Intra-Individual Comparison of 124I-PET/CT and 124I-PET/MR Hybrid Imaging of Patients with Resected Differentiated Thyroid Carcinoma: Aspects of Attenuation Correction
Abstract
:Simple Summary
Abstract
1. Introduction
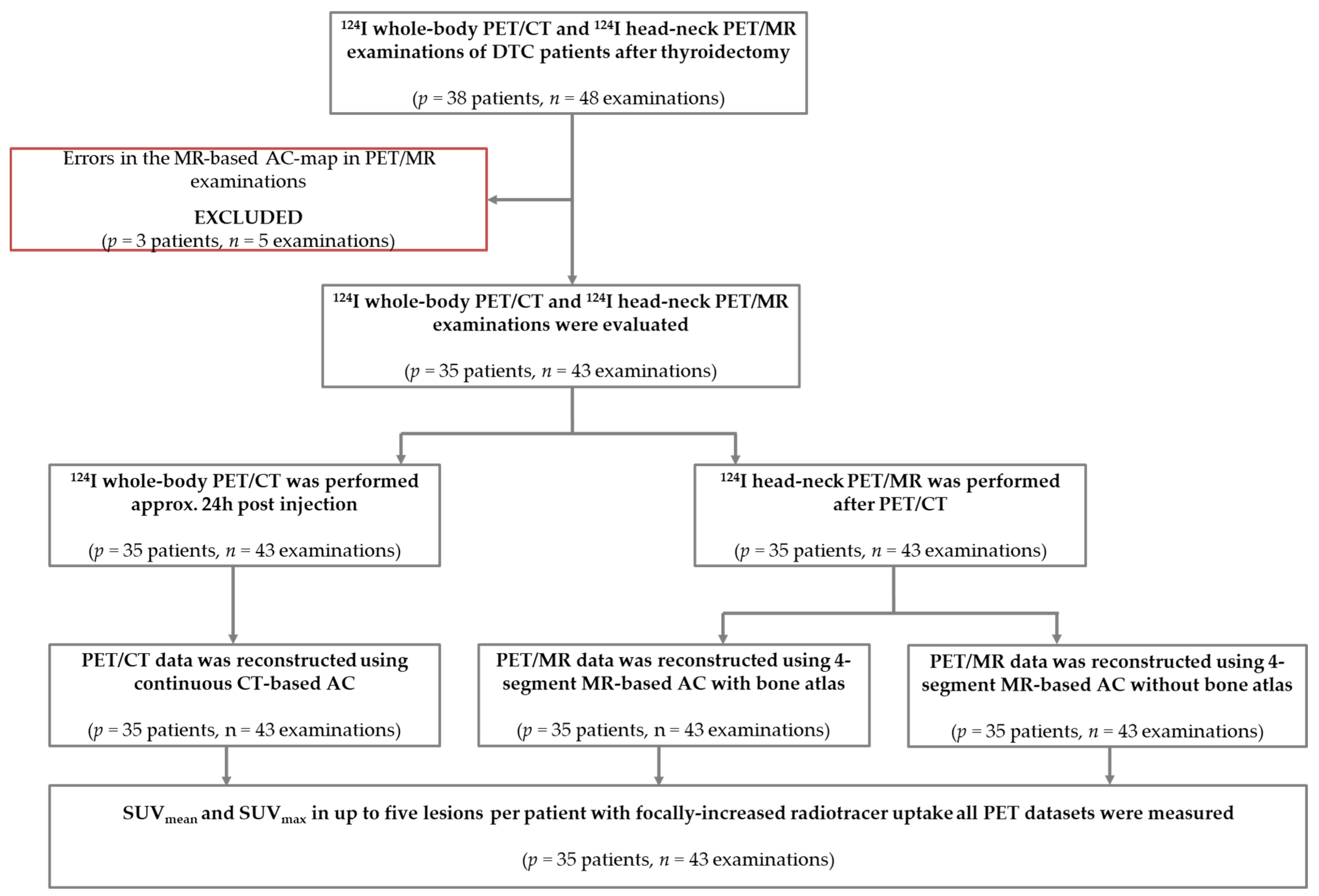
2. Materials and Methods
2.1. Patient Population
2.2. Hybrid Image Acquisition
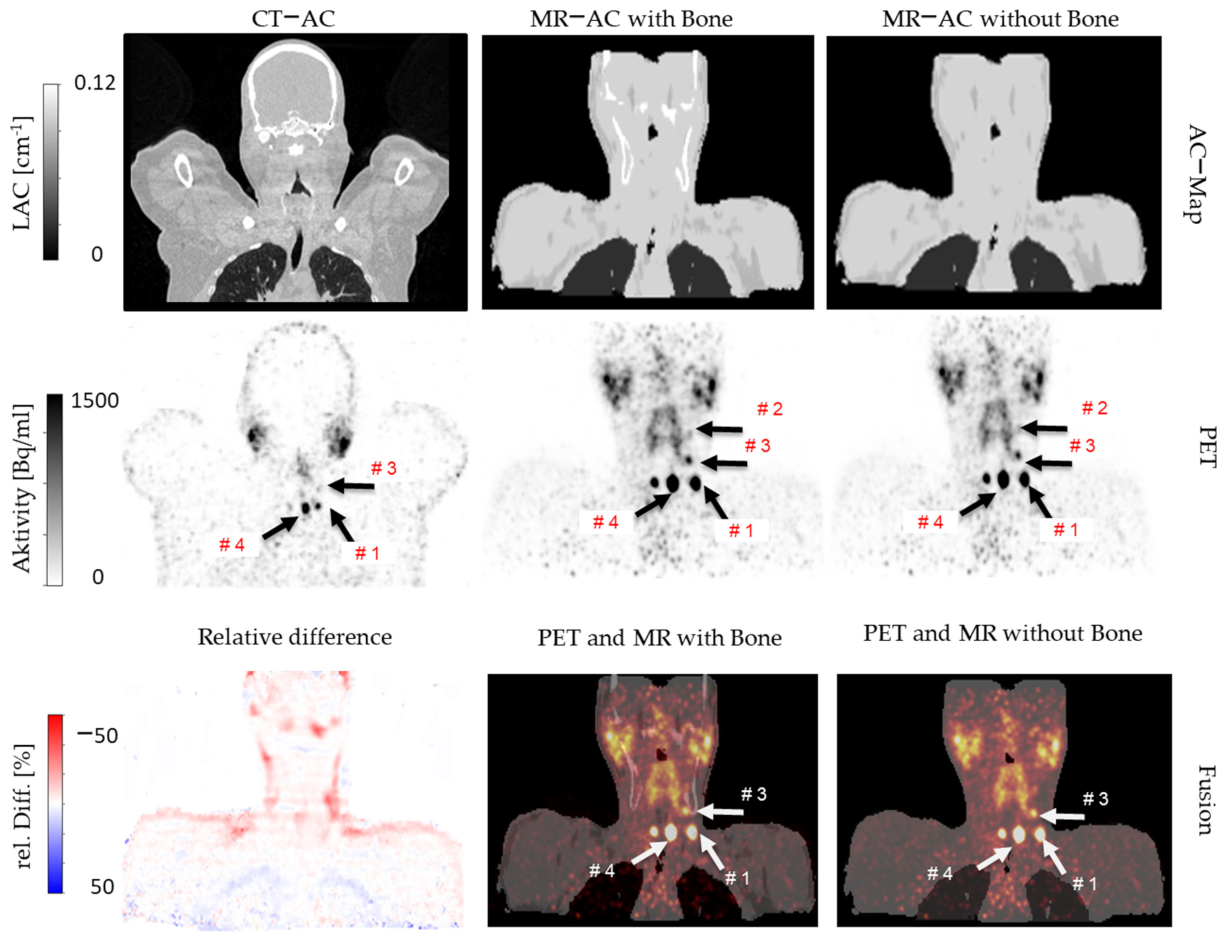
2.3. Attenuation Correction
2.4. Image Reconstruction and Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Beyer, T.; Townsend, D.W.; Brun, T.; Kinahan, P.; Charron, M.; Roddy, R.; Jerin, J.; Young, J.; Byars, L.; Nutt, R. A combined PET/CT scanner for clinical oncology. J. Nucl. Med. 2000, 41, 1369–1379. [Google Scholar] [PubMed]
- Pichler, B.J.; Kolb, A.; Nägele, T.; Schlemmer, H.-P. PET/MRI: Paving the Way for the Next Generation of Clinical Multimodality Imaging Applications. J. Nucl. Med. 2010, 51, 333–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delso, G.; Fürst, S.; Jakoby, B.; Ladebeck, R.; Ganter, C.; Nekolla, S.G.; Schwaiger, M.; Ziegler, S.I. Performance Measurements of the Siemens mMR Integrated Whole-Body PET/MR Scanner. J. Nucl. Med. 2011, 52, 1914–1922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quick, H.H. Integrated PET/MR. J. Magn. Reson. Imaging 2013, 39, 243–258. [Google Scholar] [CrossRef] [PubMed]
- Quick, H.H.; von Gall, C.; Zeilinger, M.; Wiesmüller, M.; Braun, H.; Ziegler, S.; Kuwert, T.; Uder, M.; Dörfler, A.; Kalender, W.A.; et al. Integrated Whole-Body PET/MR Hybrid Imaging: Clinical experience. Investig. Radiol. 2013, 48, 280–289. [Google Scholar] [CrossRef]
- Jentzen, W.; Hoppenbrouwers, J.; van Leeuwen, P.; van der Velden, D.; van de Kolk, R.; Poeppel, T.D.; Nagarajah, J.; Brandau, W.; Bockisch, A.; Rosenbaum-Krumme, S. Assessment of Lesion Response in the Initial Radioiodine Treatment of Differentiated Thyroid Cancer Using 124I PET Imaging. J. Nucl. Med. 2014, 55, 1759–1765. [Google Scholar] [CrossRef] [Green Version]
- Jentzen, W.; Freudenberg, L.; Eising, E.G.; Sonnenschein, W.; Knust, J.; Bockisch, A. Optimized 124I PET Dosimetry Protocol for Radioiodine Therapy of Differentiated Thyroid Cancer. J. Nucl. Med. 2008, 49, 1017–1023. [Google Scholar] [CrossRef] [Green Version]
- Freudenberg, L.S.; Jentzen, W.; Stahl, A.; Bockisch, A.; Rosenbaum-Krumme, S.J. Clinical applications of 124I-PET/CT in patients with differentiated thyroid cancer. Eur. J. Nucl. Med. Mol. Imaging Pediatr. 2011, 38, 48–56. [Google Scholar] [CrossRef]
- Nagarajah, J.; Jentzen, W.; Hartung, V.; Rosenbaum-Krumme, S.; Mikat, C.; Heusner, T.A.; Antoch, G.; Bockisch, A.; Stahl, A. Diagnosis and dosimetry in differentiated thyroid carcinoma using 124I PET: Comparison of PET/MRI vs PET/CT of the neck. Eur. J. Nucl. Med. Mol. Imaging Pediatr. 2011, 38, 1862–1868. [Google Scholar] [CrossRef]
- Song, Y.; Liu, F.; Ruan, W.; Hu, F.; Younis, M.; Gao, Z.; Ming, J.; Huang, T.; Cai, W.; Lan, X. Head-to-Head Comparison of Neck 18F-FDG PET/MR and PET/CT in the Diagnosis of Differentiated Thyroid Carcinoma Patients after Comprehensive Treatment. Cancers 2021, 13, 3436. [Google Scholar] [CrossRef]
- Binse, I.; Poeppel, T.D.; Rühlmann, M.; Gomez, B.; Umutlu, L.; Bockisch, A.; Rosenbaum-Krumme, S.J. Imaging with 124I in differentiated thyroid carcinoma: Is PET/MRI superior to PET/CT? Eur. J. Nucl. Med. Mol. Imaging Pediatr. 2015, 43, 1011–1017. [Google Scholar] [CrossRef] [PubMed]
- Carney, J.P.J.; Townsend, D.W.; Rappoport, V.; Bendriem, B. Method for transforming CT images for attenuation correction in PET/CT imaging. Med. Phys. 2006, 33, 976–983. [Google Scholar] [CrossRef] [PubMed]
- Kinahan, P.; Hasegawa, B.H.; Beyer, T. X-ray-based attenuation correction for positron emission tomography/computed tomography scanners. Semin. Nucl. Med. 2003, 33, 166–179. [Google Scholar] [CrossRef] [PubMed]
- Beyer, T.; Lassen, M.L.; Boellaard, R.; Delso, G.; Yaqub, M.; Sattler, B.; Quick, H.H. Investigating the state-of-the-art in whole-body MR-based attenuation correction: An intra-individual, inter-system, inventory study on three clinical PET/MR systems. Magn. Reson. Mater. Phys. Biol. Med. 2016, 29, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Möller, A.; Souvatzoglou, M.; Delso, G.; Bundschuh, R.A.; Chefd’hotel, C.; Ziegler, S.I.; Navab, N.; Schwaiger, M.; Nekolla, S.G. Tissue Classification as a Potential Approach for Attenuation Correction in Whole-Body PET/MRI: Evaluation with PET/CT Data. J. Nucl. Med. 2009, 50, 520–526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samarin, A.; Burger, C.; Wollenweber, S.D.; Crook, D.W.; Burger, I.A.; Schmid, D.T.; Von Schulthess, G.K.; Kuhn, F. PET/MR imaging of bone lesions–implications for PET quantification from imperfect attenuation correction. Eur. J. Nucl. Med. Mol. Imaging Pediatr. 2012, 39, 1154–1160. [Google Scholar] [CrossRef] [Green Version]
- Paulus, D.H.; Quick, H.H.; Geppert, C.; Fenchel, M.; Zhan, Y.; Hermosillo, G.; Faul, D.; Boada, F.; Friedman, K.P.; Koesters, T. Whole-Body PET/MR Imaging: Quantitative Evaluation of a Novel Model-Based MR Attenuation Correction Method Including Bone. J. Nucl. Med. 2015, 56, 1061–1066. [Google Scholar] [CrossRef] [Green Version]
- Koesters, T.; Friedman, K.P.; Fenchel, M.; Zhan, Y.; Hermosillo, G.; Babb, J.; Jelescu, I.O.; Faul, D.; Boada, F.E.; Shepherd, T.M. Dixon Sequence with Superimposed Model-Based Bone Compartment Provides Highly Accurate PET/MR Attenuation Correction of the Brain. J. Nucl. Med. 2016, 57, 918–924. [Google Scholar] [CrossRef] [Green Version]
- Grafe, H.; Lindemann, M.E.; Ruhlmann, V.; Oehmigen, M.; Hirmas, N.; Umutlu, L.; Herrmann, K.; Quick, H.H. Evaluation of improved attenuation correction in whole-body PET/MR on patients with bone metastasis using various radiotracers. Eur. J. Nucl. Med. Mol. Imaging Pediatr. 2020, 47, 2269–2279. [Google Scholar] [CrossRef] [Green Version]
- Oehmigen, M.; Lindemann, M.E.; Gratz, M.; Kirchner, J.; Ruhlmann, V.; Umutlu, L.; Blumhagen, J.O.; Fenchel, M.; Quick, H.H. Impact of improved attenuation correction featuring a bone atlas and truncation correction on PET quantification in whole-body PET/MR. Eur. J. Nucl. Med. Mol. Imaging Pediatr. 2017, 45, 642–653. [Google Scholar] [CrossRef]
- Wu, D.; Plyku, D.; Kulkarni, K.; Garcia, C.; Atkins, F.; Tefera, E.; Burman, K.D.; Wartofsky, L.; Van Nostrand, D. Optimal Time for 124I PET/CT Imaging in Metastatic Differentiated Thyroid Cancer. Clin. Nucl. Med. 2021, 46, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Ruhlmann, M.; Jentzen, W.; Ruhlmann, V.; Pettinato, C.; Rossi, G.; Binse, I.; Bockisch, A.; Rosenbaum-Krumme, S.J. High Level of Agreement Between Pretherapeutic 124I PET and Intratherapeutic 131I Imaging in Detecting Iodine-Positive Thyroid Cancer Metastases. J. Nucl. Med. 2016, 57, 1339–1342. [Google Scholar] [CrossRef] [Green Version]
- Rausch, I.; Rischka, L.; Ladefoged, C.N.; Furtner, J.; Fenchel, M.; Hahn, A.; Lanzenberger, R.; Mayerhoefer, M.E.; Traub-Weidinger, T.; Beyer, T. PET/MRI for Oncologic Brain Imaging: A Comparison of Standard MR-Based Attenuation Corrections with a Model-Based Approach for the Siemens mMR PET/MR System. J. Nucl. Med. 2017, 58, 1519–1525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eiber, M.; Takei, T.; Souvatzoglou, M.; Mayerhoefer, M.E.; Fürst, S.; Gaertner, F.C.; Loeffelbein, D.J.; Rummeny, E.J.; Ziegler, S.I.; Schwaiger, M.; et al. Performance of Whole-Body Integrated 18F-FDG PET/MR in Comparison to PET/CT for Evaluation of Malignant Bone Lesions. J. Nucl. Med. 2013, 55, 191–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heusch, P.; Buchbender, C.; Beiderwellen, K.; Nensa, F.; Hartung-Knemeyer, V.; Lauenstein, T.C.; Bockisch, A.; Forsting, M.; Antoch, G.; Heusner, T.A. Standardized uptake values for [(1)(8)F] FDG in normal organ tissues: Comparison of whole-body PET/CT and PET/MRI. Eur. J. Radiol. 2013, 82, 870–876. [Google Scholar] [CrossRef] [PubMed]
- Wiesmüller, M.; Quick, H.H.; Navalpakkam, B.; Lell, M.M.; Uder, M.; Ritt, P.; Schmidt, D.; Beck, M.; Kuwert, T.; Von Gall, C.C. Comparison of lesion detection and quantitation of tracer uptake between PET from a simultaneously acquiring whole-body PET/MR hybrid scanner and PET from PET/CT. Eur. J. Nucl. Med. Mol. Imaging Pediatr. 2012, 40, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Jentzen, W.; Phaosricharoen, J.; Gomez, B.; Hetkamp, P.; Stebner, V.; Binse, I.; Kinner, S.; Herrmann, K.; Sabet, A.; Nagarajah, J. Quantitative performance of 124I PET/MR of neck lesions in thyroid cancer patients using 124I PET/CT as reference. EJNMMI Phys. 2018, 5, 13. [Google Scholar] [CrossRef]
- Wierts, R.; Jentzen, W.; Quick, H.H.; Wisselink, H.J.; Pooters, I.N.A.; Wildberger, J.E.; Herrmann, K.; Kemerink, G.J.; Backes, W.H.; Mottaghy, F.M. Quantitative performance evaluation of124I PET/MRI lesion dosimetry in differentiated thyroid cancer. Phys. Med. Biol. 2017, 63, 015014. [Google Scholar] [CrossRef]
- Lindemann, M.E.; Nensa, F.; Quick, H.H. Impact of improved attenuation correction on 18F-FDG PET/MR hybrid imaging of the heart. PLoS ONE 2019, 14, e0214095. [Google Scholar] [CrossRef]
- Boss, A.; Stegger, L.; Bisdas, S.; Kolb, A.; Schwenzer, N.; Pfister, M.; Claussen, C.D.; Pichler, B.J.; Pfannenberg, C. Feasibility of simultaneous PET/MR imaging in the head and upper neck area. Eur. Radiol. 2011, 21, 1439–1446. [Google Scholar] [CrossRef] [Green Version]
- Kamel, E.M.; Burger, C.; Buck, A.; Von Schulthess, G.K.; Goerres, G.W. Impact of metallic dental implants on CT-based attenuation correction in a combined PET/CT scanner. Eur. Radiol. 2003, 13, 724–728. [Google Scholar] [CrossRef] [PubMed]
- Binse, I.; Rosenbaum-Krumme, S.J.; Bockisch, A. Imaging of differentiated thyroid carcinoma: 124I-PET/MRI may not be superior to 124I-PET/CT. Eur. J. Nucl. Med. Mol. Imaging Pediatr. 2016, 43, 1185–1186. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.; Binse, I.; Nagarajah, J.; Bockisch, A.; Herrmann, K.; Jentzen, W. The role of 124I PET/CT lesion dosimetry in differentiated thyroid cancer. Q. J. Nucl. Med. Mol. Imaging 2019, 63, 235–252. [Google Scholar] [CrossRef] [PubMed]
- Jentzen, W.; Verschure, F.; van Zon, A.; van de Kolk, R.; Wierts, R.; Schmitz, J.; Bockisch, A.; Binse, I. 124I PET Assessment of Response of Bone Metastases to Initial Radioiodine Treatment of Differentiated Thyroid Cancer. J. Nucl. Med. 2016, 57, 1499–1504. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Patient No | Exam No | Sex | Age [Years] | BMI [kg/m2] | Tracer | Administered Activity [MBq] | Post Administration Time [hh:mm] PET/CT | Post Administration Time [hh:mm] PET/MR |
|---|---|---|---|---|---|---|---|---|
| 35 | 43 | 23 f, 12 m | 52 ± 18 | 27.2 ± 5.5 | 124I-NaI | 34.2 ± 9.9 | 24:35 ± 2:47 | 28:53 ± 5:07 |
| CT- and MR-Based AC | Bone Volume * [%] Skull/Spine | LAC [cm−1] (Min–Max) | LAC [cm−1] (Mean ± SD) | LAC max ** (>0.250 cm−1) [% vom Vtot] |
|---|---|---|---|---|
| CT-based AC | 4.483 ± 1.082 | 0.111–0.266 | 0.134 ± 0.021 | mean: 0.023 |
| MR-based AC | 3.994 ± 0.961 | 0.101–0.247 | 0.128 ± 0.002 | - |
| Meassured Values | Comparison between PET/CT vs. PET/MR with Bone AC | Comparison between PET/MR with vs. without Bone AC | ||||
|---|---|---|---|---|---|---|
| PET/CT | PET/MR with Bone | Relative Difference [%] | PET/MR with Bone | PET/MR without Bone | Relative Difference [%] | |
| Measured No. of lesions/No. of 124I-avid lesions | 98/111 | 98/132 | 18.9 | 98/132 | 98/132 | 0 |
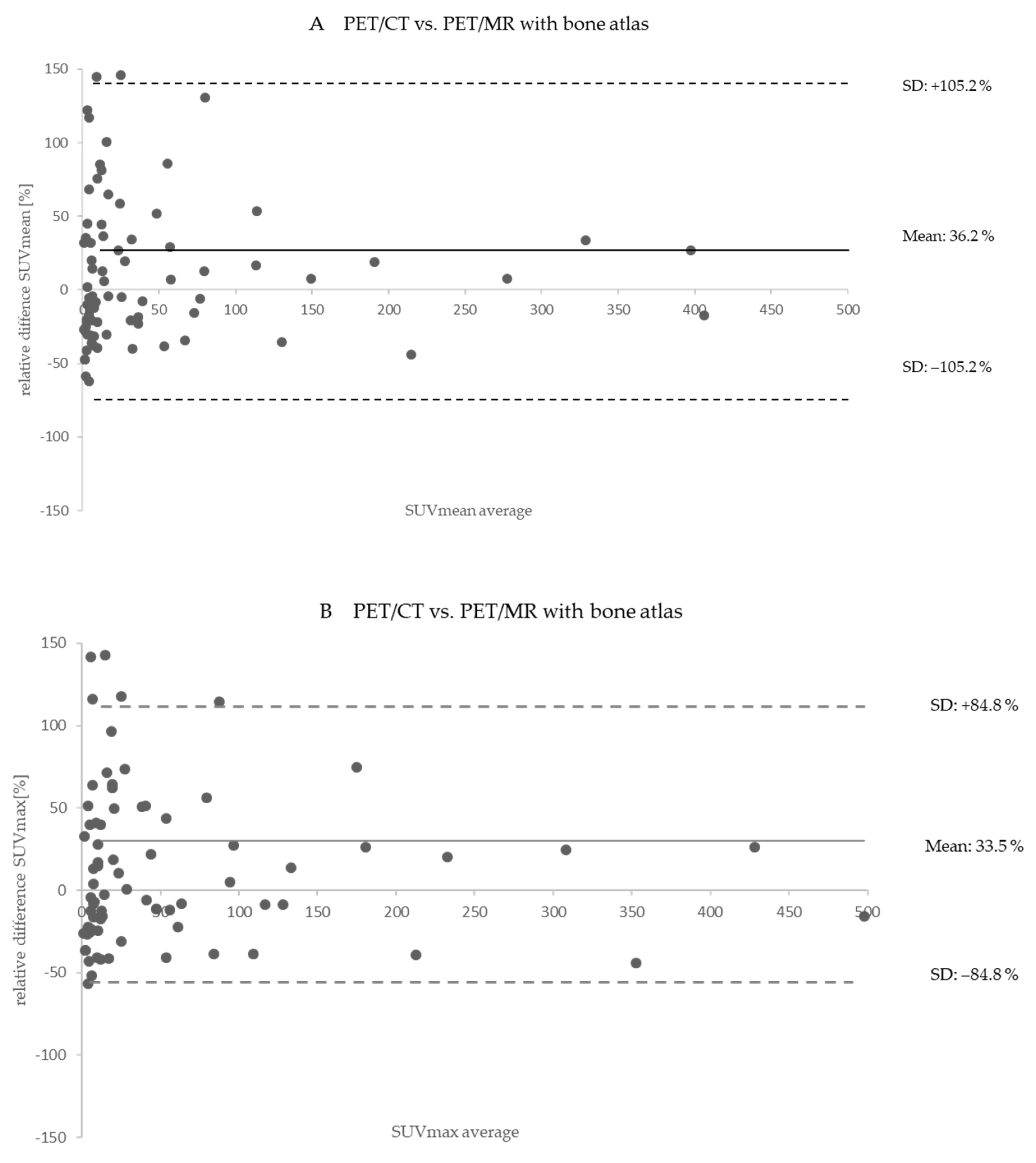
| SUVmean mean ± SD (range) median | 184.1 ± 472.3 (0.8–2669.8) 12.3 | 235.8 ± 575.0 (0.6–3036.7) 20.8 | 36.2 ± 105.2 (−67.9–720.4) 6.3 | 235.8 ± 575.0 (0.6–3036.7) 20.8 | 231.0 ± 564.6 (0.6–3065.3) 18.8 | −2.2 ± 7.2 (−63.6–9.2) −1.1 |
| SUVmax mean ± SD (range) median | 296.8 ± 754.2 (1.5–4640.2) 20.6 | 393.6 ± 970.6 (1.1–5153.5) 34.6 | 36.3 ± 84.9 (−69.8–347.4) 13.3 | 393.6 ± 970.6 (1.1–5153.5) 34.6 | 386.3 ± 953.3 (1.1–5217.9) 35.0 | −1.4 ± 3.4 (−11.9–8.7) −1.0 |
| Detected Lesions Locations | PET/CT | PET/MR with Bone Atlas | ||||
|---|---|---|---|---|---|---|
| No. of Lesions | Suvmean Mean ± SD (Range) Median | SUVmax Mean ± SD (Range) Median | No. of Lesions | Suvmean Mean ± SD (Range) Median | SUVmax Mean ± SD (Range) Median | |
| Bone | 8 | 4.73 ± 3.30 (1.94–11.85) 3.42 | 8.22 ± 5.90 (3.13–21.13) 6.12 | 8 | 3.95 ± 2.35 (1.55–7.37) 2.63 | 6.81 ± 3.84 (2.80–12.45) 4.91 |
| Lung | 8 | 68.49 ± 91.56 (4.49–275.12) 29.45 | 113.26 ± 150.85 (7.35–452.28) 48.12 | 8 | 44.30 ± 51.58 (4.24–154.46) 22.89 | 71.96 ± 83.58 (7.62–253.31) 36.98 |
| Lymph nodes | 46 | 61.97 ± 141.68 (0.81–775.43) 11.94 | 90.70 ± 192.81 (1.50–944.31) 18.10 | 46 | 97.76 ± 321.15 (0.59–2154.96) 22.69 | 165.02 ± 558.39 (1.11–3763.73) 37.23 |
| Thyroid | 36 | 405.64 ± 708.19 (0.97–2669.82) 30.64 | 665.13 ± 1131.25 (1.67–4640.19) 49.88 | 36 | 506.18 ± 806.35 (0.86–3036.71) 37.60 | 843.15 ± 1356.21 (1.60–5153.51) 63.79 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grafe, H.; Lindemann, M.E.; Weber, M.; Kirchner, J.; Binse, I.; Umutlu, L.; Herrmann, K.; Quick, H.H. Intra-Individual Comparison of 124I-PET/CT and 124I-PET/MR Hybrid Imaging of Patients with Resected Differentiated Thyroid Carcinoma: Aspects of Attenuation Correction. Cancers 2022, 14, 3040. https://doi.org/10.3390/cancers14133040
Grafe H, Lindemann ME, Weber M, Kirchner J, Binse I, Umutlu L, Herrmann K, Quick HH. Intra-Individual Comparison of 124I-PET/CT and 124I-PET/MR Hybrid Imaging of Patients with Resected Differentiated Thyroid Carcinoma: Aspects of Attenuation Correction. Cancers. 2022; 14(13):3040. https://doi.org/10.3390/cancers14133040
Chicago/Turabian StyleGrafe, Hong, Maike E. Lindemann, Manuel Weber, Julian Kirchner, Ina Binse, Lale Umutlu, Ken Herrmann, and Harald H. Quick. 2022. "Intra-Individual Comparison of 124I-PET/CT and 124I-PET/MR Hybrid Imaging of Patients with Resected Differentiated Thyroid Carcinoma: Aspects of Attenuation Correction" Cancers 14, no. 13: 3040. https://doi.org/10.3390/cancers14133040
APA StyleGrafe, H., Lindemann, M. E., Weber, M., Kirchner, J., Binse, I., Umutlu, L., Herrmann, K., & Quick, H. H. (2022). Intra-Individual Comparison of 124I-PET/CT and 124I-PET/MR Hybrid Imaging of Patients with Resected Differentiated Thyroid Carcinoma: Aspects of Attenuation Correction. Cancers, 14(13), 3040. https://doi.org/10.3390/cancers14133040





