Impact of Pelvic Anatomical Changes Caused by Radical Prostatectomy
Abstract
:Simple Summary
Abstract
1. Introduction
2. Penile Length after Radical Prostatectomy
2.1. Penile Length Measurement
2.2. Chronological Changes of Penile Length after Radical Prostatectomy
2.3. Penile Length Changes and Sexual Function
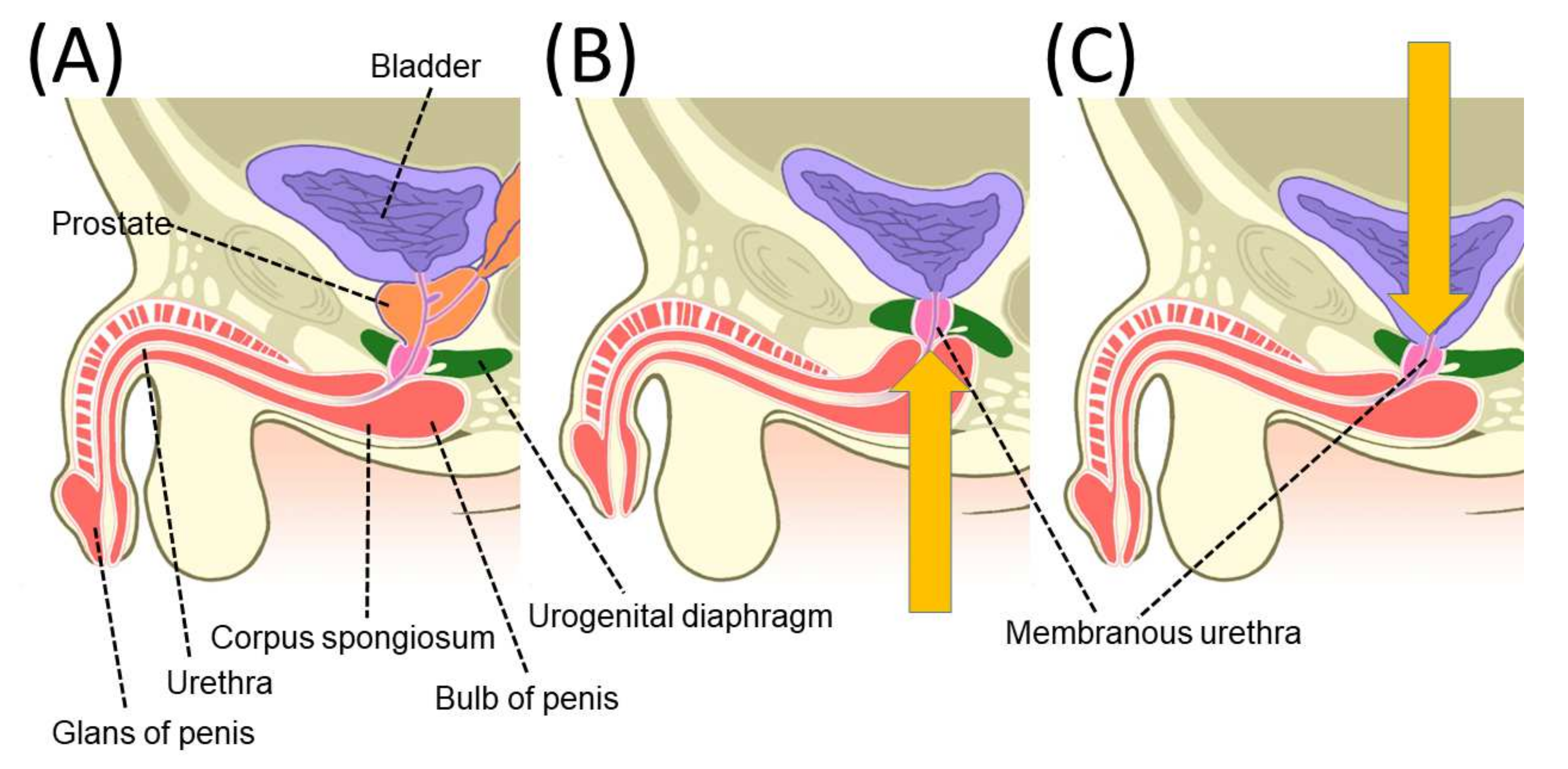
2.4. Mechanism of Penile Length Change after Radical Prostatectomy
3. Erectile Dysfunction after Radical Prostatectomy
3.1. Pathophysiology and Anatomy of Erectile Function
3.2. Surgical Technique including Nerve Sparing
4. Urinary Incontinence after Radical Prostatectomy
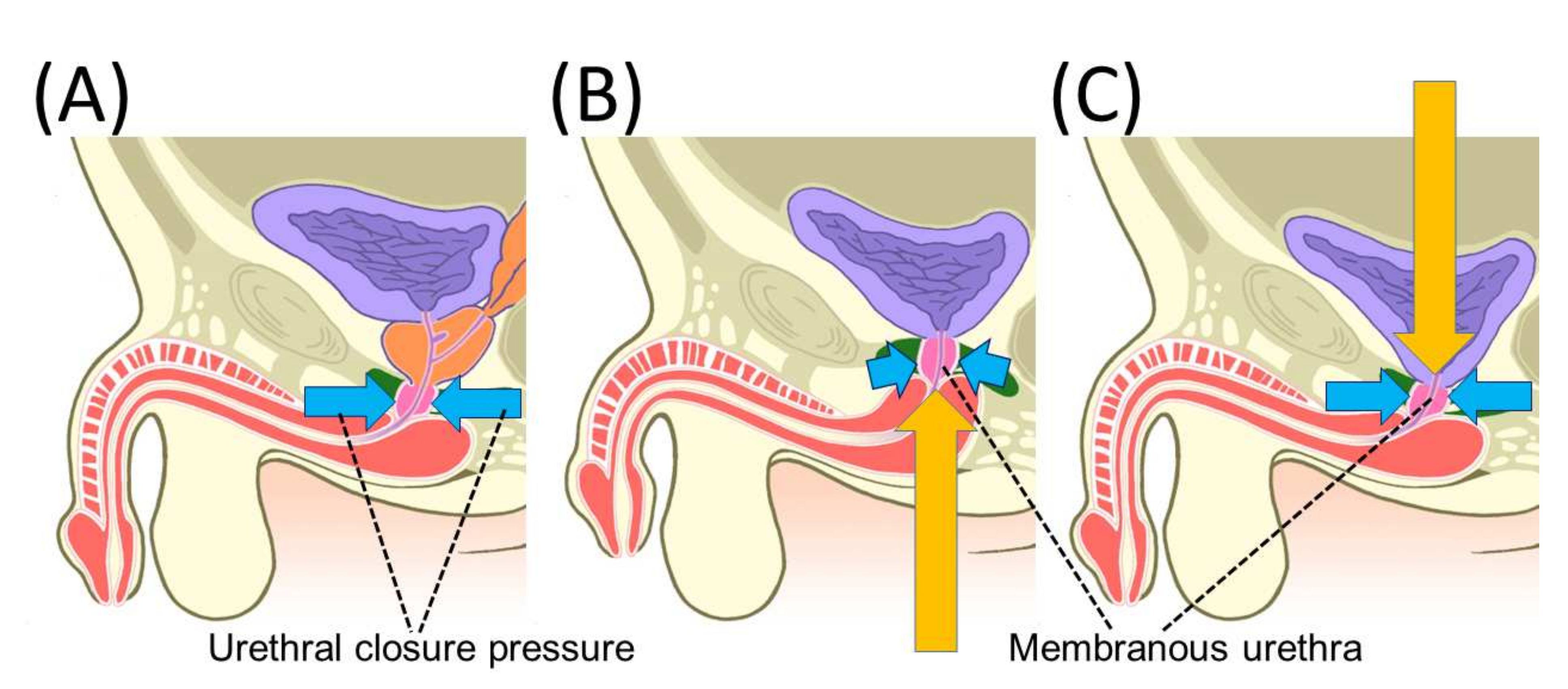
4.1. Pelvic Anatomy Affecting Urinary Incontinence after Radical Prostatectomy
4.2. Mechanism of Urinary Incontinence after Radical Prostatectomy
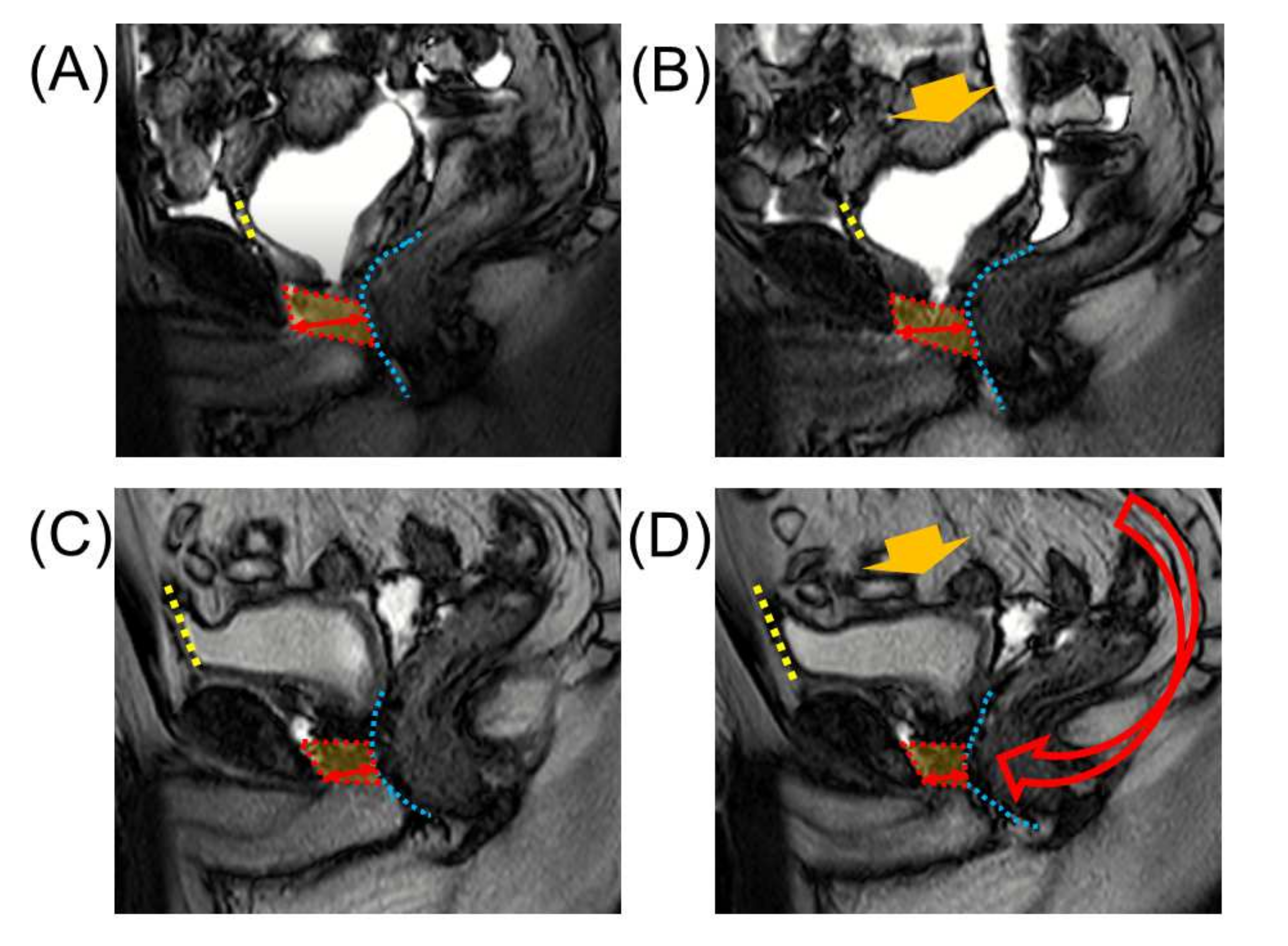
4.3. Maximum Urethral Preservation
4.4. Nerve-Sparing Procedure
4.5. Retzius Sparing Procedure
4.6. Anterior and Posterior Reconstruction
5. Inguinal Hernia
5.1. Mechanisms and Risk Factors for the Development of Inguinal Hernia
5.2. Inguinal Hernia Prevention Techniques
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Walsh, P.C.; Lepor, H.; Eggleston, J.C. Radical prostatectomy with preservation of sexual function: Anatomical and pathological considerations. Prostate 1983, 4, 473–485. [Google Scholar] [CrossRef] [PubMed]
- Reiner, W.G.; Walsh, P.C. An anatomical approach to the surgical management of the dorsal vein and Santorini’s plexus during radical retropubic surgery. J. Urol. 1979, 121, 198–200. [Google Scholar] [CrossRef]
- Guillonneau, B.; Vallancien, G. Laparoscopic radical prostatectomy: The Montsouris technique. J. Urol. 2000, 163, 1643–1649. [Google Scholar] [CrossRef]
- Menon, M.; Shrivastava, A.; Tewari, A.; Sarle, R.; Hemal, A.; Peabody, J.O.; Vallancien, G. Laparoscopic and robot assisted radical prostatectomy: Establishment of a structured program and preliminary analysis of outcomes. J. Urol. 2002, 168, 945–949. [Google Scholar] [CrossRef]
- Galfano, A.; Ascione, A.; Grimaldi, S.; Petralia, G.; Strada, E.; Bocciardi, A.M. A new anatomic approach for robot-assisted laparoscopic prostatectomy: A feasibility study for completely intrafascial surgery. Eur. Urol. 2010, 58, 457–461. [Google Scholar] [CrossRef]
- Wessells, H.; Lue, T.F.; McAninch, J.W. Penile length in the flaccid and erect states: Guidelines for penile augmentation. J. Urol. 1996, 156, 995–997. [Google Scholar] [CrossRef]
- Munding, M.D.; Wessells, H.B.; Dalkin, B.L. Pilot study of changes in stretched penile length 3 months after radical retropubic prostatectomy. Urology 2001, 58, 567–569. [Google Scholar] [CrossRef]
- Savoie, M.; Kim, S.S.; Soloway, M.S. A prospective study measuring penile length in men treated with radical prostatectomy for prostate cancer. J. Urol. 2003, 169, 1462–1464. [Google Scholar] [CrossRef]
- Gontero, P.; Galzerano, M.; Bartoletti, R.; Magnani, C.; Tizzani, A.; Frea, B.; Mondaini, N. New insights into the pathogenesis of penile shortening after radical prostatectomy and the role of postoperative sexual function. J. Urol. 2007, 178, 602–607. [Google Scholar] [CrossRef]
- Vasconcelos, J.S.; Figueiredo, R.T.; Nascimento, F.L.; Damiao, R.; da Silva, E.A. The natural history of penile length after radical prostatectomy: A long-term prospective study. Urology 2012, 80, 1293–1296. [Google Scholar] [CrossRef]
- Engel, J.D.; Sutherland, D.E.; Williams, S.B.; Wagner, K.R. Changes in penile length after robot-assisted laparoscopic radical prostatectomy. J. Endourol. 2011, 25, 65–69. [Google Scholar] [CrossRef] [Green Version]
- Berookhim, B.M.; Nelson, C.J.; Kunzel, B.; Mulhall, J.P.; Narus, J.B. Prospective analysis of penile length changes after radical prostatectomy. BJU Int. 2014, 113, E131–E136. [Google Scholar] [CrossRef] [PubMed]
- Kadono, Y.; Machioka, K.; Nakashima, K.; Iijima, M.; Shigehara, K.; Nohara, T.; Narimoto, K.; Izumi, K.; Kitagawa, Y.; Konaka, H.; et al. Changes in penile length after radical prostatectomy: Investigation of the underlying anatomical mechanism. BJU Int. 2017, 120, 293–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mulhall, J. Can penile size be preserved after radical prostatectomy? Eur. Urol. 2007, 52, 626–628. [Google Scholar] [CrossRef]
- Aydogdu, O.; Gokce, M.I.; Burgu, B.; Baltaci, S.; Yaman, O. Tadalafil rehabilitation therapy preserves penile size after bilateral nerve sparing radical retropubic prostatectomy. Int. Braz. J. Urol. 2011, 37, 336–344. [Google Scholar] [CrossRef] [Green Version]
- Montorsi, F.; Brock, G.; Stolzenburg, J.U.; Mulhall, J.; Moncada, I.; Patel, H.R.; Chevallier, D.; Krajka, K.; Henneges, C.; Dickson, R.; et al. Effects of tadalafil treatment on erectile function recovery following bilateral nerve-sparing radical prostatectomy: A randomised placebo-controlled study (REACTT). Eur. Urol. 2014, 65, 587–596. [Google Scholar] [CrossRef]
- Brock, G.; Montorsi, F.; Costa, P.; Shah, N.; Martinez-Jabaloyas, J.M.; Hammerer, P.; Ludovico, G.M.; Lee, J.C.; Henneges, C.; Hamidi, K.; et al. Effect of Tadalafil Once Daily on Penile Length Loss and Morning Erections in Patients After Bilateral Nerve-sparing Radical Prostatectomy: Results From a Randomized Controlled Trial. Urology 2015, 85, 1090–1096. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, S.; Nilsson, A.E.; Johansson, E.; Nyberg, T.; Akre, O.; Steineck, G. Self-perceived penile shortening after radical prostatectomy. Int. J. Impot. Res. 2012, 24, 179–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mulhall, J.P. Penile length changes after radical prostatectomy. BJU Int. 2005, 96, 472–474. [Google Scholar] [CrossRef]
- Park, K.K.; Lee, S.H.; Chung, B.H. The effects of long-term androgen deprivation therapy on penile length in patients with prostate cancer: A single-center, prospective, open-label, observational study. J. Sex. Med. 2011, 8, 3214–3219. [Google Scholar] [CrossRef]
- Haliloglu, A.; Baltaci, S.; Yaman, O. Penile length changes in men treated with androgen suppression plus radiation therapy for local or locally advanced prostate cancer. J. Urol. 2007, 177, 128–130. [Google Scholar] [CrossRef] [PubMed]
- Kadono, Y.; Nohara, T.; Kawaguchi, S.; Sakamoto, J.; Makino, T.; Nakashima, K.; Iijima, M.; Shigehara, K.; Izumi, K.; Mizokami, A. Changes in penile length after radical prostatectomy: Effect of neoadjuvant androgen deprivation therapy. Andrology 2018, 6, 903–908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kohler, T.S.; Pedro, R.; Hendlin, K.; Utz, W.; Ugarte, R.; Reddy, P.; Makhlouf, A.; Ryndin, I.; Canales, B.K.; Weiland, D.; et al. A pilot study on the early use of the vacuum erection device after radical retropubic prostatectomy. BJU Int. 2007, 100, 858–862. [Google Scholar] [CrossRef]
- Anatomy and physiology of erection: Pathophysiology of erectile dysfunction. Int. J. Impot. Res. 2003, 15 (Suppl. 7), S5–S8. [CrossRef] [Green Version]
- Tewari, A.; Peabody, J.O.; Fischer, M.; Sarle, R.; Vallancien, G.; Delmas, V.; Hassan, M.; Bansal, A.; Hemal, A.K.; Guillonneau, B.; et al. An operative and anatomic study to help in nerve sparing during laparoscopic and robotic radical prostatectomy. Eur. Urol. 2003, 43, 444–454. [Google Scholar] [CrossRef]
- Panebianco, V.; Barchetti, F.; Sciarra, A.; Marcantonio, A.; Zini, C.; Salciccia, S.; Collettini, F.; Gentile, V.; Hamm, B.; Catalano, C. In vivo 3D neuroanatomical evaluation of periprostatic nerve plexus with 3T-MR Diffusion Tensor Imaging. Eur. J. Radiol. 2013, 82, 1677–1682. [Google Scholar] [CrossRef]
- Sievert, K.D.; Hennenlotter, J.; Dillenburg, T.; Toomey, P.; Wollner, J.; Zweers, P.; Pannek, J.; Andersson, K.E.; Amend, B. Extended periprostatic nerve distributions on the prostate surface confirmed using diffusion tensor imaging. BJU Int. 2019, 123, 995–1004. [Google Scholar] [CrossRef]
- Walsh, P.C.; Donker, P.J. Impotence following radical prostatectomy: Insight into etiology and prevention. J. Urol. 1982, 128, 492–497. [Google Scholar] [CrossRef]
- Ficarra, V.; Novara, G.; Ahlering, T.E.; Costello, A.; Eastham, J.A.; Graefen, M.; Guazzoni, G.; Menon, M.; Mottrie, A.; Patel, V.R.; et al. Systematic review and meta-analysis of studies reporting potency rates after robot-assisted radical prostatectomy. Eur. Urol. 2012, 62, 418–430. [Google Scholar] [CrossRef]
- Moran, P.S.; O’Neill, M.; Teljeur, C.; Flattery, M.; Murphy, L.A.; Smyth, G.; Ryan, M. Robot-assisted radical prostatectomy compared with open and laparoscopic approaches: A systematic review and meta-analysis. Int. J. Urol. 2013, 20, 312–321. [Google Scholar] [CrossRef] [Green Version]
- Du, Y.; Long, Q.; Guan, B.; Mu, L.; Tian, J.; Jiang, Y.; Bai, X.; Wu, D. Robot-Assisted Radical Prostatectomy Is More Beneficial for Prostate Cancer Patients: A System Review and Meta-Analysis. Med. Sci. Monit. 2018, 24, 272–287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walz, J.; Burnett, A.L.; Costello, A.J.; Eastham, J.A.; Graefen, M.; Guillonneau, B.; Menon, M.; Montorsi, F.; Myers, R.P.; Rocco, B.; et al. A critical analysis of the current knowledge of surgical anatomy related to optimization of cancer control and preservation of continence and erection in candidates for radical prostatectomy. Eur. Urol. 2010, 57, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Stolzenburg, J.U.; Kallidonis, P.; Do, M.; Dietel, A.; Hafner, T.; Rabenalt, R.; Sakellaropoulos, G.; Ganzer, R.; Paasch, U.; Horn, L.C.; et al. A comparison of outcomes for interfascial and intrafascial nerve-sparing radical prostatectomy. Urology 2010, 76, 743–748. [Google Scholar] [CrossRef]
- Tewari, A.K.; Srivastava, A.; Huang, M.W.; Robinson, B.D.; Shevchuk, M.M.; Durand, M.; Sooriakumaran, P.; Grover, S.; Yadav, R.; Mishra, N.; et al. Anatomical grades of nerve sparing: A risk-stratified approach to neural-hammock sparing during robot-assisted radical prostatectomy (RARP). BJU Int. 2011, 108, 984–992. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.R.; Schatloff, O.; Chauhan, S.; Sivaraman, A.; Valero, R.; Coelho, R.F.; Rocco, B.; Palmer, K.J.; Kameh, D. The role of the prostatic vasculature as a landmark for nerve sparing during robot-assisted radical prostatectomy. Eur. Urol. 2012, 61, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Menon, M.; Shrivastava, A.; Kaul, S.; Badani, K.K.; Fumo, M.; Bhandari, M.; Peabody, J.O. Vattikuti Institute prostatectomy: Contemporary technique and analysis of results. Eur. Urol. 2007, 51, 648–657. [Google Scholar] [CrossRef]
- Umari, P.; Eden, C.; Cahill, D.; Rizzo, M.; Eden, D.; Sooriakumaran, P. Retzius-Sparing versus Standard Robot-Assisted Radical Prostatectomy: A Comparative Prospective Study of Nearly 500 Patients. J. Urol. 2021, 205, 780–790. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, J.; Yang, Z.; Liu, Q.; Zhang, W.; Qing, Z.; Wang, D. Comparison of Retzius-sparing and conventional robot-assisted laparoscopic radical prostatectomy regarding continence and sexual function: An updated meta-analysis. Prostate Cancer Prostatic Dis. 2021, 25, 47–54. [Google Scholar] [CrossRef]
- Xu, J.N.; Xu, Z.Y.; Yin, H.M. Comparison of Retzius-Sparing Robot-Assisted Radical Prostatectomy vs. Conventional Robot-Assisted Radical Prostatectomy: An Up-to-Date Meta-Analysis. Front. Surg. 2021, 8, 738421. [Google Scholar] [CrossRef]
- Barakat, B.; Othman, H.; Gauger, U.; Wolff, I.; Hadaschik, B.; Rehme, C. Retzius Sparing Radical Prostatectomy Versus Robot-assisted Radical Prostatectomy: Which Technique Is More Beneficial for Prostate Cancer Patients (MASTER Study)? A Systematic Review and Meta-analysis. Eur. Urol. Focus 2021, S2405–S4569. [Google Scholar] [CrossRef]
- Secin, F.P.; Karanikolas, N.; Touijer, A.K.; Salamanca, J.I.; Vickers, A.J.; Guillonneau, B. Anatomy of accessory pudendal arteries in laparoscopic radical prostatectomy. J. Urol. 2005, 174, 523–526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rogers, C.G.; Trock, B.P.; Walsh, P.C. Preservation of accessory pudendal arteries during radical retropubic prostatectomy: Surgical technique and results. Urology 2004, 64, 148–151. [Google Scholar] [CrossRef] [PubMed]
- Henry, B.M.; Pekala, P.A.; Vikse, J.; Sanna, B.; Skinningsrud, B.; Saganiak, K.; Walocha, J.A.; Tomaszewski, K.A. Variations in the Arterial Blood Supply to the Penis and the Accessory Pudendal Artery: A Meta-Analysis and Review of Implications in Radical Prostatectomy. J. Urol. 2017, 198, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Koyanagi, T. Studies on the sphincteric system located distally in the urethra: The external urethral sphincter revisited. J. Urol. 1980, 124, 400–406. [Google Scholar] [CrossRef]
- Koraitim, M.M. The male urethral sphincter complex revisited: An anatomical concept and its physiological correlate. J. Urol. 2008, 179, 1683–1689. [Google Scholar] [CrossRef]
- Rocco, F.; Carmignani, L.; Acquati, P.; Gadda, F.; Dell’Orto, P.; Rocco, B.; Casellato, S.; Gazzano, G.; Consonni, D. Early continence recovery after open radical prostatectomy with restoration of the posterior aspect of the rhabdosphincter. Eur. Urol. 2007, 52, 376–383. [Google Scholar] [CrossRef]
- Schlomm, T.; Heinzer, H.; Steuber, T.; Salomon, G.; Engel, O.; Michl, U.; Haese, A.; Graefen, M.; Huland, H. Full functional-length urethral sphincter preservation during radical prostatectomy. Eur. Urol. 2011, 60, 320–329. [Google Scholar] [CrossRef]
- Steiner, M.S. The puboprostatic ligament and the male urethral suspensory mechanism: An anatomic study. Urology 1994, 44, 530–534. [Google Scholar] [CrossRef]
- Richardson, A.C. The rectovaginal septum revisited: Its relationship to rectocele and its importance in rectocele repair. Clin. Obstet. Gynecol. 1993, 36, 976–983. [Google Scholar] [CrossRef]
- Zhang, C.; Ding, Z.H.; Li, G.X.; Yu, J.; Wang, Y.N.; Hu, Y.F. Perirectal fascia and spaces: Annular distribution pattern around the mesorectum. Dis. Colon Rectum 2010, 53, 1315–1322. [Google Scholar] [CrossRef]
- Gosling, J.A.; Dixon, J.S.; Critchley, H.O.; Thompson, S.A. A comparative study of the human external sphincter and periurethral levator ani muscles. Br. J. Urol. 1981, 53, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Machioka, K.; Kadono, Y.; Naito, R.; Nakashima, K.; Iijima, M.; Kawaguchi, S.; Shigehara, K.; Nohara, T.; Izumi, K.; Mizokami, A. Evaluating urinary incontinence before and after radical prostatectomy using the international consultation on incontinence questionnaire-short form. Neurourol. Urodyn. 2019, 38, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Dubbelman, Y.D.; Groen, J.; Wildhagen, M.F.; Rikken, B.; Bosch, J.L. Urodynamic quantification of decrease in sphincter function after radical prostatectomy: Relation to postoperative continence status and the effect of intensive pelvic floor muscle exercises. Neurourol. Urodyn. 2012, 31, 646–651. [Google Scholar] [CrossRef] [PubMed]
- Hammerer, P.; Huland, H. Urodynamic evaluation of changes in urinary control after radical retropubic prostatectomy. J. Urol. 1997, 157, 233–236. [Google Scholar] [CrossRef]
- Song, C.; Lee, J.; Hong, J.H.; Choo, M.S.; Kim, C.S.; Ahn, H. Urodynamic interpretation of changing bladder function and voiding pattern after radical prostatectomy: A long-term follow-up. BJU Int. 2010, 106, 681–686. [Google Scholar] [CrossRef]
- Porena, M.; Mearini, E.; Mearini, L.; Vianello, A.; Giannantoni, A. Voiding dysfunction after radical retropubic prostatectomy: More than external urethral sphincter deficiency. Eur. Urol. 2007, 52, 38–45. [Google Scholar] [CrossRef]
- Kadono, Y.; Ueno, S.; Yaegashi, H.; Ofude, M.; Izumi, K.; Maeda, Y.; Mizokami, A.; Miwa, S.; Miyagi, T.; Namiki, M. Urodynamic evaluation before and immediately after robot-assisted radical prostatectomy. Urology 2014, 84, 106–111. [Google Scholar] [CrossRef] [Green Version]
- Kadono, Y.; Ueno, S.; Iwamoto, D.; Takezawa, Y.; Nohara, T.; Izumi, K.; Mizokami, A.; Namiki, M. Chronological Urodynamic Evaluation of Changing Bladder and Urethral Functions After Robot-assisted Radical Prostatectomy. Urology 2015, 85, 1441–1447. [Google Scholar] [CrossRef] [Green Version]
- Kadono, Y.; Ueno, S.; Kadomoto, S.; Iwamoto, H.; Takezawa, Y.; Nakashima, K.; Nohara, T.; Izumi, K.; Mizokami, A.; Gabata, T.; et al. Use of preoperative factors including urodynamic evaluations and nerve-sparing status for predicting urinary continence recovery after robot-assisted radical prostatectomy: Nerve-sparing technique contributes to the reduction of postprostatectomy incontinence. Neurourol. Urodyn. 2016, 35, 1034–1039. [Google Scholar]
- Kadono, Y.; Nohara, T.; Kawaguchi, S.; Naito, R.; Urata, S.; Nakashima, K.; Iijima, M.; Shigehara, K.; Izumi, K.; Gabata, T.; et al. Investigating the mechanism underlying urinary continence recovery after radical prostatectomy: Effectiveness of a longer urethral stump to prevent urinary incontinence. BJU Int. 2018, 122, 456–462. [Google Scholar] [CrossRef] [Green Version]
- Stafford, R.E.; Ashton-Miller, J.A.; Constantinou, C.E.; Hodges, P.W. Novel insight into the dynamics of male pelvic floor contractions through transperineal ultrasound imaging. J. Urol. 2012, 188, 1224–1230. [Google Scholar] [CrossRef] [Green Version]
- Kumagai, S.; Muraki, O.; Yoshimura, Y. Evaluation of the effect of levator ani muscle contraction on post-prostatectomy urinary incontinence using cine MRI. Neurourol. Urodyn. 2022, 41, 616–625. [Google Scholar] [CrossRef] [PubMed]
- Stafford, R.E.; Coughlin, G.; Hodges, P.W. Comparison of dynamic features of pelvic floor muscle contraction between men with and without incontinence after prostatectomy and men with no history of prostate cancer. Neurourol. Urodyn. 2020, 39, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Stafford, R.E.; van den Hoorn, W.; Coughlin, G.; Hodges, P.W. Postprostatectomy incontinence is related to pelvic floor displacements observed with trans-perineal ultrasound imaging. Neurourol. Urodyn. 2018, 37, 658–665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mungovan, S.F.; Sandhu, J.S.; Akin, O.; Smart, N.A.; Graham, P.L.; Patel, M.I. Preoperative Membranous Urethral Length Measurement and Continence Recovery Following Radical Prostatectomy: A Systematic Review and Meta-analysis. Eur. Urol. 2017, 71, 368–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hakimi, A.A.; Faleck, D.M.; Agalliu, I.; Rozenblit, A.M.; Chernyak, V.; Ghavamian, R. Preoperative and intraoperative measurements of urethral length as predictors of continence after robot-assisted radical prostatectomy. J. Endourol. 2011, 25, 1025–1030. [Google Scholar] [CrossRef]
- Ko, Y.H.; Coelho, R.F.; Chauhan, S.; Sivaraman, A.; Schatloff, O.; Cheon, J.; Patel, V.R. Factors affecting return of continence 3 months after robot-assisted radical prostatectomy: Analysis from a large, prospective data by a single surgeon. J. Urol. 2012, 187, 190–194. [Google Scholar] [CrossRef]
- Suardi, N.; Moschini, M.; Gallina, A.; Gandaglia, G.; Abdollah, F.; Capitanio, U.; Bianchi, M.; Tutolo, M.; Passoni, N.; Salonia, A.; et al. Nerve-sparing approach during radical prostatectomy is strongly associated with the rate of postoperative urinary continence recovery. BJU Int. 2013, 111, 717–722. [Google Scholar] [CrossRef]
- Reeves, F.; Preece, P.; Kapoor, J.; Everaerts, W.; Murphy, D.G.; Corcoran, N.M.; Costello, A.J. Preservation of the neurovascular bundles is associated with improved time to continence after radical prostatectomy but not long-term continence rates: Results of a systematic review and meta-analysis. Eur. Urol. 2015, 68, 692–704. [Google Scholar] [CrossRef]
- Srivastava, A.; Chopra, S.; Pham, A.; Sooriakumaran, P.; Durand, M.; Chughtai, B.; Gruschow, S.; Peyser, A.; Harneja, N.; Leung, R.; et al. Effect of a risk-stratified grade of nerve-sparing technique on early return of continence after robot-assisted laparoscopic radical prostatectomy. Eur. Urol. 2013, 63, 438–444. [Google Scholar] [CrossRef]
- Sayyid, R.K.; Simpson, W.G.; Lu, C.; Terris, M.K.; Klaassen, Z.; Madi, R. Retzius-Sparing Robotic-Assisted Laparoscopic Radical Prostatectomy: A Safe Surgical Technique with Superior Continence Outcomes. J. Endourol. 2017, 31, 1244–1250. [Google Scholar] [CrossRef] [PubMed]
- Menon, M.; Dalela, D.; Jamil, M.; Diaz, M.; Tallman, C.; Abdollah, F.; Sood, A.; Lehtola, L.; Miller, D.; Jeong, W. Functional Recovery, Oncologic Outcomes and Postoperative Complications after Robot-Assisted Radical Prostatectomy: An Evidence-Based Analysis Comparing the Retzius Sparing and Standard Approaches. J. Urol. 2018, 199, 1210–1217. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, H.Y.; Goh, H.J.; Heo, J.E.; Almujalhem, A.; Alqahtani, A.A.; Chung, D.Y.; Chang, K.; Choi, Y.D.; Rha, K.H. Retzius Sparing Robot-Assisted Radical Prostatectomy Conveys Early Regain of Continence over Conventional Robot-Assisted Radical Prostatectomy: A Propensity Score Matched Analysis of 1863 Patients. J. Urol. 2020, 203, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Kadono, Y.; Nohara, T.; Kawaguchi, S.; Kadomoto, S.; Iwamoto, H.; Iijima, M.; Shigehara, K.; Izumi, K.; Yoshida, K.; Gabata, T.; et al. Investigating the mechanism underlying urinary continence using dynamic MRI after Retzius-sparing robot-assisted radical prostatectomy. Sci. Rep. 2022, 12, 3975. [Google Scholar] [CrossRef] [PubMed]
- Jafri, S.M.; Nguyen, L.N.; Sirls, L.T. Recovery of urinary function after robotic-assisted laparoscopic prostatectomy versus radical perineal prostatectomy for early-stage prostate cancer. Int. Urol. Nephrol. 2018, 50, 2187–2191. [Google Scholar] [CrossRef]
- Burnett, A.L.; Mostwin, J.L. In situ anatomical study of the male urethral sphincteric complex: Relevance to continence preservation following major pelvic surgery. J. Urol. 1998, 160, 1301–1306. [Google Scholar] [CrossRef]
- Rocco, F.; Carmignani, L.; Acquati, P.; Gadda, F.; Dell’Orto, P.; Rocco, B.; Bozzini, G.; Gazzano, G.; Morabito, A. Restoration of posterior aspect of rhabdosphincter shortens continence time after radical retropubic prostatectomy. J. Urol. 2006, 175, 2201–2206. [Google Scholar] [CrossRef]
- Tewari, A.K.; Bigelow, K.; Rao, S.; Takenaka, A.; El-Tabi, N.; Te, A.; Vaughan, E.D. Anatomic restoration technique of continence mechanism and preservation of puboprostatic collar: A novel modification to achieve early urinary continence in men undergoing robotic prostatectomy. Urology 2007, 69, 726–731. [Google Scholar] [CrossRef]
- Joshi, N.; de Blok, W.; van Muilekom, E.; van der Poel, H. Impact of posterior musculofascial reconstruction on early continence after robot-assisted laparoscopic radical prostatectomy: Results of a prospective parallel group trial. Eur. Urol. 2010, 58, 84–89. [Google Scholar] [CrossRef]
- Sutherland, D.E.; Linder, B.; Guzman, A.M.; Hong, M.; Frazier, H.A., 2nd; Engel, J.D.; Bianco, F.J., Jr. Posterior rhabdosphincter reconstruction during robotic assisted radical prostatectomy: Results from a phase II randomized clinical trial. J. Urol. 2011, 185, 1262–1267. [Google Scholar] [CrossRef]
- Jeong, C.W.; Lee, J.K.; Oh, J.J.; Lee, S.; Jeong, S.J.; Hong, S.K.; Byun, S.S.; Lee, S.E. Effects of new 1-step posterior reconstruction method on recovery of continence after robot-assisted laparoscopic prostatectomy: Results of a prospective, single-blind, parallel group, randomized, controlled trial. J. Urol. 2015, 193, 935–942. [Google Scholar] [CrossRef] [PubMed]
- Stolzenburg, J.U.; Nicolaus, M.; Kallidonis, P.; Do, M.; Dietel, A.; Hafner, T.; Sakellaropoulos, G.; Hicks, J.; Nikoleishvili, D.; Liatsikos, E. Influence of bladder neck suspension stitches on early continence after radical prostatectomy: A prospective randomized study of 180 patients. Asian J. Androl. 2011, 13, 806–811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hurtes, X.; Roupret, M.; Vaessen, C.; Pereira, H.; Faivre d’Arcier, B.; Cormier, L.; Bruyere, F. Anterior suspension combined with posterior reconstruction during robot-assisted laparoscopic prostatectomy improves early return of urinary continence: A prospective randomized multicentre trial. BJU Int. 2012, 110, 875–883. [Google Scholar] [CrossRef] [PubMed]
- Menon, M.; Muhletaler, F.; Campos, M.; Peabody, J.O. Assessment of early continence after reconstruction of the periprostatic tissues in patients undergoing computer assisted (robotic) prostatectomy: Results of a 2 group parallel randomized controlled trial. J. Urol. 2008, 180, 1018–1023. [Google Scholar] [CrossRef]
- Student, V., Jr.; Vidlar, A.; Grepl, M.; Hartmann, I.; Buresova, E.; Student, V. Advanced Reconstruction of Vesicourethral Support (ARVUS) during Robot-assisted Radical Prostatectomy: One-year Functional Outcomes in a Two-group Randomised Controlled Trial. Eur. Urol. 2017, 71, 822–830. [Google Scholar] [CrossRef] [Green Version]
- Alder, R.; Zetner, D.; Rosenberg, J. Incidence of Inguinal Hernia after Radical Prostatectomy: A Systematic Review and Meta-Analysis. J. Urol. 2020, 203, 265–274. [Google Scholar] [CrossRef] [Green Version]
- Lodding, P.; Bergdahl, C.; Nyberg, M.; Pileblad, E.; Stranne, J.; Hugosson, J. Inguinal hernia after radical retropubic prostatectomy for prostate cancer: A study of incidence and risk factors in comparison to no operation and lymphadenectomy. J. Urol. 2001, 166, 964–967. [Google Scholar] [CrossRef]
- Ichioka, K.; Kohei, N.; Yoshimura, K.; Arai, Y.; Terai, A. Impact of retraction of vas deferens in postradical prostatectomy inguinal hernia. Urology 2007, 70, 511–514. [Google Scholar] [CrossRef]
- Kadono, Y.; Nohara, T.; Kawaguchi, S.; Sakamoto, J.; Iwamoto, H.; Yaegashi, H.; Nakashima, K.; Iijima, M.; Shigehara, K.; Izumi, K.; et al. Novel Prevention Procedure for Inguinal Hernia after Robot-Assisted Radical Prostatectomy: Results from a Prospective Randomized Trial. J. Endourol. 2019, 33, 302–308. [Google Scholar] [CrossRef]
- Matsubara, A.; Yoneda, T.; Nakamoto, T.; Maruyama, S.; Koda, S.; Goto, K.; Teishima, J.; Shiina, H.; Igawa, M.; Usui, T. Inguinal hernia after radical perineal prostatectomy: Comparison with the retropubic approach. Urology 2007, 70, 1152–1156. [Google Scholar] [CrossRef]
- Chang, K.D.; Abdel Raheem, A.; Santok, G.D.R.; Kim, L.H.C.; Lum, T.G.H.; Lee, S.H.; Ham, W.S.; Choi, Y.D.; Rha, K.H. Anatomical Retzius-space preservation is associated with lower incidence of postoperative inguinal hernia development after robot-assisted radical prostatectomy. Hernia J. Hernias Abdom. Wall Surg. 2017, 21, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Stranne, J.; Lodding, P. Inguinal hernia after radical retropubic prostatectomy: Risk factors and prevention. Nat. Rev. Urol. 2011, 8, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Ortiz, R.F.; Andrade-Geigel, C.; Lopez-Huertas, H.; Cadillo-Chavez, R.; Soto-Aviles, O. Preoperative International Prostate Symptom Score Predictive of Inguinal Hernia in Patients Undergoing Robotic Prostatectomy. J. Urol. 2016, 195, 1744–1747. [Google Scholar] [CrossRef]
- Fujii, Y.; Yamamoto, S.; Yonese, J.; Masuda, H.; Urakami, S.; Kitsukawa, S.; Sakura, M.; Yuasa, T.; Kihara, K.; Fukui, I. The processus vaginalis transection method to prevent postradical prostatectomy inguinal hernia: Long-term results. Urology 2014, 83, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Stranne, J.; Aus, G.; Bergdahl, S.; Damber, J.E.; Hugosson, J.; Khatami, A.; Lodding, P. Post-radical prostatectomy inguinal hernia: A simple surgical intervention can substantially reduce the incidence--results from a prospective randomized trial. J. Urol. 2010, 184, 984–989. [Google Scholar] [CrossRef]
- Kurokawa, S.; Umemoto, Y.; Mizuno, K.; Okada, A.; Nakane, A.; Nishio, H.; Hamamoto, S.; Ando, R.; Kawai, N.; Tozawa, K.; et al. New steps of robot-assisted radical prostatectomy using the extraperitoneal approach: A propensity-score matched comparison between extraperitoneal and transperitoneal approach in Japanese patients. BMC Urol. 2017, 17, 106. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.H.; Koo, K.C.; Lee, S.H.; Chung, B.H. A simple procedure to prevent postoperative inguinal hernia after robot-assisted laparoscopic radical prostatectomy: A plugging method of the internal inguinal floor for patients with patent processus vaginalis. J. Urol. 2014, 191, 468–472. [Google Scholar] [CrossRef]
- Shimbo, M.; Endo, F.; Matsushita, K.; Iwabuchi, T.; Fujisaki, A.; Kyono, Y.; Hishiki, K.; Muraishi, O.; Hattori, K. Incidence, Risk Factors and a Novel Prevention Technique for Inguinal Hernia after Robot-Assisted Radical Prostatectomy. Urol. Int. 2017, 98, 54–60. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kadono, Y.; Nohara, T.; Kawaguchi, S.; Iwamoto, H.; Yaegashi, H.; Shigehara, K.; Izumi, K.; Mizokami, A. Impact of Pelvic Anatomical Changes Caused by Radical Prostatectomy. Cancers 2022, 14, 3050. https://doi.org/10.3390/cancers14133050
Kadono Y, Nohara T, Kawaguchi S, Iwamoto H, Yaegashi H, Shigehara K, Izumi K, Mizokami A. Impact of Pelvic Anatomical Changes Caused by Radical Prostatectomy. Cancers. 2022; 14(13):3050. https://doi.org/10.3390/cancers14133050
Chicago/Turabian StyleKadono, Yoshifumi, Takahiro Nohara, Shohei Kawaguchi, Hiroaki Iwamoto, Hiroshi Yaegashi, Kazuyoshi Shigehara, Kouji Izumi, and Atsushi Mizokami. 2022. "Impact of Pelvic Anatomical Changes Caused by Radical Prostatectomy" Cancers 14, no. 13: 3050. https://doi.org/10.3390/cancers14133050
APA StyleKadono, Y., Nohara, T., Kawaguchi, S., Iwamoto, H., Yaegashi, H., Shigehara, K., Izumi, K., & Mizokami, A. (2022). Impact of Pelvic Anatomical Changes Caused by Radical Prostatectomy. Cancers, 14(13), 3050. https://doi.org/10.3390/cancers14133050





