Comprehensive Comparison of 22C3 and SP263 PD-L1 Expression in Non-Small-Cell Lung Cancer Using Routine Clinical and Conditioned Archives
Abstract
:Simple Summary
Abstract
1. Introduction
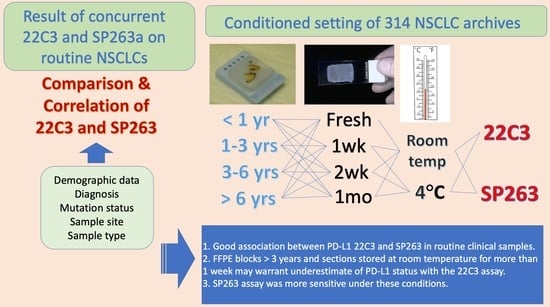
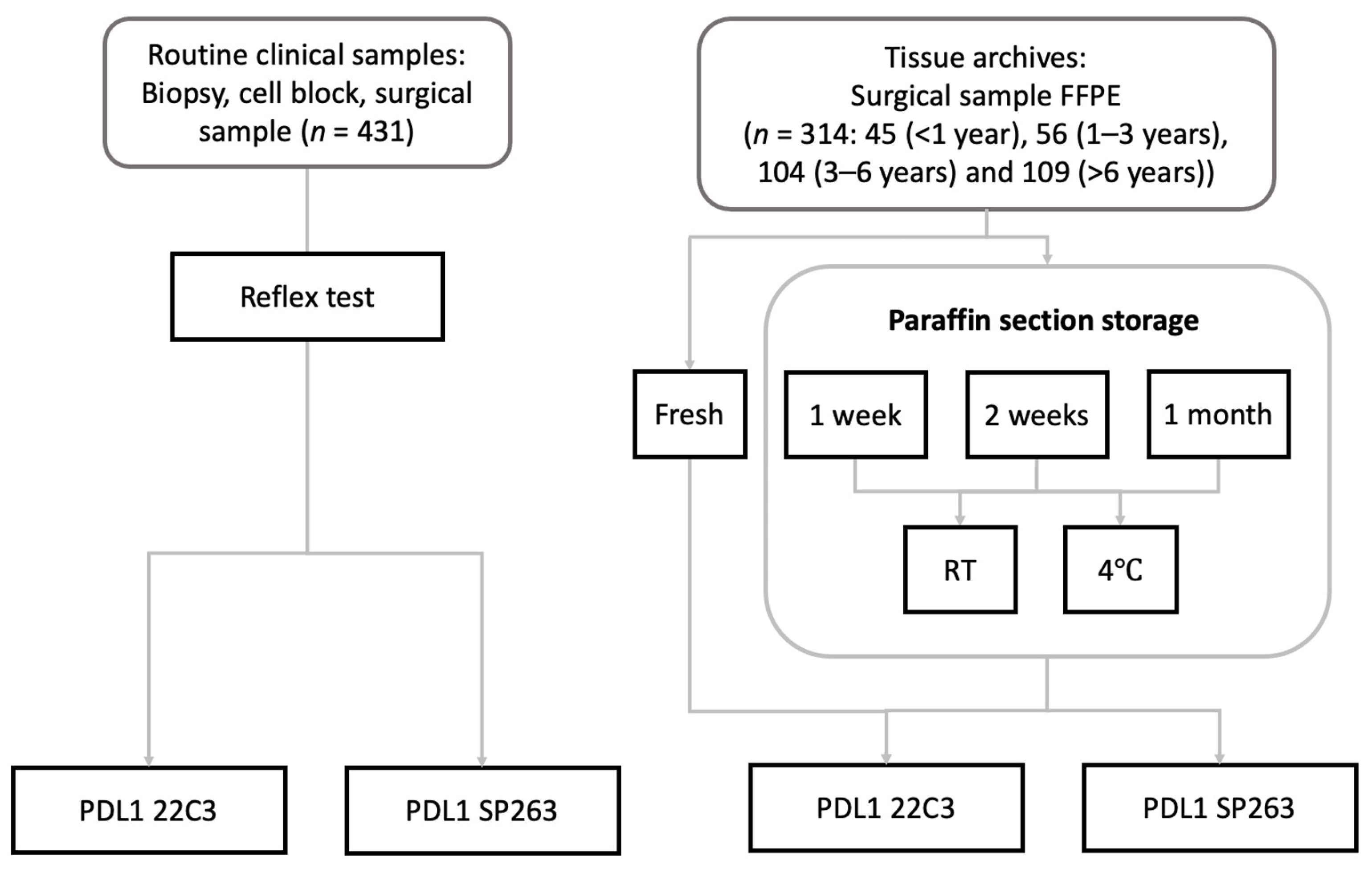
2. Materials and Methods
2.1. Patients and Routine Samples
2.2. Immunohistochemistry Preanalytic Conditions
2.3. PD-L1 Immunohistochemistry
2.4. Statistics
3. Results
3.1. Characteristics of Routine Clinical Cohort
3.2. Overall Comparison of PD-L1 Assays
3.3. Comparison of PD-L1 Assays According to Specimen Types
3.4. Associations of Preanalytic Variables and PD-L1 Staining
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Herbst, R.S.; Baas, P.; Kim, D.W.; Felip, E.; Pérez-Gracia, J.L.; Han, J.Y.; Molina, J.; Kim, J.H.; Arvis, C.D.; Ahn, M.J.; et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): A randomised controlled trial. Lancet 2016, 387, 1540–1550. [Google Scholar] [CrossRef]
- Brahmer, J.; Reckamp, K.L.; Baas, P.; Crinò, L.; Eberhardt, W.E.; Poddubskaya, E.; Antonia, S.; Pluzanski, A.; Vokes, E.E.; Holgado, E.; et al. Nivolumab versus docetaxel in advanced squamous-cell non–small-cell lung cancer. N. Engl. J. Med. 2015, 373, 123–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borghaei, H.; Paz-Ares, L.; Horn, L.; Spigel, D.R.; Steins, M.; Ready, N.E.; Chow, L.Q.; Vokes, E.E.; Felip, E.; Holgado, E.; et al. Nivolumab versus docetaxel in advanced nonsquamous non–small-cell lung cancer. N. Engl. J. Med. 2015, 373, 1627–1639. [Google Scholar] [CrossRef] [PubMed]
- Garon, E.B.; Rizvi, N.A.; Hui, R.; Leighl, N.; Balmanoukian, A.S.; Eder, J.P.; Patnaik, A.; Aggarwal, C.; Gubens, M.; Horn, L.; et al. Pembrolizumab for the treatment of non–small-cell lung cancer. N. Engl. J. Med. 2015, 372, 2018–2028. [Google Scholar] [CrossRef]
- Fehrenbacher, L.; Spira, A.; Ballinger, M.; Kowanetz, M.; Vansteenkiste, J.; Mazieres, J.; Park, K.; Smith, D.; Artal-Cortes, A.; Lewanski, C.; et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): A multicentre, open-label, phase 2 randomised controlled trial. Lancet 2016, 387, 1837–1846. [Google Scholar] [CrossRef]
- Rizvi, N.A.; Mazières, J.; Planchard, D.; Stinchcombe, T.E.; Dy, G.K.; Antonia, S.J.; Horn, L.; Lena, H.; Minenza, E.; Mennecier, B.; et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): A phase 2, single-arm trial. Lancet Oncol. 2015, 16, 257–265. [Google Scholar] [CrossRef] [Green Version]
- Hirsch, F.R.; McElhinny, A.; Stanforth, D.; Ranger-Moore, J.; Jansson, M.; Kulangara, K.; Richardson, W.; Towne, P.; Hanks, D.; Vennapusa, B.; et al. PD-L1 immunohistochemistry assays for lung cancer: Results from phase 1 of the blueprint PD-L1 IHC assay comparison project. J. Thorac. Oncol. 2017, 12, 208–222. [Google Scholar] [CrossRef] [Green Version]
- Scheel, A.H.; Dietel, M.; Heukamp, L.C.; Jöhrens, K.; Kirchner, T.; Reu, S.; Rüschoff, J.; Schildhaus, H.U.; Schirmacher, P.; Tiemann, M.; et al. Harmonized PD-L1 immunohistochemistry for pulmonary squamous-cell and adenocarcinomas. Mod. Pathol. 2016, 29, 1165–1172. [Google Scholar] [CrossRef]
- Rimm, D.L.; Han, G.; Taube, J.M.; Yi, E.S.; Bridge, J.A.; Flieder, D.B.; Homer, R.; West, W.W.; Wu, H.; Roden, A.C.; et al. A prospective, multi-institutional assessment of four assays for PD-L1 expression in NSCLC by immunohistochemistry. JAMA Oncol. 2017, 3, 1051. [Google Scholar] [CrossRef]
- Adam, J.; Le Stang, N.; Rouquette, I.; Cazes, A.; Badoual, C.; Pinot-Roussel, H.; Tixier, L.; Danel, C.; Damiola, F.; Damotte, D.; et al. Multicenter harmonization study for PD-L1 IHC testing in non-small-cell lung cancer. Ann. Oncol. 2018, 29, 953–958. [Google Scholar] [CrossRef]
- Tsao, M.S.; Kerr, K.M.; Kockx, M.; Beasley, M.B.; Borczuk, A.C.; Botling, J.; Bubendorf, L.; Chirieac, L.; Chen, G.; Chou, T.Y.; et al. PD-L1 immunohistochemistry comparability study in real-life clinical samples: Results of blueprint phase 2 project. J. Thorac. Oncol. 2018, 13, 1302–1311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ratcliffe, M.J.; Sharpe, A.; Midha, A.; Barker, C.; Scott, M.; Scorer, P.; Al-Masri, H.; Rebelatto, M.C.; Walker, J. Agreement between programmed cell death ligand-1 diagnostic assays across multiple protein expression cutoffs in non–small cell lung cancer. Clin. Cancer Res. 2017, 23, 3585–3591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palmer-Toy, D.E.; Krastins, B.; Sarracino, D.A.; Nadol, J.B.; Merchant, S.N. Efficient method for the proteomic analysis of fixed and embedded tissues. J. Proteome Res. 2005, 4, 2404–2411. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, M.T.P.; Haselkorn, T.; Bunce, M.; Sanchez, J.J.; Lucas, S.B.; Jewell, L.D.; Marck, E.V.; Worobey, M. The isolation of nucleic acids from fixed, paraffin-embedded tissues–which methods are useful when? PLoS ONE 2007, 2, e537. [Google Scholar] [CrossRef]
- Roy, U.B.; Jacobson, M.; Ferris, A. MA07. 09 Willingness to perform multiple biopsies to improve quality of lung cancer care: Understanding the oncologists’ perspective. J. Thorac. Oncol. 2018, 13, S380. [Google Scholar]
- Groelz, D.; Viertler, C.; Pabst, D.; Dettmann, N.; Zatloukal, K. Impact of storage conditions on the quality of nucleic acids in paraffin embedded tissues. PLoS ONE 2018, 13, e0203608. [Google Scholar] [CrossRef]
- Bussolati, G.; Leonardo, E. Technical pitfalls potentially affecting diagnoses in immunohistochemistry. J. Clin. Pathol. 2008, 61, 1184–1192. [Google Scholar] [CrossRef]
- Neuman, T.; London, M.; Kania-Almog, J.; Litvin, A.; Zohar, Y.; Fridel, L.; Sandbank, J.; Barshak, I.; Vainer, G.W. A harmonization study for the use of 22C3 PD-L1 immunohistochemical staining on Ventana’s platform. J. Thorac. Oncol. 2016, 11, 1863–1868. [Google Scholar] [CrossRef] [Green Version]
- Sughayer, M.A.; Alnaimy, F.; Alsughayer, A.M.; Qamhia, N. Comparison of 22C3 PharmDx and SP263 assays to test PD-L1 expression in NSCLC. Appl. Immunohistochem. Mol. Morphol. 2019, 27, 663–666. [Google Scholar] [CrossRef]
- Skov, B.G.; Skov, T. Paired comparison of PD-L1 expression on cytologic and histologic specimens from malignancies in the lung assessed with PD-L1 IHC 28-8pharmDx and PD-L1 IHC 22C3pharmDx. Appl. Immunohistochem. Mol. Morphol. 2017, 25, 453–459. [Google Scholar] [CrossRef]
- Gagné, A.; Wang, E.; Bastien, N.; Orain, M.; Desmeules, P.; Pagé, S.; Trahan, S.; Couture, C.; Joubert, D.; Joubert, P. Impact of specimen characteristics on PD-L1 testing in non–small cell lung cancer: Validation of the IASLC PD-L1 testing recommendations. J. Thorac. Oncol. 2019, 14, 2062–2070. [Google Scholar] [CrossRef] [PubMed]
- Büttner, R.; Gosney, J.R.; Skov, B.G.; Adam, J.; Motoi, N.; Bloom, K.J.; Dietel, M.; Longshore, J.W.; López-Ríos, F.; Penault-Llorca, F.; et al. Programmed death-ligand 1 immunohistochemistry testing: A review of analytical assays and clinical implementation in non–small-cell lung cancer. J. Clin. Oncol. 2017, 35, 3867–3876. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Fang, W.; Zhang, Y.; Hong, S.; Kang, S.; Yan, Y.; Chen, N.; Zhan, J.; He, X.; Qin, T.; et al. The association between PD-L1 and EGFR status and the prognostic value of PD-L1 in advanced non-small cell lung cancer patients treated with EGFR-TKIs. Oncotarget. 2015, 6, 14209. [Google Scholar] [CrossRef] [Green Version]
- Chang, G.; Yang, T.; Chen, K.; Hsu, K.; Huang, Y.; Su, K.; Yu, S.; Tseng, J. ALK variants, PD-L1 expression, and their association with outcomes in ALK-positive NSCLC patients. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef]
- Tsimafeyeu, I.; Imyanitov, E.; Zavalishina, L.; Raskin, G.; Povilaitite, P.; Savelov, N.; Kharitonova, E.; Rumyantsev, A.; Pugach, I.; Andreeva, Y.; et al. Agreement between PDL1 immunohistochemistry assays and polymerase chain reaction in non-small cell lung cancer: CLOVER comparison study. Sci. Rep. 2020, 10, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, T.; Hong, S.; Kim, O.; Kim, S.; Yang, J.; Joung, E.; Kang, J.; Hong, S. Changes in PD-L1 expression according to tumor infiltrating lymphocytes of acquired EGFR-TKI resistant EGFR-mutant non-small-cell lung cancer. Oncotarget 2017, 8, 107630. [Google Scholar] [CrossRef] [Green Version]
- Schats, K.A.; Van Vré, E.A.; De Schepper, S.; Boeckx, C.; Schrijvers, D.M.; Waelput, W.; Fransen, E.; Vanden Bempt, I.; Neyns, B.; De Meester, I.; et al. Validated programmed cell death ligand 1 immunohistochemistry assays (E1L3N and SP 142) reveal similar immune cell staining patterns in melanoma when using the same sensitive detection system. Histopathology 2017, 70, 253–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uruga, H.; Bozkurtlar, E.; Huynh, T.G.; Muzikansky, A.; Goto, Y.; Gomez-Caraballo, M.; Hata, A.N.; Gainor, J.F.; Mark, E.J.; Engelman, J.A.; et al. Programmed cell death ligand (PD-L1) expression in stage II and III lung adenocarcinomas and nodal metastases. J. Thorac. Oncol. 2017, 12, 458–466. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.R.; Cha, Y.J.; Hong, M.H.; Gandhi, M.; Levinson, S.; Jung, I.; Lee, J.G.; Lee, C.Y.; Cho, B.C.; Ha, S.J.; et al. Concordance of programmed death-ligand 1 expression between primary and metastatic non-small cell lung cancer by immunohistochemistry and RNA in situ hybridization. Oncotarget 2017, 8, 87234. [Google Scholar] [CrossRef] [Green Version]
- Mansfield, A.; Aubry, M.; Moser, J.; Harrington, S.; Dronca, R.; Park, S.; Dong, H. Temporal and spatial discordance of programmed cell death-ligand 1 expression and lymphocyte tumor infiltration between paired primary lesions and brain metastases in lung cancer. Ann. Oncol. 2016, 27, 1953–1958. [Google Scholar] [CrossRef]
- Munari, E.; Rossi, G.; Zamboni, G.; Lunardi, G.; Marconi, M.; Sommaggio, M.; Netto, G.J.; Hoque, M.O.; Brunelli, M.; Martignoni, G.; et al. PD-L1 assays 22C3 and SP263 are not interchangeable in non–small cell lung cancer when considering clinically relevant cutoffs. Am. J. Surg. Pathol. 2018, 42, 1384–1389. [Google Scholar] [CrossRef] [PubMed]
- Tsao, M.S.; Kerr, K.M.; Dacic, S.; Yatabe, Y.; Hirsch, F. IASLC Atlas of PD-L1 Immunohistochemistry Testing in Lung Cancer; International Association for the Study of Lung Cancer: Aurora, CO, USA, 2017. [Google Scholar]
- Masuda, N.; Ohnishi, T.; Kawamoto, S.; Monden, M.; Okubo, K. Analysis of chemical modification of RNA from formalin-fixed samples and optimization of molecular biology applications for such samples. Nucleic Acids Res. 1999, 27, 4436–4443. [Google Scholar] [CrossRef] [PubMed]
- Hendry, S.; Byrne, D.J.; Wright, G.M.; Young, R.J.; Sturrock, S.; Cooper, W.A.; Fox, S.B. Comparison of four PD-L1 immunohistochemical assays in lung cancer. J. Thorac. Oncol. 2018, 13, 367–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karim, L.A.; Wang, P.; Chahine, J.; Kallakury, B. Harmonization of PD-L1 immunohistochemistry assays for lung cancer: A working progress. J. Thorac. Oncol. 2017, 12, e45. [Google Scholar] [CrossRef] [Green Version]
- Marchetti, A.; Barberis, M.; Franco, R.; De Luca, G.; Pace, M.V.; Staibano, S.; Volante, M.; Buttitta, F.; Guerini-Rocco, E.; Righi, L.; et al. Multicenter comparison of 22C3 PharmDx (Agilent) and SP263 (Ventana) assays to test PD-L1 expression for NSCLC patients to be treated with immune checkpoint inhibitors. J. Thorac. Oncol. 2017, 12, 1654–1663. [Google Scholar] [CrossRef] [Green Version]



| 22C3 TPS ≥ 50% | SP263 TPS ≥ 50% | 22C3 TPS ≥ 1% | SP263 TPS ≥ 1% | ||||||
|---|---|---|---|---|---|---|---|---|---|
| (n = 108) | (n = 91) | (n = 307) | (n = 299) | ||||||
| Varable | Total (%) | n(%) | p | n(%) | p | n(%) | p | n(%) | p |
| Age, mean (SD) | 68.6 (10.3) | 67.9 (8.7) | 0.258 | 67.8 (9.0) | 0.248 | 68.4 (9.9) | 0.377 | 68.2 (10.3) | 0.294 |
| Sex | |||||||||
| Male | 291 (67.5) | 74 (68.5) | 0.179 | 61 (67.0) | 0.919 | 214 (69.7) | 0.158 | 208 (69.6) | 0.182 |
| Female | 140 (32.5) | 34 (31.5) | 30 (33.0) | 93 (30.3) | 91 (30.4) | ||||
| Smoking | |||||||||
| Never smoker | 215 (49.9) | 51 (47.2) | 0.598 | 46 (50.5) | 0.89 | 154 (50.2) | 0.94 | 149 (49.8) | 1 |
| Ever smoker | 216 (50.1) | 57 (52.8) | 45 (49.5) | 153 (49.8) | 150 (50.2) | ||||
| Histology | |||||||||
| ADC | 282 (65.4) | 69 (63.9) | 0.786 | 56 (61.5) | 0.380 | 193 (62.9) | 0.099 | 185 (61.9) | 0.021 |
| SCC | 133 (30.9) | 33 (30.6) | 29 (31.9) | 102 (33.2) | 102 (34.1) | ||||
| other NSCLC | 16 (3.7) | 6 (5.6) | 6 (6.6) | 12 (3.9) | 12 (4.0) | ||||
| Mutation | |||||||||
| EGFR | 101 (23.4) | 18 (16.7) | 0.306 | 14 (15.4) | 0.276 | 68 (22.1) | 0.371 | 68 (22.7) | 0.813 |
| ALK | 13 (3.0) | 6 (5.5) | 6 (6.6) | 9 (2.9) | 10 (3.3) | ||||
| EGFR ALK WT | 317 (73.5) | 84 (77.8) | 71 (78.0) | 230 (74.9) | 221 (73.9) | ||||
| Stage | |||||||||
| I–II | 148 (34.3) | 33 (30.7) | 0.970 | 30 (32.9) | 0.818 | 97 (31.6) | 0.576 | 97 (32.4) | 0.825 |
| III | 176 (40.9) | 50 (46.2) | 41(45.1) | 134 (43.6) | 140 (43.5) | ||||
| IV | 107 (24.8) | 25 (23.1) | 20 (22.0) | 76 (24.8) | 72 (24.1) | ||||
| Sample | |||||||||
| Biopsy | 386 (89.6) | 95 (88) | 0.656 | 78 (85.7) | 0.247 | 279 (91) | 0.503 | 269 (90) | 0.733 |
| Surgical resection | 45 (10.4) | 13 (12) | 13 (14.3) | 28 (9) | 30 (10) | ||||
| Assay | 22C3 | TPS ≥ 50% | SP263 | TPS ≥ 50% | 22C3 | TPS ≥ 1% | SP263 | TPS ≥ 1% |
|---|---|---|---|---|---|---|---|---|
| 22C3 | 108/108 | 100% | 86/91 | 94.50% | 307/307 | 100% | 276/299 | 92.30% |
| SP263 | 86/108 | 79.60% | 91/91 | 100% | 276/307 | 89.90% | 299/299 | 100% |
| Concordant | Discordant | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 22C3 TPS | 1–49% | <1% | ≥50% | <1% | ≥50% | 1–49% | |||
| Sample N (%) | Total | SP263 TPS | <1% | 1–49% | <1% | ≥50% | 1–49% | ≥50% | p |
| Bronchoscopic biopsy | 83 | 68 (81.9) | 7 (8.4) | 3 (3.6) | 0 | 0 | 3 (3.6) | 2 (2.4) | <0.001 |
| PCNB | 256 | 208 (81.3) | 20 (7.8) | 14 (5.5) | 0 | 0 | 12 (4.7) | 2 (0.8) | |
| Cell block | 6 | 4 (66.7) | 1 (16.7) | 0 | 0 | 1 (16.7) | |||
| EBUS | 15 | 12 (80.0) | 1 (6.7) | 0 | 0 | 2 (13.3) | |||
| Metastatic site biopsy | 26 | 18 (69.2) | 2 (7.7) | 2 (7.7) | 0 | 0 | 3 (11.5) | 1 (3.8) | |
| Lobectomy | 41 | 37 (90.2) | 1 (2.4) | 3 (7.3) | 0 | 0 | |||
| Wedge resection | 4 | 4 (100.0) | 0 | 0 | |||||
| FFPE block age | |||||||||
| <6 mo | 418 | 349 | 31 | 16 | 0 | 0 | 22 | 5 | 0.003 |
| ≥6 mo | 13 | 6 | 0 | 7 | 0 | 0 | 0 | 0 | |
| Total | 431 | 351 | 31 | 23 | 0 | 0 | 21 | 5 |
| PD-L1 TPS | ||||||
|---|---|---|---|---|---|---|
| FFPE Block Age | Assay | <1% | 1–49% | ≥50% | p | |
| <1yr | 22C3 | 17 | 15 | 13 | 0.224 | |
| 1-3 yr | 22C3 | 26 | 14 | 16 | 0.007 | |
| 3-6 yr | 22C3 | 60 | 28 | 16 | ||
| >6 yr | 22C3 | 60 | 29 | 20 | ||
| <1yr | SP263 | 15 | 17 | 13 | 0.184 | |
| 1-3 yr | SP263 | 26 | 14 | 16 | 0.009 | |
| 3-6 yr | SP263 | 58 | 30 | 16 | ||
| >6 yr | SP263 | 58 | 30 | 21 | ||
| FFPE section storage condition | ||||||
| Duration | Temp | |||||
| Fresh | 22C3 | RT | 163 | 86 | 65 | 0.004e |
| 1 wk | 22C3 | RT | 177 | 81 | 56 | 0.033 |
| 2 wk | 22C3 | RT | 195 | 68 | 51 | 0.469 |
| 1 mo | 22C3 | RT | 209 | 63 | 42 | 0.002 |
| 1 wk | 22C3 | 4 °C | 169 | 84 | 61 | 0.046 |
| 2 wk | 22C3 | 4 °C | 169 | 84 | 61 | 0.253 |
| 1 mo | 22C3 | 4 °C | 178 | 77 | 59 | 0.467 |
| Fresh | SP263 | RT | 157 | 91 | 66 | 0.467 |
| 1 wk | SP263 | RT | 157 | 91 | 66 | 0.8 |
| 2 wk | SP263 | RT | 157 | 91 | 66 | 0.8 |
| 1 mo | SP263 | RT | 157 | 91 | 66 | 1 |
| 1 wk | SP263 | 4 °C | 157 | 91 | 66 | |
| 2 wk | SP263 | 4 °C | 157 | 91 | 66 | |
| 1 mo | SP263 | 4 °C | 157 | 91 | 66 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.Y.; Kim, T.-E.; Park, C.K.; Yoon, H.-K.; Sa, Y.J.; Kim, H.R.; Woo, I.S.; Kim, T.-J. Comprehensive Comparison of 22C3 and SP263 PD-L1 Expression in Non-Small-Cell Lung Cancer Using Routine Clinical and Conditioned Archives. Cancers 2022, 14, 3138. https://doi.org/10.3390/cancers14133138
Kim SY, Kim T-E, Park CK, Yoon H-K, Sa YJ, Kim HR, Woo IS, Kim T-J. Comprehensive Comparison of 22C3 and SP263 PD-L1 Expression in Non-Small-Cell Lung Cancer Using Routine Clinical and Conditioned Archives. Cancers. 2022; 14(13):3138. https://doi.org/10.3390/cancers14133138
Chicago/Turabian StyleKim, Sue Youn, Tae-Eun Kim, Chan Kwon Park, Hyoung-Kyu Yoon, Young Jo Sa, Hyo Rim Kim, In Sook Woo, and Tae-Jung Kim. 2022. "Comprehensive Comparison of 22C3 and SP263 PD-L1 Expression in Non-Small-Cell Lung Cancer Using Routine Clinical and Conditioned Archives" Cancers 14, no. 13: 3138. https://doi.org/10.3390/cancers14133138
APA StyleKim, S. Y., Kim, T.-E., Park, C. K., Yoon, H.-K., Sa, Y. J., Kim, H. R., Woo, I. S., & Kim, T.-J. (2022). Comprehensive Comparison of 22C3 and SP263 PD-L1 Expression in Non-Small-Cell Lung Cancer Using Routine Clinical and Conditioned Archives. Cancers, 14(13), 3138. https://doi.org/10.3390/cancers14133138