The Increasing Role of CT-Guided Cryoablation for the Treatment of Liver Cancer: A Single-Center Report
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
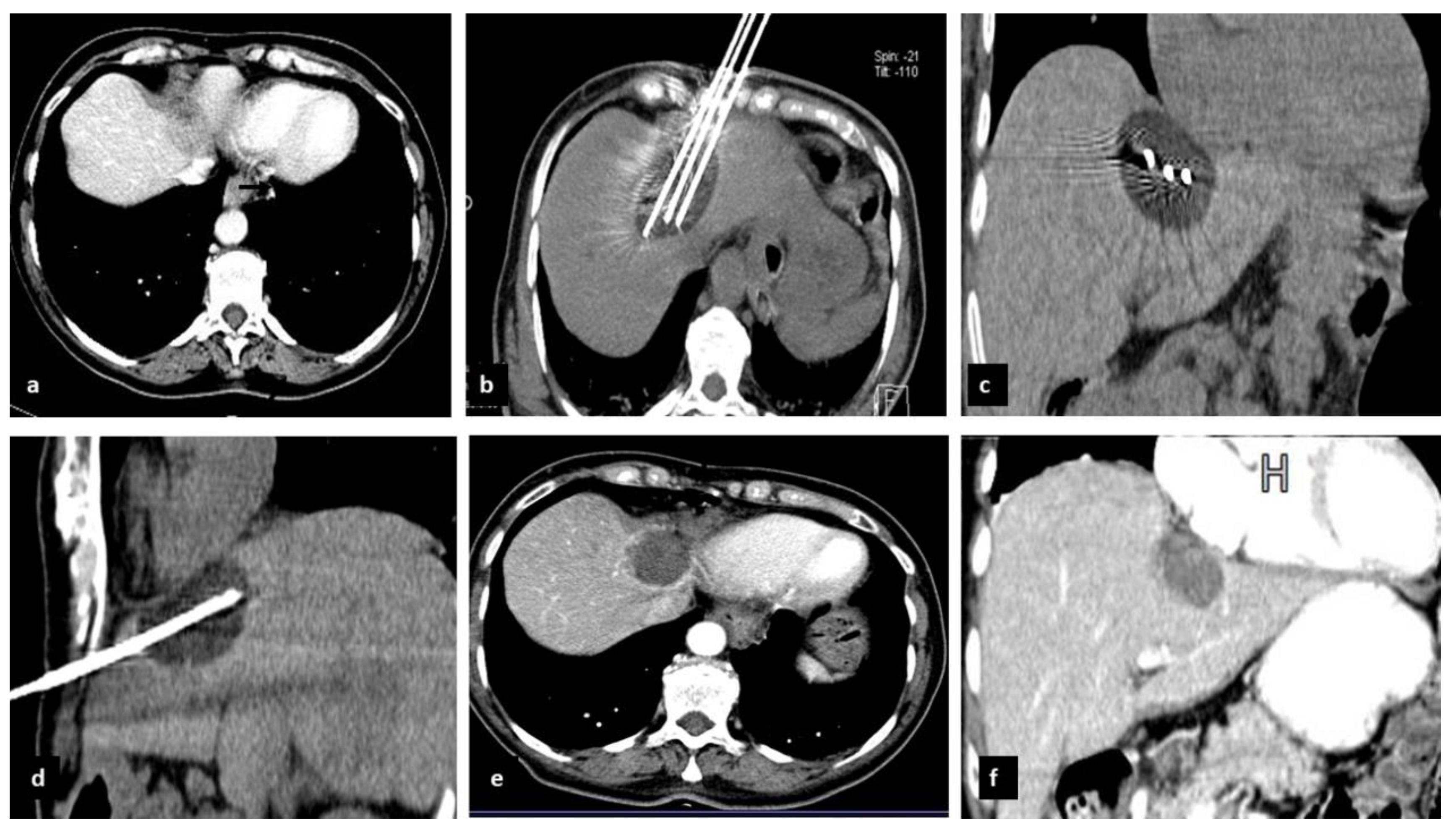
2.2. Preoperative Evaluation and CrA Procedure
2.3. Definitions and Follow-Up
2.4. Study Endpoints and Statistical Analysis
3. Results
3.1. Demographic and Operative Outcomes
3.2. Subgroup of Patients with HCC
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, W. Recent update on comprehensive therapy for advanced hepatocellular carcinoma. World J. Gastrointest. Oncol. 2021, 13, 845–855. [Google Scholar] [CrossRef] [PubMed]
- Fancellu, A.; Rosman, A.S.; Sanna, V.; Nigri, G.R.; Zorcolo, L.; Pisano, M.; Melis, M. Meta-Analysis of Trials Comparing Minimally-Invasive and Open Liver Resections for Hepatocellular Carcinoma. J. Surg. Res. 2011, 171, e33–e45. [Google Scholar] [CrossRef] [PubMed]
- Lencioni, R.; De Baere, T.; Martin, R.C.; Nutting, C.W.; Narayanan, G. Image-Guided Ablation of Malignant Liver Tumors: Recommendations for Clinical Validation of Novel Thermal and Non-Thermal Technologies-A Western Perspective. Liver Cancer 2015, 4, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Benson, A.B.; Venook, A.P.; Al-Hawary, M.M.; Arain, M.A.; Chen, Y.-J.; Ciombor, K.K.; Cohen, S.; Cooper, H.S.; Deming, D.; Farkas, L.; et al. Colon Cancer, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2021, 19, 329–359. [Google Scholar] [CrossRef]
- Glazer, D.I.; Tatli, S.; Shyn, P.B.; Vangel, M.G.; Tuncali, K.; Silverman, S.G. Percutaneous Image-Guided Cryoablation of Hepatic Tumors: Single-Center Experience With Intermediate to Long-Term Outcomes. AJR Am. J. Roentgenol. 2017, 209, 1381–1389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, L.; Qi, H.; Chen, S.; Cao, F.; Xie, L.; Wu, Y.; Ma, W.; Song, Z.; Yuan, H.; Zhang, T.; et al. Cryoablation combined with transarterial infusion of pembrolizumab (CATAP) for liver metastases of melanoma: An ambispective, proof-of-concept cohort study. Cancer Immunol. Immunother. 2020, 69, 1713–1724. [Google Scholar] [CrossRef]
- Arellano, R.S. What’s New in Percutaneous Ablative Strategies for Hepatocellular Carcinoma and Colorectal Hepatic Metastases? 2020 Update. Curr. Oncol. Rep. 2020, 22, 105. [Google Scholar] [CrossRef]
- Wu, S.; Hou, J.; Ding, Y.; Wu, F.; Hu, Y.; Jiang, Q.; Mao, P.; Yang, Y. Cryoablation Versus Radiofrequency Ablation for Hepatic Malignancies: A Systematic Review and Literature-Based Analysis. Medicine 2015, 94, e2252. [Google Scholar] [CrossRef]
- Filippiadis, D.K.; Velonakis, G.; Kelekis, A.; Sofocleous, C.T. The Role of Percutaneous Ablation in the Management of Colorectal Cancer Liver Metastatic Disease. Diagnostics 2021, 11, 308. [Google Scholar] [CrossRef]
- McCarley, J.R.; Soulen, M.C. Percutaneous ablation of hepatic tumors. Semin. Interv. Radiol. 2010, 27, 255–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pusceddu, C.; Melis, L.; Ballicu, N.; Sotgia, B.; Sanna, V.; Meloni, G.B.; Porcu, A.; Fancellu, A. Percutaneous Microwave Ablation under CT Guidance for Hepatocellular Carcinoma: A Single Institutional Experience. J. Gastrointest. Cancer 2018, 49, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Pusceddu, C.; Paliogiannis, P.; Nigri, G.; Fancellu, A. Cryoablation in The Management of Breast Cancer: Evidence to Date. Breast Cancer 2019, 11, 283–292. [Google Scholar] [CrossRef] [Green Version]
- Ng, K.M.; Chua, T.C.; Saxena, A.; Zhao, J.; Chu, F.; Morris, D.L. Two Decades of Experience with Hepatic Cryotherapy for Advanced Colorectal Metastases. Ann. Surg. Oncol. 2012, 19, 1276–1283. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Gao, X.; Sun, J.; Cheng, J.; Hu, Y.; Dong, Z.; Kong, H.; Zhang, H.; Wang, C.; Yang, Y. Percutaneous Argon-Helium Cryoablation for Small Hepatocellular Carcinoma Located Adjacent to a Major Organ or Viscus: A Retrospective Study of 92 Patients at a Single Center. Med. Sci. Monit. 2021, 27, e931473-1. [Google Scholar] [CrossRef] [PubMed]
- Baust, J.G.; Snyder, K.K.; Santucci, K.L.; Robilotto, A.T.; Van Buskirk, R.G. Cryoablation: Physical and molecular basis with putative immunological consequences. Int. J. Hyperth. 2019, 36, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Littrup, P.J.; Aoun, H.D.; Adam, B.; Krycia, M.; Prus, M.; Shields, A. Percutaneous cryoablation of hepatic tumors: Long-term experience of a large U.S. series. Abdom. Radiol. 2016, 41, 767–780. [Google Scholar] [CrossRef]
- NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Hepatobiliary Cancers Version 1. 2022. Available online: https://www.nccn.org/professionals/physician_gls/pdf/hepatobiliary.pdf (accessed on 31 March 2022).
- Kalra, N.; Gupta, P.; Jugpal, T.; Naik, S.S.; Gorsi, U.; Chaluvashetty, S.B.; Bhujade, H.; Duseja, A.; Singh, V.; Dhiman, R.K.; et al. Percutaneous Cryoablation of Liver Tumors: Initial Experience from a Tertiary Care Center in India. J. Clin. Exp. Hepatol. 2021, 11, 305–311. [Google Scholar] [CrossRef]
- Feng, Q.; Chi, Y.; Liu, Y.; Zhang, L.; Liu, Q. Efficacy and safety of percutaneous radiofrequency ablation versus surgical resection for small hepatocellular carcinoma: A meta-analysis of 23 studies. J. Cancer Res. Clin. Oncol. 2015, 141, 1–9. [Google Scholar] [CrossRef]
- Peng, Z.-W.; Lin, X.-J.; Zhang, Y.; Liang, H.-H.; Guo, R.; Shi, M.; Chen, M.-S. Radiofrequency ablation versus hepatic resection for the treatment of hepatocellular carcinomas 2 cm or smaller: A retrospective comparative study. Radiology 2012, 262, 1022–1033. [Google Scholar] [CrossRef]
- Shibata, T.; Iimuro, Y.; Yamamoto, Y.; Maetani, Y.; Ametani, F.; Itoh, K.; Konishi, J. Small hepatocellular carcinoma: Comparison of radio-frequency ablation and percutaneous microwave coagulation therapy. Radiology 2002, 223, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Sun, Q.; Wang, Y.; Jing, X.; Ding, J.; Yuan, Q.; Ren, C.; Shan, S.; Wang, Y.; Du, Z. Comparison of microwave ablation and surgical resection for treatment of hepatocellular carcinomas conforming to Milan Criteria. J. Gastroenterol. Hepatol. 2014, 29, 1500–1507. [Google Scholar] [CrossRef] [PubMed]
- Camacho, J.C.; Petre, E.N.; Sofocleous, C.T. Thermal Ablation of Metastatic Colon Cancer to the Liver. Semin. Interv. Radiol. 2019, 36, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Sofocleous, C.T.; Sideras, P.; Petre, E.N. “How We Do it”—A Practical Approach to Hepatic Metastases Ablation Techniques. Tech. Vasc. Interv. Radiol. 2013, 16, 219–229. [Google Scholar] [CrossRef]
- Wang, F.; Ma, J.; Wu, L.; Li, N.; Luo, R.; Wei, G.; Yang, J. Percutaneous cryoablation of subcapsular hepatocellular carcinoma: A retrospective study of 57 cases. Diagn. Interv. Radiol. 2020, 26, 34–39. [Google Scholar] [CrossRef]
- Niu, L.; Zhou, L.; Xu, K.; Mu, F. Cryosurgery for colorectal liver metastases. Ann. Palliat. Med. 2013, 2, 130–140. [Google Scholar] [CrossRef]
- Schmit, C.H.; Callstrom, M.R.; Boorjian, S.A.; Johnson, M.P.; Atwell, T.D.; Kurup, A.N.; Schultz, G.R.; Thompson, R.H.; Schmit, G.D. A Comparison of Bleeding Complications in Patients Undergoing Percutaneous Renal Cryoablation Using Cryoprobes with and without Heat-Based Track Ablation. J. Vasc. Interv. Radiol. 2018, 29, 874–879. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Lu, S.-N.; Hung, C.-H.; Wang, J.-H.; Chen, C.-H.; Yen, Y.-H.; Kuo, Y.-H.; Kee, K.-M. Predicting outcomes for recurrent hepatocellular carcinoma within Milan criteria after complete radiofrequency ablation. PLoS ONE 2020, 15, e0242113. [Google Scholar] [CrossRef]
- Ko, S.E.; Lee, M.W.; Rhim, H.; Kang, T.W.; Song, K.D.; Cha, D.I.; Lim, H.K. Comparison of procedure-related complications between percutaneous cryoablation and radiofrequency ablation for treating periductal hepatocellular carcinoma. Int. J. Hyperth. 2020, 37, 1354–1361. [Google Scholar] [CrossRef]
- Kamarinos, N.V.; Kaye, E.A.; Sofocleous, C.T. Image-Guided Thermal Ablation for Colorectal Liver Metastases. Tech. Vasc. Interv. Radiol. 2020, 23, 100672. [Google Scholar] [CrossRef]
- Cha, S.Y.; Kang, T.W.; Min, J.H.; Song, K.D.; Lee, M.W.; Rhim, H.; Lim, H.K.; Sinn, D.H.; Park, M.S. RF Ablation Versus Cryoablation for Small Perivascular Hepatocellular Carcinoma: Propensity Score Analyses of Mid-Term Outcomes. Cardiovasc. Interv. Radiol. 2020, 43, 434–444. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, Y.; Wu, Y.; Chen, J. Efficacy and safety of percutaneous argon-helium cryoablation for hepatocellular carcinoma abutting the diaphragm. J. Vasc. Interv. Radiol. 2020, 31, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, H.; Yang, W.; Hu, K.; Xie, H.; Hu, K.-Q.; Bai, W.; Dong, Z.; Lu, Y.; Zeng, Z.; et al. Multicenter randomized controlled trial of percutaneous cryoablation versus radiofrequency ablation in hepatocellular carcinoma. Hepatology 2015, 61, 1579–1590. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Lou, Y.; Liu, P.; Xu, L.X. Tumor-related HSP70 released after cryo-thermal therapy targeted innate immune initiation in the antitumor immune response. Int. J. Hyperth. 2020, 37, 843–853. [Google Scholar] [CrossRef]

| Characteristic | Total Cohort (N = 49) | Patients with HCC (N = 11) | Patients with Liver Metastases (N = 38) |
|---|---|---|---|
| Gender | |||
| Male | 23 (47%) | 9 (82%) | 14 (37%) |
| Female | 26 (53%) | 2 (18%) | 24 (63%) |
| Age (mean ± SD) | 67.5 ± 10.4 | 67.8 ± 9.6 | 67.4 ± 10.8 |
| Total no. of tumor lesions treated with CrA | 64 (100%) | 14 (22%) | 50 (78%) |
| 1 lesion | 54 (84%) | 12 (86%) | 42 (84%) |
| 2 lesions | 5 (16%) | 1 (14%) | 4 (16%) |
| Tumor diameter, in mm (mean ± SD) | 21.5 ± 10.7 | 19.0 ± 3.8 | 22.2 ± 11.9 |
| Tumor location | |||
| Right liver | 24 (37.5%) | 5 (36%) | 19 (38%) |
| Left liver | 40 (62.5%) | 9 (64%) | 31 (62%) |
| Outcome | HCC (14 Lesions in 11 Patients) | Metastases (50 Lesions in 38 Patients) | p Value |
|---|---|---|---|
| Technical success | 14 (100%) | 50 (100%) | - |
| Complete ablation at 1 month | 11 (79%) | 48 (96%) | 0.032 * |
| Patients with local recurrence | 5 (45%) | 11(29%) | 0.477 |
| Total number of Local Recurrence | 6 (43%) | 14 (28%) | 0.289 |
| Patients with local tumor progression | 1 (7%) | 5 (10%) | 0.105 |
| Patients with distant metastases | 1 (7%) | 6 (12%) | 0.607 |
| Patients with minor complications | 1 (9%) | 5 (13%) | 0.730 |
| Redo liver CrA | 2 (14%) | 8 (16%) | 0.864 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pusceddu, C.; Mascia, L.; Ninniri, C.; Ballicu, N.; Zedda, S.; Melis, L.; Deiana, G.; Porcu, A.; Fancellu, A. The Increasing Role of CT-Guided Cryoablation for the Treatment of Liver Cancer: A Single-Center Report. Cancers 2022, 14, 3018. https://doi.org/10.3390/cancers14123018
Pusceddu C, Mascia L, Ninniri C, Ballicu N, Zedda S, Melis L, Deiana G, Porcu A, Fancellu A. The Increasing Role of CT-Guided Cryoablation for the Treatment of Liver Cancer: A Single-Center Report. Cancers. 2022; 14(12):3018. https://doi.org/10.3390/cancers14123018
Chicago/Turabian StylePusceddu, Claudio, Luigi Mascia, Chiara Ninniri, Nicola Ballicu, Stefano Zedda, Luca Melis, Giulia Deiana, Alberto Porcu, and Alessandro Fancellu. 2022. "The Increasing Role of CT-Guided Cryoablation for the Treatment of Liver Cancer: A Single-Center Report" Cancers 14, no. 12: 3018. https://doi.org/10.3390/cancers14123018
APA StylePusceddu, C., Mascia, L., Ninniri, C., Ballicu, N., Zedda, S., Melis, L., Deiana, G., Porcu, A., & Fancellu, A. (2022). The Increasing Role of CT-Guided Cryoablation for the Treatment of Liver Cancer: A Single-Center Report. Cancers, 14(12), 3018. https://doi.org/10.3390/cancers14123018








