Simple Summary
Leiomyosarcoma (LMS) is an aggressive soft tissue sarcoma with a poor prognosis. Approximately 40% of patients will develop metastatic disease. The optimal treatment for patients with metastatic LMS is not well established, and there are no randomized controlled trials regarding metastasectomy. This systematic review and pooled survival analysis aims to assess the survival in patients undergoing a metastasectomy for LMS and compare the outcomes based on the site of metastasectomy. We identified that patients with LMS metastases in the lungs, liver, spine, and brain can undergo metastasectomy with acceptable survival. Two studies have compared survival outcomes between patients treated and not treated with metastasectomy; despite their low quality, these studies support a survival benefit associated with metastasectomy.
Abstract
This study assesses the survival in patients undergoing metastasectomy for leiomyosarcoma (LMS) and compares the outcomes by the site of metastasectomy. We conducted a systematic review and pooled survival analysis of patients undergoing metastasectomy for LMS. Survival was compared between sites of metastasectomy. We identified 23 studies including 573 patients undergoing metastasectomy for LMS. The pooled median survival was 59.6 months (95% CI 33.3 to 66.0). The pooled median survival was longest for lung metastasectomy (72.8 months 95% CI 63.0 to 82.5), followed by liver (34.8 months 95% CI 22.3 to 47.2), spine (14.1 months 95% CI 8.6 to 19.7), and brain (14 months 95% CI 6.7 to 21.3). Two studies compared the survival outcomes between patients who did, versus who did not undergo metastasectomy; both demonstrated a significantly improved survival with metastasectomy. We conclude that surgery is currently being utilized for LMS metastases to the lung, liver, spine, and brain with acceptable survival. Although low quality, comparative studies support a survival benefit with metastasectomy. In the absence of randomized studies, it is impossible to determine whether the survival benefit associated with metastasectomy is due to careful patient selection rather than a surgical advantage; limited data were included about patient selection.
1. Introduction
Leiomyosarcoma (LMS) is a malignant mesenchymal tumor arising from smooth muscle cells that accounts for 10–20% of soft tissue sarcomas [1,2]. LMSs most commonly occur in the uterus, followed by the abdomen, the retroperitoneum, and larger blood vessels [3]. LMSs are principally tumors of adults and are more common in women [3]. Most LMSs are sporadic, but some may be associated with hereditary syndromes, such as retinoblastoma and Li-Fraumeni. Compared to other histologic types of soft tissue sarcomas (STS), LMSs are inherently aggressive, with 90% of patients diagnosed with grade two or three tumors [4,5]. LMSs have a poorer prognosis with a tendency for distant recurrence and a decreased disease-free survival [6,7].
Surgery to achieve negative margins remains the only curative treatment modality for patients presenting with localized LMS. Adjunctive therapies, such as radiotherapy and systemic treatment, are used in only specific cases [8,9,10]. Despite optimal local treatment, the risk of developing metastatic disease is approximately 40% [11]. The optimal treatment for patients with metastatic LMS is not well established, and there are no randomized controlled trials regarding metastasectomy. Many studies on this topic include multiple sarcoma histologies, limiting generalizability to distinct individual histologies, which can vary in clinical course, outcome, and sensitivity to radiotherapy and systemic therapy. Most patients with metastatic LMSs are not curable, and palliative systemic or radiotherapy is the mainstay of management. Retrospective studies have demonstrated an association with improved survival in carefully selected patients. The role of metastasectomy is most well accepted for patients with oligometastatic pulmonary metastases, but other sites of metastasectomy are increasingly reported in the literature [12,13,14]. This study aims to assess the survival in patients undergoing metastasectomy for LMS and compare the outcomes based on the site of metastasectomy.
2. Materials and Methods
This study is a part of a series systematically summarizing survival outcomes for patients with soft tissue and bone sarcoma undergoing metastasectomy. This study focuses on survival outcomes of patients who underwent metastasectomy for LMS. Details on information sources, search strategy, eligibility criteria, study screening and selection, data collection, and extraction can be found elsewhere [15]. The protocol is registered within the prospective international register of systematic reviews (PROSPERO) database (registration ID: CRD42019126906), and this study is reported in compliance with PRISMA 2020 statement [16].
2.1. Search Strategy
The literature search was developed by a research librarian (D.S.). The search included Medline, Embase, Cochrane Central Register of Controlled Trials, and ClinicalTrials.gov from inception to 28 May 2021, and a PubMed search for studies not yet indexed or not found in Medline. The search strategy was tailored to each database. Conference abstracts for the last three years from three major sarcoma conferences were also searched: the Connective Tissue Oncology Society, the American Society of Clinical Oncology, and the European Society of Clinical Oncology. Reference lists of all included studies and relevant systematic reviews were reviewed for additional references.
2.2. Selection Process
We included studies that evaluated metastasectomy for LMS with survival outcomes, were peer-reviewed in the English language, and had a minimum of five patients with LMS undergoing metastasectomy. Studies that included a broad range of cancer histologies (sarcoma and non-sarcoma histologies) and reported the survival outcomes for the subgroup of patients undergoing metastasectomy for LMS were included. These studies did not have to report the sociodemographic and clinical data for the subgroup of LMS patients to be included. Four reviewers (working in pairs—B.A., M.D., A.S., and Y.W.) screened titles and abstracts independently and in duplicate in the first stage, then reviewed the full texts of potentially eligible studies in a second stage to determine the final eligible studies. Disagreements were resolved by referring to a third reviewer if necessary.
2.3. Data Collection
Data were extracted by two individual members (B.A. and M.D.) and compared for accuracy. A third member (A.S.) reviewed the data extraction and resolved inconsistencies where necessary. When patients undergoing metastasectomy for LMS were a subgroup of the entire study population, two attempts at contacting primary authors were made to obtain LMS-specific patient and treatment data. If still unavailable, these data were extracted for the entire study population.
2.4. Data Synthesis and Analysis
The details of the included articles are presented in table format. The LMS-specific baseline data were included when studies reported the sociodemographic and clinical characteristics of patients diagnosed with LMS undergoing metastasectomy [17,18,19,20,21,22,23,24]. Among studies with a broad range of cancer types, of which LMS was included, the sociodemographic and clinical characteristics of patients with LMS undergoing metastasectomy were not consistently reported [11,13,14,25,26,27,28,29,30,31,32,33,34,35,36]. Thus, these characteristics are reported for the entire study population to provide details despite representing multiple cancer histologies. The LMS-specific survival outcomes were reported by all studies and are summarized in table format.
The yearly Kaplan–Meier estimated survival rates and numbers at risk for LMS patients were extracted from each study. For studies where these data were not reported, if the Kaplan–Meier curves indicated the time at which patients were censored or a risk table was provided, this was used to derive the patient-level data from the study. For studies reporting Kaplan–Meier curves of overall survival, WebPlotDigitizer v4.5 was used to identify the follow-up time and estimated survival rate at each “step” of the curve [37]. If censoring times were not available, then IPDfromKM web-based Shiny application was utilized to reconstruct individual patient data from published Kaplan–Meier curves [38]. The numbers of deaths and numbers at risk at each year of the follow-up period were then used to calculate standard errors for the yearly survival estimates and median overall survival. If only median overall survival was reported and Kaplan–Meier curves or risk tables were not available, the standard error was calculated using methods described by Hozo et al. [39]. Median overall survival and yearly survival estimates were then pooled across studies using inverse-variance weighted random-effects meta-analysis models [40].
2.5. Risk of Bias Assessment and Certainty of Evidence
Risk of bias assessments were completed by two individual members (B.A. and K.N.), with a third member (M.D.) resolving disagreements where necessary. First, the study design was determined using accepted definitions [41]. Studies reporting survival for both metastasectomy and non-metastasectomy patients were defined as cohort studies. Studies reporting survival for only metastasectomy patients were defined as case series. Patients who did not undergo metastasectomy may have received other treatments, such as chemotherapy or radiation.
The Joanna Briggs Institute (JBI) Critical Appraisal Checklist for Case Series and the Newcastle-Ottawa Quality Assessment Scale (NOS) were selected as the methodological quality assessment tools based on expert recommendations [42,43,44]. Specific decision trees were developed and agreed upon by all authors to adjudicate each criterion.
The constructs of the GRADE (Grading of Recommendation, Assessment, Development, and Evaluation) approach to assess the certainty of evidence were applied [45]. Although we did not perform a comparative meta-analysis, the components of GRADE can still be used to address evidence synthesis of quantitative estimates of effect (and thus summarized narratively) [46].
3. Results
3.1. Study Characteristics
Out of 37,241 articles, 23 studies published between 1998 and 2020 were included (Supplementary Figure S1, Table 1) [11,13,14,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36]. Twenty-one studies were case series, [13,14,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35] and two were cohort studies [11,36]. Collectively, the articles included 1970 patients diagnosed between 1976 and 2018, of which 656 (33%) were diagnosed with metastatic LMS and 573 (29%) underwent metastasectomy for LMS (Supplementary Table S1).

Table 1.
Study details.
Eight studies reported the sociodemographic and clinical data for patients diagnosed with LMS undergoing metastasectomy (Table 2A) [17,18,19,20,21,22,23,24]. The other 15 studies included a broad range of cancer types, of which metastatic LMS was a subgroup and the survival outcomes for patients undergoing metastasectomy for LMS were explicitly reported (Table 2B) [11,13,14,25,26,27,28,29,30,31,32,33,34,35,36]. The proportion of patients undergoing metastasectomy for LMS in these studies ranged from 8% [25] to 60% [34].

Table 2.
Sociodemographic and clinical characteristics of included patients from studies reporting (A) and not reporting (B) these details for patients with LMS undergoing metastasectomy.
3.2. Sociodemographic and Clinical Characteristics of Patients Undergoing Metastasectomy for LMS
The sociodemographic and clinical data for patients with LMS undergoing metastasectomy were available for 113 patients from eight studies and will be discussed here (Table 2A) [17,18,19,20,21,22,23,24]. The mean or median age was between 47 and 58, with individual patient age ranges between 23 and 76. Fifty-eight (51%) patients were male. The most common site of origin of LMS was gastrointestinal (n = 34, 30%), uterine/adnexal (n = 33, 29%), retroperitoneal (n = 23, 20%), extremity/trunk (n = 17, 15%), other (n = 6, 5%), and vena cava (n = 1, 1%). The primary tumor in patients undergoing metastasectomy was reported to be well controlled (no additional details provided) in six studies [17,18,19,20,21,23].
Seven studies reported either the disease-free interval (DFI) or the proportion of patients presenting with synchronous versus metachronous metastatic disease [17,18,19,20,21,22,23]. Fourteen patients (23%) had synchronous disease and 47 (77%) had metachronous disease. The median DFI was between 15 and 50 months, with an individual patient range between zero and 204 months. The most common sites of metastases included liver (n = 59, 42%), lung (n = 47, 33%), spine (n = 18, 13%), peritoneum (n = 7, 5%), lymph nodes (n = 5, 4%), other (n = 4, 3%), bone (n = 1, 1%), and adrenal (n = 1, 1%).
3.3. Management of Patients Undergoing Metastasectomy for LMS
Out of 656 patients with metastatic LMS included in all 23 studies, 573 (87%) underwent at least one metastasectomy (Table 3). The most commonly reported site of metastasectomy for LMS was lung (n = 353, 62%) followed by liver (n = 165, 29%), spine (n = 39, 7%), and brain (n = 5, 1%). The site of metastasectomy was not specified for 11 (2%) patients. Nine studies reported the intent for metastasectomy, and the criteria used to select patients for metastasectomy were reported by ten studies (Table 4).

Table 3.
Management of metastatic disease in studies reporting (A) and not reporting (B) these details for the LMS patients undergoing metastasectomy.

Table 4.
Intent and criteria for metastasectomy reported by studies.
Six studies reported whether perioperative systemic therapy was used in patients undergoing metastasectomy for LMS, of which 48 (52%) received perioperative systemic treatment [11,17,18,19,20,24]. Only three studies reported the type of systemic therapy used [11,18,19]. Van Cann et al. reported that seven out of 28 patients received systemic treatment before their first metastasectomy, of which four received an anthracycline combined with an alkylating agent regimen, two received a single-agent anthracycline, and one received the oral tyrosine kinase inhibitor, pazopanib [11]. Chen et al. reported that four out of 11 patients received perioperative systemic therapy; one patient received adriamycin, dacarbazine, and etoposide preoperatively, and, postoperatively, one patient received doxorubicin, dacarbazine, ifosfamide, and mesna, another received doxorubicin, dacarbazine, and etoposide, and a third received cytoxan and vincristine [18]. Faraj et al. reported that two out of five patients with synchronous disease who underwent the simultaneous resection of all disease received postoperative chemotherapy [19]. One patient received doxorubicin and ifosfamide and another received doxorubicin alone [19].
Five studies reported whether perioperative radiotherapy was used in patients undergoing metastasectomy for LMS, of which 18 (20%) received perioperative radiotherapy [11,17,18,20,24]. The details of the radiotherapy’s type, dose, and frequency were not consistently reported.
3.4. Post-Metastasectomy Outcomes
For the assessment of overall survival, the median follow-up time ranged from 14 to 60 months across the studies (Supplementary Table S2). All 23 studies reported either a median overall survival or a one-year, three-year, or five-year overall survival for patients with LMS undergoing metastasectomy (Supplementary Table S2).
Kaplan–Meier curves or risk tables were available in 14 studies, allowing for individual patient data to be extracted and pooled yearly survival estimates to be calculated [13,17,18,19,20,21,22,23,24,25,28,29,34,36]. Two additional studies reported the median overall survival and range, from which the standard error could be calculated, and were included in the pooled median overall survival analysis [11,14].
The pooled median survival was 59.6 (95% CI 33.3 to 66.0) months. The pooled median overall survival was longest for patients undergoing lung metastasectomy (72.8 months 95% CI 63.0 to 82.5), followed by liver (34.8 months 95% CI 22.3 to 47.2), spine (14.1 months 95% CI 8.6 to 19.7), and brain (14 months 95% CI 6.7 to 21.3). The yearly pooled overall survival estimates are available in Table 5, and the yearly pooled estimates by the site of metastasectomy are displayed in Figure 1. Patients undergoing lung and liver metastasectomy did better than those undergoing brain and spine metastasectomy (Figure 1).

Table 5.
Pooled overall survival estimates.
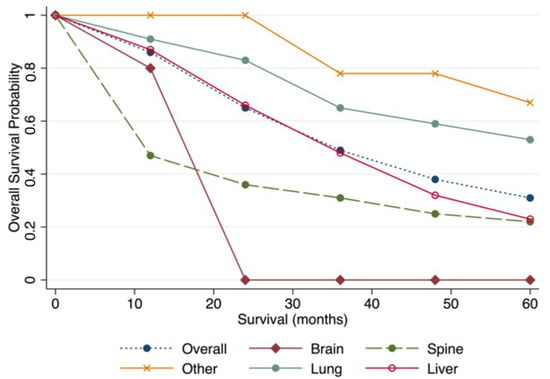
Figure 1.
Pooled overall survival by site of metastasectomy.
Two studies compared survival outcomes for patients with metastatic LMS versus those who did not undergo metastasectomy [11,36]. Both these studies reported metastasectomy was for curative intent; however, neither presented the criteria used to select patients for metastasectomy. Van Cann et al. found that among patients who underwent metastasectomy, the median overall survival was 83 months (range 4–127) compared to 16 months (range 0–83) among those who did not undergo metastasectomy (multivariable analysis HR 0.4 95% CI 0.2–0.8 p = 0.01) [11]. Farid et al. found that among patients who underwent metastasectomy, the median overall survival was 205 months (range 45–205) compared to 40 months (range 5–140) among those who did not [36]. On univariable analysis, those who did not undergo metastasectomy were at a significantly higher risk of death compared to those who did (HR 5.30 95% CI 1.52–18.49 p = 0.004), and this risk was even higher in a subgroup analysis of patients with lung metastases (HR 9.09 95% CI 1.16–100 p = 0.012) [36].
3.5. Prognostic Factors Associated with Post-Metastasectomy Outcomes
3.5.1. Lung
Burt et al. identified that patients with a longer DFI had an improved overall survival on multivariable analysis (DFI included as a monthly continuous variable, HR 0.97 95% CI 0.94–0.99 p = 0.001) [17]. Paramanathan et al. identified that patients with a more favorable International Registry of Lung Metastases prognostic group (i.e., those with a completely resectable single metastasis with a DFI greater than 36 months) had improved survival (survival outcomes not reported quantitatively by authors) [47].
3.5.2. Liver
Chen et al. identified that patients undergoing an R0 resection had a significantly longer median overall survival (median overall survival not reached, range 19–55 months) than those undergoing an R1/2 resection (median overall survival 25 months range 18–39 p = 0.03) [18]. Chen et al. also found no difference in survival between high- versus low-grade LMS, the number of liver metastases, the size of liver metastases, or the extent of liver resection [18]. Lang et al. found a prolonged survival among those undergoing first liver resections for metastatic disease who achieved an R0 resection (median overall survival 32 months range 1–84, five-year overall survival 20%) compared to an R1/2 resection (median overall survival 21 months range 1–49 p = 0.31, five-year overall survival 0%) [22]. Lang et al. also identified that patients undergoing liver resection for synchronous disease had a lower median overall survival than those with metachronous disease (22 versus 32 months, respectively, p = 0.61) [22]. Lang et al. did not find the presence of an extra-hepatic tumor to be associated with worse survival if they were able to achieve an R0 resection [22].
3.5.3. Spine
Kato et al. assessed for various prognostic factors in univariable analyses and found postoperative Eastern Cooperative Oncology Group (ECOG) status was the only significant predictor of three-year overall survival after spine metastasectomy [20]. The three-year overall survival of patients with a postoperative ECOG status greater than three was 0% compared to 78% among those with an ECOG less than three (p = 0.003) [20].
3.6. Recurrence Post-Metastasectomy
Six studies reported recurrence post-metastasectomy for patients with LMS [17,19,20,21,23,24]. Of those, including patients who underwent lung metastasectomy, Burt et al. identified that 25 out of 31 patients recurred, of which 11 were managed with repeat metastasectomy [17]. Paramanthan et al. reported that eight out of 13 developed a recurrence [23]. Only one underwent repeat metastasectomy [23]. Of patients undergoing liver metastasectomy for LMS, Faraj et al. reported that all patients included in their study died of metastatic disease; the site of recurrence and management of recurrence was not specified [19]. Kim et al. reported that five out of 10 patients developed a recurrence. Two of these patients were managed with additional surgery. Among patients who underwent spine metastasectomy, Kato et al. reported that all patients included in their study died of metastatic disease, but the site of recurrence and the management of recurrence was not specified [20]. Ziewacz et al. reported that five out of eight patients recurred in their spine, of which, four underwent additional surgery and experienced improvement in their symptoms [24].
The outcomes of patients undergoing repeat metastasectomy were only reported by Lang et al.; the five-year overall survival was 0% and the median overall survival was 31 months (range 5–51) among the nine patients undergoing a second and third liver metastasectomy [22].
3.7. Risk of Bias and Certainty of Evidence
The risk of bias assessments are available in the supplementary material (Supplementary Tables S3 and S4). All included studies were at risk of bias. Based on the risk of bias assessments and review of the studies, the certainty of the bias was deemed very low (Supplementary Table S5).
4. Discussion
The role of metastasectomy in LMS is not currently well described in the literature. This study is the first to systematically synthesize and critique the available literature on this topic, thereby providing specific data that clinicians can generalize to LMS patients with metastases. We identified only two studies comparing the survival outcomes between patients who did, versus who did not undergo metastasectomy, which suggested an improved survival associated with surgery. In the absence of randomized studies, it is impossible to determine whether these findings are due to careful patient selection and favorable biology rather than a surgical advantage, as limited data was included in the publications about patient selection. However, most metastatic LMS are caused by high-grade tumors that are not indolent in their clinical behavior, and patients with metastatic LMS often have a poor prognosis without treatment.
Among patients undergoing metastasectomy for LMS, we found a pooled five-year overall survival of 31% (95% CI 18–44%) and a median overall survival of 59.6 months (95% CI 33.3 to 66.0). Before our study, the survival outcomes of patients undergoing metastasectomy for LMS were derived from large retrospective cohort studies with diverse histologies and were mostly limited to lung metastasectomy [27,48,49]. In these studies, the five-year overall survival post-lung metastasectomy ranged between 34 and 40%, with a median overall survival of 33 months. Compared to other histologic types of STSs, lung metastasectomy for LMS is suggested to be associated with a more favorable prognosis, and our results confirm this [27]. We estimated the pooled five-year overall survival among patients undergoing lung metastasectomy was 53% (95% CI 39–67%) and the median overall was 72.8 months (95% CI 63.0 to 82.5). Considerably less evidence exists describing the outcomes of patients undergoing metastasectomy for LMS at other sites. Our results suggest that patients with liver metastasectomy may also experience acceptable survival post-metastasectomy. In contrast, spine and brain metastasectomy may be more appropriately considered in palliative situations to improve quality of life.
We aimed to identify criteria that could be used to guide clinicians in the selection of patients with LMS appropriate for metastasectomy. The criteria used to select patients and the intent of metastasectomy were not uniformly reported by all studies. It was often not detailed enough to be used or replicated in clinical practice when reported. For example, the authors most commonly described selecting patients for metastasectomy if they had a long DFI, limited sites of metastatic disease, and demonstrated disease stability on chemotherapy. Additional considerations were noted to guide the selection of patients undergoing spine and brain metastasectomy, including their estimated prognosis, current performance status, and symptom burden. However, the specific details of how these criteria were evaluated or defined were not available, limiting the ability of clinicians to use these meaningfully in their clinical practice.
We identified that some patients undergoing liver (13, 34%), spine (1, 10%), and brain (2, 40%) metastasectomy had synchronous disease compared to none undergoing lung metastasectomy. In addition, patients undergoing liver (DFI range 16–50 months) and brain (DFI range 9–89 months) metastasectomy had a shorter median DFI compared to those undergoing lung (DFI range 26–48 months) and spine (DFI range 32–50 months) metastasectomies. Patients with brain and spine metastases are more prone to experience symptoms that impair their quality of life and could be eased by metastasectomy. For these reasons, patients with unfavorable prognostic characteristics, such as a short DFI and a synchronous presentation, may be more likely to be evaluated for metastasectomy if the treatment can improve their quality of life. However, it is unclear why there are more patients with synchronous disease and a shorter DFI undergoing liver compared to lung metastasectomies. It may be to decrease the systemic tumor burden, which may be associated with improved survival when resection of the primary tumor site is also performed. This difference in patient characteristics for those undergoing liver versus lung metastasectomy may partly explain why patients with lung metastasectomy had the most prolonged survival on pooled analysis. Developing more rigorous criteria for selecting patients who can benefit from metastasectomy is a priority for future research.
We found that few prognostic factors were evaluated quantitatively. Metachronous disease, a longer DFI, and R0 metastasectomy were favorable prognostic factors among lung and liver metastasectomy patients. The study by Paramanathan et al. was the only one to define a long DFI (i.e., 36 months) based on the International Registry of Lung Metastases prognostic group [23]. Patients undergoing lung metastasectomy were less likely to have additional sites of metastases compared to those undergoing liver metastasectomy. Interestingly, patients undergoing liver metastasectomy with extrahepatic disease who achieved complete resection of all disease had comparable survival to those without extrahepatic disease. This is an important finding, as patients with multiple sites of metastatic disease are often less likely to be considered for metastasectomy. For patients undergoing spine metastasectomy, post-metastasectomy performance status was the only significant prognostic factor. This has limited clinical utility as it is often difficult to predict how patients will respond to surgery. Additional research is required to determine which patients should be selected and who are most likely to benefit from metastasectomy.
We found that perioperative systemic and radiotherapy were infrequently utilized among patients undergoing metastasectomy for LMS. There is currently no evidence to support these treatment modalities in the perioperative metastatic setting. On the other hand, in the context of unresectable, metastatic STS, there is evidence to support cytotoxic chemotherapy. Anthracyclines, with or without ifosfamide, are regarded as an acceptable first-line treatment in this setting [50,51,52,53]. Many of the patients included in this systematic review were treated when our understanding of the various histologic types of STS was limited and before the practice of histology-driven treatment [10,53,54]. LMS has moderate sensitivity to ifosfamide-based regimens. As single therapies, doxorubicin and ifosfamide have demonstrated response rates of between 10% and 25% in LMS [10]. Dacarbazine had an overall response rate of 16% as a single agent, and retrospective data indicate overall response rates of nearly 37% when used in combination with doxorubicin [55,56]. In addition, gemcitabine and docetaxel also have demonstrated activity in LMS and this combination is used as a first-line therapy in the metastatic setting in some jurisdictions [57,58]. Newer treatments, including trabectedin, pazopanib and eribulin, have shown promising results in metastatic, unresectable LMS in later line settings [59,60,61,62,63,64,65,66,67,68,69,70,71]. It is imperative to evaluate the role of metastasectomy in the era of these modern systemic therapy regimens, even for all STS. Furthermore, because the majority of patients undergoing metastasectomy for LMS experience disease recurrence within a short interval, it is imperative to apply new treatment modalities for these metastases.
There is increasing evidence to support the feasibility and effectiveness of local interventional treatments, such as radiofrequency ablation, cryoablation, and stereotactic body radiation therapy [72,73,74,75,76]. Hepatic artery embolization with or without chemotherapy and radioembolization are further interventional treatments for liver metastases that can now be used in conjunction with other treatments. None of the studies included in this systematic review compared these local treatments to metastasectomy. As with many other rare diseases, retrospective data constitute the strongest available evidence, and decision-making around the management of these complex patients should be based on patient preferences in the context of multidisciplinary management.
Despite the promising survival outcomes, our results show that patients undergoing metastasectomy for LMS experienced high recurrence rates. For example, the five-year disease-free survival of patients undergoing lung metastasectomy was 9%, and the median disease-free survival was reported to be between 6 and 40 months. The five-year disease-free survival of patients undergoing liver metastasectomy was 22%, with a median disease-free survival between 13 and 16 months. The disease-free survival was not reported for patients undergoing spine and brain metastasectomies. Some patients who experienced recurrences underwent additional metastasectomies; this was performed for patients with lung, liver, and spine metastases. Currently, repeat metastasectomy is most well described and accepted for patients with lung metastases from various STS histologies, with the median overall survival after repeat metastasectomy reported to range between 25 and 65 months [77,78,79,80]. Prognostic factors associated with an improved median overall survival after repeat lung metastasectomy in these studies include achieving R0 margins, low-grade tumors, one or two sites of metastatic nodules, and the largest size of metastases less than 2 cm. Our results suggest that repeat liver metastasectomy results in comparable survival to repeat lung metastasectomy, and repeat spine metastasectomy may be warranted to improve symptoms [22]. Additional information on the criteria used to select patients for repeat metastasectomy and more data on survival outcomes are required to understand the feasibility.
Limitations
Limitations of the evidence in this review include the retrospective nature of the existing case series and cohort studies. These non-randomized studies introduce potential biases due to careful patient selection. Most of the survival outcomes reported were not stratified or adjusted based on important prognostic factors. Given the small sample size of many included, it is unlikely such a stratified analysis would have been possible. Being limited to small study samples also increases the risk of the “small-study effects,” where smaller studies are more likely to be published if they report larger or more significant effects [81]. This is particularly important if unadjusted or unstratified estimates are reported. Another important limitation is that some studies included patients before the widespread use of the c-kit receptor for differentiation of gastrointestinal stromal tumors (GIST) versus LMS, which can otherwise have similarities on histopathology [82,83]. This is important as the outcomes for patients with GISTs are much better compared to LMS, which may have biased the results, particularly for the cohort of LMS arising from the gastrointestinal tract undergoing liver metastasectomy, as this is commonly the presentation of GISTs [84].
5. Conclusions
Surgery is currently being utilized to manage LMS metastases to the lung, liver, spine, and brain. Although low quality, comparative studies support a survival benefit, but patient selection and tumor biology are likely to have influenced these results. Recommendations regarding which patients should be considered for metastasectomy are limited by the variability in the criteria used to select patients for metastasectomy across studies and the sites of metastases. The majority of patients undergoing metastasectomy experience disease recurrence within a short interval. Additional research is required to establish the role of metastasectomy in the era of modern systemic therapy regimens and local ablative techniques. Leveraging international collaborations and registry data is one way to move forward with more robust and nuanced patient assessments in this rare disease [85].
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers14133055/s1, Figure S1: PRISMA Flow Diagram, Table S1: Total Patients Included in Each Study, Table S2: Post-Metastasectomy LMS-Specific Outcomes, Table S3: JBI Critical Appraisal Checklist for Case Series, Table S4: NOS for Cohort Studies, Table S5: Components of GRADE (Grading of Recommendation, Assessment, Development and Evaluation) to Assess the Certainty of Evidence.
Author Contributions
Conceptualization, M.D., Y.W., D.S. and A.S.; methodology, M.D., Y.W., D.S. and A.S.; validation, M.D., B.A., K.N. and A.S.; formal analysis, M.D., K.N. and B.A.; resources, Y.W. and A.S.; data curation, M.D., B.A., K.N. and A.S.; writing—original draft preparation, M.D.; writing—review and editing, M.D., B.A. and A.S.; visualization, M.D., B.A., K.N. and A.S.; supervision, Y.W. and A.S.; project administration, M.D., Y.W. and A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
Acknowledgments
The authors would like to acknowledge Ranjeeta Mallick and the Ottawa Methods Center for the expert advice regarding the statistical analysis. The researchers would like to thank the Deanship of Scientific Research, Qassim University for funding the publication of this project.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| ASPS | Alveolar soft part sarcoma |
| CI | Confidence interval |
| CSS | Cancer specific survival |
| DFI | Disease free interval |
| ECOG | Eastern Cooperative Oncology Group |
| GI | Gastrointestinal |
| GIST | Gastrointestinal stromal tumor |
| GTR | Gross total removal |
| GU | Genitourinary |
| IQR | Interquartile range |
| JBI | Joanna Briggs Institute |
| LMS | Leiomyosarcoma |
| LPS | Liposarcoma |
| MFH | Malignant fibrous histiocytoma |
| MPNST | Malignant peripheral nerve sheath tumor |
| NOS | Newcastle-Ottawa Quality Assessment Scale |
| NR | Not reported |
| OS | Overall survival |
| RMS | Rhabdomyosarcoma |
| SD | Standard deviation |
| SFT | Solitary fibrous tumor |
| SS | Synovial sarcoma |
| STR | Subtotal removal |
| STS | Soft tissue sarcoma |
| UPS | Undifferentiated pleomorphic sarcoma |
| WBRT | Whole brain radiation therapy |
References
- George, S.; Serrano, C.; Hensley, M.L.; Ray-Coquard, I. Soft Tissue and Uterine Leiomyosarcoma. J. Clin. Oncol. 2017, 36, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Bathan, A.J.; Constantinidou, A.; Pollack, S.M.; Jones, R.L. Diagnosis, Prognosis, and Management of Leiomyosarcoma: Recognition of Anatomic Variants. Curr. Opin. Oncol. 2013, 25, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Goldblum, J.; Volpe, A.; Weiss, S. Leiomyosarcoma. In Enzinger & Weiss’s Soft Tissue Tumors; Goldblum, J., Folpe, A., Weiss, S., Eds.; Elsevier: Philadelphia, PA, USA, 2020; pp. 591–613. [Google Scholar]
- Brennan, M.F.; Antonescu, C.R.; Moraco, N.; Singer, S. Lessons Learned from the Study of 10,000 Patients with Soft Tissue Sarcoma. Ann. Surg. 2014, 260, 416–422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yadav, U.; Mangla, A. Leiomyosarcoma. Available online: https://www.ncbi.nlm.nih.gov/books/NBK551667/ (accessed on 19 February 2022).
- Pisters, P.W.; Leung, D.H.; Woodruff, J.; Shi, W.; Brennan, M.F. Analysis of Prognostic Factors in 1,041 Patients with Localized Soft Tissue Sarcomas of the Extremities. J. Clin. Oncol. 1996, 14, 1679–1689. [Google Scholar] [CrossRef]
- Gronchi, A.; Strauss, D.C.; Miceli, R.; Bonvalot, S.; Swallow, C.J.; Hohenberger, P.; van Coevorden, F.; Rutkowski, P.; Callegaro, D.; Hayes, A.J.; et al. Variability in Patterns of Recurrence after Resection of Primary Retroperitoneal Sarcoma (RPS). A Report on 1007 Patients from the Multi-Institutional Collaborative RPS Working Group. Ann. Surg. 2016, 263, 1002–1009. [Google Scholar] [CrossRef]
- Yang, J.C.; Chang, A.E.; Baker, A.R.; Sindelar, W.F.; Danforth, D.N.; Topalian, S.L.; DeLaney, T.; Glatstein, E.; Steinberg, S.M.; Merino, M.J.; et al. Randomized Prospective Study of the Benefit of Adjuvant Radiation Therapy in the Treatment of Soft Tissue Sarcomas of the Extremity. J. Clin. Oncol. 1998, 16, 197–203. [Google Scholar] [CrossRef] [Green Version]
- Bonvalot, S.; Gronchi, A.; le Péchoux, C.; Swallow, C.J.; Strauss, D.; Meeus, P.; van Coevorden, F.; Stoldt, S.; Stoeckle, E.; Rutkowski, P.; et al. Preoperative Radiotherapy plus Surgery versus Surgery Alone for Patients with Primary Retroperitoneal Sarcoma (EORTC-62092: STRASS): A Multicentre, Open-Label, Randomised, Phase 3 Trial. Lancet Oncol. 2020, 21, 1366–1377. [Google Scholar] [CrossRef]
- Gamboa, A.C.; Gronchi, A.; Cardona, K. Soft-tissue Sarcoma in Adults: An Update on the Current State of Histiotype-specific Management in an Era of Personalized Medicine. CA Cancer J. Clin. 2020, 70, 200–229. [Google Scholar] [CrossRef] [Green Version]
- Van Cann, T.; Cornillie, J.; Wozniak, A.; Debiec-Rychter, M.; Sciot, R.; Hompes, D.; Vergote, I.; Schöffski, P. Retrospective Analysis of Outcome of Patients with Metastatic Leiomyosarcoma in a Tertiary Referral Center. Oncol. Res. Treat. 2018, 41, 206–213. [Google Scholar] [CrossRef]
- Tirotta, F.; Hodson, J.; Parente, A.; Pasquali, S.; Sutcliffe, R.; Desai, A.; Muiesan, P.; Ford, S.J.; Fiore, M.; Gronchi, A.; et al. Liver Resection for Sarcoma Metastases: A Systematic Review and Experience from Two European Centres. Eur. J. Surg. Oncol. 2020, 46, 1807–1813. [Google Scholar] [CrossRef]
- Deguchi, S.; Nakasu, Y.; Sakaida, T.; Akimoto, J.; Tanahashi, K.; Natsume, A.; Takahashi, M.; Okuda, T.; Asakura, H.; Mitsuya, K.; et al. Surgical Outcome and Graded Prognostic Assessment of Patients with Brain Metastasis from Adult Sarcoma: Multi-Institutional Retrospective Study in Japan. Int. J. Clin. Oncol. 2020, 25, 1995–2005. [Google Scholar] [CrossRef] [PubMed]
- Rao, G.; Suki, D.; Chakrabarti, I.; Feiz-Erfan, I.; Mody, M.G.; McCutcheon, I.E.; Gokaslan, Z.; Patel, S.; Rhines, L.D. Surgical Management of Primary and Metastatic Sarcoma of the Mobile Spine. J. Neurosurg. Spine 2008, 9, 120–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Delisle, M.; Smith, D.; Srikanthan, A. Survival by Histology among Patients with Bone and Soft Tissue Sarcoma Who Undergo Metastasectomy: Protocol for a Systematic Review and Meta-Analysis. Syst. Rev. 2020, 9, 189. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef] [PubMed]
- Burt, B.M.; Ocejo, S.; Mery, C.M.; Dasilva, M.; Bueno, R.; Sugarbaker, D.J.; Jaklitsch, M.T. Repeated and Aggressive Pulmonary Resections for Leiomyosarcoma Metastases Extends Survival. Ann. Thorac. Surg. 2011, 92, 1202–1207. [Google Scholar] [CrossRef] [PubMed]
- Chen, H. Complete Hepatic Resection of Metastases from Leiomyosarcoma Prolongs Survival. J. Gastrointest. Surg. 1998, 2, 151–155. [Google Scholar] [CrossRef]
- Faraj, W.; El-Kehdy, J.; el Nounou, G.; Deeba, S.; Fakih, H.; Jabbour, M.; Haydar, A.; el Naaj, A.A.; Abou-Alfa, G.K.; O’Reilly, E.M.; et al. Liver Resection for Metastatic Colorectal Leiomyosarcoma: A Single Center Experience. J. Gastrointest. Oncol. 2015, 6, E70–E76. [Google Scholar] [CrossRef]
- Kato, S.; Demura, S.; Shinmura, K.; Yokogawa, N.; Yonezawa, N.; Shimizu, T.; Oku, N.; Kitagawa, R.; Murakami, H.; Kawahara, N.; et al. Clinical Outcomes and Survivals after Total En Bloc Spondylectomy for Metastatic Leiomyosarcoma in the Spine. Eur. Spine J. 2020, 29, 3237–3244. [Google Scholar] [CrossRef]
- Kim, Y.W.; Lee, J.H.; Kim, J.E.; Kang, J. Surgical Resection of Liver Metastasis of Leiomyosarcoma. Korean J. Clin. Oncol. 2017, 13, 143–146. [Google Scholar] [CrossRef]
- Lang, H.; Nußbaum, K.-T.; Kaudel, P.; Frü Hauf, N.; Flemming, P.; Raab, R. Hepatic Metastases from Leiomyosarcoma A Single-Center Experience with 34 Liver Resections During a 15-Year Period. Ann. Surg. 2000, 231, 500–505. [Google Scholar] [CrossRef]
- Paramanathan, A.; Wright, G. Pulmonary Metastasectomy for Sarcoma of Gynaecologic Origin. Heart Lung Circ. 2013, 22, 270–275. [Google Scholar] [CrossRef]
- Ziewacz, J.E.; Lau, D.; la Marca, F.; Park, P. Outcomes after Surgery for Spinal Metastatic Leiomyosarcoma. J. Neurosurg. Spine 2012, 17, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Anraku, M.; Yokoi, K.; Nakagawa, K.; Fujisawa, T.; Nakajima, J.; Akiyama, H.; Nishimura, Y.; Kobayashi, K. Pulmonary Metastases from Uterine Malignancies: Results of Surgical Resection in 133 Patients. J. Thorac. Cardiovasc. Surg. 2004, 127, 1107–1112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blackmon, S.H.; Shah, N.; Roth, J.A.; Correa, A.M.; Vaporciyan, A.A.; Rice, D.C.; Hofstetter, W.; Walsh, G.L.; Benjamin, R.; Pollock, R.; et al. Resection of Pulmonary and Extrapulmonary Sarcomatous Metastases Is Associated with Long-Term Survival. Ann. Thorac. Surg. 2009, 88, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Chudgar, N.P.; Brennan, M.F.; Munhoz, R.R.; Bucciarelli, P.R.; Tan, K.S.; D’Angelo, S.P.; Bains, M.S.; Bott, M.; Huang, J.; Park, B.J.; et al. Pulmonary Metastasectomy with Therapeutic Intent for Soft-Tissue Sarcoma. J. Thorac. Cardiovasc. Surg. 2017, 154, 319–330.e1. [Google Scholar] [CrossRef] [Green Version]
- Ercolani, G.; Grazi, G.L.; Ravaioli, M.; Ramacciato, G.; Cescon, M.; Varotti, G.; del Gaudio, M.; Vetrone, G.; Pinna, A.D. The Role of Liver Resections for Noncolorectal, Nonneuroendocrine Metastases: Experience with 142 Observed Cases. Ann. Surg. Oncol. 2005, 12, 459–466. [Google Scholar] [CrossRef]
- Goumard, C.; Marcal, L.P.; Wang, W.L.; Somaiah, N.; Okuno, M.; Roland, C.L.; Tzeng, C.W.D.; Chun, Y.S.; Feig, B.W.; Vauthey, J.N.; et al. Long-Term Survival According to Histology and Radiologic Response to Preoperative Chemotherapy in 126 Patients Undergoing Resection of Non-GIST Sarcoma Liver Metastases. Ann. Surg. Oncol. 2018, 25, 107–116. [Google Scholar] [CrossRef]
- Liebl, L.S.; Elson, F.; Quaas, A.; Gawad, K.A.; Izbicki, J.R. Value of Repeat Resection for Survival in Pulmonary Metastases from Soft Tissue Sarcoma. Anticancer Res. 2007, 27, 2897–2902. [Google Scholar]
- Lin, A.Y.; Kotova, S.; Yanagawa, J.; Elbuluk, O.; Wang, G.; Kar, N.; Elashoff, D.; Grogan, T.; Cameron, R.B.; Singh, A.; et al. Risk Stratification of Patients Undergoing Pulmonary Metastasectomy for Soft Tissue and Bone Sarcomas. J. Thorac. Cardiovasc. Surg. 2015, 149, 85–92. [Google Scholar] [CrossRef] [Green Version]
- Marudanayagam, R.; Sandhu, B.; Perera, M.T.P.R.; Bramhall, S.R.; Mayer, D.; Buckels, J.A.C.; Mirza, D.F. Liver Resection for Metastatic Soft Tissue Sarcoma: An Analysis of Prognostic Factors. Eur. J. Surg. Oncol. 2010, 37, 87–92. [Google Scholar] [CrossRef] [Green Version]
- Smith, R.; Pak, Y.; Kraybill, W.; Kane, J.M. Factors Associated with Actual Long-Term Survival Following Soft Tissue Sarcoma Pulmonary Metastasectomy. Eur. J. Surg. Oncol. 2009, 35, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Zacherl, M.; Bernhardt, G.A.; Zacherl, J.; Gruber, G.; Kornprat, P.; Bacher, H.; Mischinger, H.J.; Windhager, R.; Jakesz, R.; Grünberger, T. Surgery for Liver Metastases Originating from Sarcoma-Case Series. Langenbeck’s Arch. Surg. 2011, 396, 1083–1091. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, J. Clinical Features of Surgical Resection for Liver Metastasis from Extremity Soft Tissue Sarcoma. Hepatogastroenterology 2015, 62, 677–682. [Google Scholar]
- Farid, M.; Ong, W.S.; Tan, M.H.; Foo, L.S.S.; Lim, Y.K.; Chia, W.K.; Soh, L.T.; Poon, D.; Lee, M.J.F.; Ho, Z.C.; et al. The Influence of Primary Site on Outcomes in Leiomyosarcoma. Am. J. Clin. Oncol. 2013, 36, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Rohatgi, A. WebPlotDigitizer; Version 4.5; 2021. [Google Scholar]
- Liu, N.; Zhou, Y.; Lee, J.J. IPDfromKM: Reconstruct Individual Patient Data from Published Kaplan-Meier Survival Curves. BMC Med. Res. Methodol. 2021, 21, 111. [Google Scholar] [CrossRef]
- Hozo, S.P.; Djulbegovic, B.; Hozo, I. Estimating the Mean and Variance from the Median, Range, and the Size of a Sample. BMC Med. Res. Methodol. 2005, 5, 13. [Google Scholar] [CrossRef] [Green Version]
- Deeks, J.; Higgins, J.; Altman, D. Random-Effects Methods for Meta-Analysis. In Cochrane Handbook for Systematic Reviews of Interventions; Thomas, J., Higgins, J., Eds.; Wiley-Blackwell: Chicester, UK, 2022. [Google Scholar]
- Mathes, T.; Pieper, D. Study Design Classification of Registry-Based Studies in Systematic Reviews. J. Clin. Epidemiol. 2018, 93, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Moola, S.; Munn, Z.; Tufanaru, C.; Aromataris, E.; Sears, K.; Sfetcu, R.; Currie, M.; Qureshi, R.; Mattis, P.; Lisy, K.; et al. Systematic Reviews of Etiology and Risk. In Joanna Briggs Institute Reviewer’s Manual; Aromataris, E., Munn, Z., Eds.; 2017; Available online: https://synthesismanual.jbi.global (accessed on 20 February 2022).
- The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 20 February 2022).
- Ma, L.-L.; Wang, Y.-Y.; Yang, Z.-H.; Huang, D.; Weng, H.; Zeng, X.-T. Methodological Quality (Risk of Bias) Assessment Tools for Primary and Secondary Medical Studies: What Are They and Which Is Better? Mil. Med. Res. 2020, 7, 7. [Google Scholar] [CrossRef] [Green Version]
- Santesso, N.; Glenton, C.; Dahm, P.; Garner, P.; Akl, E.A.; Alper, B.; Brignardello-Petersen, R.; Carrasco-Labra, A.; de Beer, H.; Hultcrantz, M.; et al. GRADE Guidelines 26: Informative Statements to Communicate the Findings of Systematic Reviews of Interventions. J. Clin. Epidemiol. 2020, 119, 126–135. [Google Scholar] [CrossRef] [Green Version]
- Murad, M.H.; Mustafa, R.A.; Schünemann, H.J.; Sultan, S.; Santesso, N. Rating the Certainty in Evidence in the Absence of a Single Estimate of Effect. Evid.-Based Med. 2017, 22, 85–87. [Google Scholar] [CrossRef] [Green Version]
- Pastorino, U.; Buyse, M.; Friedel, S.G.; Ginsberg, R.J.; Girard, P.; Goldstraw, P.; Johnston, M.; Mccormack, P.; Pass, H.; Putnam, J.B. Long-Term Results of Lung Metastasectomy: Prognostic Analyses Based on 5206 Cases. J. Thorac. Cardiovasc. Surg. 1997, 113, 37–49. [Google Scholar] [CrossRef] [Green Version]
- Van Geel, A.N.; Pastorino, U.; Jauch, K.; Judson, I.R.; van Coevorden, F.; Buesa, J.M.; Nieisen, S.; Boudinet, A.; Tursz, T.; Schmitz, P.I.M.; et al. Surgical Treatment of Lung Metastases the European Organization for Research and Treatment of Cancer-Soft Tissue and Bone Sarcoma Group Study of 255 Patients. Cancer 1996, 77, 675–682. [Google Scholar] [CrossRef]
- Choong, P.F.M.; Pritchard, D.J.; Rock, M.G.; Sim, F.H.; Frassica, F.J. Survival after Pulmonary Metastasectomy in Soft Tissue Sarcoma: Prognostic Factors in 214 Patients. Acta Orthop. Scand. 1995, 66, 561–568. [Google Scholar] [CrossRef]
- Maurel, J.; López-Pousa, A.; de Las Peñas, R.; Fra, J.; Martín, J.; Cruz, J.; Casado, A.; Poveda, A.; Martínez-Trufero, J.; Balañá, C.; et al. Efficacy of Sequential High-Dose Doxorubicin and Ifosfamide Compared with Standard-Dose Doxorubicin in Patients with Advanced Soft Tissue Sarcoma: An Open-Label Randomized Phase II Study of the Spanish Group for Research on Sarcomas. J. Clin. Oncol. 2009, 27, 1893–1898. [Google Scholar] [CrossRef] [PubMed]
- Tap, W.D.; Papai, Z.; van Tine, B.A.; Attia, S.; Ganjoo, K.N.; Jones, R.L.; Schuetze, S.; Reed, D.; Chawla, S.P.; Riedel, R.F.; et al. Doxorubicin plus Evofosfamide versus Doxorubicin Alone in Locally Advanced, Unresectable or Metastatic Soft-Tissue Sarcoma (TH CR-406/SARC021): An International, Multicentre, Open-Label, Randomised Phase 3 Trial. Lancet Oncol. 2017, 18, 1089–1103. [Google Scholar] [CrossRef]
- Judson, I.; Verweij, J.; Gelderblom, H.; Hartmann, J.T.; Schöffski, P.; Blay, J.-Y.; Kerst, J.M.; Sufliarsky, J.; Whelan, J.; Hohenberger, P.; et al. Doxorubicin Alone versus Intensified Doxorubicin plus Ifosfamide for First-Line Treatment of Advanced or Metastatic Soft-Tissue Sarcoma: A Randomised Controlled Phase 3 Trial. Lancet Oncol. 2014, 15, 415–423. [Google Scholar] [CrossRef]
- Edmonson, J.H.; Ryan, L.M.; Blum, R.H.; Brooks, J.S.; Shiraki, M.; Frytak, S.; Parkinson, D.R. Randomized Comparison of Doxorubicin Alone versus Ifosfamide plus Doxorubicin or Mitomycin, Doxorubicin, and Cisplatin against Advanced Soft Tissue Sarcomas. J. Clin. Oncol. 1993, 11, 1269–1275. [Google Scholar] [CrossRef] [Green Version]
- Oosten, A.W.; Seynaeve, C.; Schmitz, P.I.M.; den Bakker, M.A.; Verweij, J.; Sleijfer, S. Outcomes of First-Line Chemotherapy in Patients with Advanced or Metastatic Leiomyosarcoma of Uterine and Non-Uterine Origin. Sarcoma 2009, 2009, 348910. [Google Scholar] [CrossRef] [Green Version]
- Talbot, S.M.; Keohan, M.L.; Hesdorffer, M.; Orrico, R.; Bagiella, E.; Troxel, A.B.; Taub, R.N. A Phase II Trial of Temozolomide in Patients with Unresectable or Metastatic Soft Tissue Sarcoma. Cancer 2003, 98, 1942–1946. [Google Scholar] [CrossRef]
- D’Ambrosio, L.; Touati, N.; Blay, J.; Grignani, G.; Flippot, R.; Czarnecka, A.M.; Piperno-Neumann, S.; Martin-Broto, J.; Sanfilippo, R.; Katz, D.; et al. Doxorubicin plus Dacarbazine, Doxorubicin plus Ifosfamide, or Doxorubicin Alone as a First-line Treatment for Advanced Leiomyosarcoma: A Propensity Score Matching Analysis from the European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group. Cancer 2020, 126, 2637–2647. [Google Scholar] [CrossRef]
- Hensley, M.L.; Blessing, J.A.; Mannel, R.; Rose, P.G. Fixed-Dose Rate Gemcitabine plus Docetaxel as First-Line Therapy for Metastatic Uterine Leiomyosarcoma: A Gynecologic Oncology Group Phase II Trial. Gynecol. Oncol. 2008, 109, 329–334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hensley, M.L.; Blessing, J.A.; DeGeest, K.; Abulafia, O.; Rose, P.G.; Homesley, H.D. Fixed-Dose Rate Gemcitabine plus Docetaxel as Second-Line Therapy for Metastatic Uterine Leiomyosarcoma: A Gynecologic Oncology Group Phase II Study. Gynecol. Oncol. 2008, 109, 323–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schöffski, P.; Chawla, S.; Maki, R.G.; Italiano, A.; Gelderblom, H.; Choy, E.; Grignani, G.; Camargo, V.; Bauer, S.; Rha, S.Y.; et al. Eribulin versus Dacarbazine in Previously Treated Patients with Advanced Liposarcoma or Leiomyosarcoma: A Randomised, Open-Label, Multicentre, Phase 3 Trial. Lancet 2016, 387, 1629–1637. [Google Scholar] [CrossRef]
- Blay, J.-Y.; Schöffski, P.; Bauer, S.; Krarup-Hansen, A.; Benson, C.; D’Adamo, D.R.; Jia, Y.; Maki, R.G. Eribulin versus Dacarbazine in Patients with Leiomyosarcoma: Subgroup Analysis from a Phase 3, Open-Label, Randomised Study. Br. J. Cancer 2019, 120, 1026–1032. [Google Scholar] [CrossRef] [Green Version]
- Hirbe, A.C.; Eulo, V.; Moon, C.I.; Luo, J.; Myles, S.; Seetharam, M.; Toeniskoetter, J.; Kershner, T.; Haarberg, S.; Agulnik, M.; et al. A Phase II Study of Pazopanib as Front-Line Therapy in Patients with Non-Resectable or Metastatic Soft-Tissue Sarcomas Who Are Not Candidates for Chemotherapy. Eur. J. Cancer 2020, 137, P1–P9. [Google Scholar] [CrossRef] [PubMed]
- Grünwald, V.; Karch, A.; Schuler, M.; Schöffski, P.; Kopp, H.-G.; Bauer, S.; Kasper, B.; Lindner, L.H.; Chemnitz, J.-M.; Crysandt, M.; et al. Randomized Comparison of Pazopanib and Doxorubicin as First-Line Treatment in Patients with Metastatic Soft Tissue Sarcoma Age 60 Years or Older: Results of a German Intergroup Study. J. Clin. Oncol. 2020, 38, 3555–3564. [Google Scholar] [CrossRef] [PubMed]
- Grosso, F.; D’Ambrosio, L.; Zucchetti, M.; Ibrahim, T.; Tamberi, S.; Matteo, C.; Rulli, E.; Comandini, D.; Palmerini, E.; Baldi, G.G.; et al. Pharmacokinetics, Safety, and Activity of Trabectedin as First-Line Treatment in Elderly Patients Who Are Affected by Advanced Sarcoma and Are Unfit to Receive Standard Chemotherapy: A Phase 2 Study (TR1US Study) from the Italian Sarcoma Group. Cancer 2020, 126, 4726–4734. [Google Scholar] [CrossRef]
- Kawai, A.; Araki, N.; Sugiura, H.; Ueda, T.; Yonemoto, T.; Takahashi, M.; Morioka, H.; Hiraga, H.; Hiruma, T.; Kunisada, T.; et al. Trabectedin Monotherapy after Standard Chemotherapy versus Best Supportive Care in Patients with Advanced, Translocation-Related Sarcoma: A Randomised, Open-Label, Phase 2 Study. Lancet Oncol. 2015, 16, 406–416. [Google Scholar] [CrossRef]
- Garcia-Carbonero, R.; Supko, J.G.; Maki, R.G.; Manola, J.; Ryan, D.P.; Harmon, D.; Puchalski, T.A.; Goss, G.; Seiden, M.V.; Waxman, A.; et al. Ecteinascidin-743 (ET-743) for Chemotherapy-Naive Patients with Advanced Soft Tissue Sarcomas: Multicenter Phase II and Pharmacokinetic Study. J. Clin. Oncol. 2005, 23, 5484–5492. [Google Scholar] [CrossRef]
- Garcia-Carbonero, R.; Supko, J.G.; Manola, J.; Seiden, M.V.; Harmon, D.; Ryan, D.P.; Quigley, M.T.; Merriam, P.; Canniff, J.; Goss, G.; et al. Phase II and Pharmacokinetic Study of Ecteinascidin 743 in Patients with Progressive Sarcomas of Soft Tissues Refractory to Chemotherapy. J. Clin. Oncol. 2004, 22, 1480–1490. [Google Scholar] [CrossRef]
- Le Cesne, A.; Blay, J.Y.; Judson, I.; van Oosterom, A.; Verweij, J.; Radford, J.; Lorigan, P.; Rodenhuis, S.; Ray-Coquard, I.; Bonvalot, S.; et al. Phase II Study of ET-743 in Advanced Soft Tissue Sarcomas: A European Organisation for the Research and Treatment of Cancer (EORTC) Soft Tissue and Bone Sarcoma Group Trial. J. Clin. Oncol 2005, 23, 576–584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yovine, A.; Riofrio, M.; Blay, J.Y.; Brain, E.; Alexandre, J.; Kahatt, C.; Taamma, A.; Jimeno, J.; Martin, C.; Salhi, Y.; et al. Phase II Study of Ecteinascidin-743 in Advanced Pretreated Soft Tissue Sarcoma Patients. J. Clin. Oncol. 2004, 22, 890–899. [Google Scholar] [CrossRef] [PubMed]
- Martin-Broto, J.; Pousa, A.L.; de Las Peñas, R.; García Del Muro, X.; Gutierrez, A.; Martinez-Trufero, J.; Cruz, J.; Alvarez, R.; Cubedo, R.; Redondo, A.; et al. Randomized Phase II Study of Trabectedin and Doxorubicin Compared with Doxorubicin Alone as First-Line Treatment in Patients with Advanced Soft Tissue Sarcomas: A Spanish Group for Research on Sarcoma Study. J. Clin. Oncol. 2016, 34, 2294–2302. [Google Scholar] [CrossRef]
- Patel, S.; von Mehren, M.; Reed, D.R.; Kaiser, P.; Charlson, J.; Ryan, C.W.; Rushing, D.; Livingston, M.; Singh, A.; Seth, R.; et al. Overall Survival and Histology-Specific Subgroup Analyses from a Phase 3, Randomized Controlled Study of Trabectedin or Dacarbazine in Patients with Advanced Liposarcoma or Leiomyosarcoma. Cancer 2019, 125, 2610–2620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demetri, G.D.; von Mehren, M.; Jones, R.L.; Hensley, M.L.; Schuetze, S.M.; Staddon, A.; Milhem, M.; Elias, A.; Ganjoo, K.; Tawbi, H.; et al. Efficacy and Safety of Trabectedin or Dacarbazine for Metastatic Liposarcoma or Leiomyosarcoma After Failure of Conventional Chemotherapy: Results of a Phase III Randomized Multicenter Clinical Trial. J. Clin. Oncol. 2016, 34, 786–793. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.L.; McCall, J.; Adam, A.; O’Donnell, D.; Ashley, S.; Al-Muderis, O.; Thway, K.; Fisher, C.; Judson, I.R. Radiofrequency Ablation Is a Feasible Therapeutic Option in the Multi Modality Management of Sarcoma. Eur. J. Surg. Oncol. 2010, 36, 477–482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berber, E.; Ari, E.; Herceg, N.; Siperstein, A. Laparoscopic Radiofrequency Thermal Ablation for Unusual Hepatic Tumors: Operative Indications and Outcomes. Surg. Endosc. 2005, 19, 1613–1617. [Google Scholar] [CrossRef]
- Dhakal, S.; Corbin, K.S.; Milano, M.T.; Philip, A.; Sahasrabudhe, D.; Jones, C.; Constine, L.S. Stereotactic Body Radiotherapy for Pulmonary Metastases from Soft-Tissue Sarcomas: Excellent Local Lesion Control and Improved Patient Survival. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 940–945. [Google Scholar] [CrossRef]
- Navarria, P.; Ascolese, A.M.; Cozzi, L.; Tomatis, S.; D’Agostino, G.R.; de Rose, F.; de Sanctis, R.; Marrari, A.; Santoro, A.; Fogliata, A.; et al. Stereotactic Body Radiation Therapy for Lung Metastases from Soft Tissue Sarcoma. Eur. J. Cancer 2015, 51, 668–674. [Google Scholar] [CrossRef]
- Nakamura, T.; Matsumine, A.; Yamakado, K.; Matsubara, T.; Takaki, H.; Nakatsuka, A.; Takeda, K.; Abo, D.; Shimizu, T.; Uchida, A. Lung Radiofrequency Ablation in Patients with Pulmonary Metastases from Musculoskeletal Sarcomas. Cancer 2009, 115, 3774–3781. [Google Scholar] [CrossRef]
- Wigge, S.; Heißner, K.; Steger, V.; Ladurner, R.; Traub, F.; Sipos, B.; Bösmüller, H.; Kanz, L.; Mayer, F.; Kopp, H.-G. Impact of Surgery in Patients with Metastatic Soft Tissue Sarcoma: A Monocentric Retrospective Analysis. J. Surg. Oncol. 2018, 118, 167–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weiser, M.R.; Downey, R.J.; Leung, D.H.; Brennan, M.F. Repeat Resection of Pulmonary Metastases in Patients with Soft-Tissue Sarcoma. J. Am. Coll. Surg. 2000, 191, 184–190. [Google Scholar] [CrossRef]
- Casson, A.G.; Putnam, J.B.; Natarajan, G.; Johnston, D.A.; Mountain, C.; McMurtrey, M.; Roth, J.A. Efficacy of Pulmonary Metastasectomy for Recurrent Soft Tissue Sarcoma. J. Surg. Oncol. 1991, 47, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Pogrebniak, H.W.; Roth, J.A.; Steinberg, S.M.; Rosenberg, S.A.; Pass, H.I. Reoperative Pulmonary Resection in Patients with Metastatic Soft Tissue Sarcoma. Ann. Thorac. Surg. 1991, 52, 197–203. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Sutton, A.J.; Ioannidis, J.P.A.; Terrin, N.; Jones, D.R.; Lau, J.; Carpenter, J.; Rucker, G.; Harbord, R.M.; Schmid, C.H.; et al. Recommendations for Examining and Interpreting Funnel Plot Asymmetry in Meta-Analyses of Randomised Controlled Trials. BMJ 2011, 343, d4002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirota, S.; Isozaki, K.; Moriyama, Y.; Hashimoto, K.; Nishida, T.; Ishiguro, S.; Kawano, K.; Hanada, M.; Kurata, A.; Takeda, M.; et al. Gain-of-Function Mutations of c- Kit in Human Gastrointestinal Stromal Tumors. Science 1998, 279, 577–580. [Google Scholar] [CrossRef]
- Newman, P.L.; Wadden, C.; Fletcher, C.D. Gastrointestinal Stromal Tumours: Correlation of Immunophenotype with Clinicopathological Features. J. Pathol. 1991, 164, 107–117. [Google Scholar] [CrossRef]
- Van Glabbeke, M.; van Oosterom, A.T.; Oosterhuis, J.W.; Mouridsen, H.; Crowther, D.; Somers, R.; Verweij, J.; Santoro, A.; Buesa, J.; Tursz, T. Prognostic Factors for the Outcome of Chemotherapy in Advanced Soft Tissue Sarcoma: An Analysis of 2,185 Patients Treated with Anthracycline-Containing First-Line Regimens—A European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group Study. J. Clin. Oncol. 1999, 17, 150–157. [Google Scholar] [CrossRef]
- Van Houdt, W.J.; Raut, C.P.; Bonvalot, S.; Swallow, C.J.; Haas, R.; Gronchi, A. New Research Strategies in Retroperitoneal Sarcoma. The Case of TARPSWG, STRASS and RESAR: Making Progress through Collaboration. Curr. Opin. Oncol. 2019, 31, 310–316. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).