Development and Validation of a Risk Score for Post-Transplant Lymphoproliferative Disorders among Solid Organ Transplant Recipients
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Primary Outcome
2.3. Data Sources, Risk Factors, and Definitions
2.4. Model Derivation
2.5. External Model Validation
2.6. Calculation of an Individual’s Risk of Developing PTLD—An Example
2.7. Sensitivity Analyses
3. Results
3.1. Patient Characteristics
3.2. Development of the Best Model to Predict PTLD in the Derivation Cohort
3.3. Validation Cohort
3.4. Calculation of an Individual’s PTLD Risk Score
3.5. Sensitivity Analyses in Derivation Cohort
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dierickx, D.; Habermann, T.M. Post-Transplantation Lymphoproliferative Disorders in Adults. N. Engl. J. Med. 2018, 378, 549–562. [Google Scholar] [CrossRef] [PubMed]
- Petrara, M.R.; Giunco, S.; Serraino, D.; Dolcetti, R.; De Rossi, A. Post-transplant lymphoproliferative disorders: From epidemiology to pathogenesis-driven treatment. Cancer Lett. 2015, 369, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Nalesnik, M.A. The diverse pathology of post-transplant lymphoproliferative disorders: The importance of a standardized approach. Transpl. Infect. Dis. 2001, 3, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Dierickx, D.; Tousseyn, T.; Gheysens, O. How I treat posttransplant lymphoproliferative disorders. Blood 2015, 126, 2274–2283. [Google Scholar] [CrossRef] [Green Version]
- Al-Mansour, Z.; Nelson, B.P.; Evens, A.M. Post-transplant lymphoproliferative disease (PTLD): Risk factors, diagnosis, and current treatment strategies. Curr. Hematol. Malig. Rep. 2013, 8, 173–183. [Google Scholar] [CrossRef] [Green Version]
- Wareham, N.E.; Mocroft, A.; Sengeløv, H.; Cunha-Bang, D.; Gustafsson, F.; Heilmann, C.; Iversen, M.; Kirkby, N.S.; Rasmussen, A.; Sørensen, S.S.; et al. The value of EBV DNA in early detection of post-transplant lymphoproliferative disorders among solid organ and hematopoietic stem cell transplant recipients. J. Cancer Res. Clin. Oncol. 2018, 144, 1569–1580. [Google Scholar] [CrossRef]
- Green, M.; Michaels, M.G. Epstein-Barr virus infection and posttransplant lymphoproliferative disorder. Am. J. Transplant. 2013, 13 (Suppl. 3), 341–354. [Google Scholar] [CrossRef]
- Ok, C.Y.; Li, L.; Young, K.H. EBV-driven B-cell lymphoproliferative disorders: From biology, classification and differential diagnosis to clinical management. Exp. Mol. Med. 2015, 47, e132. [Google Scholar] [CrossRef] [Green Version]
- Parker, A.; Bowles, K.; Bradley, J.A.; Emery, V.; Featherstone, C.; Gupte, G.; Marcus, R.; Parameshwar, J.; Ramsay, A.; Newstead, C. Diagnosis of post-transplant lymphoproliferative disorder in solid organ transplant recipients-BCSH and BTS Guidelines. Br. J. Haematol. 2010, 149, 675–692. [Google Scholar] [CrossRef]
- Lodding, I.; Sengelov, H.; da Cunha-Bang, C.; Iversen, M.; Rasmussen, A.; Gustafsson, F.; Downing, J.; Grarup, J.; Kirkby, N.; Frederiksen, C.; et al. Clinical Application of Variation in Replication Kinetics During Episodes of Post-transplant Cytomegalovirus Infections. EBioMedicine 2015, 2, 699–705. [Google Scholar] [CrossRef] [Green Version]
- Koller, M.T.; Van Delden, C.; Müller, N.J.; Baumann, P.; Lovis, C.; Marti, H.-P.; Fehr, T.; Binet, I.; De Geest, S.; Bucher, H.C.; et al. Design and methodology of the Swiss Transplant Cohort Study (STCS): A comprehensive prospective nationwide long-term follow-up cohort. Eur. J. Epidemiol. 2013, 28, 347–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabattini, E.; Bacci, F.; Sagramoso, C.; Pileri, S.A. WHO classification of tumours of haematopoietic and lymphoid tissues in 2008: An overview. Pathologica 2010, 102, 83–87. [Google Scholar] [PubMed]
- Aagaard, T.; Roen, A.; Reekie, J.; Daugaard, G.; Brown, P.D.N.; Specht, L.; Sengeløv, H.; Mocroft, A.; Lundgren, J.; Helleberg, M. Development and Validation of a Risk Score for Febrile Neutropenia After Chemotherapy in Patients With Cancer: The FENCE Score. JNCI Cancer Spectr. 2018, 2, pky053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ekenberg, C.; da Cunha-Bang, C.; Lodding, I.P.; Sørensen, S.S.; Sengeløv, H.; Perch, M.; Rasmussen, A.; Gustafsson, F.; Wareham, N.E.; Kirkby, N.; et al. Evaluation of an electronic, patient-focused management system aimed at preventing cytomegalovirus disease following solid organ transplantation. Transpl. Infect. Disease 2020, 22, e13252. [Google Scholar] [CrossRef]
- Van Delden, C.; Stampf, S.; Hirsch, H.H.; Manuel, O.; Meylan, P.; Cusini, A.; Hirzel, C.; Khanna, N.; Weisser, M.; Garzoni, C.; et al. Burden and Timeline of Infectious Diseases in the First Year after Solid Organ Transplantation in the Swiss Transplant Cohort Study. Clin. Infect. Dis. 2020, 71, e159–e169. [Google Scholar] [CrossRef] [Green Version]
- Koff, A.; Azar, M.M. Diagnosing peritoneal tuberculosis. BMJ Case Rep. 2020, 13, e233131. [Google Scholar] [CrossRef]
- Akaike, H. A New Look at the Statistical Model Identification. In Selected Papers of Hirotugu Akaike; Parzen, E., Tanabe, K., Kitagawa, G., Eds.; Springer: New York, NY, USA, 1998; pp. 215–222. [Google Scholar]
- Harrell, F.E., Jr.; Lee, K.L.; Mark, D.B. Multivariable prognostic models: Issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat. Med. 1996, 15, 361–387. [Google Scholar] [CrossRef]
- Caetano, S.J.; Sonpavde, G.; Pond, G.R. C-statistic: A brief explanation of its construction, interpretation and limitations. Eur. J. Cancer 2018, 90, 130–132. [Google Scholar] [CrossRef]
- Morton, M.; Coupes, B.; Roberts, S.A.; Johnson, S.L.; Klapper, P.; Vallely, P.; Picton, M.L. Epstein-Barr virus infection in adult renal transplant recipients. Am. J. Transplant. 2014, 14, 1619–1629. [Google Scholar] [CrossRef]
- Schaffer, K.; Hassan, J.; Staines, A.; Coughlan, S.; Holder, P.; Tuite, G.; McCormick, A.P.; Traynor, O.; Hall, W.W.; Connell, J. Surveillance of Epstein-Barr virus loads in adult liver transplantation: Associations with age, sex, posttransplant times, and transplant indications. Liver Transplant. 2011, 17, 1420–1426. [Google Scholar] [CrossRef]
- Liu, M.; Husain, S.; Famure, O.; Li, Y.; Kim, S.J. Incidence, Risk Factors, Clinical Management, and Outcomes of Posttransplant Lymphoproliferative Disorder in Kidney Transplant Recipients. Prog. Transplant. 2019, 29, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Wasson, S.; Zafar, M.N.; Best, J.; Reddy, H.K. Post-transplantation lymphoproliferative disorder in heart and kidney transplant patients: A single-center experience. J. Cardiovasc. Pharmacol. Ther. 2006, 11, 77–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quinlan, S.C.; Pfeiffer, R.M.; Morton, L.M.; Engels, E.A. Risk factors for early-onset and late-onset post-transplant lymphoproliferative disorder in kidney recipients in the United States. Am. J. Hematol. 2011, 86, 206–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dharnidharka, V.R.; Tejani, A.H.; Ho, P.-L.; Harmon, W.E. Post-Transplant Lymphoproliferative Disorder in the United States: Young Caucasian Males are at Highest Risk. Am. J. Transplant. 2002, 2, 993–998. [Google Scholar] [CrossRef] [PubMed]
- Franceschini, E.; Plessi, J.; Zona, S.; Santoro, A.; Digaetano, M.; Fontana, F.; Alfano, G.; Guaraldi, G.; Comoli, P.; Facchini, F.; et al. Clinical Utility of Epstein-Barr Virus Viral Load Monitoring and Risk Factors for Posttransplant Lymphoproliferative Disorders After Kidney Transplantation: A Single-Center, 10-Year Observational Cohort Study. Transpl. Direct 2017, 3, e182. [Google Scholar] [CrossRef] [Green Version]
- Brunner, B.; Kropshofer, G.; Ellemunter, H.; Brunner, A.; Mueller, T.; Margreiter, R.; Tzankov, A. Severe cold agglutinin disease caused by recurrent monomorphic Epstein-Barr virus (EBV)-associated post-transplant lymphoproliferative disorder (PTLD), clonally related to an EBV-negative plasmacytic hyperplasia in a pediatric multivisceral organ transplant recipient. Pediatr. Transplant. 2007, 11, 547–551. [Google Scholar]
- Siegel, A.; Boike, J.; Trivedi, I.; Yadlapati, R. Posttransplant Lymphoproliferative Disorder of the Small Bowel as an Unexpected Cause of Iron Deficiency Anemia Decades after Heart Transplantation. ACG Case Rep. J. 2017, 4, e86. [Google Scholar] [CrossRef]
- Leyssens, A.; Dierickx, D.; Verbeken, E.K.; Tousseyn, T.; Verleden, S.; Vanaudenaerde, B.; Dupont, L.J.; Yserbyt, J.; Verleden, G.M.; Van Raemdonck, D.; et al. Post-transplant lymphoproliferative disease in lung transplantation: A nested case-control study. Clin. Transplant. 2017, 31, e12983. [Google Scholar] [CrossRef]
- Gandhi, S.; Behling, E.; Behrens, D.; Ferber, A.; Schwarting, R.; Budak-Alpdogan, T. Late-Onset Posttransplant Lymphoproliferative Disorders after Solid Organ Transplantation in Adults: A Case Series and Review of the Literature. Case Rep. Transplant. 2020, 2020, 8247308. [Google Scholar] [CrossRef]
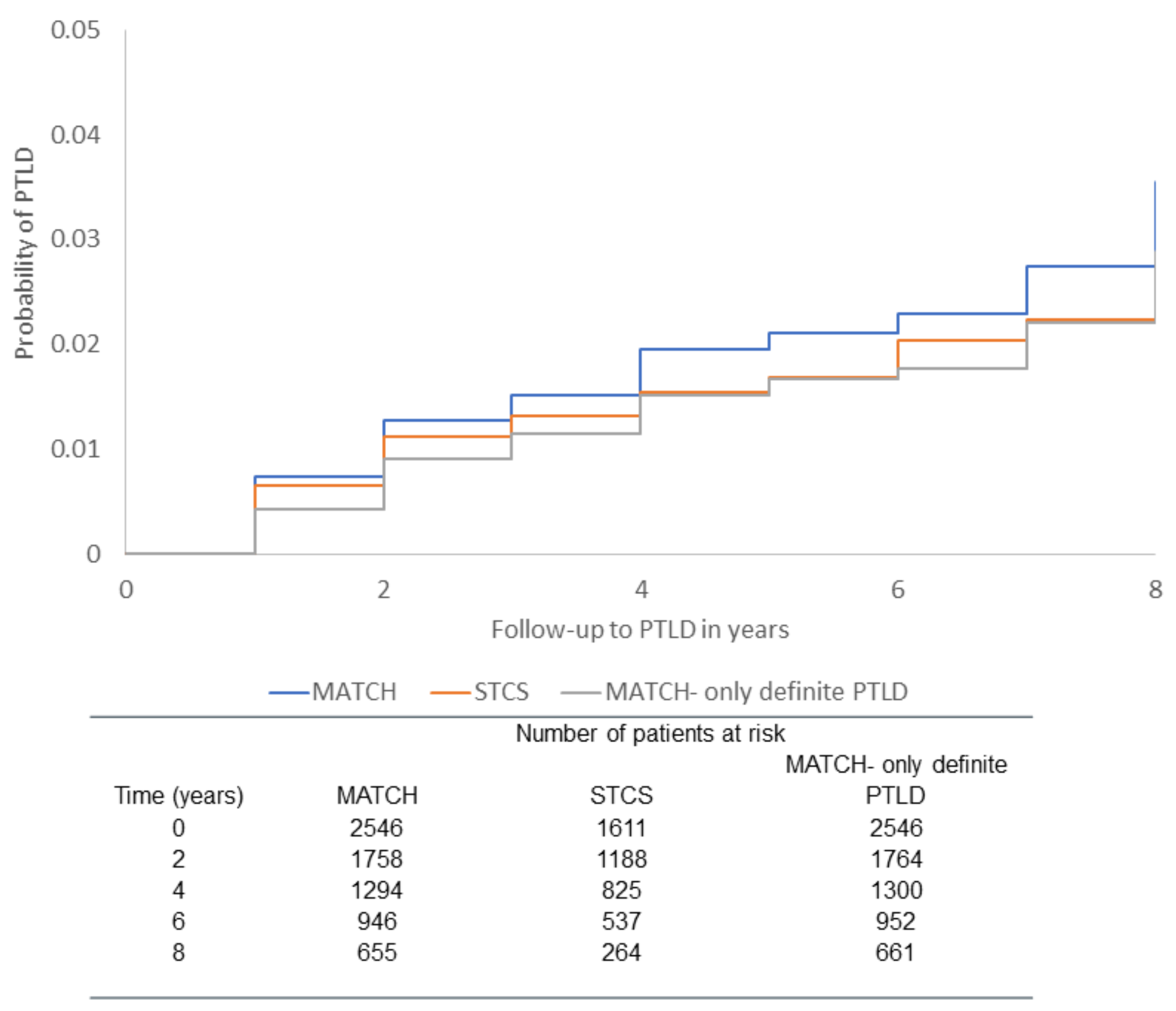
| MATCH (Derivation) | STCS (Validation) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All | Did Not Develop PTLD | Developed PTLD | All | Did Not Develop PTLD | Developed PTLD | |||||||
| Characteristics | N or Median | % or IQR | N or Median | % or IQR | N or Median | % or IQR | N or Median | % or IQR | N or Median | % or IQR | N or Median | % or IQR |
| All | 2546 | 100 | 2489 | 97.8 | 57 | 2.2 | 1611 | 100% | 1587 | 98.5 | 24 | 1.5 |
| Total follow-up (PYFU) | 13,026.45 | 12,810.67 | 215.78 | 7218.53 | 7168.42 | 50.11 | ||||||
| Gender | ||||||||||||
| Male | 1507 | 59.2 | 1473 | 59.5 | 34 | 59.6 | 1010 | 62.7 | 994 | 62.6 | 16 | 66.6 |
| Female | 1039 | 40.8 | 1016 | 41.5 | 23 | 40.4 | 601 | 37.3 | 593 | 37.4 | 8 | 33.4 |
| Age group | ||||||||||||
| ≤16 years | 156 | 6.1 | 143 | 5.7 | 13 | 23.0 | 72 | 4.5 | 71 | 4.5 | 1 | 4.2 |
| >16 years | 2390 | 93.9 | 2346 | 94.3 | 44 | 77.0 | 1539 | 95.5 | 1516 | 95.5 | 24 | 95.8 |
| Year of transplant | 2012 | (2008–2016) | 2012 | (2008–2016) | 2010 | (2007–2012) | 2013 | (2011–2016) | 2013 | (2011–2016) | 2011 | (2010–2014) |
| Type of transplant | ||||||||||||
| Heart | 204 | 8.0 | 199 | 8.1 | 5 | 8.8 | 120 | 7.5 | 119 | 7.5 | 1 | 4.2 |
| Lung | 458 | 18.0 | 447 | 17.9 | 11 | 19.3 | 250 | 15.5 | 241 | 15.3 | 9 | 37.6 |
| Kidney and/or pancreas 1 | 1201 | 47.2 | 1175 | 47.2 | 26 | 45.6 | 838 | 52.0 | 831 | 52.3 | 7 | 29.1 |
| Liver | 683 | 26.8 | 668 | 26.8 | 15 | 26.3 | 403 | 25.0 | 396 | 24.9 | 7 | 29.1 |
| Donor/recipient EBV risk | ||||||||||||
| Low risk | 2467 | 96.9 | 2418 | 97.1 | 49 | 86.0 | 1499 | 93.0 | 1480 | 93.2 | 19 | 79.2 |
| High risk | 79 | 3.1 | 71 | 2.9 | 8 | 14.0 | 112 | 7.0 | 107 | 6.8 | 5 | 20.8 |
| Lab Tests | ||||||||||||
| Hemoglobin mmol/L | 6.0 | (5.4–6.6) | 6.0 | (5.4–6.6) | 6.0 | (5.4–6.5) | 5.4 | (4.8–6.1) | 5.4 | (4.8–6.1) | 5.2 | (4.8–5.7) |
| C-reactive protein mg/L | 12.0 | (5.0–32.0) | 12.0 | (5.0–32.0) | 23.0 | (6.0–59.0) | 21.0 | (8.0–48.0) | 21.0 | (8.0–48.0) | 30.5 | (13.5–52.5) |
| Platelets × 109/L | 203.0 | (138.0–266.0) | 203.0 | (138.0–266.0) | 188.0 | (82.0–283.0) | 187.0 | (117.0–263.0) | 187.0 | (117.0–263.0) | 198.5 | (103.0–277.0) |
| PTLD Characteristics | Derivation Cohort | Validation Cohort |
|---|---|---|
| Lymphocyte lineage: N (%) | B cell: 32 (56.1) Non-B cell: 14 (24.6) Missing a: 11 (19.3) | B cell: 13 (54.2) Non-B cell: 11 (45.8) |
| Ann Arbor classification of Lymphoma: N (%) | Stage I: 13 (22.8) Stage II: 9 (15.8) Stage III: 5 (8.8) Stage IV: 13 (22.8) NA b: 17 (29.8) | Stage I: 2 (8.3) Stage II: 2 (8.3) Stage III: 0 (0) Stage IV: 20 (83.4) |
| EBV DNA at time of PTLD diagnosis: N (%) | Negative: 6 (10.6) Positive: 28 (49.1) Missing: 23 (40.3) | Negative: 5 (20.8) Positive: 18 (75.0) Missing: 1 (4.2) |
| Periodicity in MATCH 2 | Periodicity in STCS 3 | |||
|---|---|---|---|---|
| Laboratory Parameters | First Year after Transplant Median and IQR | Follow-up after First Year Median and IQR | First Year after Transplant Median and IQR | Follow-Up after the First Year Median and IQR |
| EBV DNA | 16 days (7–44) | 54 days (14–196) | 8 days (6–14) | 18 days (7–35) |
| CRP | 2 days (1–10) | 7 days (1–39) | 1 day (1–6) | 7 days (1–29) |
| Hemoglobin | 3 days (1–13) | 8 days (1–36) | 1 day (1–6) | 7 days (1–33) |
| Platelets | 2 days (1–13) | 10 days (1–45) | 1 day (1–6) | 7 days (1–33) |
| Parameter a | N of PTLD/Total PYFU | IRR WITH 95% CI | Exact Coefficient | Coefficient Used in Score Calculation |
|---|---|---|---|---|
| Intercept b | −12.529 | |||
| Variables at baseline: | ||||
| Donor/recipient serostatus risk | ||||
| Low donor/recipient risk | 49/12,676.98 | 1 | 0 (ref) | 0 |
| High donor/recipient risk | 8/291.31 | 3.58 (1.32; 9.64) | 1.275 | 19 |
| Type of transplant | ||||
| Kidney or and pancreas transplant | 26/7177.53 | 1 | 0 (ref) | 0 |
| Heart transplant | 5/1133.55 | 1.81 (0.69; 4.68) | 0.593 | 9 |
| Liver transplant | 15/2879.61 | 1.33 (0.68; 2.60) | 0.286 | 4 |
| Lung transplant | 11/1777.59 | 1.06 (0.47; 2.38) | 0.066 | 1 |
| Time-updated variables: | ||||
| Hemoglobin levels (mmol/L) c | ||||
| Hemoglobin§ < normal ranges d | 33/4826.75 | 1.98 (1.05; 3.71) | 0.683 | 10 |
| Hemoglobin normal | 15/6167.38 | 1 | 0 (ref) | 0 |
| Hemoglobin > normal or missing | 9/1974.16 | 2.12 (0.74; 6.02) | 0.753 | 11 |
| C-reactive protein levels (mg/L) c | ||||
| C-reactive protein (<10 mg/L) or missing | 37/10,934.90 | 1 | 0 (ref) | 0 |
| C-reactive protein ≥ 10 mg/L | 20/2033.39 | 2.39 (1.35; 4.23) | 0.873 | 13 |
| Platelets levels (×109/L) c | ||||
| Platelets < normal or missing | 15/2802.98 | 1.43 (0.64; 3.17) | 0.360 | 5 |
| Platelets normal | 35/9554.03 | 1 | 0 (ref) | 0 |
| Platelets > normal | 7/611.27 | 1.77 (0.78; 3.99) | 0.571 | 9 |
| EBV DNA categories c | ||||
| Negative EBV DNA | 25/7419.02 | 1 | 0 (ref) | 0 |
| EBV DNA-missing | 13/5304.36 | 0.69 (0.34; 1.38) | −0.364 | −6 |
| Positive EBV DNA | 19/244.91 | 16.34 (8.07; 33.10) | 2.794 | 42 |
| Variable | Derivation Cohort | Validation Cohort |
|---|---|---|
| Developed PTLD/no. | 57/2546 | 24/1611 |
| Incidence of PTLD per 1000 PYFU (95% CI) | 4.40 (3.25–5.54) | 3.32 (1.99–4.65) |
| Total follow-up (PYFU) | 13,026.45 | 7218.53 |
| Risk score model | ||
| Score, median (IQR) | 10.0 (0–14.0) | 10.0 (1–16.0) |
| Score for those who developed PTLD, median (IQR) | 20.0 (10.0–55.0) | 22.0 (14.0–62.0) |
| N of PTLD by risk score group, low/high risk | 24/33 | 8/16 |
| N of PYFU by risk score group, low/high risk | 10,776.14/2192.17 | 5644.28/1574.26 |
| Incidence of PTLD per 1000 PYFU (95% CI) | ||
| Low risk (score ≤ 17) | 2.23 (1.34–3.12) | 1.42 (0.44–2.40) |
| High risk (score > 17) | 15.05 (9.92–20.19) | 10.16 (5.18–15.14) |
| Incidence rate ratio (95% CI) | ||
| Low risk (score ≤ 17) | 1 (ref) | 1 (ref) |
| High risk (score > 17) | 6.75 (4.00–11.41) | 7.17 (3.05–16.82) |
| Incidence rate ratio per 1-point increase in score | 1.06 (1.05–1.08) | 1.06 (1.04–1.08) |
| Harrell’s C-statistic | 0.82 (0.76–0.88) | 0.82 (0.72–0.92) |
| Brier score | 0.000084 | 0.000064 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
dos Santos, Q.; Wareham, N.E.; Mocroft, A.; Rasmussen, A.; Gustafsson, F.; Perch, M.; Sørensen, S.S.; Manuel, O.; Müller, N.J.; Lundgren, J.; et al. Development and Validation of a Risk Score for Post-Transplant Lymphoproliferative Disorders among Solid Organ Transplant Recipients. Cancers 2022, 14, 3279. https://doi.org/10.3390/cancers14133279
dos Santos Q, Wareham NE, Mocroft A, Rasmussen A, Gustafsson F, Perch M, Sørensen SS, Manuel O, Müller NJ, Lundgren J, et al. Development and Validation of a Risk Score for Post-Transplant Lymphoproliferative Disorders among Solid Organ Transplant Recipients. Cancers. 2022; 14(13):3279. https://doi.org/10.3390/cancers14133279
Chicago/Turabian Styledos Santos, Quenia, Neval Ete Wareham, Amanda Mocroft, Allan Rasmussen, Finn Gustafsson, Michael Perch, Søren Schwartz Sørensen, Oriol Manuel, Nicolas J. Müller, Jens Lundgren, and et al. 2022. "Development and Validation of a Risk Score for Post-Transplant Lymphoproliferative Disorders among Solid Organ Transplant Recipients" Cancers 14, no. 13: 3279. https://doi.org/10.3390/cancers14133279
APA Styledos Santos, Q., Wareham, N. E., Mocroft, A., Rasmussen, A., Gustafsson, F., Perch, M., Sørensen, S. S., Manuel, O., Müller, N. J., Lundgren, J., & Reekie, J. (2022). Development and Validation of a Risk Score for Post-Transplant Lymphoproliferative Disorders among Solid Organ Transplant Recipients. Cancers, 14(13), 3279. https://doi.org/10.3390/cancers14133279






