.; Harnett, A.N.; Ah-See, M.-L.; Simcock, R.; Rea, D.; et al. 6 vs. 12 months of adjuvant trastuzumab for HER2-positive early breast cancer (PERSEPHONE): 4-year disease-free survival results of a randomised phase 3 non-inferiority trial. Lancet 2019, 393, 2599–2612. [Google Scholar] [CrossRef] [Green Version]
- Tolaney, S.M.; Barry, W.T.; Dang, C.T.; Yardley, D.A.; Moy, B.; Marcom, P.K.; Albain, K.S.; Rugo, H.S.; Ellis, M.; Shapira, I.; et al. Adjuvant Paclitaxel and Trastuzumab for Node-Negative, HER2-Positive Breast Cancer. N. Engl. J. Med. 2015, 372, 134–141. [Google Scholar] [CrossRef] [Green Version]
- Cortazar, P.; Zhang, L.; Untch, M.; Mehta, K.; Costantino, J.P.; Wolmark, N.; Bonnefoi, H.; Cameron, D.; Gianni, L.; Valagussa, P.; et al. Pathological complete response and long-term clinical benefit in breast cancer: The CTNeoBC pooled analysis. Lancet 2014, 384, 164–172. [Google Scholar] [CrossRef] [Green Version]
- van Ramshorst, M.S.; van der Voort, A.; van Werkhoven, E.D.; Mandjes, I.A.; Kemper, I.; Dezentj, V.O.; Oving, I.M.; Honkoop, A.H.; Tick, L.W.; van de Wouw, A.J.; et al. Neoadjuvant chemotherapy with or without anthracyclines in the presence of dual HER2 blockade for HER2-positive breast cancer (TRAIN-2): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2018, 19, 1630–1640. [Google Scholar] [CrossRef]
- van der Voort, A.; van Ramshorst, M.S.; van Werkhoven, E.D.; Mandjes, I.A.; Kemper, I.; Vulink, A.J.; Oving, I.M.; Honkoop, A.H.; Tick, L.W.; van de Wouw, A.J.; et al. Three-Year Fol-low-up of Neoadjuvant Chemotherapy with or without Anthracyclines in the Presence of Dual ERBB2 Blockade in Patients with ERBB2-Positive Breast Cancer: A Secondary Analysis of the TRAIN-2 Randomized, Phase 3 Trial. JAMA Oncol. 2021, 7, 978–984. [Google Scholar] [CrossRef]
- Nitz, U.A.; Gluz, O.; Christgen, M.; Grischke, E.M.; Augustin, D.; Kuemmel, S.; Braun, M.; Potenberg, J.; Kohls, A.; Krauss, K.; et al. De-escalation strategies in HER2-positive early breast cancer (EBC): Final analysis of the WSG-ADAPT HER2+/HR- phase II trial: Efficacy, safety, and predictive markers for 12 weeks of neoadjuvant dual blockade with trastuzumab and pertuzumab ± weekly paclitaxel. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2017, 28, 2768–2772. [Google Scholar]
- Hurvitz, S.A.; Martin, M.; Symmans, W.F.; Jung, K.H.; Huang, C.-S.; Thompson, A.M.; Harbeck, N.; Valero, V.; Stroyakovskiy, D.; Wildiers, H.; et al. Neoadjuvant trastuzumab, pertuzumab, and chemotherapy versus trastuzumab emtansine plus pertuzumab in patients with HER2-positive breast cancer (KRISTINE): A randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 2018, 19, 115–126. [Google Scholar] [CrossRef]
- Hurvitz, S.A.; Martin, M.; Jung, K.H.; Huang, C.-S.; Harbeck, N.; Valero, V.; Stroyakovskiy, D.; Wildiers, H.; Campone, M.; Boileau, J.-F.; et al. Neoadjuvant Trastuzumab Emtansine and Pertuzumab in Human Epidermal Growth Factor Receptor 2–Positive Breast Cancer: Three-Year Outcomes from the Phase III KRISTINE Study. J. Clin. Oncol. 2019, 37, 2206–2216. [Google Scholar] [CrossRef]
- Rimawi, M.F.; Niravath, P.; Wang, T.; Rexer, B.N.; Forero, A.; Wolff, A.C.; Nanda, R.; Storniolo, A.M.; Krop, I.; Goetz, M.P.; et al. TBCRC023: A randomized phase II neoad-juvant trial of lapatinib plus trastuzumab without chemotherapy for 12 versus 24 weeks in patients with HER2-positive breast cancer. Clin. Cancer Res. 2020, 26, 821–827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prat, A.; Pascual, T.; DE Angelis, C.; Gutierrez, C.; Llombart-Cussac, A.; Wang, T.; Cortés, J.; Rexer, B.; Pare, L.; Forero, A.; et al. HER2-Enriched Subtype and ERBB2 Expression in HER2-Positive Breast Cancer Treated with Dual HER2 Blockade. JNCI: J. Natl. Cancer Inst. 2019, 112, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Gianni, L.; Pienkowski, T.; Im, Y.-H.; Tseng, L.-M.; Liu, M.-C.; Lluch, A.; Starosławska, E.; De La Haba-Rodríguez, J.R.; Im, S.-A.; Pedrini, J.L.; et al. 5-year analysis of neoadjuvant pertuzumab and trastuzumab in patients with locally advanced, inflammatory, or early-stage HER2-positive breast cancer (NeoSphere): A multicentre, open-label, phase 2 randomised trial. Lancet Oncol. 2016, 17, 791–800. [Google Scholar] [CrossRef]
- Pérez-García, J.M.; Gebhart, G.; Borrego, M.R.; Stradella, A.; Bermejo, B.; Schmid, P.; Marmé, F.; Escrivá-de-Romani, S.; Calvo, L.; Ribelles, N.; et al. Chemotherapy de-escalation using an 18 F-FDG-PET-based pathological response-adapted strategy in patients with HER2-positive early breast cancer (PHER-Gain): A multicentre, randomised, open-label, non-comparative, phase 2 trial. Lancet Oncol. 2021, 22, 858–871. [Google Scholar] [CrossRef]
- Piccart, M.; Procter, M.; Fumagalli, D.; de Azambuja, E.; Clark, E.; Ewer, M.S.; Restuccia, E.; Jerusalem, G.; Dent, S.; Reaby, L.; et al. Adjuvant Pertuzumab and Trastuzumab in Early HER2-Positive Breast Cancer in the APHINITY Trial: 6 Years’ Follow-Up. J. Clin. Oncol. 2021, 39, 1448–1457. [Google Scholar] [CrossRef] [PubMed]
- Piccart-Gebhart, M.; Holmes, E.; Baselga, J.; de Azambuja, E.; Dueck, A.A.; Viale, G.; Zujewski, J.A.; Goldhirsch, A.; Armour, A.A.; Pritchard, K.I.; et al. Adjuvant Lapatinib and Trastuzumab for Early Human Epidermal Growth Factor Receptor 2–Positive Breast Cancer: Results from the Randomized Phase III Adjuvant Lapatinib and/or Trastuzumab Treatment Optimization Trial. J. Clin. Oncol. 2016, 34, 1034–1042. [Google Scholar] [CrossRef] [Green Version]
- Krop, I.E.; Im, S.-A.; Barrios, C.; Bonnefoi, H.; Gralow, J.; Toi, M.; Ellis, P.A.; Gianni, L.; Swain, S.M.; Im, Y.-H.; et al. Trastuzumab Emtansine Plus Pertuzumab Versus Taxane Plus Trastuzumab Plus Pertuzumab After Anthracycline for High-Risk Human Epidermal Growth Factor Receptor 2–Positive Early Breast Cancer: The Phase III KAITLIN Study. J. Clin. Oncol. 2022, 40, 438–448. [Google Scholar] [CrossRef]
- Swain, S.M.; Ewer, M.S.; Viale, G.; Delaloge, S.; Ferrero, J.M.; Verrill, M.; Colomer, R.; Vieira, C.; Werner, T.L.; Douthwaite, H.; et al. Pertuzumab, trastuzumab, and standard an-thracycline- and taxane-based chemotherapy for the neoadjuvant treatment of patients with HER2-positive localized breast cancer (BERENICE): A phase II, open-label, multicenter, multinational cardiac safety study. Ann. Oncol. 2018, 29, 646–653. [Google Scholar] [CrossRef]
- Schneeweiss, A.; Chia, S.; Hickish, T.; Harvey, V.; Eniu, A.; Hegg, R.; Tausch, C.; Seo, J.H.; Tsai, Y.-F.; Ratnayake, J.; et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: A randomized phase II cardiac safety study (TRYPHAENA). Ann. Oncol. 2013, 24, 2278–2284. [Google Scholar] [CrossRef]
- Guarneri, V.; Griguolo, G.; Miglietta, F.; Conte, P.; Dieci, M.; Girardi, F. Survival after neoadjuvant therapy with trastuzumab–lapatinib and chemotherapy in patients with HER2-positive early breast cancer: A meta-analysis of randomized trials. ESMO Open 2022, 7, 100433. [Google Scholar] [CrossRef]
- Von Minckwitz, G.; Huang, C.-S.; Mano, M.S.; Loibl, S.; Mamounas, E.P.; Untch, M.; Wolmark, N.; Rastogi, P.; Schneeweiss, A.; Redondo, A.; et al. Trastuzumab Emtansine for Residual Invasive HER2-Positive Breast Cancer. N. Engl. J. Med. 2019, 380, 617–628. [Google Scholar] [CrossRef]
- Martin, M.; Holmes, F.A.; Ejlertsen, B.; Delaloge, S.; Moy, B.; Iwata, H.; von Minckwitz, G.; Chia, S.K.L.; Mansi, J.; Barrios, C.H.; et al. Neratinib after trastuzumab-based adjuvant therapy in HER2-positive breast cancer (ExteNET): 5-year analysis of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2017, 18, 1688–1700. [Google Scholar] [CrossRef]
- Chan, A.; Delaloge, S.; Holmes, F.A.; Moy, B.; Iwata, H.; Harvey, V.J.; Robert, N.J.; Silovski, T.; Gokmen, E.; von Minckwitz, G.; et al. Neratinib after trastuzumab-based adjuvant therapy in patients with HER2-positive breast cancer (ExteNET): A multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2016, 17, 367–377. [Google Scholar] [CrossRef]
- Chan, A.; Moy, B.; Mansi, J.; Ejlertsen, B.; Holmes, F.A.; Chia, S.; Iwata, H.; Gnant, M.; Loibl, S.; Barrios, C.H.; et al. Final Efficacy Results of Neratinib in HER2-positive Hormone Receptor-positive Early-stage Breast Cancer from the Phase III ExteNET Trial. Clin. Breast Cancer 2020, 21, 80–91.e7. [Google Scholar] [CrossRef]
- Barcenas, C.H.; Hurvitz, S.A.; Di Palma, J.A.; Bose, R.; Chan, A.; Chien, A.J.; Farrell, C.; Hunt, D.; McCulloch, L.; Kupic, A.; et al. Effect of prophylaxis on neratinib-associated diarrhea and tolerability in patients with HER2+ early-stage breast cancer: Phase II CONTROL trial. J. Clin. Oncol. 2019, 37, 548. [Google Scholar] [CrossRef]
- Swain, S.M.; Miles, D.; Kim, S.-B.; Im, Y.-H.; Im, S.-A.; Semiglazov, V.; Ciruelos, E.; Schneeweiss, A.; Loi, S.; Monturus, E.; et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA): End-of-study results from a double-blind, randomised, placebo-controlled, phase 3 study. Lancet Oncol. 2020, 21, 519–530. [Google Scholar] [CrossRef]
- Verma, S.; Miles, D.; Gianni, L.; Krop, I.E.; Welslau, M.; Baselga, J.; Pegram, M.; Oh, D.-Y.; Diéras, V.; Guardino, E.; et al. Trastuzumab Emtansine for HER2-Positive Advanced Breast Cancer. New Engl. J. Med. 2012, 367, 1783–1791. [Google Scholar] [CrossRef] [Green Version]
- Geyer, C.E.; Forster, J.; Lindquist, D.; Chan, S.; Romieu, C.G.; Pienkowski, T.; Jagiello-Gruszfeld, A.; Crown, J.; Chan, A.; Kaufman, B.; et al. Lapatinib plus Capecitabine for HER2-Positive Advanced Breast Cancer. N. Engl. J. Med. 2006, 355, 2733–2743. [Google Scholar] [CrossRef] [Green Version]
- Cortés, J.; Kim, S.-B.; Chung, W.-P.; Im, S.-A.; Park, Y.H.; Hegg, R.; Kim, M.H.; Tseng, L.-M.; Petry, V.; Chung, C.-F.; et al. Trastuzumab Deruxtecan versus Trastuzumab Emtansine for Breast Cancer. New Engl. J. Med. 2022, 386, 1143–1154. [Google Scholar] [CrossRef]
- Ogitani, Y.; Aida, T.; Hagihara, K.; Yamaguchi, J.; Ishii, C.; Harada, N.; Soma, M.; Okamoto, H.; Oitate, M.; Arakawa, S.; et al. DS-8201a, A Novel HER2-Targeting ADC with a Novel DNA Topoisomerase I Inhibitor, Demonstrates a Promising Antitumor Efficacy with Differentiation from T-DM1. Clin. Cancer Res. 2016, 22, 5097–5108. [Google Scholar] [CrossRef] [Green Version]
- Kumagai, K.; Aida, T.; Tsuchiya, Y.; Kishino, Y.; Kai, K.; Mori, K. Interstitial pneumonitis related to trastuzumab deruxtecan, a human epidermal growth factor receptor 2-targeting Ab-drug conjugate, in monkeys. Cancer Sci. 2020, 111, 4636–4645. [Google Scholar] [CrossRef] [PubMed]
- Curigliano, G.; Mueller, V.; Borges, V.F.; Hamilton, E.P.; Hurvitz, S.A.; Loi, S.; Murthy, R.K.; Okines, A.F.C.; Paplomata, E.; Cameron, D.A.; et al. Updated results of tucatinib versus placebo added to trastuzumab and capecitabine for patients with pretreated HER2+ metastatic breast cancer with and without brain metastases (HER2CLIMB). J. Clin. Oncol. 2021, 39, 1043. [Google Scholar] [CrossRef]
- Rugo, H.S.; Im, S.A.; Cardoso, F.; Corts, J.; Curigliano, G.; Musolino, A.; Pegram, M.D.; Wright, G.S.; Saura, C.; Escriv De, R. Efficacy of Margetuximab vs. Trastuzumab in Patients with Pretreated ERBB2-Positive Advanced Breast Cancer: A Phase 3 Randomized Clinical Trial. JAMA Oncol. 2021, 7, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Manich, C.S.; O’Shaughnessy, J.; Aftimos, P.G.; van den Tweel, E.; Oesterholt, M.; de Escriv, R. LBA15 Primary outcome of the phase III SYD985.002/TULIP trial comparing vic- trastuzumab duocarmazine to physician’s choice treatment in patients with pre-treated HER2-positive locally advanced or metastatic breast cancer. Ann. Oncol. 2021, 32, S1288. [Google Scholar] [CrossRef]
- Blackwell, K.L.; Burstein, H.J.; Storniolo, A.M.; Rugo, H.S.; Sledge, G.; Aktan, G.; Ellis, C.; Florance, A.; Vukelja, S.; Bischoff, J.; et al. Overall Survival Benefit with Lapatinib in Combination with Trastuzumab for Patients with Human Epidermal Growth Factor Receptor 2–Positive Metastatic Breast Cancer: Final Results From the EGF104900 Study. J. Clin. Oncol. 2012, 30, 2585–2592. [Google Scholar] [CrossRef]
- Schettini, F.; Pascual, T.; Conte, B.; Chic, N.; Brasó-Maristany, F.; Galván, P.; Martínez, O.; Adamo, B.; Vidal, M.; Muñoz, M.; et al. HER2-enriched subtype and pathological complete response in HER2-positive breast cancer: A systematic review and meta-analysis. Cancer Treat. Rev. 2020, 84, 101965. [Google Scholar] [CrossRef]
- Von Minckwitz, G.; Untch, M.; Blohmer, J.-U.; Costa, S.D.; Eidtmann, H.; Fasching, P.A.; Gerber, B.; Eiermann, W.; Hilfrich, J.; Huober, J.; et al. Definition and Impact of Pathologic Complete Response on Prognosis After Neoadjuvant Chemotherapy in Various Intrinsic Breast Cancer Subtypes. J. Clin. Oncol. 2012, 30, 1796–1804. [Google Scholar] [CrossRef] [Green Version]
- Wallden, B.; Storhoff, J.; Nielsen, T.; Dowidar, N.; Schaper, C.; Ferree, S.; Liu, S.; Leung, S.; Geiss, G.; Snider, J.; et al. Development and verification of the PAM50-based Prosigna breast cancer gene signature assay. BMC Med. Genom. 2015, 8, 54. [Google Scholar] [CrossRef] [Green Version]
- Schettini, F.; Prat, A. Dissecting the biological heterogeneity of HER2-positive breast cancer. Breast 2021, 59, 339–350. [Google Scholar] [CrossRef]
- Prat, A.; Bianchini, G.; Thomas, M.; Belousov, A.; Cheang, M.C.; Koehler, A.; Gómez, P.; Semiglazov, V.; Eiermann, W.; Tjulandin, S.; et al. Research-Based PAM50 Subtype Predictor Identifies Higher Responses and Improved Survival Outcomes in HER2-Positive Breast Cancer in the NOAH Study. Clin. Cancer Res. 2014, 20, 511–521. [Google Scholar] [CrossRef] [Green Version]
- Conte, P.F.; Griguolo, G.; Dieci, M.V.; Bisagni, G.; Brandes, A.A.; Frassoldati, A.; Cavanna, L.; Musolino, A.; Giotta, F.; Rimanti, A.; et al. PAM50 HER2-enriched subtype as an independent prognostic factor in early-stage HER2+ breast cancer following adjuvant chemotherapy plus trastuzumab in the ShortHER trial. J. Clin. Oncol. 2019, 37, 544. [Google Scholar] [CrossRef]
- Salgado, R.; Denkert, C.; Campbell, C.; Savas, P.; Nuciforo, P.; Aura, C.; de Azambuja, E.; Eidtmann, H.; Ellis, C.E.; Baselga, J.; et al. Tumor-Infiltrating Lymphocytes and Associations with Pathological Complete Response and Event-Free Survival in HER2-Positive Early-Stage Breast Cancer Treated with Lapatinib and Trastuzumab: A Secondary Analysis of the NeoALTTO Trial. JAMA Oncol. 2015, 1, 448–455. [Google Scholar] [CrossRef]
- Dieci, M.V.; Prat, A.; Tagliafico, E.; Paré, L.; Ficarra, G.; Bisagni, G.; Piacentini, F.; Generali, D.G.; Conte, P.; Guarneri, V. Integrated evaluation of PAM50 subtypes and immune modulation of pCR in HER2-positive breast cancer patients treated with chemotherapy and HER2-targeted agents in the CherLOB trial. Ann. Oncol. 2016, 27, 1867–1873. [Google Scholar] [CrossRef]
- Denkert, C.; von Minckwitz, G.; Brase, J.; Sinn, B.; Gade, S.; Kronenwett, R.; Pfitzner, B.; Salat, C.; Loi, S.; Schmitt, W.; et al. Tumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy with or without carboplatin in human epidermal growth factor receptor 2-positive and tri-ple-negative primary breast cancers. J. Clin. Oncol. 2015, 33, 983–991. [Google Scholar] [CrossRef]
- Prat, A.; Guarneri, V.; Paré, L.; Griguolo, G.; Pascual, T.; Dieci, M.V.; Chic, N.; González-Farré, B.; Frassoldati, A.; Sanfeliu, E.; et al. A multivariable prognostic score to guide systemic therapy in early-stage HER2-positive breast cancer: A retrospective study with an external evaluation. Lancet Oncol. 2020, 21, 1455–1464. [Google Scholar] [CrossRef]
- Rimawi, M.; Ferrero, J.M.; de la Haba-Rodriguez, J.; Poole, C.; De Placido, S.; Osborne, C.K.; Hegg, R.; Easton, V.; Wohlfarth, C.; Arpino, G. First-Line Trastuzumab Plus an Aromatase Inhibitor, With or Without Pertuzumab, in Human Epidermal Growth Factor Receptor 2-Positive and Hormone Receptor-Positive Metastatic or Locally Advanced Breast Cancer (PERTAIN): A Randomized, Open-Label Phase II Trial. J. Clin. Oncol. 2018, 36, 2826–2835. [Google Scholar]
- Giuliano, M.; Trivedi, M.V.; Schiff, R. Bidirectional Crosstalk between the Estrogen Receptor and Human Epidermal Growth Factor Receptor 2 Signaling Pathways in Breast Cancer: Molecular Basis and Clinical Implications. Breast Care 2013, 8, 256–262. [Google Scholar] [CrossRef] [Green Version]
- Osborne, C.K.; Schiff, R.; Fuqua, S.A.; Shou, J. Estrogen receptor: Current understanding of its activation and modulation. Clin. Cancer Res. 2001, 7, 4338s–4342s. [Google Scholar]
- Nemere, I.; Pietras, R.; Blackmore, P.F. Membrane receptors for steroid hormones: Signal transduction and physiological significance. J. Cell. Biochem. 2003, 88, 438–445. [Google Scholar] [CrossRef]
- Arpino, G.; Wiechmann, L.; Osborne, C.K.; Schiff, R. Crosstalk between the Estrogen Receptor and the HER Tyrosine Kinase Receptor Family: Molecular Mechanism and Clinical Implications for Endocrine Therapy Resistance. Endocr. Rev. 2008, 29, 217–233. [Google Scholar] [CrossRef] [Green Version]
- Konecny, G.; Pauletti, G.; Pegram, M.; Untch, M.; Dandekar, S.; Aguilar, Z.; Wilson, C.; Rong, H.M.; Bauerfeind, I.; Felber, M.; et al. Quantitative association between HER-2/neu and steroid hormone receptors in hormone receptor-positive primary breast cancer. J. Natl. Cancer Inst. 2003, 95, 142–153. [Google Scholar] [CrossRef]
- Ferrari, A.; Vincent-Salomon, A.; Pivot, X.; Sertier, A.S.; Thomas, E.; Tonon, L.; Boyault, S.; Mulugeta, E.; Treilleux, I.; MacGrogan, G.; et al. A whole-genome sequence and tran-scriptome perspective on HER2-positive breast cancers. Nat. Commun. 2016, 7, 12222. [Google Scholar] [CrossRef] [Green Version]
- Loibl, S.; Majewski, I.; Guarneri, V.; Nekljudova, V.; Holmes, E.; Bria, E.; Denkert, C.; Schem, C.; Sotiriou, C.; Loi, S.; et al. PIK3CA mutations are associated with reduced pathological complete response rates in primary HER2-positive breast cancer: Pooled analysis of 967 patients from five pro-spective trials investigating lapatinib and trastuzumab. Ann. Oncol. 2016, 27, 1519–1525. [Google Scholar] [CrossRef]
- Hwang, K.-T.; Kim, J.; Jung, J.; Chang, J.H.; Chai, Y.J.; Oh, S.W.; Oh, S.; Kim, Y.A.; Park, S.B.; Hwang, K.R. Impact of Breast Cancer Subtypes on Prognosis of Women with Operable Invasive Breast Cancer: A Population-based Study Using SEER Database. Clin. Cancer Res. 2019, 25, 1970–1979. [Google Scholar] [CrossRef] [Green Version]
- Brandão, M.; Caparica, R.; Malorni, L.; Prat, A.; Carey, L.A.; Piccart, M. What Is the Real Impact of Estrogen Receptor Status on the Prognosis and Treatment of HER2-Positive Early Breast Cancer? Clin. Cancer Res. 2020, 26, 2783–2788. [Google Scholar] [CrossRef]
- Kavarthapu, R.; Anbazhagan, R.; Dufau, M.L. Crosstalk between PRLR and EGFR/HER2 Signaling Pathways in Breast Cancer. Cancers 2021, 13, 4685. [Google Scholar] [CrossRef]
- Carver, K.C.; Arendt, L.M.; Schuler, L.A. Complex prolactin crosstalk in breast cancer: New therapeutic implications. Mol. Cell. Endocrinol. 2009, 307, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Flågeng, M.H.; Moi, L.L.; Dixon, J.M.; Geisler, J.; Lien, E.A.; Miller, W.R.; Lønning, P.E.; Mellgren, G. Nuclear receptor co-activators and HER-2/neu are upregulated in breast cancer patients during neo-adjuvant treatment with aromatase inhibitors. Br. J. Cancer. 2009, 101, 1253–1260. [Google Scholar] [CrossRef] [Green Version]
- Massarweh, S.; Osborne, C.K.; Jiang, S.; Wakeling, A.E.; Rimawi, M.; Mohsin, S.K.; Hilsenbeck, S.; Schiff, R. Mechanisms of tumor regression and resistance to estrogen deprivation and fulvestrant in a model of estrogen receptor-positive, HER-2/neu-positive breast cancer. Cancer Res. 2006, 66, 8266–8273. [Google Scholar] [CrossRef] [Green Version]
- Bianchini, G.; Kiermaier, A.; Bianchi, G.V.; Im, Y.-H.; Pienkowski, T.; Liu, M.-C.; Tseng, L.-M.; Dowsett, M.; Zabaglo, L.; Kirk, S.; et al. Biomarker analysis of the NeoSphere study: Pertuzumab, trastuzumab, and docetaxel versus trastuzumab plus docetaxel, pertuzumab plus trastuzumab, or pertuzumab plus docetaxel for the neoadjuvant treatment of HER2-positive breast cancer. Breast Cancer Res. 2017, 19, 16. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.-C.; Morrison, G.; Gillihan, R.; Guo, J.; Ward, R.M.; Fu, X.; Botero, M.F.; Healy, N.A.; Hilsenbeck, S.G.; Phillips, G.L.; et al. Different mechanisms for resistance to trastuzumab versus lapatinib in HER2- positive breast cancers-role of estrogen receptor and HER2 reactivation. Breast Cancer Res. 2011, 13, R121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fumagalli, D.; Venet, D.; Ignatiadis, M.; Azim, H.A., Jr.; Maetens, M.; Rothé, F.; Salgado, R.; Bradbury, I.; Pusztai, L.; Harbeck, N.; et al. RNA Sequencing to Predict Response to Neoadjuvant Anti-HER2 Therapy: A Secondary Analysis of the NeoALTTO Randomized Clinical Trial. JAMA Oncol. 2017, 3, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Pogue-Geile, K.L.; Kim, C.; Jeong, J.-H.; Tanaka, N.; Bandos, H.; Gavin, P.G.; Fumagalli, D.; Goldstein, L.C.; Sneige, N.; Burandt, E.; et al. Predicting Degree of Benefit from Adjuvant Trastuzumab in NSABP Trial B-31. JNCI: J. Natl. Cancer Inst. 2013, 105, 1782–1788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, G.; Zhao, F.; Huo, X.; Ren, D.; Du, F.; Zheng, F.; Zhao, J. Meta-Analysis of HER2-Enriched Subtype Predicting the Patho-logical Complete Response Within HER2-Positive Breast Cancer in Patients Who Received Neoadjuvant Treatment. Front. Oncol. 2021, 11, 632357. [Google Scholar] [CrossRef]
- Maristany, F.B.; Griguolo, G.; Pascual, T.; Pare, L.; Nuciforo, P.; Llombart-Cussac, A.; Bermejo, B.; Oliveira, M.; Morales, S.; Martínez, N.; et al. Phenotypic changes of HER2-positive breast cancer during and after dual HER2 blockade. Nat. Commun. 2020, 11, 385. [Google Scholar] [CrossRef]
- Risi, E.; Grilli, A.; Migliaccio, I.; Biagioni, C.; McCartney, A.; Guarducci, C.; Bonechi, M.; Benelli, M.; Vitale, S.; Biganzoli, L.; et al. A gene expression signature of Retinoblastoma loss-of-function predicts resistance to neoadjuvant chemotherapy in ER-positive/HER2-positive breast cancer patients. Breast Cancer Res. Treat. 2018, 170, 329–341. [Google Scholar] [CrossRef]
- Denkert, C.; von Minckwitz, G.; Darb-Esfahani, S.; Lederer, B.; Heppner, B.I.; Weber, K.E.; Budczies, J.; Huober, J.; Klauschen, F.; Furlanetto, J.; et al. Tumour-infiltrating lym-phocytes and prognosis in different subtypes of breast cancer: A pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol. 2018, 19, 40–50. [Google Scholar] [CrossRef]
- Martínez-Sáez, O.; Chic, N.; Pascual, T.; Adamo, B.; Vidal, M.; González-Farré, B.; Sanfeliu, E.; Schettini, F.; Conte, B.; Brasó-Maristany, F.; et al. Frequency and spectrum of PIK3CA somatic mutations in breast cancer. Breast Cancer Res. 2020, 22, 45. [Google Scholar] [CrossRef]
- André, F.; Ciruelos, E.M.; Juric, D.; Loibl, S.; Campone, M.; Mayer, I.A.; Rubovszky, G.; Yamashita, T.; Kaufman, B.; Lu, Y.S.; et al. Alpelisib plus fulvestrant for PIK3CA-mutated, hormone receptor-positive, human epidermal growth factor receptor-2-negative advanced breast cancer: Final overall survival results from SOLAR-1. Ann. Oncol. 2021, 32, 208–217. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, P.; Spangle, J.M.; Von, T.; Roberts, T.M.; Lin, N.U.; Krop, I.E.; Winer, E.P.; Zhao, J.J. PI3K-p110α mediates resistance to HER2-targeted therapy in HER2+, PTEN-deficient breast cancers. Oncogene 2016, 35, 3607–3612. [Google Scholar] [CrossRef] [Green Version]
- Berns, K.; Horlings, H.M.; Hennessy, B.T.; Madiredjo, M.; Hijmans, E.M.; Beelen, K.; Linn, S.C.; Gonzalez-Angulo, A.M.; Stemke-Hale, K.; Hauptmann, M.; et al. A Functional Genetic Approach Identifies the PI3K Pathway as a Major Determinant of Trastuzumab Resistance in Breast Cancer. Cancer Cell 2007, 12, 395–402. [Google Scholar] [CrossRef] [Green Version]
- Jain, S.; Shah, A.N.; Santa-Maria, C.A.; Siziopikou, K.; Rademaker, A.; Helenowski, I.; Cristofanilli, M.; Gradishar, W.J. Phase I study of alpelisib (BYL-719) and trastuzumab emtansine (T-DM1) in HER2-positive metastatic breast cancer (MBC) after trastuzumab and taxane therapy. Breast Cancer Res. Treat. 2018, 171, 371–381. [Google Scholar] [CrossRef]
- Acevedo-Gadea, C.; Hatzis, C.; Chung, G.; Fishbach, N.; Lezon-Geyda, K.; Zelterman, D.; DiGiovanna, M.P.; Harris, L.; Abu-Khalaf, M.M. Sirolimus and trastuzumab combination therapy for HER2-positive metastatic breast cancer after progression on prior trastuzumab therapy. Breast Cancer Res. Treat. 2015, 150, 157–167. [Google Scholar] [CrossRef]
- Saura, C.; Bendell, J.; Jerusalem, G.; Su, S.; Ru, Q.; De Buck, S.; Mills, D.; Ruquet, S.; Bosch, A.; Urruticoechea, A.; et al. Phase Ib Study of Buparlisib plus Trastuzumab in Patients with HER2-Positive Advanced or Metastatic Breast Cancer That Has Progressed on Trastuzumab-Based Therapy. Clin. Cancer Res. 2014, 20, 1935–1945. [Google Scholar] [CrossRef] [Green Version]
- Jhaveri, K.; Drago, J.Z.; Shah, P.D.; Wang, R.; Pareja, F.; Ratzon, F.; Iasonos, A.; Patil, S.; Rosen, N.; Fornier, M.N.; et al. A Phase I Study of Alpelisib in Combination with Trastuzumab and LJM716 in Patients with PIK3CA-Mutated HER2-Positive Metastatic Breast Cancer. Clin. Cancer Res. 2021, 27, 3867–3875. [Google Scholar] [CrossRef]
- Morrow, P.K.; Wulf, G.M.; Ensor, J.; Booser, D.J.; Moore, J.A.; Flores, P.R.; Xiong, Y.; Zhang, S.; Krop, I.E.; Winer, E.P.; et al. Phase I/II Study of Trastuzumab in Combination With Everolimus (RAD001) in Patients With HER2-Overexpressing Metastatic Breast Cancer Who Progressed on Trastuzumab-Based Therapy. J. Clin. Oncol. 2011, 29, 3126–3132. [Google Scholar] [CrossRef]
- Hurvitz, S.A.; Andre, F.; Jiang, Z.; Shao, Z.; Mano, M.S.; Neciosup, S.P.; Tseng, L.-M.; Zhang, Q.; Shen, K.; Liu, D.; et al. Combination of everolimus with trastuzumab plus paclitaxel as first-line treatment for patients with HER2-positive advanced breast cancer (BOLERO-1): A phase 3, randomised, double-blind, multicentre trial. Lancet Oncol. 2015, 16, 816–829. [Google Scholar] [CrossRef] [Green Version]
- Hortobagyi, G.N.; Stemmer, S.M.; Burris, H.A.; Yap, Y.-S.; Sonke, G.S.; Paluch-Shimon, S.; Campone, M.; Blackwell, K.L.; André, F.; Winer, E.P.; et al. Ribociclib as First-Line Therapy for HR-Positive, Advanced Breast Cancer. N. Engl. J. Med. 2016, 375, 1738–1748. [Google Scholar] [CrossRef]
- Finn, R.S.; Martin, M.; Rugo, H.S.; Jones, S.; Im, S.-A.; Gelmon, K.; Harbeck, N.; Lipatov, O.N.; Walshe, J.M.; Moulder, S.; et al. Palbociclib and Letrozole in Advanced Breast Cancer. N. Engl. J. Med. 2016, 375, 1925–1936. [Google Scholar] [CrossRef]
- Goetz, M.P.; Toi, M.; Campone, M.; Sohn, J.; Paluch-Shimon, S.; Huober, J.; Park, I.H.; Trédan, O.; Chen, S.-C.; Manso, L.; et al. MONARCH 3: Abemaciclib as Initial Therapy for Advanced Breast Cancer. J. Clin. Oncol. 2017, 35, 3638–3646. [Google Scholar] [CrossRef]
- Slamon, D.; Neven, P.; Chia, S.; Jerusalem, G.; De Laurentiis, M.; Im, S.; Petrakova, K.; Bianchi, G.V.; Martín, M.; Nusch, A.; et al. Ribociclib plus fulvestrant for postmenopausal women with hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer in the phase III randomized MONALEESA-3 trial: Updated overall survival. Ann. Oncol. 2021, 32, 1015–1024. [Google Scholar] [CrossRef] [PubMed]
- Goel, S.; DeCristo, M.J.; Watt, A.C.; BrinJones, H.; Sceneay, J.; Li, B.B.; Khan, N.; Ubellacker, J.M.; Xie, S.; Metzger-Filho, O.; et al. CDK4/6 inhibition triggers anti-tumour immunity. Nature 2017, 548, 471–475. [Google Scholar] [CrossRef] [PubMed]
- Goel, S.; Wang, Q.; Watt, A.C.; Tolaney, S.M.; Dillon, D.A.; Li, W.; Ramm, S.; Palmer, A.C.; Yuzugullu, H.; Varadan, V.; et al. Overcoming Therapeutic Resistance in HER2-Positive Breast Cancers with CDK4/6 Inhibitors. Cancer Cell 2016, 29, 255–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prat, A.; Chaudhury, A.; Solovieff, N.; Paré, L.; Martinez, D.; Chic, N.; Martínez-Sáez, O.; Brasó-Maristany, F.; Lteif, A.; Taran, T.; et al. Correlative Biomarker Analysis of Intrinsic Subtypes and Efficacy Across the MONALEESA Phase III Studies. J. Clin. Oncol. 2021, 39, 1458–1467. [Google Scholar] [CrossRef]
- Spencer, S.; Mark, D.; Koei, C.; Zuzana, T.; Moqing, L.; Tiera, L.; Wallace, T.; Rebecca, S.; Michel, N.; Elmar, B.; et al. Microenvironment-Mediated Mechanisms of Resistance to HER2 Inhibitors Differ between HER2+ Breast Cancer Subtypes. Cell Syst. 2018, 6, 329–342.e6. [Google Scholar]
- Griguolo, G.; Pascual, T.; Dieci, M.V.; Guarneri, V.; Prat, A. Interaction of host immunity with HER2-targeted treatment and tumor heterogeneity in HER2-positive breast cancer. J. Immunother. Cancer 2019, 7, 90. [Google Scholar] [CrossRef]
- Stanton, S.E.; Adams, S.; Disis, M.L. Variation in the Incidence and Magnitude of Tumor-Infiltrating Lymphocytes in Breast Cancer Subtypes: A Systematic Review. JAMA Oncol. 2016, 2, 1354–1360. [Google Scholar] [CrossRef]
- Miller, L.D.; Chou, J.A.; Black, M.A.; Print, C.; Chifman, J.; Alistar, A.; Putti, T.; Zhou, X.; Bedognetti, D.; Hendrickx, W.; et al. Immunogenic Subtypes of Breast Cancer Delineated by Gene Classifiers of Immune Responsiveness. Cancer Immunol. Res. 2016, 4, 600–610. [Google Scholar] [CrossRef] [Green Version]
- Bianchini, G.; Gianni, L. The immune system and response to HER2-targeted treatment in breast cancer. Lancet Oncol. 2014, 15, e58–e68. [Google Scholar] [CrossRef]
- Denkert, C.; Loibl, S.; Noske, A.; Roller, M.; Müller, B.M.; Komor, M.; Budczies, J.; Darb-Esfahani, S.; Kronenwett, R.; Hanusch, C.; et al. Tumor-Associated Lymphocytes as an Independent Predictor of Response to Neoadjuvant Chemotherapy in Breast Cancer. J. Clin. Oncol. 2010, 28, 105–113. [Google Scholar] [CrossRef]
- Hannesdóttir, L.; Tymoszuk, P.; Parajuli, N.; Wasmer, M.-H.; Philipp, S.; Daschil, N.; Datta, S.; Koller, J.-B.; Tripp, C.H.; Stoitzner, P.; et al. Lapatinib and doxorubicin enhance the Stat1-dependent antitumor immune response. Eur. J. Immunol. 2013, 43, 2718–2729. [Google Scholar] [CrossRef] [Green Version]
- Powles, R.L.; Redmond, D.; Sotiriou, C.; Loi, S.; Fumagalli, D.; Nuciforo, P.; Harbeck, N.; de Azambuja, E.; Sarp, S.; Di Cosimo, S.; et al. Association of T-Cell Receptor Repertoire Use with Response to Combined Trastuzumab-Lapatinib Treatment of HER2-Positive Breast Cancer: Secondary Analysis of the NeoALTTO Randomized Clinical Trial. JAMA Oncol. 2018, 4, e181564. [Google Scholar] [CrossRef]
- Ledys, F.; Kalfeist, L.; Galland, L.; Limagne, E.; Ladoire, S. Therapeutic Associations Comprising Anti-PD-1/PD-L1 in Breast Cancer: Clinical Challenges and Perspectives. Cancers 2021, 13, 5999. [Google Scholar] [CrossRef]
- Dieci, M.; Miglietta, F.; Guarneri, V. Immune Infiltrates in Breast Cancer: Recent Updates and Clinical Implications. Cells 2021, 10, 223. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Z. The history and advances in cancer immunotherapy: Understanding the characteristics of tu-mor-infiltrating immune cells and their therapeutic implications. Cell. Mol. Immunol. 2020, 17, 807–821. [Google Scholar] [CrossRef]
- Gianni, L.; Pienkowski, T.; Im, Y.-H.; Roman, L.; Tseng, L.-M.; Liu, M.-C.; Lluch, A.; Staroslawska, E.; De La Haba-Rodriguez, J.; Im, S.-A.; et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): A randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 2012, 13, 25–32. [Google Scholar] [CrossRef]
- de Azambuja, E.; Holmes, A.P.; Piccart-Gebhart, M.; Holmes, E.; Di Cosimo, S.; Swaby, R.F.; Untch, M.; Jackisch, C.; Lang, I.; Smith, I.; et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): Survival outcomes of a randomised, open-label, multicentre, phase 3 trial and their association with pathological complete response. Lancet Oncol. 2014, 15, 1137–1146. [Google Scholar] [CrossRef] [Green Version]
- Loi, S.; Michiels, S.; Salgado, R.; Sirtaine, N.; Jose, V.; Fumagalli, D.; Kellokumpu-Lehtinen, P.-L.; Bono, P.; Kataja, V.; Desmedt, C.; et al. Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: Results from the FinHER trial. Ann. Oncol. 2014, 25, 1544–1550. [Google Scholar] [CrossRef]
- Barroso-Sousa, R.; Barry, W.; Guo, H.; Dillon, D.; Tan, Y.; Fuhrman, K.; Osmani, W.; Getz, A.; Baltay, M.; Dang, C.; et al. The immune profile of small HER2-positive breast cancers: A secondary analysis from the APT trial. Ann. Oncol. 2019, 30, 575–581. [Google Scholar] [CrossRef]
- Perez, E.A.; Ballman, K.V.; Tenner, K.S.; Thompson, E.A.; Badve, S.S.; Bailey, H.; Baehner, F.L. Association of Stromal Tu-mor-Infiltrating Lymphocytes with Recurrence-Free Survival in the N9831 Adjuvant Trial in Patients with Early-Stage HER2-Positive Breast Cancer. JAMA Oncol. 2016, 2, 56–64. [Google Scholar] [CrossRef]
- McArthur, H.L.; Diab, A.; Page, D.B.; Yuan, J.; Solomon, S.B.; Sacchini, V.; Comstock, C.; Durack, J.C.; Maybody, M.; Sung, J.; et al. A Pilot Study of Preoperative Single-Dose Ipilimumab and/or Cryoablation in Women with Early-Stage Breast Cancer with Comprehensive Immune Profiling. Clin. Cancer Res. 2016, 22, 5729–5737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tolaney, S.M.; Barroso-Sousa, R.; Keenan, T.; Li, T.; Trippa, L.; Vaz-Luis, I.; Wulf, G.; Spring, L.; Sinclair, N.F.; Andrews, C.; et al. Effect of Eribulin with or Without Pem-brolizumab on Progression-Free Survival for Patients with Hormone Receptor-Positive, ERBB2-Negative Metastatic Breast Cancer: A Randomized Clinical Trial. JAMA Oncol. 2020, 6, 1598–1605. [Google Scholar] [CrossRef] [PubMed]
- Dirix, L.Y.; Takacs, I.; Jerusalem, G.; Nikolinakos, P.; Arkenau, H.T.; Forero-Torres, A.; Boccia, R.; Lippman, M.E.; Somer, R.; Smakal, M.; et al. Avelumab, an anti-PD-L1 antibody, in patients with locally advanced or metastatic breast cancer: A phase 1b JAVELIN Solid Tumor study. Breast Cancer Res. Treat. 2018, 167, 671–686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chia, S.; Bedard, P.L.; Hilton, J.; Amir, E.; Gelmon, K.; Goodwin, R.; Villa, D.; Cabanero, M.; Tu, D.; Tsao, M.; et al. A Phase Ib Trial of Durvalumab in Combination with Trastuzumab in HER2-Positive Metastatic Breast Cancer (CCTG IND.229). Oncologist 2019, 24, 1439–1445. [Google Scholar] [CrossRef] [Green Version]
- Costa, R.L.B.; Soliman, H.; Czerniecki, B.J. The clinical development of vaccines for HER2 + breast cancer: Current landscape and future perspectives. Cancer Treat. Rev. 2017, 61, 107–115. [Google Scholar] [CrossRef]
- Ayoub, N.M.; Al-Shami, K.M.; Yaghan, R.J. Immunotherapy for HER2-positive breast cancer: Recent advances and combi-nation therapeutic approaches. Breast Cancer Targets Ther. 2019, 11, 53–69. [Google Scholar] [CrossRef] [Green Version]
| Trial | Phase | Treatment | n | pCR | DFS | OS |
|---|---|---|---|---|---|---|
| TRAIN-2 | III | A: FEC + H + P → Pac + CBDA + H + P B: Pac + CBDA | 438 | A: 67 (60–73) B: 68 (61–74) | A: 3 y EFS: 92.7 (89.3–96.2) B: 3 y EFS: 93.6 (90.4–96.9) | A: 3 y: 97.7 (95.7–99.7) B: 3 y: 98.2 (96.4–100) |
| WSG-ADAPT | II | A: (H + P) B: (H + P) + Pac | 134 | A: 34.4 (24.7–45.2) B: 90.5 (77.4–97.3) | ||
| KRISTINE | III | A: T-DM1 + P B: TCH + P | 444 | A: 44 B: 56 | A: 3 y IDFS: 93 (89.4–96.7) B: 3 y IDFS: 92 (86.7–97.3) | A: 3 y: 97 (94.6–99.4) B: 3 y: 97.6 (95.5–99.7) |
| TBCRC006 | II | Lapatinib + H | 64 | 27 | ||
| TBCRC023 | II | A: Lapatinib + H B: Lapatinib + H | 97 | A: 12 B: 28 | ||
| PER-ELISA | II | A: Letrozole + H + P B: Pac + H + P | 61 | A: 20.5 (11.1–34.5) B: 81 (57–93.4) | ||
| SOLTI-PAMELA | II | Lapatinib + H | 151 | 30 (23–39) | ||
| NeoSphere | II | H + P | 107 | 16.8 (10.3–25.3) | 5 y DFS: 80 (70–86) | |
| PHERGain | II | A: TCHP B: HP | 356 | A: 57.7 (47.4–69.4) B: 35.4 (29.9–41.3) | ||
| NeoSphere | II | A: H + T B: P + H + T C: P + H D: P + T | 417 | A: 29 (20.6–38.5) B: 45.8 (36.1–55.7) * C: 16.8 (10.3–25.3) † D: 24.0 (15.8–33.7) ‡ * p = 0.0141 vs. A † p = 0.0198 vs. A ‡ p = 0.003 vs. B | 5 y DFS A: 81 (71–87) B: 86 (77–91) C: 73 (64–81) D: 73 (63–81) | |
| TRYPHAENA | II | A: FEC + H + P→ THP B: FEC→ THP C: TCHP | 225 | A: 56.2 B: 54.7 C: 63.6 | ||
| BERENICE | II | A: ddAC→Pac + H + P B: FEC → THP | 400 | A: 61.8 (54.7–68.6) B: 60.7 (53.6–67.5) | ||
| Meta-analysis (CALGB 40601, Cher-LOB, NSABP-B41, NeoALTTO) | II/III | A: ChT + H B: ChT + H + Lapatinib | 1410 | RFS Pooled HR 0.62 (0.46–0.85) | OS Pooled HR 0.65 (0.43–0.98) | |
| Cher-LOB | II | A: ChT + H B: ChT + Lapatinib C: ChT + H + Lapatinib ChT: Pac → FEC | 121 | A: 25 (13.1–36.9) B: 26.3 (14.5–38.1) C: 46.7 (34.4–58.9) | A: 5 y RFS: 77.8 B: 5 y RFS: 77.1 C: 5 y RFS: 85.5 HR 0.52 (0.23–1.15) (A vs. C) | HR 1.00 (0.31–3.27) (A vs. C) |
| NSABP-B41 | III | A: ChT + H B: ChT + Lapatinib C: ChT + H + Lapatinib ChT: AC → Pac | 529 | A: 52.5 (44.9–59.5) B: 53.2 (45.4–60.3) C: 62 (54.3–68.8) | A: 5 y RFI: 84.3 B: 5 y RFI: 78.6 C: 5 y RFI: 90 EFS: HR 0.66 (0.34–1.25) (A vs. C) | A: 5 y: 94.5 B: 5 y: 89.4 C: 5 y: 95.7 HR 1.00 (0.24–1.67) (A vs. C) |
| NeoALTTO | III | A: H + ChT B: Lapatinib + ChT C: H + Lapatinib + ChT ChT: Pac | 455 | 29.5 (22.4–37.5) 24.7 (18.1–32.3) 51.3 (43.1–59.5) | 6 y EFS: 67 6 y EFS: 67 6 y EFS: 74 EFS: HR 0.98 (0.64–1.91) (A vs. C) | 6 y: 82 6 y: 79 6 y: 85 HR 0.85 (0.49–1.86) (A vs. C) |
| CALGB 40601 | III | A: H + ChT B: Lapatinib + ChT C: H + Lapatinib + ChT ChT: Pac | 305 | 46 (37–55) 32 (22–45) 56 (47–65) | 7 y EFS: 79 7 y EFS: 69 7 y EFS: 93 EFS: HR 0.32 (0.14–0.71) (A vs. C) | 7 y: 88 7 y: 84 7 y: 96 HR 0.34 (0.12–0.94) (A vs. C) |
| Trial | Phase | Treatment | n | DFS | OS |
|---|---|---|---|---|---|
| HERA | III | A: 1 y H B: 2 y H C: Observation | 3389 | A: 2 y DFS: 85.8 C: 2 y DFS: 77.4 p < 0.0001 A: 10 y DFS: 69 B: 10 y DFS: 69 C: 10y DFS: 63 HR 0.76 (0.68–0.86) (A vs. C) | A: 2 y: 96 C: 2 y: 95.1 p = 0.26 A: 12 y: 79 B: 12 y: 80 C: 12 y: 73 HR 0.74 (0.64–0.86) (A vs. C) |
| BCIRG-006 | III | A: AC →T B: AC → T → 1 y H C: TC → 1 y H | 3222 | A: 5 y DFS: 75 B: 5 y DFS: 84 C: 5 y DFS: 81 p < 0.001 (A vs. B) p = 0.04 (A vs. C) A: 10 y DFS: 67.9 B: 10 y DFS: 74.6 C: 10 y DFS: 73 p < 0.0001 (A vs. B) p = 0.0011 (A vs. C) | A: 5 y: 87 B: 5 y: 92 C: 5 y: 91 p < 0.001 (A vs. B) p = 0.04 (A vs. C) A: 10 y: 78.7 B: 10 y: 85.9 C: 10 y: 83.3 p < 0.0001 (A vs. B) p = 0.0075 (A vs. C) |
| NSABP B-31 | III | A: AC →Pac B: AC → Pac + 1 y H | 3351 | A: 10 y DFS: 62.2 B/C: 10 y DFS: 73.7 p ≤ 0.001 HR 0.6 (0.53–0.68) | A: 10 y OS: 75.2 B/C: 10 y OS: 85 p = 0.001 HR 0.63 (0.54–0.73) |
| NCCTG N9831 | III | A: AC → Pac B: AC → Pac + 1 y H C: AC → Pac → 1 y H | |||
| Short-HER | III | A: AC or EC → T or Pac → 1 y H B: T → FEC → 9 w H | 1254 | A: 5 year-DFS: 88.5 B: 5 year-DFS: 85.5HR 1.13 (90% CI, 0.89–1.42) 8.7 y DFS: HR 1.09 (90% CI, 0.88–1.35) | A: 5 y: 95.2 B: 5 y: 95 HR 1.07 (CI 90%, 0.74–1.56) A: 9 y: 90 B: 9 y: 91 HR 1.18 (CI 90%, 0.86–1.62) |
| SOLD | III | A: T + 9 w H →FEC B: T → FEC → 1 y H | 2174 | A: 5 y DFS: 88 B: 5 y DFS: 90.5 HR 1.39 (CI 90%, 1.12–1.72) | A: 5 y: 94.7 B: 5 y: 95.9 HR 1.36 (CI 90%, 0.98–1.89) |
| PHARE | III | A: 6 m H B: 12 m H | 3384 | A: 7.5 y DFS: 78.8 B: 7.5 y DFS: 79.6 p = 0.39 HR 1.08 (0.93–1.25) | 7.5 y OS: HR 1.13 (0.92–1.39) |
| PERSEPHONE | III | A: ChT + 6 m H B: ChT + 1 y H | 4089 | A: 4 y DFS: 89.4 B: 4 y DFS: 89.8 p = 0.011 HR 1.07 (CI 90%, 0.93–1.24) | A: 4 y: 93.8 B: 4 y: 94.8 HR 1.14 (CI 90%, 0.95–1.37) |
| APT | II | Pac + 12 w H → 9 m H | 406 | 7 y DFS: 93.3 (90.4–96.2) | 7 y: 95 (92.4–97.7) |
| APHINITY | III | A: ChT + 1 y H B: ChT + H + 1 y P | 4800 | A: 6 y DFS: 88 B: 6 y DFS: 91 HR 0.76 (0.64–0.91) | A: 6 y: 94 B: 6 y: 95 p = 0.17 Immature data |
| ALTTO | III | A: ChT + H → lapatinib B: ChT + H + lapatinib C: ChT + H | 8381 | A: 6.9 y DFS: 84 B: 6.9 y DFS: 85 C: 6.9 y DFS: 82 HR 0.86 (0.74–1.0) (B vs. C) HR 0.93 (0.81–1.08) (A vs. C) | A: 6 y: 92 B: 6 y: 93 C: 6 y: 91 HR 0.86 (0.7–1.06) (B vs. C) HR 0.88 (0.71–1.08) (A vs. C) |
| KAITLIN | III | A: AC →T-DM1 + P B: AC → P + H + P | 1846 | A: 3 y DFS: 93 B: 3 y DFS: 94 p = 0.827 HR 0.98 (0.72–1.32) | Immature data for OS |
| ExteNET | III | A: Neratinib 1 y after H-based therapy B: Observation after H-based therapy | 2840 | 5 y DFS in HR+/≤ 1 y post-H: A: 90.8 B: 85.7 HR 0.58 (0.41–0.82) 5 y DFS in HR+/>1 y post-H and residual disease after NA: benefit of 7.4% in group A vs. B HR 0.60 (0.33–1.07) | 8y OS in HR+/≤ 1 y post-H: A: 91.5 B: 89.4 HR 0.79 (0.55–1.13) 8y OS in HR+/>1 y of prior H and residual disease after NA: benefit of 9.1% in group A vs. B HR 0.47 (0.23–0.92) |
| KATHERINE | III | A: T-DM1 × 14 cycles B: H × 14 cycles | 1486 | A: 3 y DFS: 88.3 B: 3 y DFS: 77 HR 0.5 (0.39–0.64) | Immature data for OS |
| Trial | Phase | Treatment | n | Primary Endpoint | Results | Target |
|---|---|---|---|---|---|---|
| PATRICIA | II | Palbociclib 200 mg 2 w on 1 w off or 125 3 w on 1 w off + H 600 mg sc every 3 w ER+ patients were treated with Letrozole vs. Placebo | 71 (56 ER+) | PFS at 6 m | 6 m PFS in ER+ patients treated with Palbociclib + H 42.8% vs. 46.4% Luminal disease by PAM50 had longer PFS (10.6 m vs. 4.2 m) | Similar potency against CDK 4 than CDK 6 |
| Ribociclib, NCT02657343 | Ib/II | Ribociclib 400 mg daily (phase II) + H iv | 13 (ER + in 8) | MTD and CBR | 1 experienced stable disease >24 w PFS was 1.3 m | Greater potency against CDK 4 than CDK 6 |
| Ribociclib, NCT02657343 | Ib | Ribociclib 400 mg given on days 8–21 of a 21-day cycle with T-DM1 | 12 | MTD for phase II | PFS was 10.4 m | Greater potency against CDK 4 than CDK 6 |
| MonarchHER | II | A: Abemaciclib 150 mg/12 h + H iv B: Abemaciclib + BPC ChT C: Abemaciclib + fulvestrant im + H iv | 237 physician’s choice (all HER+/ER+) | PFS between groups | Abemaciclib + H + Fulvestrant longer PFS: 8.3 m vs. 5.7 m and 5.7 m compared with the other groups | Greater potency against CDK 4 than CDK 6, also CDK 1/2/5 inhibitor |
| PATINA | III | H + P with endocrine therapy (letrozole, anastrozole, exemestane or fulvestrant) +/- palbociclib | 496 already recruited | PFS | Not reported yet | Similar potency against CDK 4 than CDK 6 |
| ASPIRE | I/II | Palbociclib (100 and 125 mg 3 w on 1 w off) + H iv + P iv + Anastrozole | 36 planned | DLT, MTD, CBR | Not reported yet | Similar potency against CDK 4 than CDK 6 |
| NCI Identifier | Phase | Recruitment | Setting | Subtype | Immunotherapies | Combined Treatments | Target |
|---|---|---|---|---|---|---|---|
| NCT03241173 | I/II | Active, not recruiting | Metastatic or LA | All | Ipilimumab and/or nivolumab | INCAGN01949 | Anti-CTLA4 |
| NCT03126110 | I/II | Active, not recruiting | Metastatic or LA | All | Ipilimumab and/or nivolumab | INCAGN01876 | Anti-CTLA5 |
| NCT03328026 | I/II | Recruiting | Metastatic or LA | All | Ipilimumab or pembrolizumab | SV-BR-1-GM, cyclophosphamide, and interferon inoculation | Anti-CTLA6 |
| NCT02129556 | I/II | Active, not recruiting | Metastatic | HER2+ resistant to H | Pembrolizumab | H | anti-PDL1; anti-PD1 |
| NCT01772004 | I | Active, not recruiting | Metastatic | HER2+ | Avelumab | H | Anti-PD1 |
| NCT03747120 | II | Recruiting | Neoadjuvant | HER2+ | Pembrolizumab | Neoadjuvant H + P + Pac | Anti-PDL1 |
| NCT03523572 | I | Recruiting | Advanced | HER2+ | Nivolumab | Trastuzumab deruxtecan | Anti-PDL1; Anti-PD3 |
| NCT02649686 | I | Active, not recruiting | Metastatic | HER2+ | Durvalumab | H | Anti-PDL1; Anti-PD4 |
| NCT02924883 | II | Active, not recruiting | Metastatic | HER2+ | Atezolizumab | T-DM1 | Anti-PDL1; Anti-PD5 |
| NCT03125928 | II | Recruiting | Metastatic | HER2+ | Atezolizumab | Pac + H + P | Anti-PDL1; Anti-PD6 |
| NCT03620201 | I | Recruiting | Stage II–III | HER2+ | M7824 (anti-PD-L1 fusion protein) | Anti-PDL1; Anti-PD7 | |
| NCT05180006 | I | Recruiting | Neoadjuvant | HER2+ TNBC | Atezolizumab | H + P | Anti-PDL1 |
| NCT02336984 | I/II | Active, not recruiting | DCIS | HER2+ | HER2-pulsed DC1 | H + P | Vaccine |
| NCT02061423 | I | Active, not recruiting | Stage I–III | HER2+ | HER2-pulsed DC vaccine | Vaccine | |
| NCT03384914 | II | Recruiting | Stage I–III | HER2+ | DC1 vaccine | Vaccine | |
| NCT03387553 | I | Active, not recruiting | Stage II/III | HER2+ | DC1 vaccine | Vaccine | |
| NCT03113019 | I | Active, not recruiting | Stage II–IV | HER2+ TNBC | DC-based vaccine | Vaccine | |
| NCT03113019 | I | Active, not recruiting | Stage II–IV | HER2+ TNBC | DC-based vaccine | Vaccine | |
| NCT03630809 | II | Not yet recruiting | DCIS or inflammatory | HER2+ | HER2-pulsed DC1 | Vaccine | |
| NCT01376505 | I | Recruiting | Metastatic | HER2 1+, 2+, or 3+ by IHC | MVF-HER-2 (597–626)-MVF- HER-2 (266–296) peptide vaccine | Vaccine | |
| NCT03632941 | II | Recruiting | Metastatic | HER+ | VRP-HER2 immunizations plus pembrolizumab. | Vaccine |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrando-Díez, A.; Felip, E.; Pous, A.; Bergamino Sirven, M.; Margelí, M. Targeted Therapeutic Options and Future Perspectives for HER2-Positive Breast Cancer. Cancers 2022, 14, 3305. https://doi.org/10.3390/cancers14143305
Ferrando-Díez A, Felip E, Pous A, Bergamino Sirven M, Margelí M. Targeted Therapeutic Options and Future Perspectives for HER2-Positive Breast Cancer. Cancers. 2022; 14(14):3305. https://doi.org/10.3390/cancers14143305
Chicago/Turabian StyleFerrando-Díez, Angelica, Eudald Felip, Anna Pous, Milana Bergamino Sirven, and Mireia Margelí. 2022. "Targeted Therapeutic Options and Future Perspectives for HER2-Positive Breast Cancer" Cancers 14, no. 14: 3305. https://doi.org/10.3390/cancers14143305
APA StyleFerrando-Díez, A., Felip, E., Pous, A., Bergamino Sirven, M., & Margelí, M. (2022). Targeted Therapeutic Options and Future Perspectives for HER2-Positive Breast Cancer. Cancers, 14(14), 3305. https://doi.org/10.3390/cancers14143305






