An Overview on Radiation Sensitivity in Hereditary Breast and Ovarian Cancer Syndrome
Abstract
Simple Summary
Abstract
1. Hereditary Breast and Ovarian Cancer Syndrome
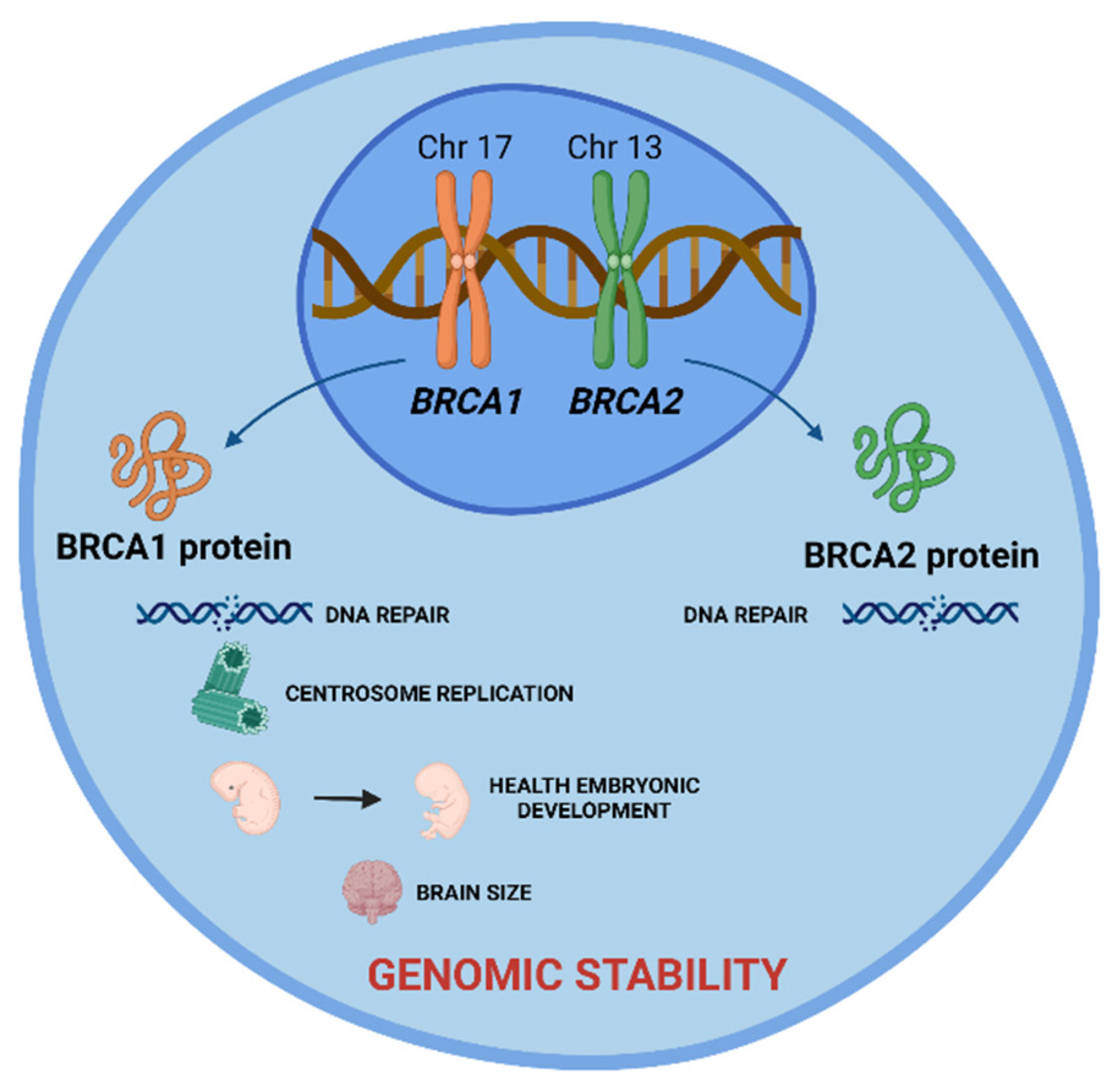
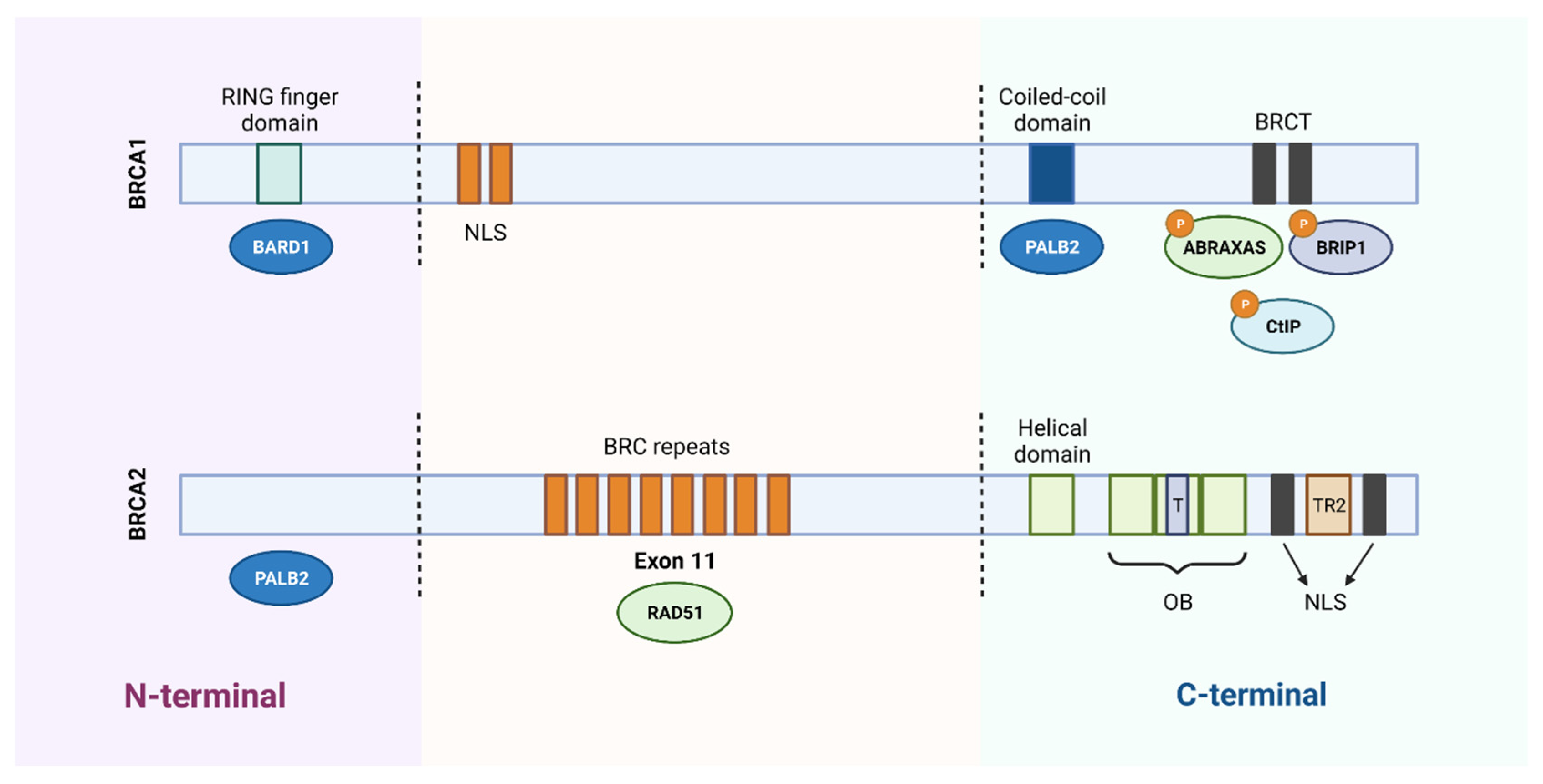
BRCA1 and BRCA2 Genes
2. Ionizing Radiation
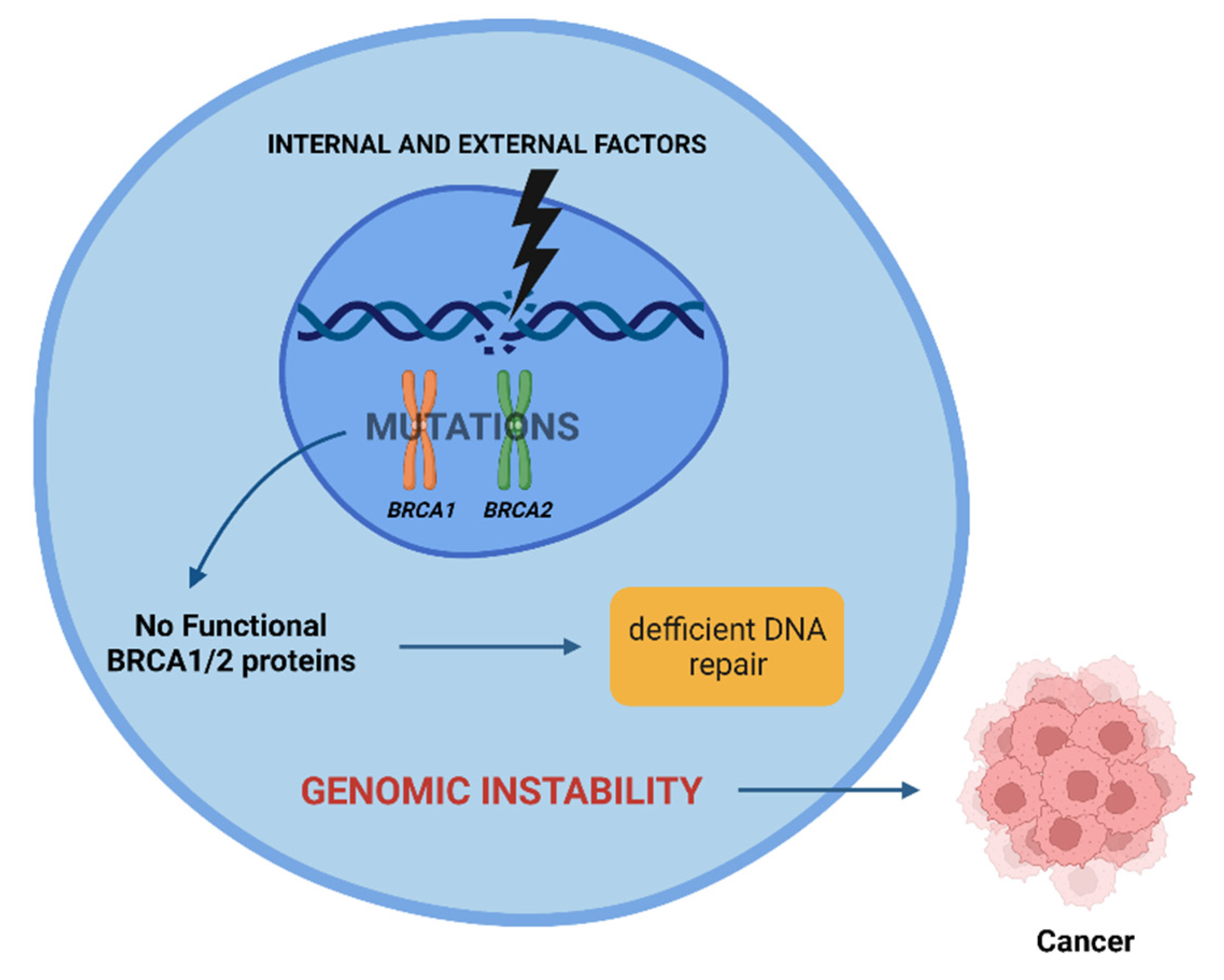
3. Ionizing Radiation and BRCA1 and BRCA2 Mutations
3.1. Diagnostic Doses
| Authors | Year | Sample Size (n) | Gene | Exposure Type | Outcome |
|---|---|---|---|---|---|
| Narold et al. [41] | 2006 | 3200 (1600 carriers with breast cancer and 1600 healthy carriers) | BRCA1 and BRCA2 | Mammography | No association was found between having a mammogram and breast cancer risk |
| Goldfrank et al. [42] | 2006 | 213 carriers | BRCA1 and BRCA2 | Mammography | No association was found between mammogram exposure and breast cancer risk |
| John et al. [43] | 2013 | 727 (454 BRCA1 and 273 BRCA2 mutation carriers aged <50 years) | BRCA1 and BRCA2 | Chest x-rays | No association was found between diagnostic chest x-rays and breast cancer risk before age 50 years |
| Giannakeas et al. [44] | 2014 | 2346 (1844 BRCA1 mutation carriers and 502 BRCA2 mutation carriers) | BRCA1 and BRCA2 | Mammography | No significant association was found between prior mammography exposure and breast cancer risk for BRCA1 or BRCA2 carriers |
| Andrieu et al. [45] | 2006 | 1601 carriers (1187 BRCA1 mutation carriers and 414 BRCA2 mutation carriers) | BRCA1 and BRCA2 | Chest x-rays | A positive association was found between diagnostic chest x-rays and breast cancer risk. In addition, the risk was increased in women aged 40 years and younger |
| Lecarpentier et al. [46] | 2011 | 990 (379 affected by breast cancer and 611 unaffected) | BRCA1 and BRCA2 | Chest x-rays | An association was found between exposure to chest x-rays and the risk of breast cancer. A positive association was found between smoking and cancer risk |
| Pijpe et al. [47] | 2012 | 1993 carriers | BRCA1 and BRCA2 | <0.0020 Gy, ≥0.0020–0.0065 Gy, ≥0.0066–0.0173 Gy, and ≥0.0174 Gy | A positive association was found between diagnostic chest x-rays before the age of 30 and breast cancer risk |
| Baert et al. [48] | 2016 | 36 (18 carriers of BRCA1 mutations and 18 non-carriers) | BRCA1 | 2 and 4 Gy | Healthy individuals with a BRCA1 mutation show a significantly increased radiosensitivity compared with healthy controls |
| Baert et al. [49] | 2017 | 35 (18 carriers of mutations in BRCA2 gene and 17 non-carriers) | BRCA2 | 2 Gy | An increased radiosensitivity was found in BRCA2 mutation carriers compared to non-carriers |
3.2. Therapeutic Doses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Petrucelli, N.; Daly, M.B.; Pal, T. BRCA1- and BRCA2-Associated Hereditary Breast and Ovarian Cancer; GeneReviews; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Yoshida, R. Hereditary breast and ovarian cancer (HBOC): Review of its molecular characteristics, screening, treatment, and prognosis. Breast Cancer 2021, 28, 1167–1180. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, A.; Turashvili, G. Pathology of Hereditary Breast and Ovarian Cancer. Front. Oncol. 2020, 10, 531790. [Google Scholar] [CrossRef]
- Okano, M.; Nomizu, T.; Tachibana, K.; Nagatsuka, M.; Matsuzaki, M.; Katagata, N.; Ohtake, T.; Yokoyama, S.; Arai, M.; Nakamura, S. The relationship between BRCA-associated breast cancer and age factors: An analysis of the Japanese HBOC consortium database. J. Hum. Genet. 2021, 66, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, F.C.; van Overeem Hansen, T.; Sørensen, C.S. Hereditary breast and ovarian cancer: New genes in confined pathways. Nat. Rev. Cancer 2016, 16, 599–612. [Google Scholar] [CrossRef] [PubMed]
- Elsayegh, N.; Barrera, A.M.G.; Muse, K.I.; Lin, H.; Kuerer, H.M.; Helm, M.; Litton, J.K.; Arun, B.K. Evaluation of BRCAPRO Risk Assessment Model in Patients with Ductal Carcinoma In Situ Who Underwent Clinical BRCA Genetic Testing. Front. Genet. 2016, 7, 71. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hart, S.N.; Polley, E.C.; Yussuf, A.; Yadav, S.; Goldgar, D.E.; Hu, C.; LaDuca, H.; Smith, L.P.; Fujimoto, J.; Li, S.; et al. Mutation prevalence tables for hereditary cancer derived from multigene panel testing. Hum. Mutat. 2020, 41. [Google Scholar] [CrossRef]
- Understanding Genetics: A New York, Mid-Atlantic Guide for Patients and Health Professionals; Genetic Alliance: Washington, DC, USA, 2009.
- Lee, E.Y.H.P.; Muller, W.J. Oncogenes and tumor suppressor genes. Cold Spring Harb. Perspect. Biol. 2010, 2, a003236. [Google Scholar] [CrossRef]
- Gorodetska, I.; Kozeretska, I.; Dubrovska, A. BRCA Genes: The Role in Genome Stability, Cancer Stemness and Therapy Resistance. J. Cancer 2019, 10, 2109–2127. [Google Scholar] [CrossRef]
- Clark, S.L.; Rodriguez, A.M.; Snyder, R.R.; Hankins, G.D.; Boehning, D. STRUCTURE-FUNCTION OF THE TUMOR SUPPRESSOR BRCA1. Comput. Struct. Biotechnol. J. 2012, 1. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. BRCA1 BRCA1 DNA Repair Associated [Homo sapiens (Human)]. 2022. Available online: https://www.ncbi.nlm.nih.gov/gene?Db=gene&Cmd=DetailsSearch&Term=672 (accessed on 8 June 2022).
- Roy, R.; Chun, J.; Powell, S.N. BRCA1 and BRCA2: Different roles in a common pathway of genome protection. Nat. Rev. Cancer 2011, 12, 68–78. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. BRCA2 BRCA2 DNA Repair Associated [Homo sapiens (Human)]. 2022. Available online: https://www.ncbi.nlm.nih.gov/gene?Db=gene&Cmd=DetailsSearch&Term=675 (accessed on 8 June 2022).
- Vidarsson, H.; Mikaelsdottir, E.; Eyfjörd, J.; Ogmundsdottir, H.; Rafnar, T.; Valgeirsdottir, S. BRCA2 interacting proteins. Breast Cancer Res. 2000, 2. [Google Scholar] [CrossRef][Green Version]
- Kelsey, C.A.; Heintz, P.H.; Sandoval, D.J.; Chambers, G.D.; Adolphi, N.L.; Paffett, K.S. Radiation Biology of Medical Imaging, 1st ed.; John Wiley & Sons, Inc: Hoboken, NJ, USA, 2014; p. 41. [Google Scholar]
- Beyzadeoglu, M.; Ozyigit, G.; Ebruli, C. Radiation Physics. In Basic Radiation Oncology; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Podgorsak, E.B. Basic Radiation Physics. In Radiation Oncology Physics: A Handbook for Teachers and Students; IAEA: Vienna, Austria, 2005; pp. 1–44. [Google Scholar]
- Donya, M.; Radford, M.; ElGuindy, A.; Firmin, D.; Yacoub, M.H. Radiation in medicine: Origins, risks and aspirations. Glob. Cardiol. Sci. Pract. 2014, 2014, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Kelsey, C.A.; Heintz, P.H.; Sandoval, D.J.; Chambers, G.D.; Adolphi, N.L.; Paffett, K.S. Radiation Interactions with Tissue. In Radiation Biology of Medical Imaging, 1st ed.; John Wiley & Sons, Inc: Hoboken, NJ, USA, 2014; pp. 61–80. [Google Scholar]
- Podgorsak, E.B. Minimum Essential Syllabus for Radiobiology. In Radiation Biology: A Handbook for Teachers and Students; IAEA: Vienna, Austria, 2010; pp. 13–56. [Google Scholar]
- Neshasteh-Riz, A.; Mahmoud Pashazadeh, A.; Mahdavi, S.R. Relative Biological Effectiveness (RBE) of 131I Radiation Relative to 60Co Gamma Rays. Cell J. 2013, 15, 224–229. [Google Scholar]
- Kelsey, C.A.; Heintz, P.H.; Sandoval, D.J.; Chambers, G.D.; Adolphi, N.L.; Paffett, K.S. Cell Survival Curves. In Radiation Biology of Medical Imaging, 1st ed.; John Wiley & Sons, Inc: Hoboken, NJ, USA, 2014; pp. 81–104. [Google Scholar]
- Desouky, O.; Ding, N.; Zhou, G. Targeted and non-targeted effects of ionizing radiation. J. Radiat. Res. Appl. Sci. 2015, 8, 247–254. [Google Scholar] [CrossRef]
- Gonçalo, M.; Botelho, M.F. Radiações e Electricidade. In Fisiopatologia—Fundamentos e Aplicações; Lidel—Edições Técnicas Lda: Lisboa, Portugal, 2007; pp. 79–102. [Google Scholar]
- Rodrigues, L.; Palma, M.; Marques, L.T.; Varela, J.B. Dietary water affects human skin hydration and biomechanics. Clin. Cosmet. Investig. Dermatol. 2015, 8, 413–421. [Google Scholar] [CrossRef]
- Kelsey, C.A.; Heintz, P.H.; Sandoval, D.J.; Chambers, G.D.; Adolphi, N.L.; Paffett, K.S. DNA and Genetics. In Radiation Biology of Medical Imaging, 1st ed.; John Wiley & Sons, Inc: Hoboken, NJ, USA, 2014; pp. 105–124. [Google Scholar]
- Kelsey, C.A.; Heintz, P.H.; Sandoval, D.J.; Chambers, G.D.; Adolphi, N.L.; Paffett, K.S. Radiation Damage and Repair of Cells. In Radiation Biology of Medical Imaging, 1st ed.; John Wiley & Sons, Inc: Hoboken, NJ, USA, 2014; pp. 125–144. [Google Scholar]
- Bai, Y.; Wang, W.; Wang, J. Targeting DNA repair pathways: Mechanisms and potential applications in cancer therapy. Genome Instab. Dis. 2020, 1, 318–338. [Google Scholar] [CrossRef]
- Friedberg, E.C. DNA damage and repair. Nature 2003, 421, 436–440. [Google Scholar] [CrossRef]
- Chatterjee, N.; Walker, G.C. Mechanisms of DNA damage, repair, and mutagenesis. Environ. Mol. Mutagen. 2017, 58, 235–263. [Google Scholar] [CrossRef]
- Pećina-Šlaus, N.; Kafka, A.; Salamon, I.; Bukovac, A. Mismatch Repair Pathway, Genome Stability and Cancer. Front. Mol. Biosci. 2020, 7, 122. [Google Scholar] [CrossRef]
- Brandsma, I.; Gent, D.C. Pathway choice in DNA double strand break repair: Observations of a balancing act. Genome Integr. 2012, 3, 9. [Google Scholar] [CrossRef]
- Cai, L. Research of the adaptive response induced by low-dose radiation: Where have we been and where should we go? Hum. Exp. Toxicol. 1999, 18, 419–425. [Google Scholar] [CrossRef]
- Short, S.C.; Bourne, S.; Martindale, C.; Woodcock, M.; Jackson, S.P. DNA Damage Responses at Low Radiation Doses. Radiat. Res. 2005, 164, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Sakane, H.; Ishida, M.; Shi, L.; Fukumoto, W.; Sakai, C.; Miyata, Y.; Ishida, T.; Akita, T.; Okada, M.; Awai, K.; et al. Biological Effects of Low-Dose Chest CT on Chromosomal DNA. Radiology 2020, 295, 439–445. [Google Scholar] [CrossRef]
- Jia, C.; Wang, Q.; Yao, X.; Yang, J. The Role of DNA Damage Induced by Low/High Dose Ionizing Radiation in Cell Carcinogenesis. Explor. Res. Hypothesis Med. 2021, 6, 177–184. [Google Scholar] [CrossRef]
- Paul, A.; Paul, S. The breast cancer susceptibility genes (BRCA) in breast and ovarian cancers. Front. Biosci. Landmark Ed. 2014, 19, 605. [Google Scholar] [CrossRef]
- Drooger, J.C.; Hooning, M.J.; Seynaeve, C.M.; Baaijens, M.H.; Obdeijn, I.M.; Sleijfer, S.; Jager, A. Diagnostic and therapeutic ionizing radiation and the risk of a first and second primary breast cancer, with special attention for BRCA1 and BRCA2 mutation carriers: A critical review of the literature. Cancer Treat. Rev. 2015, 41, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Sharan, S.K.; Morimatsu, M.; Albrecht, U.; Lim, D.-S.; Regel, E.; Dinh, C.; Sands, A.; Eichele, G.; Hasty, P.; Bradley, A. Embryonic lethality and radiation hypersensitivity mediated by Rad51 in mice lacking Brca2. Nature 1997, 386, 804–810. [Google Scholar] [CrossRef]
- Narod, S.A.; Lubinski, J.; Ghadirian, P.; Lynch, H.T.; Moller, P.; Foulkes, W.; Rosen, B.; Kim-Sing, C.; Isaacs, C.; Domcheck, S.; et al. Screening mammography and risk of breast cancer in BRCA1 and BRCA2 mutation carriers: A case-control study. Lancet Oncol. 2006, 7, 402–406. [Google Scholar] [CrossRef]
- Goldfrank, D.; Chuai, S.; Bernstein, J.L.; Cajal, T.R.Y.; Lee, J.B.; Alonso, M.C.; Diez, O.; Baiget, M.; Kauff, N.D.; Offit, K.; et al. Effect of Mammography on Breast Cancer Risk in Women with Mutations in BRCA1 or BRCA2. Cancer Epidemiol. Biomark. Prev. 2006, 15, 2311–2313. [Google Scholar] [CrossRef]
- John, E.M.; McGuire, V.; Thomas, D.; Haile, R.; Ozcelik, H.; Milne, R.L.; Felberg, A.; West, D.W.; Miron, A.; Knight, J.A.; et al. Diagnostic Chest X-rays and Breast Cancer Risk before Age 50 Years for BRCA1 and BRCA2 Mutation Carriers. Cancer Epidemiol. Biomark. Prev. 2013, 22, 1547–1556. [Google Scholar] [CrossRef]
- Giannakeas, V.; Lubinski, J.; Gronwald, J.; Moller, P.; Armel, S.; Lynch, H.T.; Foulkes, W.; Kim-Sing, C.; Singer, C.; Neuhausen, S.L.; et al. Mammography screening and the risk of breast cancer in BRCA1 and BRCA2 mutation carriers: A prospective study. Breast Cancer Res. Treat. 2014, 147, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Andrieu, N.; Easton, D.F.; Chang-Claude, J.; Rookus, M.A.; Brohet, R.; Cardis, E.; Antoniou, A.; Wagner, T.; Simard, J.; Evans, G.; et al. Effect of Chest X-Rays on the Risk of Breast Cancer Among BRCA1/2 Mutation Carriers in the International BRCA1/2 Carrier Cohort Study: A Report from the EMBRACE, GENEPSO, GEO-HEBON, and IBCCS Collaborators’ Group. J. Clin. Oncol. 2006, 24, 3361–3366. [Google Scholar] [CrossRef] [PubMed]
- Lecarpentier, J.; Nogues, C.; Mouret-Fourme, E.; Stoppa-Lyonnet, D.; Lasset, C.; Caron, O.; Fricker, J.P.; Gladieff, L.; Faivre, L.; Sobol, H.; et al. Variation in breast cancer risk with mutation position, smoking, alcohol, and chest X-ray history, in the French National BRCA1/2 carrier cohort (GENEPSO). Breast Cancer Res. Treat. 2011, 130, 927–938. [Google Scholar] [CrossRef] [PubMed]
- Pijpe, A.; Andrieu, N.; Easton, D.F.; Kesminiene, A.; Cardis, E.; Nogues, C.; Gauthier-Villars, M.; Lasset, C.; Fricker, J.P.; Peock, S.; et al. Exposure to diagnostic radiation and risk of breast cancer among carriers of BRCA1/2 mutations: Retrospective cohort study (GENE-RAD-RISK). BMJ 2012, 345, e5660. [Google Scholar] [CrossRef] [PubMed]
- Baert, A.; Depuydt, J.; Van Maerken, T.; Poppe, B.; Malfait, F.; Storm, K.; Ende, J.V.D.; Van Damme, T.; De Nobele, S.; Perletti, G.; et al. Increased chromosomal radiosensitivity in asymptomatic carriers of a heterozygous BRCA1 mutation. Breast Cancer Res. 2016, 18, 52. [Google Scholar] [CrossRef] [PubMed]
- Baert, A.; Depuydt, J.; Van Maerken, T.; Poppe, B.; Malfait, F.; Van Damme, T.; De Nobele, S.; Perletti, G.; De Leeneer, K.; Claes, K.B.; et al. Analysis of chromosomal radiosensitivity of healthy BRCA2 mutation carriers and non-carriers in BRCA families with the G2 micronucleus assay. Oncol. Rep. 2017, 37, 1379–1386. [Google Scholar] [CrossRef]
- Broeks, A.; Braaf, L.M.; Huseinovic, A.; Nooijen, A.; Urbanus, J.; Hogervorst, F.B.; Schmidt, M.K.; Klijn, J.G.; Russell, N.S.; Van Leeuwen, F.E.; et al. Identification of women with an increased risk of developing radiation-induced breast cancer: A case only study. Breast Cancer Res. 2007, 9, R26. [Google Scholar] [CrossRef]
- PierceKelly-Anne, L.J.; Phillips, K.-A.; Griffith, K.A.; Buys, S.; Gaffney, D.K.; Moran, M.S.; Haffty, B.G.; Ben-David, M.; Kaufman, B.; Garber, J.E.; et al. Local therapy in BRCA1 and BRCA2 mutation carriers with operable breast cancer: Comparison of breast conservation and mastectomy. Breast Cancer Res. Treat. 2010, 121, 389–398. [Google Scholar]
- Metcalfe, K.A.; Gershman, S.J.; Lynch, H.T.; Ghadirian, P.; Tung, N.; Kimsing, C.; Olopade, O.I.; Domchek, S.M.; McLennan, J.E.; Eisen, A.; et al. Predictors of contralateral breast cancer in BRCA1 and BRCA2 mutation carriers. Br. J. Cancer 2011, 104, 1384–1392. [Google Scholar] [CrossRef]
- Bernstein, J.L.; Thomas, D.C.; Shore, R.; Robson, M.; Boice, J.D.; Stovall, M.; Andersson, M.; Bernstein, L.; Malone, K.E.; Reiner, A.; et al. Contralateral breast cancer after radiotherapy among BRCA1 and BRCA2 mutation carriers: A WECARE Study Report. Eur. J. Cancer 2013, 49, 2979–2985. [Google Scholar] [CrossRef]
- Schlosser, S.; Rabinovitch, R.; Shatz, Z.; Galper, S.; Shahadi-Dromi, I.; Finkel, S.; Jacobson, G.; Rasco, A.; Friedman, E.; Laitman, Y.; et al. Radiation-Associated Secondary Malignancies in BRCA Mutation Carriers Treated for Breast Cancer. Int. J. Radiat. Oncol. 2020, 107, 353–359. [Google Scholar] [CrossRef] [PubMed]
| Authors | Year | Sample Size (n) | Gene | Dose | Outcome |
|---|---|---|---|---|---|
| Broeks et al. [50] | 2007 | 247 (169 treated with radiotherapy and 78 not treated) | BRCA1, BRCA2, CHEK and ATM | 30.5–76 Gy | The risk of developing contralateral breast cancer after radiotherapy was higher for individuals carrying mutations in genes involved in DNA damage repair pathways. |
| Pierce et al. [51] | 2010 | 655 carriers | BRCA1 and BRCA2 | Not disclosed | The risk of developing contralateral breast cancer was higher for individuals undergoing BCS compared to individuals undergoing mastectomy. The risk in individuals undergoing adjuvant radiotherapy was not statistically significant. |
| Metcalfe et al. [52] | 2011 | 810 carriers | BRCA1 and BRCA2 | Not disclosed | The risk of developing contralateral breast cancer decreased with age at diagnosis, increasing with the number of first-degree relatives with the same diagnosis. |
| Bernstein et al. [53] | 2013 | 1802 (603 with contralateral breast cancer and 1199 with unilateral breast cancer) | BRCA1 and BRCA2 | 1.1 Gy (range = 0.02–6.2 Gy) | The risk of developing contralateral breast cancer in carriers was four times greater, however, carriers undergoing radiation therapy for primary breast cancer did not have a significantly higher relative risk of contralateral breast cancer. |
| Schlosser et al. [54] | 2020 | 230 carriers | BRCA1 and BRCA2 | 50 Gy (25 fractions, 2Gy per fraction (fx)) or 42.4 Gy for patients treated after 2010 (16 fractions, 2.65 Gy/fx) or 50.4 Gy for reconstructed or augmented breasts (28 fractions, 1.8 Gy/fx) or 45 Gy in 1.8 Gy/fx | Women with the mutation undergoing radiation therapy for breast cancer did not have a statistically significant risk of a second primary malignancy induced by exposure to ionizing radiation. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonçalves, D.; Pires, A.S.; Marques, I.A.; Gomes, I.; Sousa, G.; Botelho, M.F.; Abrantes, A.M. An Overview on Radiation Sensitivity in Hereditary Breast and Ovarian Cancer Syndrome. Cancers 2022, 14, 3254. https://doi.org/10.3390/cancers14133254
Gonçalves D, Pires AS, Marques IA, Gomes I, Sousa G, Botelho MF, Abrantes AM. An Overview on Radiation Sensitivity in Hereditary Breast and Ovarian Cancer Syndrome. Cancers. 2022; 14(13):3254. https://doi.org/10.3390/cancers14133254
Chicago/Turabian StyleGonçalves, Diana, Ana Salomé Pires, Inês A. Marques, Inês Gomes, Gabriela Sousa, Maria Filomena Botelho, and Ana Margarida Abrantes. 2022. "An Overview on Radiation Sensitivity in Hereditary Breast and Ovarian Cancer Syndrome" Cancers 14, no. 13: 3254. https://doi.org/10.3390/cancers14133254
APA StyleGonçalves, D., Pires, A. S., Marques, I. A., Gomes, I., Sousa, G., Botelho, M. F., & Abrantes, A. M. (2022). An Overview on Radiation Sensitivity in Hereditary Breast and Ovarian Cancer Syndrome. Cancers, 14(13), 3254. https://doi.org/10.3390/cancers14133254










