Body Mass Index Is Inversely Associated with Risk of Postmenopausal Interval Breast Cancer: Results from the Women’s Health Initiative
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
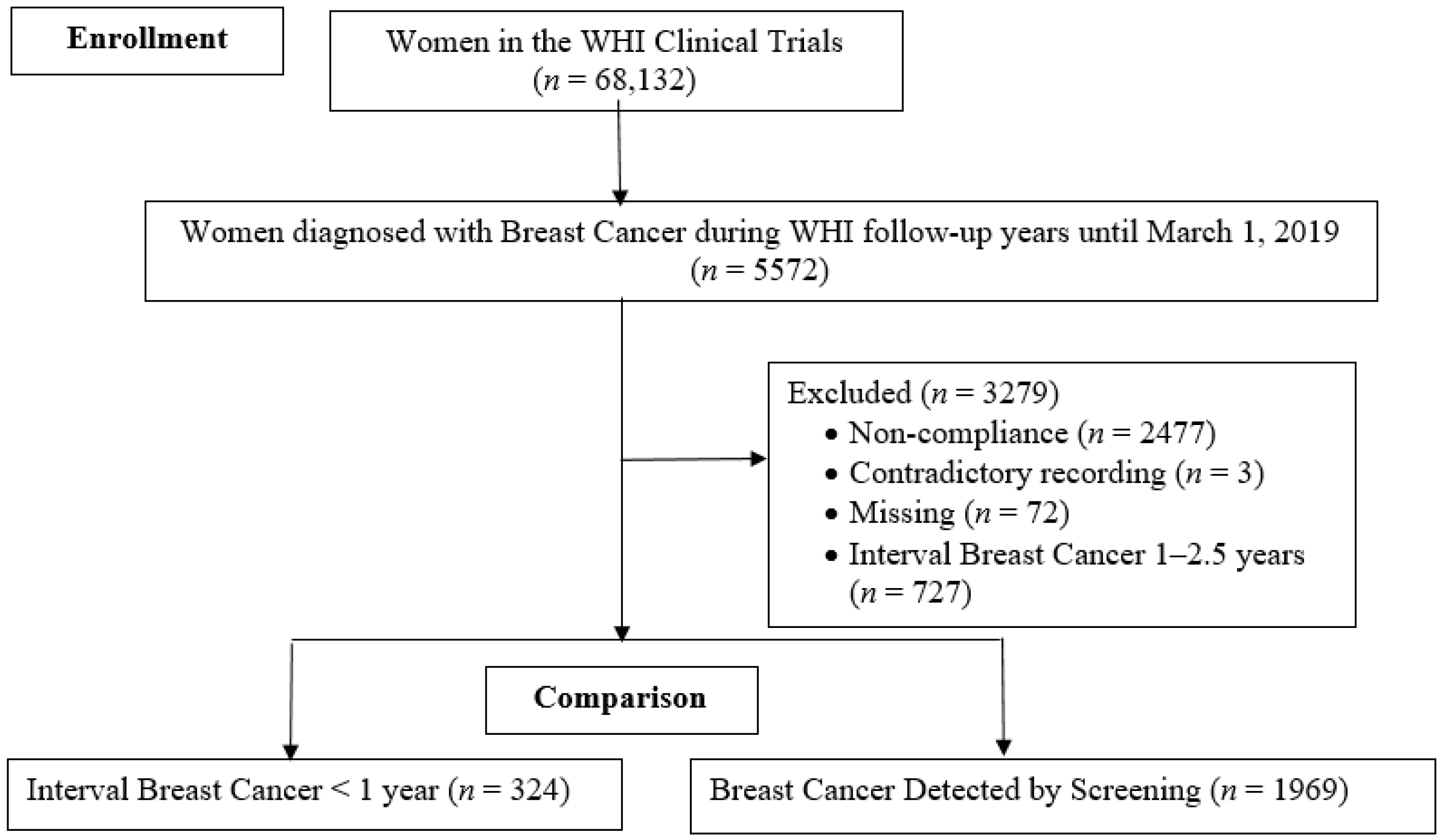
2.1. Study Population
2.2. Assessment of Interval Breast Cancer and Screening-Detected Breast Cancer
2.3. Assessment of Exposures
2.4. Assessment of Covariates
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Irvin, V.L.; Zhang, Z.; Simon, M.S.; Chlebowski, R.T.; Luoh, S.W.; Shadyab, A.H.; Krok-Schoen, J.L.; Tabung, F.K.; Qi, L.; Stefanick, M.L.; et al. Comparison of Mortality Among Participants of Women’s Health Initiative Trials With Screening-Detected Breast Cancers vs Interval Breast Cancers. JAMA Netw. Open 2020, 3, e207227. [Google Scholar] [CrossRef]
- Grassmann, F.; He, W.; Eriksson, M.; Gabrielson, M.; Hall, P.; Czene, K. Interval breast cancer is associated with other types of tumors. Nat. Commun. 2019, 10, 4648. [Google Scholar] [CrossRef] [Green Version]
- Hoff, S.R.; Samset, J.H.; Abrahamsen, A.L.; Vigeland, E.; Klepp, O.; Hofvind, S. Missed and true interval and screen-detected breast cancers in a population based screening program. Acad. Radiol. 2011, 18, 454–460. [Google Scholar] [CrossRef]
- Houssami, N.; Hunter, K. The epidemiology, radiology and biological characteristics of interval breast cancers in population mammography screening. NPJ Breast Cancer 2017, 3, 12. [Google Scholar] [CrossRef]
- Vlug, E.; Ercan, C.; van der Wall, E.; van Diest, P.J.; Derksen, P.W. Lobular breast cancer: Pathology, biology, and options for clinical intervention. Arch. Immunol. Ther. Exp. 2014, 62, 7–21. [Google Scholar] [CrossRef]
- Kobayashi, N.; Hikichi, M.; Ushimado, K.; Sugioka, A.; Kiriyama, Y.; Kuroda, M.; Utsumi, T. Differences in subtype distribution between screen-detected and symptomatic invasive breast cancer and their impact on survival. Clin. Transl. Oncol. 2017, 19, 1232–1240. [Google Scholar] [CrossRef]
- Niraula, S.; Biswanger, N.; Hu, P.; Lambert, P.; Decker, K. Incidence, Characteristics, and Outcomes of Interval Breast Cancers Compared With Screening-Detected Breast Cancers. JAMA Netw. Open 2020, 3, e2018179. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Shieh, Y.; Ziv, E.; Kerlikowske, K. Interval breast cancers-insights into a complex phenotype. Nat. Rev. Clin. Oncol. 2020, 17, 138–139. [Google Scholar] [CrossRef]
- Anderson, G.; Cummings, S.; Freedman, L.S.; Furberg, C.; Henderson, M.; Johnson, S.R.; Kuller, L.; Manson, J.; Oberman, A.; Prentice, R.L.; et al. Design of the Women’s Health Initiative clinical trial and observational study. The Women’s Health Initiative Study Group. Control. Clin. Trials 1998, 19, 61–109. [Google Scholar]
- Neuhouser, M.L.; Aragaki, A.K.; Prentice, R.L.; Manson, J.E.; Chlebowski, R.; Carty, C.L.; Ochs-Balcom, H.M.; Thomson, C.A.; Caan, B.J.; Tinker, L.F.; et al. Overweight, Obesity, and Postmenopausal Invasive Breast Cancer Risk: A Secondary Analysis of the Women’s Health Initiative Randomized Clinical Trials. JAMA Oncol. 2015, 1, 611–621. [Google Scholar] [CrossRef]
- Kerlikowske, K.; Walker, R.; Miglioretti, D.L.; Desai, A.; Ballard-Barbash, R.; Buist, D.S. Obesity, mammography use and accuracy, and advanced breast cancer risk. J. Natl. Cancer Inst. 2008, 100, 1724–1733. [Google Scholar] [CrossRef] [Green Version]
- Cui, Y.; Whiteman, M.K.; Flaws, J.A.; Langenberg, P.; Tkaczuk, K.H.; Bush, T.L. Body mass and stage of breast cancer at diagnosis. Int. J. Cancer 2002, 98, 279–283. [Google Scholar] [CrossRef] [Green Version]
- Tran, T.X.M.; Moon, S.G.; Kim, S.; Park, B. Association of the Interaction Between Mammographic Breast Density, Body Mass Index, and Menopausal Status With Breast Cancer Risk Among Korean Women. JAMA Netw. Open 2021, 4, e2139161. [Google Scholar] [CrossRef]
- Quail, D.F.; Dannenberg, A.J. The obese adipose tissue microenvironment in cancer development and progression. Nat. Rev. Endocrinol. 2019, 15, 139–154. [Google Scholar] [CrossRef]
- Chen, K.; Zhang, J.; Beeraka, N.M.; Tang, C.; Babayeva, Y.V.; Sinelnikov, M.Y.; Zhang, X.; Zhang, J.; Liu, J.; Reshetov, I.V.; et al. Advances in the Prevention and Treatment of Obesity-Driven Effects in Breast Cancers. Front. Oncol. 2022, 12. [Google Scholar] [CrossRef]
- Stephenson, G.D.; Rose, D.P. Breast cancer and obesity: An update. Nutr. Cancer 2003, 45, 1–16. [Google Scholar] [CrossRef]
- The Premenopausal Breast Cancer Collaborative Group; Schoemaker, M.J.; Nichols, H.B.; Wright, L.B.; Brook, M.N.; Jones, M.E.; O’Brien, K.M.; Adami, H.O.; Baglietto, L.; Bernstein, L.; et al. Association of Body Mass Index and Age With Subsequent Breast Cancer Risk in Premenopausal Women. JAMA Oncol. 2018, 4, e181771. [Google Scholar] [CrossRef]
- Elmore, J.G.; Carney, P.A.; Abraham, L.A.; Barlow, W.E.; Egger, J.R.; Fosse, J.S.; Cutter, G.R.; Hendrick, R.E.; D’Orsi, C.J.; Paliwal, P.; et al. The association between obesity and screening mammography accuracy. Arch. Intern. Med. 2004, 164, 1140–1147. [Google Scholar] [CrossRef] [Green Version]
- Nelson, H.D.; O’Meara, E.S.; Kerlikowske, K.; Balch, S.; Miglioretti, D. Factors Associated With Rates of False-Positive and False-Negative Results From Digital Mammography Screening: An Analysis of Registry Data. Ann. Intern. Med. 2016, 164, 226–235. [Google Scholar] [CrossRef]
- Mello-Thoms, C. Mammographic Breast Density, Body Mass Index, Menopausal Status, and Breast Cancer Risk. JAMA Netw. Open 2021, 4, e2139855. [Google Scholar] [CrossRef]
- Boyd, N.F.; Rommens, J.M.; Vogt, K.; Lee, V.; Hopper, J.L.; Yaffe, M.J.; Paterson, A.D. Mammographic breast density as an intermediate phenotype for breast cancer. Lancet Oncol. 2005, 6, 798–808. [Google Scholar] [CrossRef]
- Mandelson, M.T.; Oestreicher, N.; Porter, P.L.; White, D.; Finder, C.A.; Taplin, S.H.; White, E. Breast density as a predictor of mammographic detection: Comparison of interval- and screen-detected cancers. J. Natl. Cancer Inst. 2000, 92, 1081–1087. [Google Scholar] [CrossRef]
- Fernandez-Nogueira, P.; Mancino, M.; Fuster, G.; Bragado, P.; Puig, M.P.; Gascon, P.; Casado, F.J.; Carbo, N. Breast Mammographic Density: Stromal Implications on Breast Cancer Detection and Therapy. J. Clin. Med. 2020, 9, 776. [Google Scholar] [CrossRef] [Green Version]
- Xu, S.; Xu, H.; Wang, W.; Li, S.; Li, H.; Li, T.; Zhang, W.; Yu, X.; Liu, L. The role of collagen in cancer: From bench to bedside. J. Transl. Med. 2019, 17, 309. [Google Scholar] [CrossRef] [Green Version]
- Lethaby, A.E.; O’Neill, M.A.; Mason, B.H.; Holdaway, I.M.; Harvey, V.J. Overall survival from breast cancer in women pregnant or lactating at or after diagnosis. Auckland Breast Cancer Study Group. Int. J. Cancer 1996, 67, 751–755. [Google Scholar] [CrossRef]
- Huang, Z.; Willett, W.C.; Colditz, G.A.; Hunter, D.J.; Manson, J.E.; Rosner, B.; Speizer, F.E.; Hankinson, S.E. Waist circumference, waist:hip ratio, and risk of breast cancer in the Nurses’ Health Study. Am. J. Epidemiol. 1999, 150, 1316–1324. [Google Scholar] [CrossRef] [Green Version]
- Gold, R.; Michael, Y.L.; Whitlock, E.P.; Hubbell, F.A.; Mason, E.D.; Rodriguez, B.L.; Safford, M.M.; Sarto, G.E. Race/ethnicity, socioeconomic status, and lifetime morbidity burden in the women’s health initiative: A cross-sectional analysis. J. Womens Health (Larchmt) 2006, 15, 1161–1173. [Google Scholar] [CrossRef]
- Momenimovahed, Z.; Salehiniya, H. Epidemiological characteristics of and risk factors for breast cancer in the world. Breast Cancer 2019, 11, 151–164. [Google Scholar] [CrossRef] [Green Version]
- Manson, J.E.; Chlebowski, R.T.; Stefanick, M.L.; Aragaki, A.K.; Rossouw, J.E.; Prentice, R.L.; Anderson, G.; Howard, B.V.; Thomson, C.A.; LaCroix, A.Z.; et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA 2013, 310, 1353–1368. [Google Scholar] [CrossRef]
- Key, T.J.; Pike, M.C. The role of oestrogens and progestagens in the epidemiology and prevention of breast cancer. Eur. J. Cancer Clin. Oncol. 1988, 24, 29–43. [Google Scholar] [CrossRef]
- Bulun, S.E.; Chen, D.; Moy, I.; Brooks, D.C.; Zhao, H. Aromatase, breast cancer and obesity: A complex interaction. Trends Endocrinol. Metab. 2012, 23, 83–89. [Google Scholar] [CrossRef] [Green Version]
- Buist, D.S.; Porter, P.L.; Lehman, C.; Taplin, S.H.; White, E. Factors contributing to mammography failure in women aged 40–49 years. J. Natl. Cancer Inst. 2004, 96, 1432–1440. [Google Scholar] [CrossRef]
- Han, Y.; Berkey, C.S.; Herman, C.R.; Appleton, C.M.; Alimujiang, A.; Colditz, G.A.; Toriola, A.T. Adiposity Change Over the Life Course and Mammographic Breast Density in Postmenopausal Women. Cancer Prev. Res. 2020, 13, 475–482. [Google Scholar] [CrossRef] [Green Version]
- Soguel, L.; Durocher, F.; Tchernof, A.; Diorio, C. Adiposity, breast density, and breast cancer risk: Epidemiological and biological considerations. Eur. J. Cancer Prev. 2017, 26, 511–520. [Google Scholar] [CrossRef] [Green Version]
- Wanders, J.O.P.; Holland, K.; Karssemeijer, N.; Peeters, P.H.M.; Veldhuis, W.B.; Mann, R.M.; van Gils, C.H. The effect of volumetric breast density on the risk of screen-detected and interval breast cancers: A cohort study. Breast Cancer Res. 2017, 19, 67. [Google Scholar] [CrossRef] [Green Version]
- Heymsfield, S.B.; Scherzer, R.; Pietrobelli, A.; Lewis, C.E.; Grunfeld, C. Body mass index as a phenotypic expression of adiposity: Quantitative contribution of muscularity in a population-based sample. Int. J. Obes. (Lond.) 2009, 33, 1363–1373. [Google Scholar] [CrossRef] [Green Version]
- Houghton, S.C.; Eliassen, H.; Tamimi, R.M.; Willett, W.C.; Rosner, B.A.; Hankinson, S.E. Central Adiposity and Subsequent Risk of Breast Cancer by Menopause Status. J. Natl. Cancer Inst. 2021, 113, 900–908. [Google Scholar] [CrossRef]
- Rose, D.P.; Haffner, S.M.; Baillargeon, J. Adiposity, the metabolic syndrome, and breast cancer in African-American and white American women. Endocr. Rev. 2007, 28, 763–777. [Google Scholar] [CrossRef]
- Ronco, A.L.; Boeing, H.; De Stefani, E.; Schulz, M.; Schulze, M.; Pischon, T. A case-control study on fat-to-muscle ratio and risk of breast cancer. Nutr. Cancer 2009, 61, 466–474. [Google Scholar] [CrossRef]
- Iyengar, N.M.; Arthur, R.; Manson, J.E.; Chlebowski, R.T.; Kroenke, C.H.; Peterson, L.; Cheng, T.D.; Feliciano, E.C.; Lane, D.; Luo, J.; et al. Association of Body Fat and Risk of Breast Cancer in Postmenopausal Women With Normal Body Mass Index: A Secondary Analysis of a Randomized Clinical Trial and Observational Study. JAMA Oncol. 2019, 5, 155–163. [Google Scholar] [CrossRef]
- Arthur, R.S.; Xue, X.; Kamensky, V.; Chlebowski, R.T.; Simon, M.; Luo, J.; Shadyab, A.H.; Neuhouser, M.L.; Banack, H.; Ho, G.Y.F.; et al. The association between DXA-derived body fat measures and breast cancer risk among postmenopausal women in the Women’s Health Initiative. Cancer Med. 2020, 9, 1581–1599. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Tabung, F.K.; Jin, Q.; Curran, G.; Irvin, V.L.; Shannon, J.; Velie, E.M.; Manson, J.E.; Simon, M.S.; Vitolins, M.; et al. Diet-Driven Inflammation and Insulinemia and Risk of Interval Breast Cancer. Nutr. Cancer 2022, 1–15. [Google Scholar] [CrossRef]
| Variable | Interval Breast Cancer (n = 324) | Breast Cancer Detected by Screening (n = 1969) | p-Value |
|---|---|---|---|
| Demographic characteristics | |||
| Age at enrollment, mean (SD), y | 63.07 (7.15) | 63.24 (6.79) | 0.68 |
| BMI (kg/m2) at enrollment, mean (SD) continuous variable | 28.08 (5.35) | 29.46 (5.74) | <0.0001 |
| BMI (kg/m2) at enrollment, categorical variable | <0.0001 | ||
| Underweight | 2 (0.62) | 8 (0.41) | |
| Normal weight | 95 (29.32) | 439 (22.30) | |
| Overweight | 126 (38.89) | 675 (34.28) | |
| Obese | 101 (31.17) | 847 (43.02) | |
| Waist-to-hip ratio at enrollment, mean (SD) | 0.81 (0.07) | 0.82 (0.08) | 0.03 |
| Height (cm), mean (SD) | 161.8 (6.48) | 162.2 (6.49) | 0.25 |
| Gail 5-yr risk score, mean (SD) | 2.00 (1.22) | 1.86 (1.08) | 0.03 |
| Race/ethnicity, No. (%) | 0.24 | ||
| White | 276 (85.2) | 1688 (85.9) | |
| African American | 22 (6.8) | 160 (8.1) | |
| Hispanic | 14 (4.3) | 44 (2.2) | |
| Asian | 8 (2.5) | 45 (2.3) | |
| Other | 4 (1.2) | 28 (1.4) | |
| Missing | 0 | 4 | |
| Family history of breast cancer, No. (%) | 0.22 | ||
| Yes | 80 (25.9) | 423 (22.7) | |
| No | 229 (74.1) | 1441 (77.3) | |
| Missing | 15 | 105 | |
| Ever full-term birth, No. (%) | 0.54 | ||
| Yes | 273 (96.5) | 1732 (97.1) | |
| No | 10 (3.5) | 51 (2.9) | |
| Missing | 41 | 186 | |
| Age at first live birth, No. (%) | 0.11 | ||
| Never had any live birth | 8 (3.17) | 46 (2.87) | |
| <20 years old | 28 (11.11) | 269 (16.80) | |
| 20–29 years old | 184 (73.02) | 1122 (70.08) | |
| ≥30 years old | 32 (12.70) | 164 (10.24) | |
| Missing | 72 | 368 | |
| Age at menarche, No. (%) | 0.09 | ||
| ≤11 years old | 66 (12.38) | 467 (23.85) | |
| 12 years old | 89 (27.47) | 490 (25.03) | |
| 13 years old | 110 (33.95) | 559 (28.55) | |
| 14 years old | 35 (10.80) | 288 (14.71) | |
| ≥15 years old | 24 (7.41) | 154 (7.87) | |
| Missing | 0 | 11 | |
| Previous breast biopsy, No. (%) | 0.09 | ||
| 0 | 197 (68.17) | 1286 (73.53) | |
| 1 | 65 (22.49) | 351 (20.07) | |
| >1 | 27 (9.34) | 112 (6.40) | |
| Missing | 35 | 220 | |
| Total dietary energy intake (kcal/day) | 1683.8 (650.4) | 1733.4 (703.9) | 0.24 |
| Total energy expend from recreational physical activity (MET-hours/week) | 10.42 (10.60) | 9.74 (10.85) | 0.29 |
| HT Study group, No. (%) | 0.37 | ||
| Estrogen-alone intervention | 20 (15.27) | 131 (14.13) | |
| Estrogen-alone control | 21 (16.03) | 191 (20.60) | |
| Estrogen + progestin intervention | 57 (43.51) | 341 (36.79) | |
| Estrogen + progestin control | 33 (25.19) | 264 (28.48) | |
| Not randomized to HT | 193 | 1042 | |
| HT Study group Re-group, No. (%) | 0.95 | ||
| Estrogen-alone intervention | 20 (6.17) | 131 (6.65) | |
| Estrogen + progestin intervention | 57 (17.59) | 341 (17.32) | |
| No Estrogen or progestin Intervention | 247 (76.23) | 1497 (76.03) | |
| DM Trial group, No. (%) | 0.80 | ||
| Intervention | 92 (39.66) | 521 (38.79) | |
| Control | 140 (60.34) | 822 (61.21) | |
| Not randomized to DM | 92 | 626 | |
| Comorbidity at Enrollment, No. (%) | 0.45 | ||
| 0 | 222 (68.52) | 1364 (69.34) | |
| 1 | 66 (20.37) | 423 (21.50) | |
| 2 | 23 (7.10) | 131 (6.66) | |
| ≥3 | 13 (4.01) | 49 (2.49) | |
| Education | 0.27 | ||
| Below high school | 11 (3.42) | 87 (4.47) | |
| High school diploma/GED | 49 (15.22) | 331(16.98) | |
| Vocational or training school | 38 (11.80) | 215 (11.03) | |
| Some college or associate degree | 87 (27.02) | 585 (30.02) | |
| College degree or baccalaureate degree | 46 (14.29) | 200 (10.26) | |
| Postgraduate degree | 91 (28.26) | 531 (27.24) | |
| Missing | 2 | 20 | |
| Smoking | 0.07 | ||
| Never smokers | 169 (53.14) | 977 (50.28) | |
| Current smokers | 28 (8.81) | 120 (6.18) | |
| Ever smokers | 121 (38.05) | 846 (43.54) | |
| Missing | 6 | 26 | |
| Alcohol | 0.64 | ||
| Never drinkers | 38 (11.91) | 221 (11.34) | |
| <1 drink/month | 48 (15.05) | 277 (14.22) | |
| <1 drink/week | 63 (19.75) | 402 (20.64) | |
| 1– < 7 drinks/week | 75 (23.51) | 478 (24.54) | |
| ≥7 drinks/week | 43 (13.48) | 206 (10.57) | |
| Past drinkers | 52 (16.30) | 364 (18.69) | |
| Missing | 5 | 21 |
| Variable | Normal Weight (BMI: 18.5–24.9) (n = 534) | Overweight (BMI: >24.9–29.9) (n = 801) | Obese (BMI: >29.9) (n = 948) | p Value |
|---|---|---|---|---|
| Continuous variables | ||||
| Age at enrollment, mean (SD), y | 63.13 | 63.37 | 63.16 | 0.75 |
| Age at diagnosis, mean (SD), y | 68.29 | 68.58 | 68.60 | 0.69 |
| Growth morphometric variables, mean (SD) | ||||
| Height (cm) | 163.38 | 162.13 | 161.39 | <0.0001 |
| Waist-to-hip ratio at enrollment | 0.77 | 0.82 | 0.85 | <0.0001 |
| Gail 5-yr risk score | 1.95 | 1.92 | 1.80 | 0.02 |
| Total energy intake | 1603.95 | 1687.14 | 1832.18 | <0.0001 |
| Total energy expended from recreational physical activity (MET-hours/week) | 12.51 | 10.57 | 7.72 | <0.0001 |
| Categorical variables | p value | |||
| Race/ethnicity, No. (%) | <0.0001 | |||
| White | 479 (89.70) | 707 (88.38) | 769 (81.38) | |
| African American | 22 (4.12) | 47 (5.88) | 113 (11.96) | |
| Hispanic | 8 (1.50) | 20 (2.50) | 30 (3.17) | |
| Asian | 22 (4.12) | 15 (1.88) | 16 (1.69) | |
| Other | 3 (0.56) | 11 (1.38) | 17 (1.80) | |
| Family history of breast cancer, No. (%) | 0.95 | |||
| Yes | 121 (23.68) | 176 (23.01) | 204 (23.00) | |
| No | 390 (76.32) | 589 (76.99) | 683 (77.00) | |
| Ever full-term birth, No. (%) | ||||
| Yes | 460 (97.05) | 703 (97.10) | 835 (96.98) | 0.99 |
| No | 14 (2.95) | 21 (2.90) | 26 (3.02) | |
| Age at first live birth, No. (%) | <0.0001 | |||
| Never had any live birth | 13 (2.97) | 19 (2.92) | 22 (2.90) | |
| <20 years old | 50 (11.42) | 86 (13.23) | 161 (21.21) | |
| 20–29 years old | 326 (74.43) | 482 (74.15) | 493 (64.95) | |
| ≥30 years old | 49 (11.19) | 63 (9.69) | 83 (10.94) | |
| Age at menarche, No. (%) | <0.0001 | |||
| ≤11 years old | 89 (16.82) | 178 (22.33) | 265 (28.01) | |
| 12 years old | 121 (22.87) | 201 (25.22) | 255 (26.96) | |
| 13 years old | 176 (33.27) | 235 (29.49) | 253 (26.74) | |
| 14 years old | 93 (17.58) | 121 (15.18) | 107 (11.31) | |
| ≥15 years old | 50 (9.45) | 62 (7.78) | 66 (6.98) | |
| Previous breast biopsy, No. (%) | 0.0004 | |||
| 0 | 310 (66.67) | 506 (72.29) | 661 (76.42) | |
| 1 | 117 (25.16) | 134 (19.14) | 153 (18.84) | |
| >1 | 38 (8.17) | 60 (8.57) | 41 (4.74) | |
| HT study group, No. (%) | 0.0002 | |||
| Estrogen-alone intervention | 27 (12.74) | 36 (10.26) | 88 (17.96) | |
| Estrogen-alone control | 29 (13.68) | 65 (18.52) | 118 (24.08) | |
| Estrogen + progestin intervention | 93 (43.87) | 143 (40.74) | 159 (32.45) | |
| Estrogen + progestin control | 63 (29.72) | 107 (30.48) | 125 (25.51) | |
| Not randomized to HRT | 322 | 450 | 458 | |
| DM trial group, No. (%) | 0.12 | |||
| Intervention | 135 (37.71) | 237 (42.17) | 238 (36.62) | |
| Control | 223 (62.29) | 325 (57.83) | 412 (63.38) | |
| Not randomized to DM | 176 | 239 | 298 | |
| Comorbidity at enrollment, No. (%) | <0.0001 | |||
| 0 | 404 (75.66) | 576 (71.91) | 601 (63.40) | |
| 1 | 96 (17.98) | 160 (19.98) | 230 (24.26) | |
| 2 | 26 (4.87) | 50 (6.24) | 78 (8.23) | |
| ≥3 | 8 (1.50) | 15 (1.87) | 39 (4.11) | |
| Education | <0.0001 | |||
| Below high school | 9 (1.69) | 30 (3.78) | 59 (6.30) | |
| High school diploma/GED | 78 (14.69) | 135 (17.02) | 166 (17.72) | |
| Vocational or training school | 51 (9.60) | 88 (11.10) | 114 (12.17) | |
| Some college or associate degree | 129 (24.29) | 229 (28.88) | 311 (33.19) | |
| College degree or baccalaureate degree | 79 (14.88) | 94 (11.85) | 71 (7.58) | |
| Postgraduate degree | 185 (34.84) | 217 (27.36) | 216 (23.05) | |
| Smoking | 0.52 | |||
| Never smokers | 266 (50.96) | 394 (49.68) | 480 (51.28) | |
| Current smokers | 39 97.47) | 56 (7.06) | 51 (5.45) | |
| Ever smokers | 217 (41.57) | 343 (43.25) | 405 (43.27) | |
| Alcohol | <0.0001 | |||
| Never drink | 56 (10.63) | 87 (10.94) | 115 (12.29) | |
| <1 drink/month | 48 (9.11) | 106 (13.33) | 170 (18.16) | |
| <1 drink/week | 100 (18.98) | 174 (21.89) | 190 (20.30) | |
| 1– <7 drinks/week | 175 (33.21) | 209 (26.29) | 167 (17.84) | |
| ≥7 drinks/week | 87 (16.51) | 94 (11.82) | 66 (7.05) | |
| Past drinking | 61 (11.57) | 125 (15.72) | 228 (24.36) |
| Odds Ratio (95% Confidence Interval) 1 | ||||||
|---|---|---|---|---|---|---|
| Model 1 2 | Model 2 3 | Model 3 4 | Model 4 5 | Model 5 6 | Model 6 7 | |
| BMI Categories | ||||||
| Underweight (BMI: <18.5) | 1.16 (0.24, 5.53) | 1.14 (0.24, 5.47) | 1.15 (0.24, 5.49) | 1.13 (0.24, 5.43) | 0.98 (0.20, 4.81) | 0.95 (0.19, 4.70) |
| Normal weight (BMI: 18.5–24.9) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Overweight (BMI: >24.9–29.9) | 0.86 (0.64, 1.16) | 0.88 (0.65, 1.198) | 0.88 (0.66, 1.20) | 0.89 (0.66, 1.21) | 0.93 (0.69, 1.27) | 0.91 (0.67, 1.24) |
| Obese (BMI: >29.9) | 0.55 (0.41, 0.75) | 0.57 (0.41, 0.80) | 0.58 (0.42, 0.81) | 0.60 (0.43, 0.84) | 0.65 (0.46, 0.92) | 0.62 (0.43, 0.89) |
| BMI Continuous Variables | ||||||
| Per 1 unit increase | 0.96 (0.94, 0.98) | 0.96 (0.94, 0.98) | 0.96 (0.94, 0.98) | 0.96 (0.94, 0.99) | 0.97 (0.94, 0.99) | 0.97 (0.94, 0.99) |
| Excluding Breast Cancer Cases Diagnosed within 2 Years (n = 285 for IBC and n = 1865 for SBC) | ||||||
|---|---|---|---|---|---|---|
| Odds Ratio (95% Confidence Interval) 1 | ||||||
| Model 1 2 | Model 2 3 | Model 3 4 | Model 4 5 | Model 5 6 | Model 6 7 | |
| BMI Categories | ||||||
| Underweight (BMI: <18.5) | 1.20 (0.25, 5.75) | 1.20 (0.25, 5.73) | 1.20 (0.25, 5.75) | 1.19 (0.25, 5.70) | 1.03 (0.21, 5.10) | 0.98 (0.20, 4.87) |
| Normal weight (BMI: 18.5–24.9) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Overweight (BMI: >24.9–29.9) | 0.80 (0.59, 1.10) | 0.81 (0.59, 1.11) | 0.81 (0.59, 1.12) | 0.82 (0.59, 1.12) | 0.85 (0.61, 1.18) | 0.83 (0.60, 1.15) |
| Obese (BMI: >29.9) | 0.54 (0.39, 0.74) | 0.55 (0.39, 0.77) | 0.55 (0.39, 0.78) | 0.56 (0.39, 0.79) | 0.60 (0.42, 0.87) | 0.57 (0.39, 0.84) |
| BMI Continuous Variables | ||||||
| Per 1 unit increase | 0.96 (0.93, 0.98) | 0.96 (0.93, 0.98) | 0.96 (0.94, 0.98) | 0.96 (0.94, 0.99) | 0.97 (0.94, 0.99) | 0.96 (0.94, 0.99) |
| Excluding breast cancer cases diagnosed within 4 years (n = 212 for IBC and n = 1405 for SBC) | ||||||
| Odds Ratio (95% Confidence Interval) 1 | ||||||
| Model 1 2 | Model 2 3 | Model 3 4 | Model 4 5 | Model 5 6 | Model 6 7 | |
| BMI Categories | Odds Ratio | |||||
| Underweight (BMI: <18.5) | 1.55 (0.31, 7.85) | 1.59 (0.31, 8.04) | 1.61 (0.32, 8.18) | 1.59 (0.31, 8.08) | 1.28 (0.24, 6.88) | 1.25 (0.23, 6.79) |
| Normal weight (BMI: 18.5–24.9) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Overweight (BMI: >24.9–29.9) | 0.73 (0.51, 1.04) | 0.69 (0.48, 1.01) | 0.70 (0.48, 1.01) | 0.70 (0.48, 1.02) | 0.75 (0.51, 1.09) | 0.73 (0.50, 1.08) |
| Obese (BMI: <29.9) | 0.53 (0.37, 0.76) | 0.49 (0.33, 0.72) | 0.49 (0.33, 0.73) | 0.49 (0.33, 0.74) | 0.56 (0.37, 0.86) | 0.55 (0.35, 0.85) |
| BMI Continuous Variables | ||||||
| Per 1 unit increase | 0.96 (0.94, 0.99) | 0.96 (0.93, 0.99) | 0.96 (0.93, 0.99) | 0.96 (0.93, 0.99) | 0.97 (0.94, 1.00) | 0.97 (0.94, 1.00) |
| Excluding breast cancer cases on E + P (n = 267 for IBC, n = 1628 for SBC) | ||||||
| Odds Ratio (95% Confidence Interval) 1 | ||||||
| Model 1 2 | Model 2 3 | Model 3 4 | Model 4 5 | Model 5 6 | Model 6 7 | |
| BMI Categories | ||||||
| Underweight (BMI: <18.5) | 0.83 (0.10, 6.97) | 0.82 (0.10, 6.92) | 0.84 (0.10, 7.06) | 0.82 (0.10, 6.91) | 0.82 (0.10, 7.00) | 0.70 (0.08, 6.05) |
| Normal weight (BMI: 18.5–24.9) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Overweight (BMI: >24.9–29.9) | 0.96 (0.70, 1.33) | 0.98 (0.70, 1.36) | 0.98 (0.70, 1.37) | 0.99 (0.71, 1.38) | 1.04 (0.74, 1.46) | 1.00 (0.71, 1.40) |
| Obese (BMI: <29.9) | 0.60 (0.43, 0.84) | 0.62 (0.43, 0.89) | 0.62 (0.43, 0.90) | 0.64 (0.44, 0.93) | 0.68 (0.46, 1.01) | 0.63 (0.42, 0.94) |
| BMI Continuous Variables | ||||||
| Per 1 unit increase | 0.96 (0.94, 0.98) | 0.96 (0.94, 0.99) | 0.96 (0.94, 0.99) | 0.96 (0.93, 0.99) | 0.97 (0.94, 0.995) | 0.96 (0.94, 0.99) |
| Including early-stage breast cancer (in situ + localized) (n = 218 for IBC, n = 1599 for SBC) | ||||||
| Odds Ratio (95% Confidence Interval) 1, 8 | ||||||
| Model 1 2 | Model 2 3 | Model 3 4 | Model 4 5 | Model 5 6 | Model 6 7 | |
| BMI Categories | ||||||
| Underweight (BMI: <18.5) | 1.50 (0.31, 7.23) | 1.45 (0.30, 6.99) | 1.46 (0.30, 7.03) | 1.47 (0.31, 7.11) | 1.23 (0.24, 6.19) | 1.20 (0.24, 6.11) |
| Normal weight (BMI: 18.5–24.9) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Overweight (BMI: >24.9–29.9) | 1.03 (0.73, 1.46) | 1.11 (0.78, 1.58) | 1.11 (0.78, 1.59) | 1.12 (0.78, 1.60) | 1.18 (0.82, 1.71) | 1.17 (0.81, 1.70) |
| Obese (BMI: >29.9) | 0.54 (0.37, 0.78) | 0.62 (0.41, 0.93) | 0.62 (0.42, 0.94) | 0.63 (0.42, 0.96) | 0.71 (0.46, 1.09) | 0.70 (0.45, 1.09) |
| BMI Continuous Variables | ||||||
| Per 1 unit increase | 0.95 (0.93, 0.98) | 0.96 (0.94, 0.99) | 0.96 (0.94, 0.99) | 0.97 (0.94, 0.99) | 0.97 (0.94, 1.00) | 0.97 (0.94, 1.00) |
| Including late-stage breast cancer (regional + distant) (n = 103 for IBC, n = 349 for SBC) | ||||||
| Odds Ratio (95% Confidence Interval) 1, 8 | ||||||
| Model 1 2 | Model 2 3 | Model 3 4 | Model 4 5 | Model 5 6 | Model 6 7 | |
| BMI Categories | ||||||
| Underweight (BMI: <18.5) | NA | NA | NA | NA | NA | NA |
| Normal weight (BMI: 18.5–24.9) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Overweight (BMI: >24.9–29.9) | 0.51 (0.29, 0.93) | 0.48 (0.26, 0.88) | 0.47 (0.26, 0.87) | 0.48 (0.26, 0.89) | 0.54 (0.29, 1.02) | 0.49 (0.25, 0.93) |
| Obese (BMI: >29.9) | 0.51 (0.29, 0.87) | 0.43 (0.23, 0.80) | 0.45 (0.24, 0.84) | 0.47 (0.25, 0.88) | 0.54 (0.27, 1.08) | 0.47 (0.22, 0.97) |
| BMI Continuous Variables | ||||||
| Per 1 unit increase | 0.95 (0.91, 0.99) | 0.94 (0.90, 0.98) | 0.94 (0.90, 0.99) | 0.95 (0.90, 0.99) | 0.95 (0.91, 1.00) | 0.95 (0.90, 1.00) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Curran, G.; Shannon, J.; Velie, E.M.; Irvin, V.L.; Manson, J.E.; Simon, M.S.; Altinok Dindar, D.; Pyle, C.; Schedin, P.; et al. Body Mass Index Is Inversely Associated with Risk of Postmenopausal Interval Breast Cancer: Results from the Women’s Health Initiative. Cancers 2022, 14, 3228. https://doi.org/10.3390/cancers14133228
Zhang Z, Curran G, Shannon J, Velie EM, Irvin VL, Manson JE, Simon MS, Altinok Dindar D, Pyle C, Schedin P, et al. Body Mass Index Is Inversely Associated with Risk of Postmenopausal Interval Breast Cancer: Results from the Women’s Health Initiative. Cancers. 2022; 14(13):3228. https://doi.org/10.3390/cancers14133228
Chicago/Turabian StyleZhang, Zhenzhen, Grace Curran, Jackilen Shannon, Ellen M. Velie, Veronica L. Irvin, JoAnn E. Manson, Michael S. Simon, Duygu Altinok Dindar, Chelsea Pyle, Pepper Schedin, and et al. 2022. "Body Mass Index Is Inversely Associated with Risk of Postmenopausal Interval Breast Cancer: Results from the Women’s Health Initiative" Cancers 14, no. 13: 3228. https://doi.org/10.3390/cancers14133228
APA StyleZhang, Z., Curran, G., Shannon, J., Velie, E. M., Irvin, V. L., Manson, J. E., Simon, M. S., Altinok Dindar, D., Pyle, C., Schedin, P., & Tabung, F. K. (2022). Body Mass Index Is Inversely Associated with Risk of Postmenopausal Interval Breast Cancer: Results from the Women’s Health Initiative. Cancers, 14(13), 3228. https://doi.org/10.3390/cancers14133228







