Environmental Contaminants Modulate Breast Cancer Development and Outcome in TP53 p.R337H Carriers and Noncarriers
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Rationale for Classification of the Paraná State into Three Subregions
2.2. Long-Term Follow-Up of Women Negative and Positive for the Germline TP53 p.R337H and XAF1 p.E134* Variants in the Three Subregions of Paraná State
2.3. Body Mass Index (BMI) in Breast Cancer Women
2.4. Cross-Sectional Analysis of Breast Cancer Average Age-Specific Mortality Rate (AASMR) in the Three Subregions of Paraná State (Group 2)
2.5. Frequency of Germline TP53 p.R337H and XAF1 p.E134* Variants in Women with BC from a Paraná State Public Cancer Hospital (Group 3) from All Subregions of Paraná
2.6. TP53 p.R337H and XAF1 p.E134* Genotyping
2.7. Geographic Visualization and Statistical Analysis
3. Results
3.1. Environmental Factors
3.2. Breast Cancer Chracteristics
3.3. BC Risk among Women with Negative and Positive TP53 p.R337H Variant (Group 1)
3.4. BC Age-Specific Mortality Rates in Each Subregion (Group 2)
3.5. TP53 p.R337H and XAF1 p.E134* Allele Percentages in Women with BC
3.6. Body Mass Index (BMI) in Breast Cancer Women
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- García-Pérez, J.; Lope, V.; Pérez-Gómez, B.; Molina, A.J.; Tardón, A.; Díaz Santos, M.A.D.; Ardanaz, E.; O’Callaghan-Gordo, C.; Altzibar, J.M.; Gómez-Acebo, I.; et al. Risk of breast cancer and residential proximity to industrial installations: New findings from a multicase-control study (MCC-Spain). Environ. Pollut. 2018, 237, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, R.C.; Sandrini, F.; Figueiredo, B.; Zambetti, G.P.; Michalkiewicz, E.; Lafferty, A.R.; DeLacerda, L.; Rabin, M.; Cadwell, C.; Sampaio, G.; et al. An inherited p53 mutation that contributes in a tissue-specific manner to pediatric adrenal cortical carcinoma. Proc. Natl Acad. Sci. USA 2001, 98, 9330–9335. [Google Scholar] [CrossRef] [PubMed]
- Custódio, G.; Parise, G.A.; Kiesel Filho, N.K.; Komechen, H.; Sabbaga, C.C.; Rosati, R.; Grisa, L.; Parise, I.Z.; Pianovski, M.A.; Fiori, C.M.; et al. Impact of neonatal screening and surveillance for the TP53 R337H mutation on early detection of childhood adrenocortical tumors. J. Clin. Oncol. 2013, 31, 2619–2626. [Google Scholar] [CrossRef] [PubMed]
- Costa, T.E.J.; Gerber, V.K.Q.; Ibañez, H.C.; Melanda, V.S.; Parise, I.Z.S.; Watanabe, F.M.; Pianovski, M.A.D.; Fiori, C.M.C.M.; Fabro, A.L.M.R.; Silva, D.B.D.; et al. Penetrance of the TP53 R337H mutation and pediatric adrenocortical carcinoma incidence associated with environmental influences in a 12-year observational cohort in Southern Brazil. Cancers 2019, 11, 1804. [Google Scholar] [CrossRef]
- Tosin, K.C.F.; Legal, E.F.; Pianovski, M.A.D.; Ibañez, H.C.; Custódio, G.; Carvalho, D.S.; Figueiredo, M.M.O.; Hoffmann Filho, A.; Fiori, C.M.C.M.; Rodrigues, A.L.M.; et al. Newborn screening for the detection of the TP53 R337H variant and surveillance for early diagnosis of pediatric adrenocortical tumors: Lessons learned and way forward. Cancers 2021, 13, 6111. [Google Scholar] [CrossRef]
- Caminha, I.P. Prevalência da Mutação Germinativa TP53 p.R337H Na Região Metropolitana de Campinhas e Cidades Circunvizinhas; University Estadual de Campinas, State University of Campinas: Campinas, São Paulo, Brazil, 2015; Available online: http://repositorio.unicamp.br/jspui/bitstream/REPOSIP/316893/1/Caminha_IsabelPereira_D.pdf (accessed on 19 November 2001).
- Pinto, E.M.; Billerbeck, A.E.C.; Villares, M.C.B.F.; Domenice, S.; Mendonça, B.B.; Latronico, A.C. Founder effect for the highly prevalent R337H mutation of tumor suppressor p53 in Brazilian patients with adrenocortical tumors. Arq. Bras. Endocrinol. Metabol. 2004, 48, 647–650. [Google Scholar] [CrossRef]
- Pinto, E.M.; Figueiredo, B.C.; Chen, W.; Galvao, H.C.R.; Formiga, M.N.; Fragoso, M.C.B.V.; Ashton-Prolla, P.; Ribeiro, E.M.S.F.; Felix, G.; Costa, T.E.B.; et al. XAF1 as a modifier of p53 function and cancer susceptibility. Sci. Adv. 2020, 6, eaba3231. [Google Scholar] [CrossRef]
- Gomes, M.C.; Kotsopoulos, J.; de Almeida, G.L.; Costa, M.M.; Vieira, R.; Filho, F.d.A.; Pitombo, M.B.; Leal, P.R.F.; Royer, R.; Zhang, P.; et al. The R337H mutation in TP53 and breast cancer in Brazil. Hered. Cancer Clin. Pract. 2012, 10, 3. [Google Scholar] [CrossRef]
- Mathias, C.; Bortoletto, S.; Centa, A.; Komechen, H.; Lima, R.S.; Fonseca, A.S.; Sebastião, A.P.; Urban, C.A.; Soares, E.W.S.; Prando, C.; et al. Frequency of the TP53 R337H variant in sporadic breast cancer from Southern Brazil and its impact in genomic instability. Sci. Rep. 2020, 10, 16614. [Google Scholar] [CrossRef]
- Assumpção, J.G.; Seidinger, A.L.; Mastellaro, M.J.; Ribeiro, R.C.; Zambetti, G.P.; Ganti, R.; Srivastava, K.; Shurtleff, S.; Pei, D.; Zeferino, L.C.; et al. Association of the germline TP53 R337H mutation with breast cancer in Southern Brazil. BMC Cancer 2008, 8, 357. [Google Scholar] [CrossRef]
- Silva, F.C.; Lisboa, B.C.G.; Figueiredo, M.C.P.; Torrezan, G.T.; Santos, E.M.; Krepischi, A.C.; Rossi, B.M.; Achatz, M.I.; Carraro, D.M. Hereditary breast and ovarian cancer: Assessment of point mutations and copy number variations in Brazilian Patients. BMC Med. Genet. 2014, 15, 55. [Google Scholar] [CrossRef] [PubMed]
- Giacomazzi, J.; Graudenz, M.S.; Osorio, C.A.B.T.; Koehler-Santos, P.; Palmero, E.I.; Zagonel-Oliveira, M.; Michelli, R.A.; Scapulatempo Neto, C.; Fernandes, G.C.; Achatz, M.I.; et al. Prevalence of the TP53 p.R337H mutation in breast cancer patients in Brazil. PLoS ONE 2014, 9, e99893. [Google Scholar] [CrossRef] [PubMed]
- Tinat, J.; Bougeard, G.; Baert-Desurmont, S.; Vasseur, S.; Martin, C.; Bouvignies, E.; Caron, O.; Bressac-de Paillerets, B.; Berthet, P.; Dugast, C.; et al. 2009 version of the Chompret criteria for Li Fraumeni syndrome. J. Clin. Oncol. 2009, 27, e108–e109. [Google Scholar] [CrossRef] [PubMed]
- Panis, C.; Kawassaki, A.C.B.; Crestani, A.P.J.; Pascotto, C.R.; Bortoloti, D.S.; Vicentini, G.E.; Lucio, L.C.; Ferreira, M.O.; Prates, R.T.C.; Vieira, V.K.; et al. Evidence on human exposure to pesticides and the occurrence of health hazards in the Brazilian population: A systematic review. Front. Public Health 2022, 9, 787438. [Google Scholar] [CrossRef]
- Khanjani, N.; English, D.R.; Sim, M.R. An ecological study of organochlorine pesticides and breast cancer in rural Victoria, Australia. Arch. Environ. Contam. Toxicol. 2006, 50, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Louis, L.M.; Lerro, C.C.; Friesen, M.C.; Andreotti, G.; Koutros, S.; Sandler, D.P.; Blair, A.; Robson, M.G.; Beane Freeman, L.E. A Prospective study of cancer risk among agricultural health study farm spouses associated with personal use of organochlorine insecticides. Environ. Health 2017, 16, 95. [Google Scholar] [CrossRef]
- Andersen, Z.J.; Stafoggia, M.; Weinmayr, G.; Pedersen, M.; Galassi, C.; Jørgensen, J.T.; Oudin, A.; Forsberg, B.; Olsson, D.; Oftedal, B.; et al. Long-term exposure to ambient air pollution and incidence of postmenopausal breast cancer in 15 European cohorts within the ESCAPE project. Environ. Health Perspect. 2017, 125, 107005. [Google Scholar] [CrossRef]
- Ibañez, H.C.; Melanda, V.S.; Gerber, V.K.Q.; Licht, O.A.B.; Ibañez, M.V.C.; Aguiar Júnior, T.R.; Mello, R.G.; Komechen, H.; Andrade, D.P.; Picharski, G.L.; et al. Spatial trends in congenital malformations and stream water chemistry in Southern Brazil. Sci. Total Environ. 2019, 650, 1278–1291. [Google Scholar] [CrossRef]
- DATASUS. Win32, T.N. 3.0. Available online: http://tabnet.datasus.gov.br/cgi/tabcgi.exe?sim/cnv/obt10pr.def (accessed on 8 November 2019).
- Custódio, G.; Taques, G.R.; Figueiredo, B.C.; Gugelmin, E.S.; Oliveira Figueiredo, M.M.O.; Watanabe, F.; Pontarolo, R.; Lalli, E.; Torres, L.F. Increased incidence of choroid plexus carcinoma due to the germline TP53 R337H mutation in Southern Brazil. PLoS ONE 2011, 6, e18015. [Google Scholar] [CrossRef]
- QGIS. Welcome to the QGIS Project. Available online: https://qgis.org/en/site/ (accessed on 7 July 2020).
- Post, G.I.S. Spatial and Geographic Objects for PostgreSQL. Available online: https://postgis.net (accessed on 9 July 2020).
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. Available online: https://r-project.org/ (accessed on 13 December 2021).
- Fisher, R.A. Statistical Methods for Research Workers, 14th ed.; Oliver & Boyd: Edinburgh, London, UK, 1970; pp. 1–378. [Google Scholar]
- Agresti, A. An Introduction to Categorical Data Analysis, 2nd ed.; John Wiley & Sons: New York, NY, USA, 2007; pp. 38–50. [Google Scholar]
- Kaplan, E.L.; Meier, P. Nonparametric estimation from incomplete observations. J. Am. Stat. Assoc. 1958, 53, 457–481. [Google Scholar] [CrossRef]
- Mantel, N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother. Rep. 1966, 50, 163–170. [Google Scholar]
- Lenth, R.V.; Buerkner, P.; Herve, M.; Riebl, J.L.H.; Singmann, H. Estimated Marginal Means, Aka Least-Squares Means. R Package Version 1.6.0. Available online: https://CRAN.R-project.org/package=emmeans (accessed on 12 July 2021).
- Fritz, A.; Percy, C.; Jack, A.; Shanmugaratnam, K.; Sobin, L.; Parkin, D.M.; Whelan, S. International Classification of Diseases for Oncology (ICD-O), 3rd ed.; World Health Organization: Geneve, Switzerland, 2003. [Google Scholar]
- Naslavsky, M.S.; Yamamoto, G.L.; de Almeida, T.F.; Ezquina, S.A.M.; Sunaga, D.Y.; Pho, N.; Bozoklian, D.; Sandberg, T.O.M.; Brito, L.A.; Lazar, M.; et al. Exomic variants of an elderly cohort of Brazilians in the ABraOM database. Hum. Mutat. 2017, 38, 751–763. [Google Scholar] [CrossRef]
- Mastellaro, M.J.; Seidinger, A.L.; Kang, G.; Abrahão, R.; Miranda, E.C.M.; Pounds, S.B.; Cardinalli, I.A.; Aguiar, S.S.; Figueiredo, B.C.; Rodriguez-Galindo, C.; et al. Contribution of the TP53 R337H mutation to the cancer burden in Southern Brazil: Insights from the study of 55 families of children with adrenocortical tumors. Cancer 2017, 123, 3150–3158. [Google Scholar] [CrossRef]
- Lustbader, E.D.; Williams, W.R.; Bondy, M.L.; Strom, S.; Strong, L.C. Segregation analysis of cancer in families of childhood soft-tissue-sarcoma patients. Am. J. Hum. Genet. 1992, 51, 344–356. [Google Scholar]
- Birch, J.M.; Blair, V.; Kelsey, A.M.; Evans, D.G.; Harris, M.; Tricker, K.J.; Varley, J.M. Cancer phenotype correlates with constitutional TP53 genotype in families with the Li-Fraumeni syndrome. Oncogene 1998, 17, 1061–1068. [Google Scholar] [CrossRef]
- Birch, J.M.; Alston, R.D.; Mcnally, R.J.; Evans, D.G.; Kelsey, A.M.; Harris, M.; Eden, O.B.; Varley, J.M. Relative frequency and morphology of cancers in carriers of germline TP53 mutations. Oncogene 2001, 20, 4621–4628. [Google Scholar] [CrossRef]
- Malkin, D. Li-Fraumeni Syndrome. Genes Cancer 2011, 2, 475–484. [Google Scholar] [CrossRef]
- Wu, C.C.; Shete, S.; Amos, C.I.; Strong, L.C. Joint effects of germ-line p53 mutation and sex on cancer risk in Li-Fraumeni syndrome. Cancer Res. 2006, 66, 8287–8292. [Google Scholar] [CrossRef]
- Achatz, M.I.W.; Hainaut, P.; Ashton-Prolla, P. Highly prevalent TP53 mutation predisposing to many cancers in the Brazilian population: A case for newborn screening? Lancet Oncol. 2009, 10, 920–925. [Google Scholar] [CrossRef]
- Bougeard, G.; Renaux-Petel, M.; Flaman, J.M.; Charbonnier, C.; Fermey, P.; Belotti, M.; Gauthier-Villars, M.; Stoppa-Lyonnet, D.; Consolino, E.; Brugières, L.; et al. Revisiting Li-Fraumeni syndrome from TP53 mutation carriers. J. Clin. Oncol. 2015, 33, 2345–2352. [Google Scholar] [CrossRef]
- Olivier, M.; Goldgar, D.E.; Sodha, N.; Ohgaki, H.; Kleihues, P.; Hainaut, P.; Eeles, R.A. Li-Fraumeni and related syndromes: Correlation between tumor type, family structure, and TP53 genotype. Cancer Res. 2003, 63, 6643–6650. [Google Scholar]
- Huang, Z.; Hankinson, S.E.; Colditz, G.A.; Stampfer, M.J.; Hunter, D.J.; Manson, J.E.; Hennekens, C.H.; Rosner, B.; Speizer, F.E.; Willett, W.C. Dual effects of weight and weight gain on breast cancer risk. JAMA 1997, 278, 1407–1411. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Siegel, R.L.; Rosenberg, P.S.; Jemal, A. Emerging cancer trends among young adults in the USA: Analysis of a population-based cancer registry. Lancet Public Health 2019, 4, e137–e147. [Google Scholar] [CrossRef]
- Arnold, M.; Pandeya, N.; Byrnes, G.; Renehan, P.A.G.; Stevens, G.A.; Ezzati, P.M.; Ferlay, J.; Miranda, J.J.; Romieu, I.; Dikshit, R.; et al. Global burden of cancer attributable to high body-mass index in 2012: A population-based study. Lancet Oncol. 2015, 16, 36–46. [Google Scholar] [CrossRef]
- De Matos, J.C.; Pelloso, S.M.; De Barros Carvalho, M.D. Prevalence of risk factors for breast neoplasm in the city of Maringá, Paraná State, Brazil. Rev. Lat.-Am. Enferm. 2010, 18, 352–359. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bhattacharya, B.; Sarkar, S.K.; Mukherjee, N. Organochlorine pesticide residues in sediments of a tropical mangrove estuary, India: Implications for monitoring. Environ. Int. 2003, 29, 587–592. [Google Scholar] [CrossRef]
- Kannan, K.; Kajiwara, N.; Le Boeuf, B.J.; Tanabe, S. Organochlorine pesticides and polychlorinated biphenyls in California sea lions. Environ. Pollut. 2004, 131, 425–434. [Google Scholar] [CrossRef]
- Naso, B.; Zaccaroni, A.; Perrone, D.; Ferrante, M.C.; Severino, L.; Stracciari, G.L.; Lucisano, A. Organochlorine pesticides and polychlorinated biphenyls in European roe deer Capreolus capreolus resident in a protected area in Northern Italy. Sci. Total Environ. 2004, 328, 83–93. [Google Scholar] [CrossRef]
- Laden, F.; Collman, G.; Iwamoto, K.; Alberg, A.J.; Berkowitz, G.S.; Freudenheim, J.L.; Hankinson, S.E.; Helzlsouer, K.J.; Holford, T.R.; Huang, H.Y.; et al. 1,1-Dichloro-2,2-bis(p-chlorophenyl)ethylene and polychlorinated biphenyls and breast cancer: Combined analysis of five U.S. studies. J. Natl. Cancer Inst. 2001, 93, 768–776. [Google Scholar] [CrossRef][Green Version]
- López-Cervantes, M.; Torres-Sánchez, L.; Tobías, A.; López-Carrillo, L. Dichlorodiphenyldichloroethane burden and breast cancer risk: A meta-analysis of the epidemiologic evidence. Environ. Health Perspect. 2004, 112, 207–214. [Google Scholar] [CrossRef]
- Ingber, S.Z.; Buser, M.C.; Pohl, H.R.; Abadin, H.G.; Murray, H.E.; Scinicariello, F. DDT/DDE and breast cancer: A meta-analysis. Regul. Toxicol. Pharmacol. 2013, 67, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Weisburger, J.H.; Williams, G.M. The distinction between genotoxic and epigenetic carcinogens and implication for cancer risk. Toxicol. Sci. 2000, 57, 4–5. [Google Scholar] [CrossRef] [PubMed]
- Momen, N.C.; Ernst, A.; Arendt, L.H.; Olsen, J.; Li, J.; Gissler, M.; Rasmussen, F.; Ramlau-Hansen, C.H. Maternal cancer and congenital anomalies in children—A Danish nationwide cohort study. PLoS ONE 2017, 12, e0173355. [Google Scholar] [CrossRef] [PubMed]
- Monti, P.; Menichini, P.; Speciale, A.; Cutrona, G.; Fais, F.; Taiana, E.; Neri, A.; Bomben, R.; Gentile, M.; Gattei, V.; et al. Heterogeneity of TP53 mutations and p53 protein residual function in cancer: Does it matter? Front. Oncol. 2020, 10, 593383. [Google Scholar] [CrossRef] [PubMed]
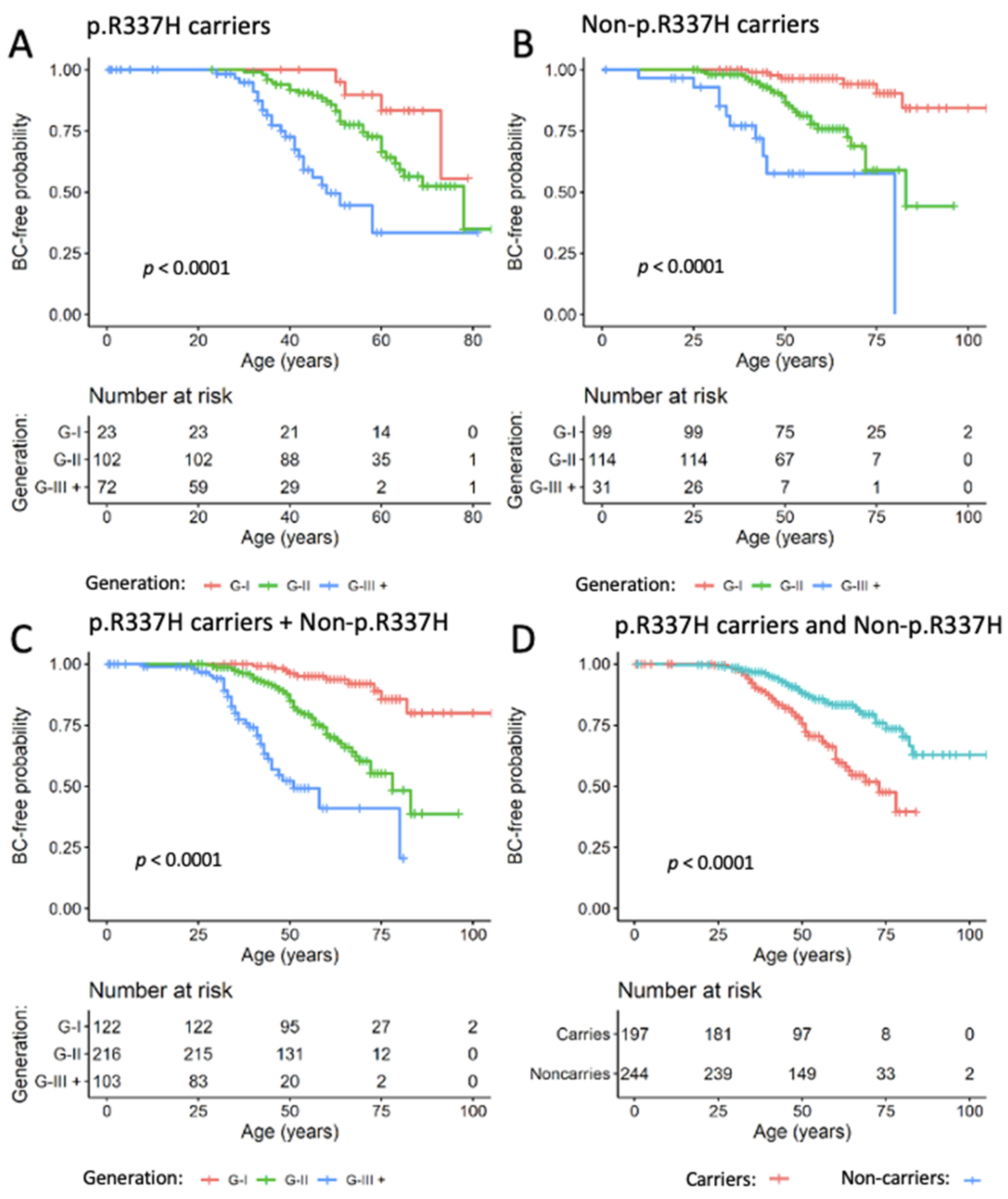
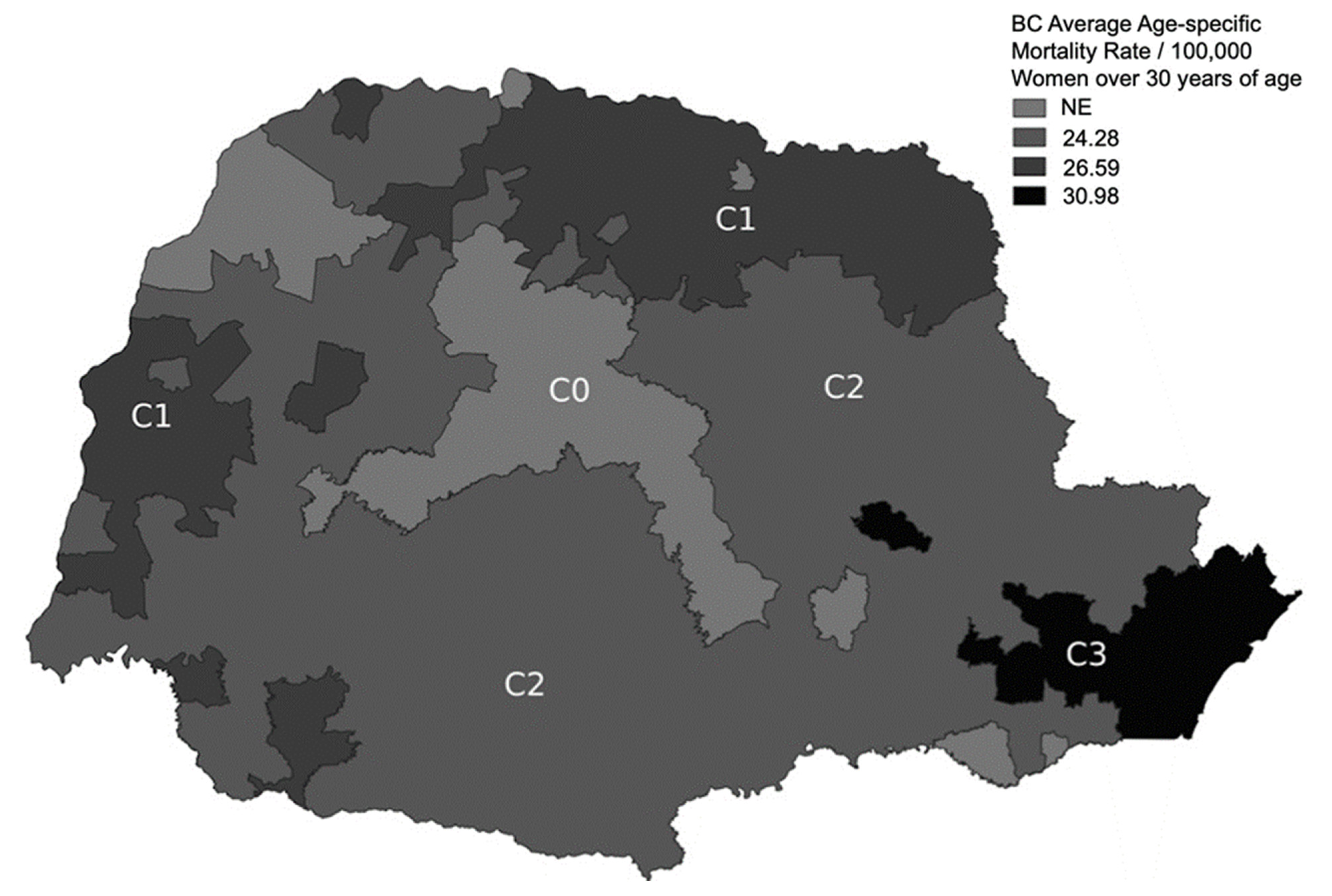
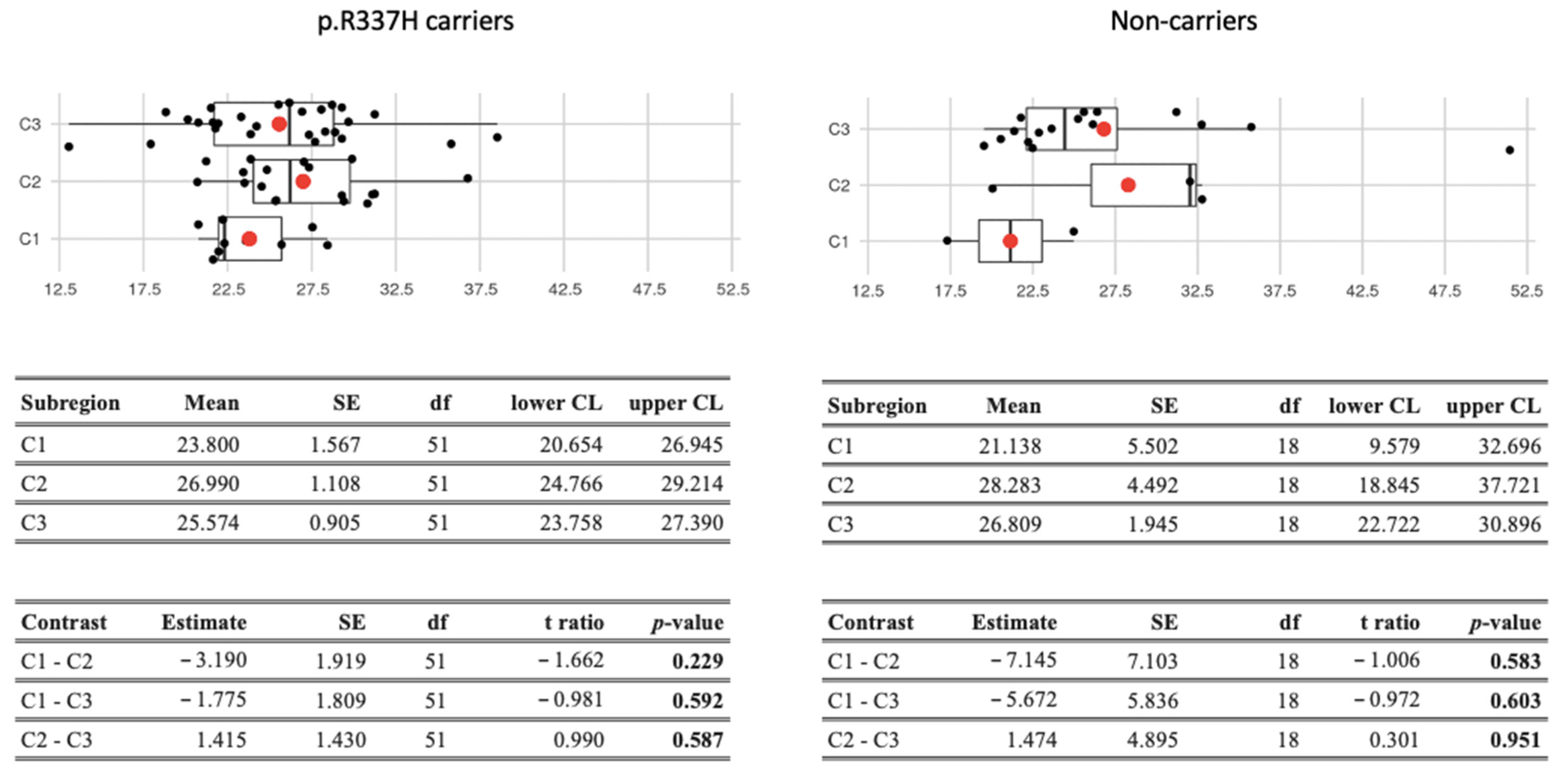
| p.R337H Carrier and Noncarrier Women | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Pedigree | C1 | C2 | C3 | Total | |||||
| Generation | Non a | p.R337H b | Non a | p.R337H b | Non a | p.R337H b | Non a | p.R337H b | |
| I | 82 | 14 | 200 | 37 | 135 | 27 | 417 | 78 | |
| II | 292 | 82 | 601 | 180 | 450 | 158 | 1343 | 420 | |
| III | III c | 307 | 102 | 691 | 218 | 524 | 190 | 1522 | 510 |
| IV | 54 | 11 | 139 | 39 | 80 | 34 | 273 | 84 | |
| V | 1 | 0 | 6 | 3 | 0 | 1 | 7 | 4 | |
| N (%) | |||||||||
| BC | 14 (1.9) | 17 (8.1) | 23 (1.4) | 25 (5.2) | 24 (2.0) | 39 (9.5) | 61 (1.7) | 81 (7.4) | |
| Total | 736 | 209 | 1637 | 477 | 1189 | 410 | 3562 | 1096 | |
| Breast Cancer | |||||||
|---|---|---|---|---|---|---|---|
| n a | Prevalence (/1000) | n | Prevalence (/1000) | Risk Ratio | p Value | ||
| X2 | |||||||
| Non-p.R337H | |||||||
| C1 | 14 | 19.02 | C2 | 23 | 14.05 | 1.354 (0.701–2.616) | 0.468 |
| C3 | 24 | 20.19 | C2 | 23 | 14.05 | 1.437 (0.395–1.227) | 0.267 |
| C3 | 24 | 20.19 | C1 | 14 | 19.02 | 1.061 (0.552–2.038) | 0.992 |
| p.R337H | |||||||
| C1 | 17 | 81.34 | C2 | 25 | 52.41 | 1.552 (0.857–2.812) | 0.200 |
| C3 | 39 | 95.12 | C2 | 25 | 52.41 | 1.815 (1.118–2.946) | 0.020 |
| C3 | 39 | 95.12 | C1 | 17 | 81.34 | 1.169 (0.678–2.016) | 0.677 |
| Total | |||||||
| C1 | 31 | 32.8 | C2 | 48 | 22.71 | 1.445 (0.926–2.255) | 0.133 |
| C3 | 63 | 39.4 | C2 | 48 | 22.71 | 1.735 (1.199–2.512) | 0.004 |
| C3 | 63 | 39.4 | C1 | 31 | 32.80 | 1.201 (0.787–1.832) | 0.457 |
| Group 1 BC Patients (Cohort from Neonatal Screening) | ||||
| Subregion | Carriers < 45 years | Carriers ≥ 45 years | Noncarriers < 45 years | Noncarriers ≥ 45 years |
| n = 81 BC | n = 61 BC | |||
| C1: BC (%) | 9 (53) 1 | 8 (47) 1 | 6 (42) 4 | 8 (58) 4 |
| C2: BC (%) | 11 (44) 2 | 14 (56) 2 | 8 (34) 5 | 15 (64) 5 |
| C3: BC (%) | 19 (49) 3 | 20 (51) 3 | 10 (41) 6 | 14 (59) 6 |
| Group 3 BC Patients (Hospital Database) | ||||
| Carriers < 45 years | Carriers ≥ 45 years | Noncarriers < 45 years | Noncarriers ≥ 45 years | |
| n = 8 | n = 506 * | |||
| Total (%) | 3 (38) 7 | 5 (62) 7 | 114 (22) 8 | 392 (78) 8 |
| Mortality Rate | ||||||||
|---|---|---|---|---|---|---|---|---|
| BC (n) a | AASMR b (/100,000) | BC (n) | AASMR (/100,000) | Relative Risk | p Value | |||
| X2 | ||||||||
| C1 | 2220 | 26.59 | C2 | 2761 | 24.28 | 1.095 (1.036–1.158) | 0.002 | |
| C3 | 2833 | 30.98 | C2 | 1.276 (1.211–1.345) | <0.001 | |||
| C3 | C1 | 2220 | 26.59 | 1.165 (1.102–1.232) | <0.001 | |||
| Municipalities in each subregion | ||||||||
| Subregions | C0 c | C1 | C2 | C3 | Total | |||
| Municipalities (n) | 66 | 114 | 200 | 19 | 399 | |||
| Women over 30 years of age in 2010 | 152,606 | 759,083 | 1,033,835 | 831,323 | 2,776,847 | |||
| Detected Alleles | p.R337H+/ p.E134*- | p.E134*+/ p.R337H- | p.R337H+/ p.E134*+ | All p.R337H+ | All p.E134*+ |
|---|---|---|---|---|---|
| n (%) | 2 (0.166) | 1 (0.083) | 6 (0.497) | 8 (0.663) | 7 (0.580) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gerber, V.K.Q.; Paraizo, M.M.; Ibañez, H.C.; Casali-da-Rocha, J.C.; Pinto, E.M.; Andrade, D.P.; Ibañez, M.V.C.; Komechen, H.; Figueiredo, M.M.O.; Custódio, G.; et al. Environmental Contaminants Modulate Breast Cancer Development and Outcome in TP53 p.R337H Carriers and Noncarriers. Cancers 2022, 14, 3014. https://doi.org/10.3390/cancers14123014
Gerber VKQ, Paraizo MM, Ibañez HC, Casali-da-Rocha JC, Pinto EM, Andrade DP, Ibañez MVC, Komechen H, Figueiredo MMO, Custódio G, et al. Environmental Contaminants Modulate Breast Cancer Development and Outcome in TP53 p.R337H Carriers and Noncarriers. Cancers. 2022; 14(12):3014. https://doi.org/10.3390/cancers14123014
Chicago/Turabian StyleGerber, Viviane K. Q., Mariana M. Paraizo, Humberto C. Ibañez, José C. Casali-da-Rocha, Emilia M. Pinto, Diancarlos P. Andrade, Marilea V. C. Ibañez, Heloisa Komechen, Mirna M. O. Figueiredo, Gislaine Custódio, and et al. 2022. "Environmental Contaminants Modulate Breast Cancer Development and Outcome in TP53 p.R337H Carriers and Noncarriers" Cancers 14, no. 12: 3014. https://doi.org/10.3390/cancers14123014
APA StyleGerber, V. K. Q., Paraizo, M. M., Ibañez, H. C., Casali-da-Rocha, J. C., Pinto, E. M., Andrade, D. P., Ibañez, M. V. C., Komechen, H., Figueiredo, M. M. O., Custódio, G., Fiori, C. M. C. M., Balbinotti, J. H. G., Nardin, J. M., Almeida, T. A., Beltrame, O. O., Yamada, P. A., de Fraga, G. S., de Brito, L. L., Martins, J., ... Figueiredo, B. C. (2022). Environmental Contaminants Modulate Breast Cancer Development and Outcome in TP53 p.R337H Carriers and Noncarriers. Cancers, 14(12), 3014. https://doi.org/10.3390/cancers14123014







