Immunohistochemical Detection of the Presence of Vitamin D Receptor in Childhood Solid Tumors
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient and Sample Selection
2.2. Tissue Microarrays
2.3. Immunohistochemical Analysis
2.4. Statistical Analysis
3. Results
3.1. Measurement of VDR Expression
3.2. VDR Expression and Survival
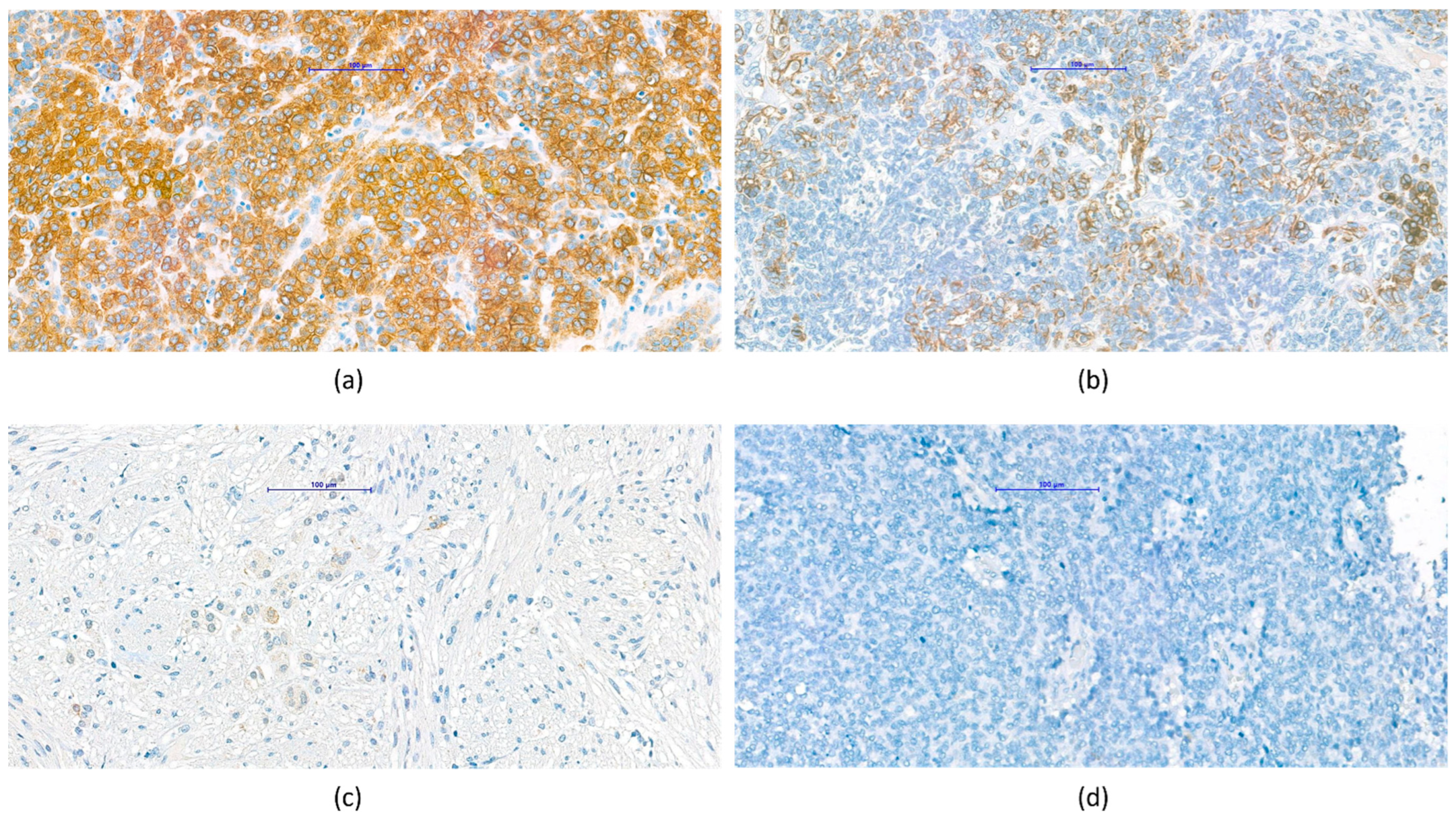
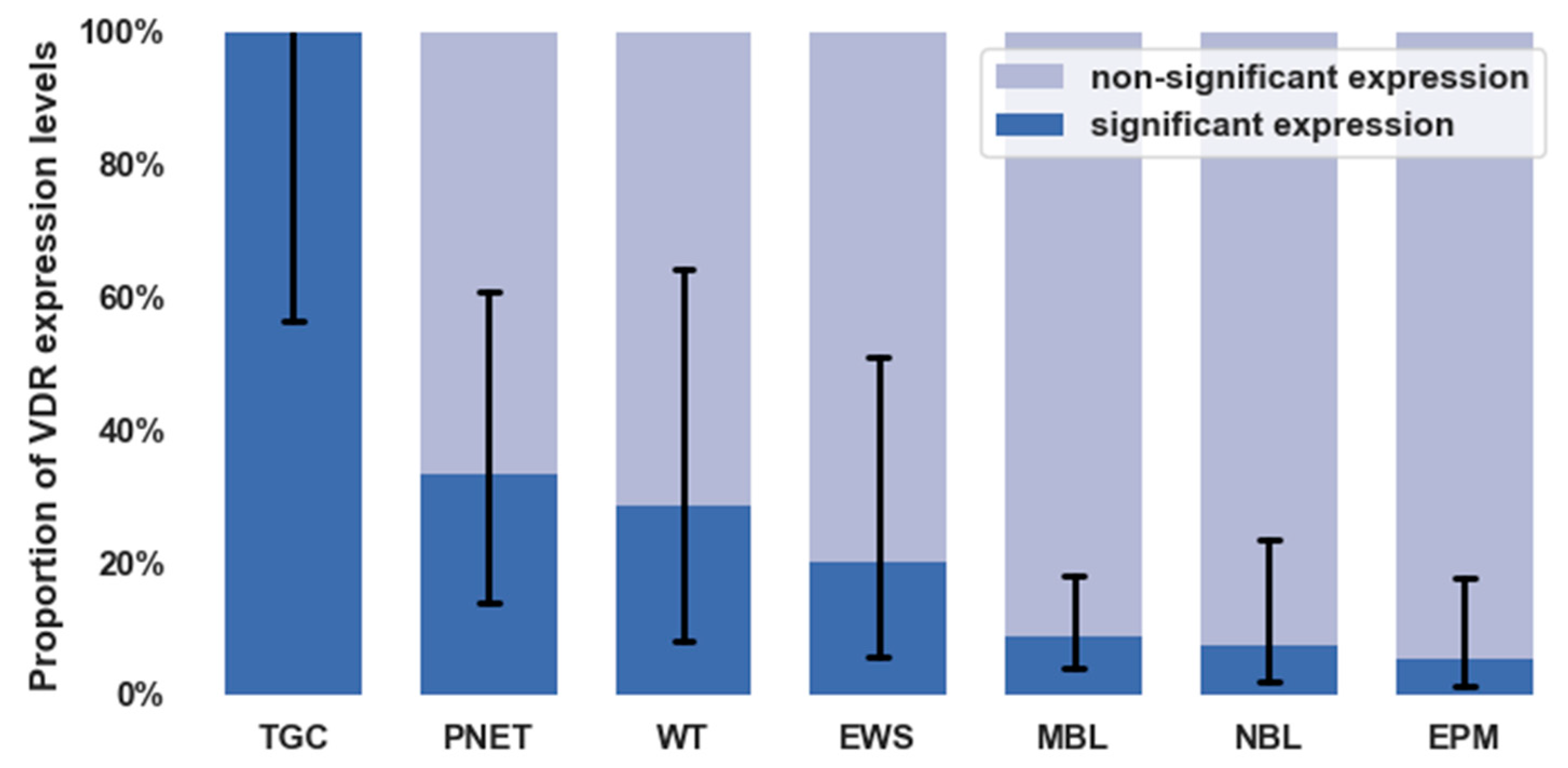
3.3. VDR Expression in Different Types of Childhood Solid Tumors
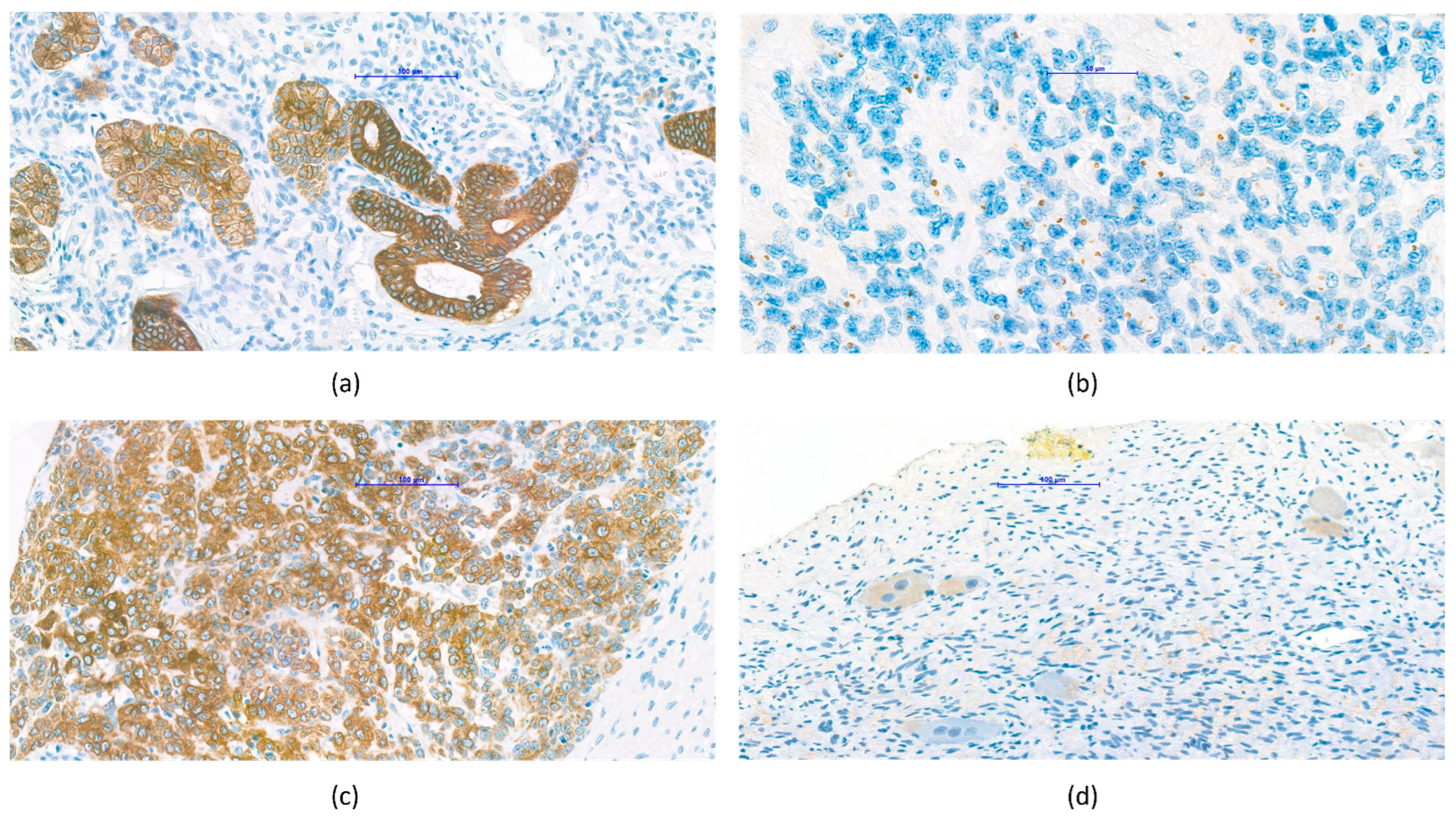
3.4. Pathological Observations Regarding VDR Characteristics of Tumor Types
3.4.1. Nephroblastoma (Wilms’ Tumor)
3.4.2. Central Nervous System Tumors
3.4.3. Hepatoblastoma
3.4.4. Neuroblastoma, Ganglioneuroma
4. Discussions
4.1. Need for New Biomarkers
4.2. Role of Vitamin D in Cancer Prevention and Therapy
4.3. VDR Expression and Survival
4.4. Pathological Observations Regarding VDR Characteristics of Tumor Types
4.4.1. Nephroblastoma (Wilms’ Tumor)
4.4.2. Central Nervous System Tumors
4.4.3. Hepatoblastoma
4.4.4. Neuroblastoma, Ganglioneuroma
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| VDR | vitamin D receptor |
| WHO | World Health Organization |
| PNET | primitive neuro-ectodermal tumor |
| TMA | tissue microarray |
| OR | odds ratio |
| DNA | deoxyribonucleic acid |
| IHC | immunohistochemistry |
| NBL | neuroblastoma |
| TGC | thyroid gland carcinoma |
| WT | Wilms’ tumor |
| EWS | Ewing sarcoma |
| MBL | medulloblastoma |
| EPM | ependymoma |
References
- Zhao, Y.; Sun, P.; Xiao, J.; Jin, L.; Ma, N.; Li, Z.; Feng, G.; Huang, H.; Deziel, N.C.; Ma, X.; et al. International patterns and trends of childhood and adolescent cancer, 1978–2012. J. Natl. Cancer Cent. 2022, 2, 78–89. [Google Scholar] [CrossRef]
- Pritchard-Jones, K.; Pieters, R.; Reaman, G.H.; Hjorth, L.; Downie, P.; Calaminus, G.; Naafs-Wilstra, M.C.; Steliarova-Foucher, E. Sustaining innovation and improvement in the treatment of childhood cancer: Lessons from high-income countries. Lancet Oncol. 2013, 14, e95–e103. [Google Scholar] [CrossRef]
- Smith, L.; Stiller, C.A.; Aitken, J.F.; Hjalgrim, L.L.; Johannesen, T.; Lahteenmaki, P.; McCabe, M.G.; Phillips, R.; Pritchard-Jones, K.; Steliarova-Foucher, E.; et al. International variation in childhood cancer mortality rates from 2001 to 2015: Comparison of trends in the International Cancer Benchmarking Partnership countries. Int. J. Cancer 2022, 150, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Gatta, G.; Botta, L.; Rossi, S.; Aareleid, T.; Bielska-Lasota, M.; Clavel, J.; Dimitrova, N.; Jakab, Z.; Kaatsch, P.; Lacour, B.; et al. Childhood cancer survival in Europe 1999–2007: Results of EUROCARE-5—A population-based study. Lancet Oncol. 2014, 15, 35–47. [Google Scholar] [CrossRef]
- Bosetti, C.; Bertuccio, P.; Chatenoud, L.; Negri, E.; Levi, F.; La Vecchia, C. Childhood cancer mortality in Europe, 1970–2007. Eur. J. Cancer 2010, 46, 384–394. [Google Scholar] [CrossRef]
- Carlberg, C.; Muñoz, A. An update on vitamin D signaling and cancer. Semin. Cancer Biol. 2022, 79, 217–230. [Google Scholar] [CrossRef]
- Jeon, S.M.; Shin, E.A. Exploring vitamin D metabolism and function in cancer. Exp. Mol. Med. 2018, 50, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Vanhevel, J.; Verlinden, L.; Doms, S.; Wildiers, H.; Verstuyf, A. The role of vitamin D in breast cancer risk and progression. Endocr.-Relat. Cancer 2022, 29, R33–R55. [Google Scholar] [CrossRef]
- Fiz, C.; Apprato, G.; Ricca, C.; Aillon, A.; Bergandi, L.; Silvagno, F. TGF Beta Induces Vitamin D Receptor and Modulates Mitochondrial Activity of Human Pancreatic Cancer Cells. Cancers 2021, 13, 2932. [Google Scholar] [CrossRef]
- Bhutia, S.K. Vitamin D in autophagy signaling for health and diseases: Insights on potential mechanisms and future perspectives. J. Nutr. Biochem. 2022, 99, 108841. [Google Scholar] [CrossRef]
- Shariev, A.; Painter, N.; Reeve, V.E.; Haass, N.K.; Rybchyn, M.S.; Ince, F.A.; Mason, R.S.; Dixon, K.M. PTEN: A novel target for vitamin D in melanoma. J. Steroid Biochem. Mol. Biol. 2022, 218, 106059. [Google Scholar] [CrossRef] [PubMed]
- Molinari, C.G. Biological Effects of Vitamin D3 Mediated by the Interaction with VDR in Different Tissues. Available online: https://core.ac.uk/download/pdf/327087351.pdf (accessed on 31 March 2022).
- Zmijewski, M.A.; Carlberg, C. Vitamin D receptor (s): In the nucleus but also at membranes? Exp. Dermatol. 2020, 29, 876–884. [Google Scholar] [CrossRef] [PubMed]
- Gil, Á.; Plaza-Diaz, J.; Mesa, M.D. Vitamin D: Classic and novel actions. Ann. Nutr. Metab. 2018, 72, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Hoshi, T.; Abe, Y.; Sugiyama, I.; Sadzuka, Y. The connection of vitamin D3 induced cytotoxicity with the vitamin D3 receptor. AACR 2019, 79, 367. [Google Scholar]
- Lo, C.S.-C.; Kiang, K.M.-Y.; Leung, G.K.-K. Anti-tumor effects of vitamin D in glioblastoma: Mechanism and therapeutic implications. Lab. Investig. 2022, 102, 118–125. [Google Scholar] [CrossRef]
- Ling, Y.; Xu, F.; Xia, X.; Dai, D.; Sun, R.; Xie, Z. Vitamin D receptor regulates proliferation and differentiation of thyroid carcinoma via the E-cadherin-β-catenin complex. J. Mol. Endocrinol. 2022, 68, 137–151. [Google Scholar] [CrossRef]
- Ding, P.; Du, X.; Wan, L.; Zhao, X.; Zhang, D.; Huang, Z.; Cao, G.; Zhou, X.; Zheng, Y.; Cao, Y. Diagnostic Value of VDR in Bone Metastasis and Prognosis of Patients with Breast Cancer and Expression Correlation between VDR and Hr. Oncol. Res. Treat. 2022, 45, 166–176. [Google Scholar] [CrossRef]
- Kurucu, N.; Şahin, G.; Sarı, N.; Ceylaner, S.; İlhan, İ.E. Association of vitamin D receptor gene polymorphisms with osteosarcoma risk and prognosis. J. Bone Oncol. 2019, 14, 100208. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO classification of tumors of the central nervous system: A summary. Neuro-Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Steliarova-Foucher, E.; Colombet, M.; Ries, L.A.; Moreno, F.; Dolya, A.; Bray, F.; Hesseling, P.; Shin, H.Y.; Stiller, C.A.; IICC-3 contributors; et al. International incidence of childhood cancer, 2001–2010: A population-based registry study. Lancet Oncol. 2017, 18, 719–731. [Google Scholar] [CrossRef]
- World Health Organization. CureAll Framework: WHO Global Initiative for Childhood Cancer: Increasing Access, Advancing Quality, Saving Lives; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Lam, C.G.; Howard, S.C.; Bouffet, E.; Pritchard-Jones, K. Science and health for all children with cancer. Science 2019, 363, 1182–1186. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.J.; Abedin, S.A. Vitamin D and cancer. Expert Rev. Endocrinol. Metab. 2006, 1, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Thorne, J.; Campbell, M.J. The vitamin D receptor in cancer: Symposium on ‘Diet and cancer’. Proc. Nutr. Soc. 2008, 67, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Martin, E.G.; Castiñeiras, A.C. Vitamin D Modulation and microRNAs in Gastric Cancer: Prognostic and Therapeutic Role. 2021. Available online: https://tcr.amegroups.com/article/view/46878/html (accessed on 31 March 2022).
- Genc, D.B.; Vural, S.; Yagar, G. The incidence of and factors associated with vitamin D deficiency in newly diagnosed children with cancer. Nutr. Cancer 2016, 68, 756–761. [Google Scholar] [CrossRef] [PubMed]
- Iniesta, R.R.; Rush, R.; Paciarotti, I.; Rhatigan, E.; Brougham, F.; McKenzie, J.; Wilson, D. Systematic review and meta-analysis: Prevalence and possible causes of vitamin D deficiency and insufficiency in pediatric cancer patients. Clin. Nutr. 2016, 35, 95–108. [Google Scholar] [CrossRef]
- Juhász, O.; Jakab, Z.; Szabó, A.; Garami, M. Examining the vitamin D status of children with solid tumors. J. Am. Coll. Nutr. 2020, 39, 128–134. [Google Scholar] [CrossRef]
- Margolis, R.N.; Christakos, S. The nuclear receptor superfamily of steroid hormones and vitamin D gene regulation: An update. Ann. N. Y. Acad. Sci. 2010, 1192, 208–214. [Google Scholar] [CrossRef]
- Deeb, K.K.; Trump, D.L.; Johnson, C.S. Vitamin D signalling pathways in cancer: Potential for anticancer therapeutics. Nat. Rev. Cancer 2007, 7, 684–700. [Google Scholar] [CrossRef]
- Feldman, D.; Krishnan, A.V.; Swami, S.; Giovannucci, E.; Feldman, B.J. The role of vitamin D in reducing cancer risk and progression. Nat. Rev. Cancer 2014, 14, 342–357. [Google Scholar] [CrossRef]
- Anderson, M.G.; Nakane, M.; Ruan, X.; Kroeger, P.E.; Wu-Wong, J.R. Expression of VDR and CYP24A1 mRNA in human tumors. Cancer Chemother. Pharmacol. 2006, 57, 234–240. [Google Scholar] [CrossRef]
- Matusiak, D.; Murillo, G.; Carroll, R.E.; Mehta, R.G.; Benya, R.V. Expression of vitamin D receptor and 25-hydroxyvitamin D3-1α-hydroxylase in normal and malignant human colon. Cancer Epidemiol. Prev. Biomark. 2005, 14, 2370–2376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, T.C.; Sakaki, T.; Yamamoto, K.; Kittaka, A. The roles of cytochrome P450 enzymes in prostate cancer development and treatment. Anticancer Res. 2012, 32, 291–298. [Google Scholar] [PubMed]
- Wang, Y.; Zhu, J.; DeLuca, H.F. Where is the vitamin D receptor? Arch. Biochem. Biophys. 2012, 523, 123–133. [Google Scholar] [CrossRef]
- Cui, X.; Pertile, R.; Liu, P.; Eyles, D. Vitamin D regulates tyrosine hydroxylase expression: N-cadherin a possible mediator. Neuroscience 2015, 304, 90–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eyles, D.W. Vitamin D: Brain and behavior. JBMR Plus 2021, 5, e10419. [Google Scholar] [CrossRef] [PubMed]
- Norlin, M. Effects of vitamin D in the nervous system: Special focus on interaction with steroid hormone signalling and a possible role in the treatment of brain cancer. J. Neuroendocr. 2020, 32, e12799. [Google Scholar] [CrossRef]
- Horváth, E.; Balla, B.; Kósa, J.; Lakatos, P.A.; Lazáry, Á.; Németh, D.; Jozilan, H.; Somorácz, Á.; Korompay, A.; Gyöngyösi, B. A D-vitamin metabolizmusa humán hepatocellularis carcinomában és az azt körülvevő tumormentes májszövetben. Orv. Hetil. 2016, 157, 1910–1918. [Google Scholar] [CrossRef] [Green Version]
- Segura, C.; Alonso, M.; Fraga, C.; García-Caballero, T.; Diéguez, C.; Pérez-Fernández, R. Vitamin D receptor ontogenesis in rat liver. Histochem. Cell Biol. 1999, 112, 163–167. [Google Scholar] [CrossRef]
- Gascon-Barré, M.; Demers, C.; Mirshahi, A.; Néron, S.; Zalzal, S.; Nanci, A. The normal liver harbors the vitamin D nuclear receptor in nonparenchymal and biliary epithelial cells. Hepatology 2003, 37, 1034–1042. [Google Scholar] [CrossRef]
- Grobe, M.; Kretzschmar, G.; Vuica, A.; Filipovic, N. Expression of vitamin D receptors in the superior cervical ganglia of rats. Biotech. Histochem. 2018, 93, 320–327. [Google Scholar] [CrossRef]
- Bini, F.; Frati, A.; Garcia-Gil, M.; Battistini, C.; Granado, M.; Martinesi, M.; Mainardi, M.; Vannini, E.; Luzzati, F.; Caleo, M.; et al. New signalling pathway involved in the anti-proliferative action of vitamin D3 and its analogues in human neuroblastoma cells. A role for ceramide kinase. Neuropharmacology 2012, 63, 524–537. [Google Scholar] [CrossRef] [PubMed]
| Favorable Prognosis | Unfavorable Prognosis | |
|---|---|---|
| Significant VDR expression | 18 | 0 |
| Non-significant VDR expression | 66 | 26 |
| Tumor Types | Significant VDR Expression | Non-Significant VDR Expression |
|---|---|---|
| Neuroblastoma | 2 | 7.4% (2.1–23.4) |
| Ewing sarcoma | 2 | 20.0% (5.7–51.0) |
| Wilms’ tumor | 2 | 28.6% (8.2–64.1) |
| Thyroid carcinoma | 5 | 100.0% (56.6–100) |
| Ependymoma | 2 | 5.4% (1.5–17.7) |
| Medulloblastoma | 6 | 8.8% (4.1–17.9) |
| PNET | 4 | 33.3% (13.8–60.9) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juhász, O.; Jákob, N.; Rajnai, H.; Imrei, M.; Garami, M. Immunohistochemical Detection of the Presence of Vitamin D Receptor in Childhood Solid Tumors. Cancers 2022, 14, 3295. https://doi.org/10.3390/cancers14143295
Juhász O, Jákob N, Rajnai H, Imrei M, Garami M. Immunohistochemical Detection of the Presence of Vitamin D Receptor in Childhood Solid Tumors. Cancers. 2022; 14(14):3295. https://doi.org/10.3390/cancers14143295
Chicago/Turabian StyleJuhász, Orsolya, Noémi Jákob, Hajnalka Rajnai, Marcell Imrei, and Miklós Garami. 2022. "Immunohistochemical Detection of the Presence of Vitamin D Receptor in Childhood Solid Tumors" Cancers 14, no. 14: 3295. https://doi.org/10.3390/cancers14143295







