Prolonged Exposition with Hyperthermic Intraperitoneal Chemotherapy (HIPEC) May Provide Survival Benefit after Cytoreductive Surgery (CRS) in Advanced Primary Epithelial Ovarian, Fallopian Tube, and Primary Peritoneal Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Study Design
2.2. Details of CRS + HIPEC
2.3. Clinical Characteristics
2.4. Statistical Analysis
3. Results
3.1. Interval vs. Upfront HIPEC
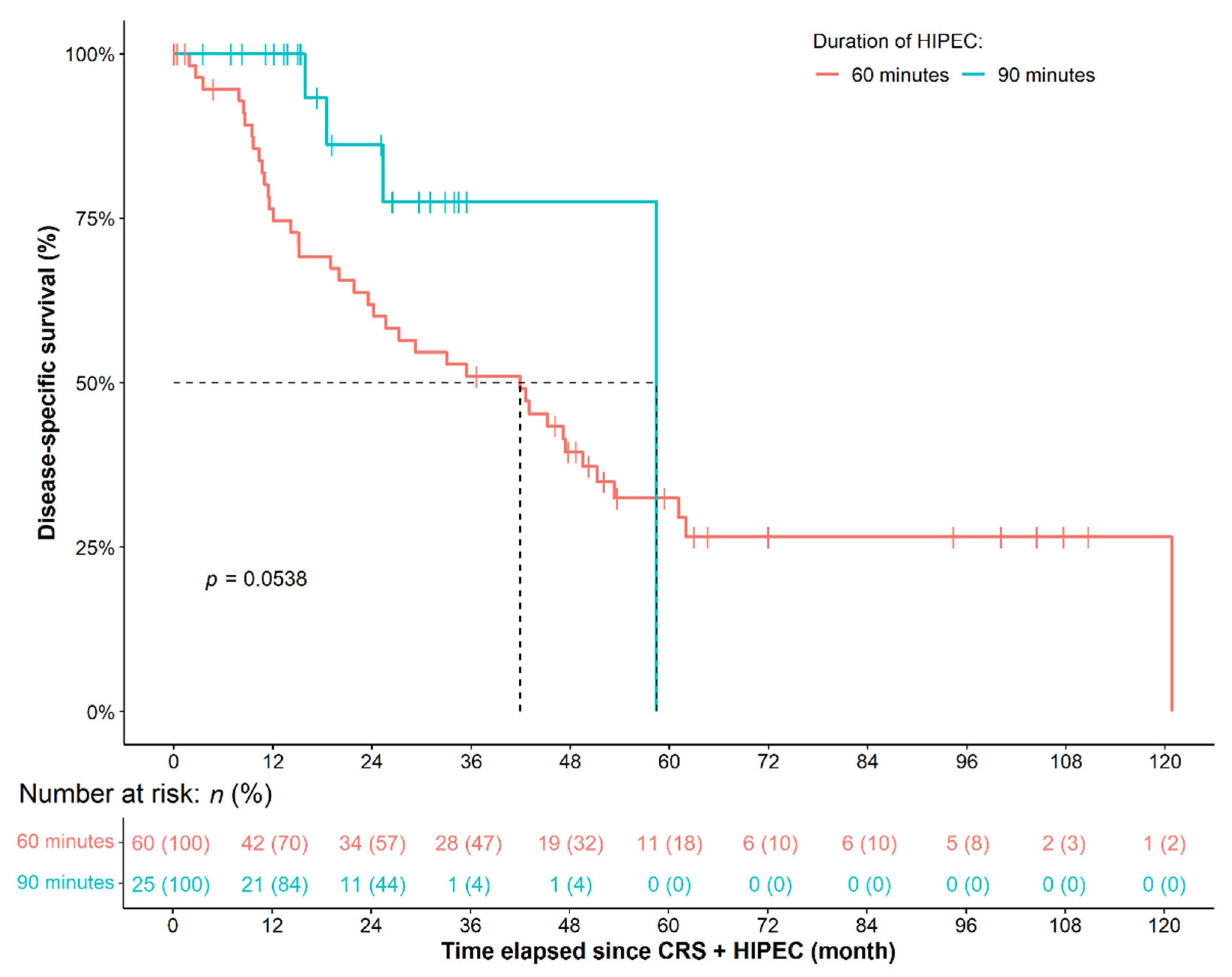
3.2. Does the Duration of HIPEC Affect Clinical Parameters and Patient Survival?
3.3. Analysis of Other Parameters on Patient Survival Data
4. Discussion
Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lowe, K.A.; Chia, V.M.; Taylor, A.; O’Malley, C.; Kelsh, M.; Mohamed, M.; Mowat, F.S.; Goff, B. An international assessment of ovarian cancer incidence and mortality. Gynecol. Oncol. 2013, 130, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Colombo, N.; Sessa, C.; du Bois, A.; Ledermann, J.; McCluggage, W.G.; McNeish, I.; Morice, P.; Pignata, S.; Ray-Coquard, I.; Vergote, I.; et al. ESMO-ESGO consensus conference recommendations on ovarian cancer: Pathology and molecular biology, early and advanced stages, borderline tumours and recurrent diseasedagger. Ann. Oncol. 2019, 30, 672–705. [Google Scholar] [CrossRef] [Green Version]
- Coccolini, F.; Fugazzola, P.; Montori, G.; Ansaloni, L.; Chiarugi, M. Intraperitoneal chemotherapy for ovarian cancer with peritoneal metastases, systematic review of the literature and focused personal experience. J. Gastrointest. Oncol. 2021, 12, S144–S181. [Google Scholar] [CrossRef]
- Friedrich, M.; Zinn, W.; Kolnsberg, L.; Kraft, C.; Kuhn, W. Hyperthermic Intraperitoneal Chemotherapy (HIPEC) for Ovarian Cancer: Evaluation of Side Effects in a Single Institution Cohort. Anticancer Res. 2020, 40, 1481–1486. [Google Scholar] [CrossRef]
- Riggs, M.J.; Pandalai, P.K.; Kim, J.; Dietrich, C.S. Hyperthermic Intraperitoneal Chemotherapy in Ovarian Cancer. Diagnostics 2020, 10, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spiliotis, J.; Iavazzo, C.; Fotiou, A.; Kopanakis, N.; Terra, A.; Efstathiou, E.; Margari, C.; Tsiatas, M. Upfront or intermediate treatment of advanced ovarian cancer patients with cytoreduction plus HIPEC: Results of a retrospective study. J. Surg. Oncol. 2021, 123, 630–637. [Google Scholar] [CrossRef]
- Le Saux, O.; Decullier, E.; Freyer, G.; Glehen, O.; Bakrin, N. Long-term survival in patients with epithelial ovarian cancer following cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC). Int. J. Hyperth. 2018, 35, 652–657. [Google Scholar] [CrossRef] [Green Version]
- Van Driel, W.J.; Koole, S.N.; Sikorska, K.; van Leeuwen, J.H.S.; Schreuder, H.W.; Hermans, R.H.; de Hingh, I.H.J.T.; van der Velden, J.; Arts, H.J.; Massuger, L.F.A.G.; et al. Hyperthermic intraperitoneal chemotherapy in ovarian cancer. N. Engl. J. Med. 2018, 378, 230–340. [Google Scholar] [CrossRef]
- Armstrong, D.K.; Alvarez, R.D.; Bakkum-Gamez, J.N.; Barroilhet, L.; Behbakht, K.; Berchuck, A.; Berek, J.S.; Chen, L.M.; Cristea, M.; DeRosa, M.; et al. NCCN Guidelines Insights: Ovarian Cancer, Version 1.2019. J. Natl. Compr. Cancer Netw. 2019, 17, 896–909. [Google Scholar] [CrossRef] [Green Version]
- Lavoue, V.; Huchon, C.; Akladios, C.; Alfonsi, P.; Bakrin, N.; Ballester, M.; Bendifallah, S.; Bolze, P.A.; Bonnet, F.; Bourgin, C.; et al. Management of epithelial cancer of the ovary, fallopian tube, primary peritoneum. Long text of the joint French clinical practice guidelines issued by FRANCOGYN, CNGOF, SFOG, GINECO-ARCAGY, endorsed by INCa. (Part 2: Systemic, intraperitoneal treatment, elderly patients, fertility preservation, follow-up). J. Gynecol. Obstet. Hum. Reprod. 2019, 48, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Tokunaga, H.; Mikami, M.; Nagase, S.; Kobayashi, Y.; Tabata, T.; Kaneuchi, M.; Satoh, T.; Hirashima, Y.; Matsumura, N.; Yokoyama, Y.; et al. The 2020 Japan Society of Gynecologic Oncology guidelines for the treatment of ovarian cancer, fallopian tube cancer, and primary peritoneal cancer. J. Gynecol. Oncol. 2021, 32, e49. [Google Scholar] [CrossRef] [PubMed]
- Redondo, A.; Guerra, E.; Manso, L.; Martin-Lorente, C.; Martinez-Garcia, J.; Perez-Fidalgo, J.A.; Varela, M.Q.; Rubio, M.J.; Barretina-Ginesta, M.P.; Gonzalez-Martin, A. SEOM clinical guideline in ovarian cancer (2020). Clin. Transl. Oncol. 2021, 23, 961–968. [Google Scholar] [CrossRef] [PubMed]
- Deutsche Krebsgesellschaft, D.K. AWMF. S3-Leitlinie Diagnostik, Therapie und Nachsorge maligner Ovarialtumoren Langversion 4.0, AWMF Registrierungsnummer: 032/035OL 2020.
- Di Giorgio, A.; Naticchioni, E.; Biacchi, D.; Sibio, S.; Accarpio, F.; Rocco, M.; Tarquini, S.; Di Seri, M.; Ciardi, A.; Montruccoli, D.; et al. Cytoreductive surgery (peritonectomy procedures) combined with hyperthermic intraperitoneal chemotherapy (HIPEC) in the treatment of diffuse peritoneal carcinomatosis from ovarian cancer. Cancer 2008, 113, 315–325. [Google Scholar] [CrossRef]
- Munoz-Casares, F.C.; Medina-Fernandez, F.J.; Arjona-Sanchez, A.; Casado-Adam, A.; Sanchez-Hidalgo, J.M.; Rubio, M.J.; Ortega-Salas, R.; Munoz-Villanueva, M.C.; Rufian-Pena, S.; Briceno, F.J. Peritonectomy procedures and HIPEC in the treatment of peritoneal carcinomatosis from ovarian cancer: Long-term outcomes and perspectives from a high-volume center. Eur. J. Surg. Oncol. 2015, 42, 224–233. [Google Scholar] [CrossRef]
- Lim, M.C.; Chang, S.J.; Yoo, H.J.; Nam, B.H.; Bristow, R.; Park, S.Y. Randomised trial of hyperthermic intraperitneal chemotherapy (HIPEC) in women with advanced peritoneal, ovarian and tubal cancer. J. Clin. Oncol. 2017, 35 (Suppl. S15), 5520. [Google Scholar] [CrossRef]
- Mutch, D.G.; Prat, J. 2014 FIGO staging for ovarian, fallopian tube and peritoneal cancer. Gynecol. Oncol. 2014, 133, 401–404. [Google Scholar] [CrossRef]
- Wright, A.A.; Bohlke, K.; Armstrong, D.K.; Bookman, M.A.; Cliby, W.A.; Coleman, R.L.; Dizon, D.S.; Kash, J.J.; Meyer, L.A.; Moore, K.N.; et al. Neoadjuvant chemotherapy for newly diagnosed, advanced ovarian cancer: Society of Gynecologic Oncology and American Society of Clinical Oncology Clinical Practice Guideline. Gynecol. Oncol. 2016, 143, 3–15. [Google Scholar] [CrossRef] [Green Version]
- Jacquet, P.; Sugarbaker, P.H. Current methodologies for clinical assessment of patients with peritoneal carcinomatosis. J. Exp. Clin. Cancer Res. 1996, 15, 49–58. [Google Scholar]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- Holm, S. A Simple Sequentially Rejective Multiple Test Procedure. Scand. J. Stat. 1979, 6, 65–70. [Google Scholar]
- Klar, M.; Hasenburg, A.; Hasanov, M.; Hilpert, F.; Meier, W.; Pfisterer, J.; Pujade-Lauraine, E.; Herrstedt, J.; Reuss, A.; du Bois, A. Prognostic factors in young ovarian cancer patients: An analysis of four prospective phase III intergroup trials of the AGO Study Group, GINECO and NSGO. Eur. J. Cancer 2016, 66, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Aletti, G.D.; Gostout, B.S.; Podratz, K.C.; Cliby, W.A. Ovarian cancer surgical resectability: Relative impact of disease, patient status, and surgeon. Gynecol. Oncol. 2006, 100, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Aletti, G.D.; Dowdy, S.C.; Gostout, B.S.; Jones, M.B.; Stanhope, C.R.; Wilson, T.O.; Podratz, K.C.; Cliby, W.A. Aggressive surgical effort and improved survival in advanced-stage ovarian cancer. Obstet. Gynecol. 2006, 107, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Torre, L.A.; Trabert, B.; DeSantis, C.E.; Miller, K.D.; Samimi, G.; Runowicz, C.D.; Gaudet, M.M.; Jemal, A.; Siegel, R.L. Ovarian cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 284–296. [Google Scholar] [CrossRef]
- Helm, C.W. The role of hyperthermic intraperitoneal chemotherapy (HIPEC) in ovarian cancer. Oncologist 2009, 14, 683–694. [Google Scholar] [CrossRef]
- Markman, M. Intraperitoneal chemotherapy in the management of malignant disease. Expert Rev. Anticancer Ther. 2001, 1, 142–148. [Google Scholar] [CrossRef]
- Armstrong, D.K.; Bundy, B.; Wenzel, L.; Huang, H.Q.; Baergen, R.; Lele, S.; Copeland, L.J.; Walker, J.L.; Burger, R.A.; Gynecologic Oncology, G. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N. Engl. J. Med. 2006, 354, 34–43. [Google Scholar] [CrossRef] [Green Version]
- Tewari, D.; Java, J.J.; Salani, R.; Armstrong, D.K.; Markman, M.; Herzog, T.; Monk, B.J.; Chan, J.K. Long-term survival advantage and prognostic factors associated with intraperitoneal chemotherapy treatment in advanced ovarian cancer: A gynecologic oncology group study. J. Clin. Oncol. 2015, 33, 1460–1466. [Google Scholar] [CrossRef]
- Wright, A.A.; Cronin, A.; Milne, D.E.; Bookman, M.A.; Burger, R.A.; Cohn, D.E.; Cristea, M.C.; Griggs, J.J.; Keating, N.L.; Levenback, C.F.; et al. Use and Effectiveness of Intraperitoneal Chemotherapy for Treatment of Ovarian Cancer. J. Clin. Oncol. 2015, 33, 2841–2847. [Google Scholar] [CrossRef]
- Istomin, Y.P.; Zhavrid, E.A.; Alexandrova, E.N.; Sergeyeva, O.P.; Petrovich, S.V. Dose enhancement effect of anticaner drugs associated with increased temperature in vitro. Exp. Oncol. 2008, 30, 56–59. [Google Scholar] [PubMed]
- Rietbroek, R.C.; van de Vaart, P.J.; Haveman, J.; Blommaert, F.A.; Geerdink, A.; Bakker, P.J.; Veenhof, C.H. Hyperthermia enhances the cytotoxicity and platinum-DNA adduct formation of lobaplatin and oxaliplatin in cultured SW 1573 cells. J. Cancer Res. Clin. Oncol. 1997, 123, 6–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Helderman, R.; Loke, D.R.; Kok, H.P.; Oei, A.L.; Tanis, P.J.; Franken, N.; Crezee, J. Variation in Clinical Application of Hyperthermic Intraperitoneal Chemotherapy: A Review. Cancers 2019, 11, 78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Ren, F.; Chen, P.; Liu, S.; Song, Z.; Ma, X. Effects of CytoReductive surgery plus hyperthermic IntraPEritoneal chemotherapy (HIPEC) versus CytoReductive surgery for ovarian cancer patients: A systematic review and meta-analysis. Eur. J. Surg. Oncol. 2019, 45, 301–309. [Google Scholar] [CrossRef]
- Acs, M.; Halmy, L.; Isgandarova, S.; Blaj, S.; Gerken, M.; Hormann, B.; Piso, P. Hyperthermic Intraperitoneal Chemotherapy with Cisplatin and Doxorubicin for 90 Minutes Versus 60 Minutes after Cytoreductive Surgery (CRS). Does the 30-Minute Difference Matter? A Comparative Study in a High Volume Centre. Anticancer Res. 2022, 42, 1019–1029. [Google Scholar] [CrossRef]
- Mueller, J.J.; Zhou, Q.C.; Iasonos, A.; O’Cearbhaill, R.E.; Alvi, F.A.; El Haraki, A.; Eriksson, A.G.; Gardner, G.J.; Sonoda, Y.; Levine, D.A.; et al. Neoadjuvant chemotherapy and primary debulking surgery utilization for advanced-stage ovarian cancer at a comprehensive cancer center. Gynecol. Oncol. 2016, 140, 436–442. [Google Scholar] [CrossRef] [Green Version]
- Fago-Olsen, C.L.; Ottesen, B.; Kehlet, H.; Antonsen, S.L.; Christensen, I.J.; Markauskas, A.; Mosgaard, B.J.; Ottosen, C.; Soegaard, C.H.; Soegaard-Andersen, E.; et al. Does neoadjuvant chemotherapy impair long-term survival for ovarian cancer patients? A nationwide Danish study. Gynecol. Oncol. 2014, 132, 292–298. [Google Scholar] [CrossRef]
- Leary, A.; Cowan, R.; Chi, D.; Kehoe, S.; Nankivell, M. Primary Surgery or Neoadjuvant Chemotherapy in Advanced Ovarian Cancer: The Debate Continues. Am. Soc. Clin. Oncol. Educ. Book 2016, 35, 153–162. [Google Scholar] [CrossRef]
- Vergote, I.; Trope, C.G.; Amant, F.; Kristensen, G.B.; Ehlen, T.; Johnson, N.; Verheijen, R.H.; van der Burg, M.E.; Lacave, A.J.; Panici, P.B.; et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N. Engl. J. Med. 2010, 363, 943–953. [Google Scholar] [CrossRef] [Green Version]
- Kehoe, S.; Hook, J.; Nankivell, M.; Jayson, G.C.; Kitchener, H.; Lopes, T.; Luesley, D.; Perren, T.; Bannoo, S.; Mascarenhas, M.; et al. Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): An open-label, randomised, controlled, non-inferiority trial. Lancet 2015, 386, 249–257. [Google Scholar] [CrossRef]
- Marrelli, D.; Petrioli, R.; Cassetti, D.; D’Ignazio, A.; Marsili, S.; Mazzei, M.A.; Lazzi, S.; Roviello, F. A novel treatment protocol with 6 cycles of neoadjuvant chemotherapy followed by cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC) in stage III primary ovarian cancer. Surg. Oncol. 2021, 37, 101523. [Google Scholar] [CrossRef] [PubMed]
- Fotopoulou, C. Neoadjuvant chemotherapy for advanced ovarian cancer: The tail of the scorpion for radical debulking surgery? Int. J. Gynecol. Cancer 2020, 30, 1665–1666. [Google Scholar] [CrossRef] [PubMed]
- Timmermans, M.; van der Hel, O.; Sonke, G.S.; Van de Vijver, K.K.; van der Aa, M.A.; Kruitwagen, R.F. The prognostic value of residual disease after neoadjuvant chemotherapy in advanced ovarian cancer; A systematic review. Gynecol. Oncol. 2019, 153, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Tate, S.; Nishikimi, K.; Kato, K.; Matsuoka, A.; Kambe, M.; Kiyokawa, T.; Shozu, M. Microscopic diseases remain in initial disseminated sites after neoadjuvant chemotherapy for stage III/IV ovarian, tubal, and primary peritoneal cancer. J. Gynecol. Oncol. 2020, 31, e34. [Google Scholar] [CrossRef] [Green Version]
- Carboni, F.; Federici, O.; Sperduti, I.; Zazza, S.; Sergi, D.; Corona, F.; Valle, M. Cytoreductive Surgery with Hyperthermic Intraperitoneal Chemotherapy for Peritoneal Carcinomatosis from Epithelial Ovarian Cancer: A 20-Year Single-Center Experience. Cancers 2021, 13, 523. [Google Scholar] [CrossRef]
- Bartels, H.C.; Rogers, A.C.; McSharry, V.; McVey, R.; Walsh, T.; O’Brien, D.; Boyd, W.D.; Brennan, D.J. A meta-analysis of morbidity and mortality in primary cytoreductive surgery compared to neoadjuvant chemotherapy in advanced ovarian malignancy. Gynecol. Oncol. 2019, 154, 622–630. [Google Scholar] [CrossRef]
- Fagotti, A.; Ferrandina, M.G.; Vizzielli, G.; Pasciuto, T.; Fanfani, F.; Gallotta, V.; Margariti, P.A.; Chiantera, V.; Costantini, B.; Alletti, S.G.; et al. Randomized trial of primary debulking surgery versus neoadjuvant chemotherapy for advanced epithelial ovarian cancer (SCORPION-NCT01461850). Int. J. Gynecol. Cancer. 2020, 30, 1657–1664. [Google Scholar] [CrossRef]
- Bristow, R.E.; Tomacruz, R.S.; Armstrong, D.K.; Trimble, E.L.; Montz, F.J. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: A meta-analysis. J. Clin. Oncol. 2002, 20, 1248–1259. [Google Scholar] [CrossRef]
- Voelker, R. Lighting the Way for Improved Detection of Ovarian Cancer. JAMA 2022, 327, 27. [Google Scholar] [CrossRef]
- Reuss, A.; du Bois, A.; Harter, P.; Fotopoulou, C.; Sehouli, J.; Aletti, G.; Guyon, F.; Greggi, S.; Mosgaard, B.J.; Reinthaller, A.; et al. TRUST: Trial of Radical Upfront Surgical Therapy in advanced ovarian cancer (ENGOT ov33/AGO-OVAR OP7). Int. J. Gynecol. Cancer 2019, 29, 1327–1331. [Google Scholar] [CrossRef]
- Liu, Y.L.; Filippova, O.T.; Zhou, Q.; Iasonos, A.; Chi, D.S.; Zivanovic, O.; Sonoda, Y.; Gardner, G.J.; Broach, V.A.; O’Cearbhaill, R.E.; et al. Characteristics and survival of ovarian cancer patients treated with neoadjuvant chemotherapy but not undergoing interval debulking surgery. J. Gynecol. Oncol. 2020, 31, e17. [Google Scholar] [CrossRef] [PubMed]



| Clinical Characteristics | Total (n = 85) | Upfront HIPEC (n = 37) | Interval HIPEC (n = 48) | Crude p-Value | p-Value | 60 min HIPEC (n = 60) | 90 min HIPEC (n = 25) | Crude p-Value | p-Value |
|---|---|---|---|---|---|---|---|---|---|
| Age (year) | 62.16 ± 10.29 | 61.98 ± 12.33 | 62.52 ± 8.57 | 1.0000 | 62.50 ± 10.87 | 61.77 ± 9.01 | 1.0000 | ||
| Body mass index (kg/m2) | 26.28 ± 5.39 | 26.74 ± 5.51 | 25.91 ± 5.29 | 1.0000 | 26.12 ± 5.00 | 26.62 ± 6.28 | 1.0000 | ||
| ASA score | 1.0000 | 1.0000 | |||||||
| 20 (23.53%) | 9 (24.32%) | 11 (22.92%) | 13 (21.67%) | 7 (28%) | ||||
| 65 (76.47%) | 28 (75.68%) | 37 (77.08%) | 47 (78.33%) | 18 (72%) | ||||
| Histology | 1.0000 | 1.0000 | |||||||
| 61 (71.76%) | 27 (72.97%) | 34 (70.83%) | 42 (70.00%) | 19 (76%) | ||||
| 21 (24.71%) | 9 (24.32%) | 12 (25%) | 15 (25.00%) | 6 (24%) | ||||
| 1 (1.18%) | 0 (0%) | 1 (2.08%) | 1 (1.67%) | 0 (0%) | ||||
| 1 (1.18%) | 1 (2.7%) | 0 (0%) | 1 (1.67%) | 0 (0%) | ||||
| 1 (1.18%) | 0 (0%) | 1 (2.08%) | 1 (1.67%) | 0 (0%) | ||||
| FIGO Stage | 0.0025 | 0.1496 | 1.0000 | ||||||
| 11 (12.94%) | 5 (13.51%) | 6 (12.5%) | 9 (15.00%) | 2 (8%) | ||||
| 55 (64.71%) | 19 (51.35%) | 36 (75%) | 36 (60.00%) | 19 (76%) | ||||
| 3 (3.53%) | 0 (0%) | 3 (6.25%) | 3 (5.00%) | 0 (0%) | ||||
| 16 (18.82%) | 13 (35.14%) | 3 (6.25%) | 12 (20.00%) | 4 (16%) | ||||
| Cause of FIGO IV | |||||||||
| 7 (8.24%) | 5 (13.51%) | 2 (4.17%) | 1.0000 | 4 (6.67%) | 3 (12%) | 1.0000 | ||
| 2 (2.35%) | 1 (2.7%) | 1 (2.08%) | 1.0000 | 2 (3.33%) | 0 (0%) | 1.0000 | ||
| 6 (7.06%) | 6 (16.22%) | 0 (0%) | 0.0053 | 0.3136 | 6 (10.00%) | 0 (0%) | 1.0000 | |
| 1 (1.18%) | 1 (2.7%) | 0 (0%) | 1.0000 | 1 (1.67%) | 0 (0%) | 1.0000 | ||
| 3 (3.53%) | 1 (2.7%) | 2 (4.17%) | 1.0000 | 3 (5.00%) | 0 (0%) | 1.0000 | ||
| 1 (1.18%) | 1 (2.7%) | 0 (0%) | 1.0000 | 0 (0%) | 1 (4%) | 1.0000 | ||
| 1 (1.18%) | 0 (0%) | 1 (2.08%) | 1.0000 | 1 (1.67%) | 0 (0%) | 1.0000 | ||
| Cycles of carboplatin/TAX NACT | – | 0.1273 | 1.0000 | ||||||
| 17 (20%) | 0 (0%) | 17 (35.42%) | 9 (15.00%) | 8 (32%) | ||||
| 9 (10.59%) | 0 (0%) | 9 (18.75%) | 7 (11.67%) | 2 (8%) | ||||
| 22 (25.88%) | 0 (0%) | 22 (45.83%) | 13 (21.67%) | 9 (36%) | ||||
| Peritoneal carcinomatosis index | 11.41 ± 6.45 | 15.00 ± 4.96 | 8.60 ± 5.79 | <0.0001 | 12.92 ± 5.65 | 7.72 ± 6.29 | 0.0009 | 0.0593 | |
| CC score | 1.0000 | 1.0000 | |||||||
| 67 (78.82%) | 30 (81.08%) | 37 (77.08%) | 44 (73.33%) | 23 (92%) | ||||
| 16 (18.82%) | 6 (16.22%) | 10 (20.83%) | 14 (23.33%) | 2 (8%) | ||||
| 2 (2.35%) | 1 (2.7%) | 1 (2.08%) | 2 (3.33%) | 0 (0%) | ||||
| Cisplatin (mg) | 132 ± 16.46 | 134.34 ± 17.37 | 130.20 ± 15.67 | 1.0000 | 130.69 ± 14.75 | 135.16 ± 19.97 | 1.0000 | ||
| Doxorubicin (mg) | 26.58 ± 3.10 | 27.01 ± 3.17 | 26.11 ± 2.91 | 1.0000 | 26.28 ± 2.54 | 27.04 ± 4.00 | 1.0000 | ||
| Duration of HIPEC (min) | 0.0298 | 1.0000 | – | ||||||
| 60 (70.59%) | 31 (83.78%) | 29 (60.42%) | – | – | ||||
| 25 (29.41%) | 6 (16.22%) | 19 (39.58%) | – | – | ||||
| Type of HIPEC | – | 0.0298 | 1.0000 | ||||||
| 37 (43.53%) | – | – | 31 (51.67%) | 6 (24%) | ||||
| 48 (56.47%) | – | – | 29 (48.33%) | 19 (76%) | ||||
| Length of surgery excl. HIPEC (min) | 317 ± 94 | 353 ± 77 | 290.38 ± 97.59 | 0.0015 | 0.0892 | 336.87 ± 74.26 | 270.80 ± 118.43 | 0.0145 | 0.8676 |
| Surgical procedures | |||||||||
| 74 (87.06%) | 34 (91.89%) | 40 (83.33%) | 1.0000 | 52 (86.67%) | 22 (88%) | 1.0000 | ||
| 74 (87.06%) | 31 (83.78%) | 43 (89.58%) | 1.0000 | 54 (90.00%) | 20 (80%) | 1.0000 | ||
| 24 (28.24%) | 15 (40.54%) | 9 (18.75%) | 0.0317 | 1.0000 | 15 (25.00%) | 9 (36%) | 1.0000 | |
| 58 (68.24%) | 30 (81.08%) | 28 (58.33%) | 0.0345 | 1.0000 | 41 (68.33%) | 17 (68%) | 1.0000 | |
| 35 (41.18%) | 19 (51.35%) | 16 (33.33%) | 1.0000 | 30 (50%) | 5 (20%) | 0.0149 | 0.8814 | |
| 12 (14.12%) | 4 (10.81%) | 8 (16.67%) | 1.0000 | 9 (15.00%) | 3 (12%) | 1.0000 | ||
| 7 (8.24%) | 3 (8.11%) | 4 (8.33%) | 1.0000 | 5 (8.33%) | 2 (8%) | 1.0000 | ||
| 2 (2.35%) | 0 (0%) | 2 (4.17%) | 1.0000 | 2 (3.33%) | 0 (0%) | 1.0000 | ||
| 25 (29.41%) | 14 (37.84%) | 11 (22.92%) | 1.0000 | 19 (31.67%) | 6 (24%) | 1.0000 | ||
| 3 (3.53%) | 3 (8.11%) | 0 (0%) | 1.0000 | 3 (5.00%) | 0 (0%) | 1.0000 | ||
| 39 (45.88%) | 21 (56.76%) | 18 (37.50%) | 1.0000 | 29 (48.33%) | 10 (40%) | 1.0000 | ||
| 7 (8.24%) | 4 (10.81%) | 3 (6.25%) | 1.0000 | 5 (8.33%) | 2 (8%) | 1.0000 | ||
| 3 (3.53%) | 3 (8.11%) | 0 (0%) | 1.0000 | 3 (5.00%) | 0 (0%) | 1.0000 | ||
| 25 (29.41%) | 16 (43.24%) | 9 (18.75%) | 0.0159 | 0.9220 | 23 (38.33%) | 2 (8%) | 0.0080 | 0.5015 |
| 46 (54.12%) | 29 (78.38%) | 17 (35.42%) | 0.0055 | 38 (63.33%) | 8 (32%) | 0.0097 | 0.5947 | |
| 15 (17.65%) | 7 (18.92%) | 8 (16.67%) | 1.0000 | 11 (18.33%) | 4 (16%) | 1.0000 | ||
| 43 (50.59%) | 27 (72.97%) | 16 (33.33%) | 0.0260 | 36 (60.00%) | 7 (28%) | 0.0091 | 0.5635 | |
| 13 (15.29%) | 7 (18.92%) | 6 (12.50%) | 1.0000 | 11 (18.33%) | 2 (8%) | 1.0000 | ||
| 1 (1.18%) | 0 (0%) | 1 (2.08%) | 1.0000 | 1 (1.67%) | 0 (0%) | 1.0000 | ||
| 48 (56.47%) | 21 (56.76%) | 27 (56.25%) | 1.0000 | 37 (61.67%) | 11 (44%) | 1.0000 | ||
| 13 (15.29%) | 8 (21.62%) | 5 (10.42%) | 1.0000 | 9 (15.00%) | 4 (16%) | 1.0000 | ||
| 1 (1.18%) | 1 (2.70%) | 0 (0%) | 1.0000 | 1 (1.67%) | 0 (0%) | 1.0000 | ||
| 76 (89.41%) | 34 (91.89%) | 42 (87.50%) | 1.0000 | 53 (88.33%) | 23 (92%) | 1.0000 | ||
| 34 (40%) | 19 (51.35%) | 15 (31.25%) | 1.0000 | 25 (41.67%) | 9 (36%) | 1.0000 | ||
| 9 (10.59%) | 6 (16.22%) | 3 (6.25%) | 1.0000 | 7 (11.67%) | 2 (8%) | 1.0000 | ||
| 6 (7.06%) | 3 (8.11%) | 3 (6.25%) | 1.0000 | 5 (8.33%) | 1 (4%) | 1.0000 | ||
| 60 (70.59%) | 25 (67.57%) | 35 (72.92%) | 1.0000 | 45 (75.00%) | 15 (60%) | 1.0000 | ||
| 65 (76.47%) | 28 (75.68%) | 37 (77.08%) | 1.0000 | 43 (71.67%) | 22 (88%) | 1.0000 | ||
| Length of hospital stay (day) | 22.76 ± 12.29 | 24.16 ± 9.33 | 21.79 ± 14.37 | 1.0000 | 24.38 ± 13.39 | 19.08 ± 8.89 | 1.0000 | ||
| Length of ICU stay (day) | 5.23 ± 5.25 | 5.46 ± 5.28 | 5.06 ± 5.37 | 1.0000 | 5.72 ± 5.72 | 4.08 ± 3.99 | 0.0361 | 1.0000 | |
| Blood transfusion | |||||||||
| 1.46 ± 2.20 | 1.30 ± 2.26 | 1.46 ± 2.00 | 1.0000 | 1.60 ± 2.28 | 0.88 ± 1.54 | 0.0511 | 1.0000 | |
| 3.45 ± 3.98 | 4.22 ± 5.03 | 2.73 ± 2.53 | 1.0000 | 3.73 ± 4.11 | 2.52 ± 3.14 | 1.0000 | ||
| 3:4:1 (3.53:4.71:1.18%) | 0:1:0 (0:2.70:0%) | 3:3:1 (6.25:6.25:2.08%) | 1.0000 | 0:0:0 (0:0:0%) | 3:4:1 (12:16:8%) | 0.0015 | ||
| Pleura punction | 8 (9.41%) | 4 (%) | 4 (8.33%) | 1.0000 | 5 (8.33%) | 3 (12%) | 1.0000 | ||
| Dialysis | 3 (3.53%) | 1 (2.70%) | 2 (4.17%) | 1.0000 | 2 (3.33%) | 1 (4%) | 1.0000 | ||
| Complication grade (Clavien–Dindo) | 1.0000 | 1.0000 | |||||||
| 37 (43.53%) | 18 (48.65%) | 19 (39.58%) | 29 (48.33%) | 8 (32%) | ||||
| 22 (25.88%) | 12 (32.43%) | 10 (20.83%) | 14 (23.33%) | 8 (32%) | ||||
| 3 (3.53%) | 1 (2.70%) | 2 (4.17%) | 2 (3.33%) | 1 (4%) | ||||
| 2 (2.35%) | 0 (0%) | 2 (4.17%) | 2 (3.33%) | 0 (0%) | ||||
| Complications | |||||||||
| 2 (2.35%) | 2 (5.41%) | 0 (0%) | 1.0000 | 1 (1.67%) | 1 (4%) | 1.0000 | ||
| 7 (8.24%) | 2 (5.41%) | 5 (10.42%) | 1.0000 | 6 (10.00%) | 1 (4%) | 1.0000 | ||
| 4 (4.71%) | 2 (5.41%) | 2 (4.17%) | 1.0000 | 4 (6.67%) | 0 (0%) | 1.0000 | ||
| 20 (23.53%) | 10 (27.03%) | 10 (20.83%) | 1.0000 | 15 (25.00%) | 5 (20%) | 1.0000 | ||
| 21 (24.71%) | 11 (29.73%) | 10 (20.83%) | 1.0000 | 16 (26.67%) | 5 (20%) | 1.0000 | ||
| 3 (3.53%) | 1 (2.70%) | 2 (4.17%) | 1.0000 | 2 (3.33%) | 1 (4%) | 1.0000 | ||
| 7 (8.24%) | 2 (5.41%) | 5 (10.42%) | 1.0000 | 3 (5.00%) | 4 (16%) | 1.0000 | ||
| 10 (11.76%) | 7 (18.92%) | 3 (6.25%) | 1.0000 | 9 (15.00%) | 1 (4%) | 1.0000 | ||
| 2 (2.35%) | 0 (0%) | 2 (4.17%) | 1.0000 | 2 (3.33%) | 0 (0%) | 1.0000 | ||
| 12 (14.12%) | 5 (13.51%) | 7 (14.58%) | 1.0000 | 8 (13.33%) | 4 (16%) | 1.0000 | ||
| Median overall survival (month) | 42.58 | 43.00 | 47.44 | – | 31.16 | 58.41 | – | ||
| Median DSS (month) | 45.24 | 45.24 | 47.44 | – | 41.92 | 58.41 | – | ||
| Median RFS (month) | – 1 | – 1 | 59.73 | – | 59.73 | – 1 | – |
| Clinical Characteristics | DSS | OS | RFS | |||
|---|---|---|---|---|---|---|
| Univariate p-Value | Multivariate p-Value | Univariate p-Value | Multivariate p-Value | Univariate p-Value | Multivariate p-Value | |
| Age (years) | 0.0307 | 0.0113 | 0.0088 | 0.0416 | 0.6850 | 0.4184 |
| Body mass index (kg/m2) | 0.0230 | 0.0004 | 0.0301 | 0.0044 | 0.6630 | 0.7968 |
| ASA score | <0.0001 | <0.0001 | 0.2821 | 0.1319 | <0.0001 | <0.0001 |
| FIGO Stage | 0.4550 | <0.0001 | 0.3085 | 0.2154 | <0.0001 | <0.0001 |
| CC score | 0.0921 | 0.1617 | 0.0888 | 0.2351 | 0.3446 | 0.1521 |
| Peritoneal carcinomatosis index | 0.0724 | < 0.0001 | 0.1040 | 0.0062 | 0.7090 | 0.1075 |
| Type of HIPEC (upfront vs. interval) | 0.3670 | < 0.0001 | 0.2410 | 0.0016 | 0.8240 | 0.1865 |
| Duration of HIPEC (60 vs. 90 min) | 0.0538 | 0.0030 | 0.0330 | 0.0012 | 0.2070 | 0.0959 |
| Complication grade (Clavien–Dindo) | 0.1340 | – 1 | 0.0018 | 0.0041 | <0.0001 | <0.0001 |
| Any incomplete tumor removal surgery prior CRS + HIPEC | 0.4300 | 0.0655 | 0.4290 | 0.2552 | 0.8740 | 0.9810 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acs, M.; Herold, Z.; Szasz, A.M.; Mayr, M.; Häusler, S.; Piso, P. Prolonged Exposition with Hyperthermic Intraperitoneal Chemotherapy (HIPEC) May Provide Survival Benefit after Cytoreductive Surgery (CRS) in Advanced Primary Epithelial Ovarian, Fallopian Tube, and Primary Peritoneal Cancer. Cancers 2022, 14, 3301. https://doi.org/10.3390/cancers14143301
Acs M, Herold Z, Szasz AM, Mayr M, Häusler S, Piso P. Prolonged Exposition with Hyperthermic Intraperitoneal Chemotherapy (HIPEC) May Provide Survival Benefit after Cytoreductive Surgery (CRS) in Advanced Primary Epithelial Ovarian, Fallopian Tube, and Primary Peritoneal Cancer. Cancers. 2022; 14(14):3301. https://doi.org/10.3390/cancers14143301
Chicago/Turabian StyleAcs, Miklos, Zoltan Herold, Attila Marcell Szasz, Max Mayr, Sebastian Häusler, and Pompiliu Piso. 2022. "Prolonged Exposition with Hyperthermic Intraperitoneal Chemotherapy (HIPEC) May Provide Survival Benefit after Cytoreductive Surgery (CRS) in Advanced Primary Epithelial Ovarian, Fallopian Tube, and Primary Peritoneal Cancer" Cancers 14, no. 14: 3301. https://doi.org/10.3390/cancers14143301
APA StyleAcs, M., Herold, Z., Szasz, A. M., Mayr, M., Häusler, S., & Piso, P. (2022). Prolonged Exposition with Hyperthermic Intraperitoneal Chemotherapy (HIPEC) May Provide Survival Benefit after Cytoreductive Surgery (CRS) in Advanced Primary Epithelial Ovarian, Fallopian Tube, and Primary Peritoneal Cancer. Cancers, 14(14), 3301. https://doi.org/10.3390/cancers14143301






