Response to Systemic Therapies in Ovarian Adult Granulosa Cell Tumors: A Literature Review
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Selection
2.2. Data Extraction
2.3. Data Analysis
3. Results
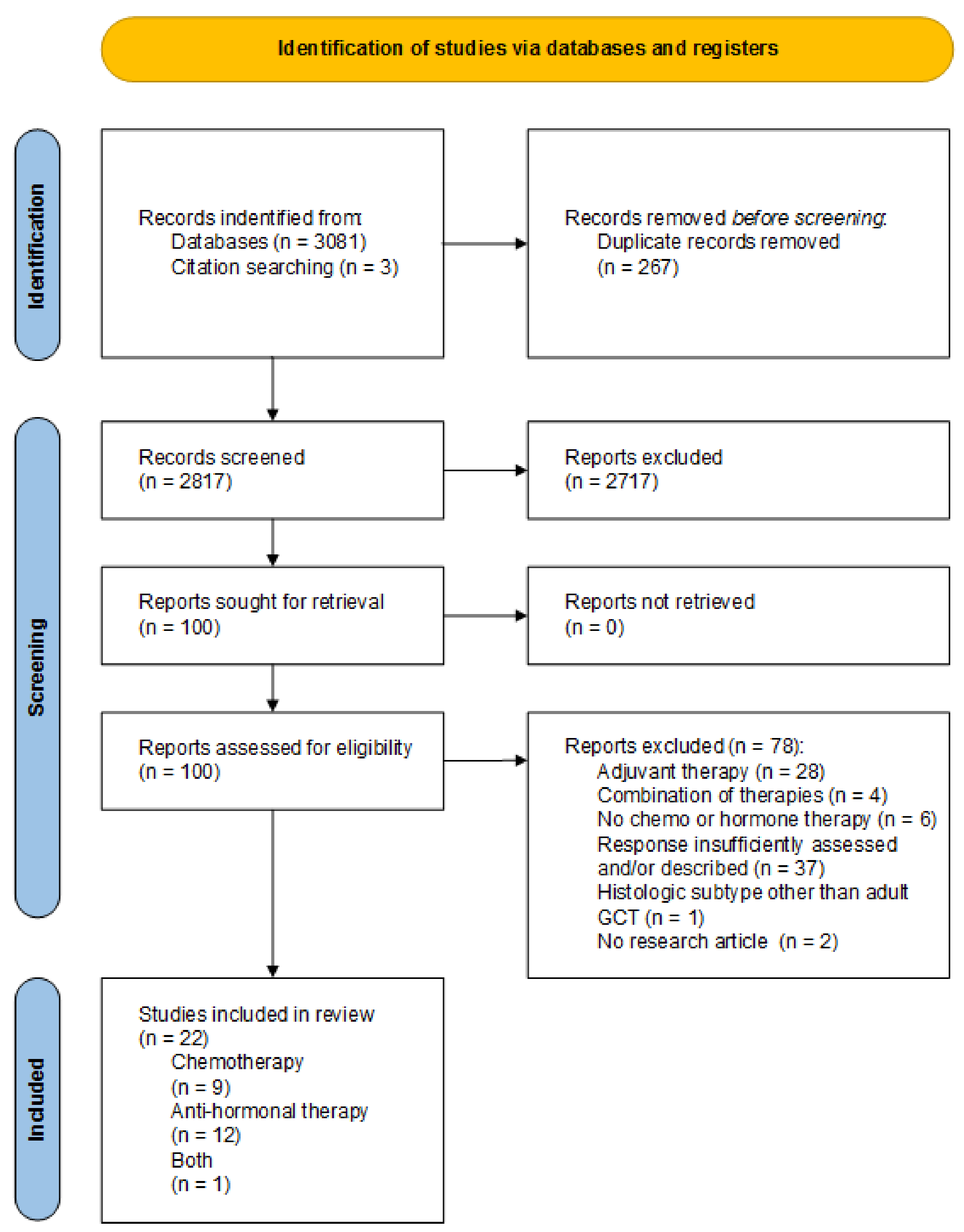
3.1. Study Selection
3.2. Study Characteristics
| Author, Year | Study Design | Study Period | Patients (n) | Stage at Diagnosis | Previous Treatment n (%) | Chemotherapy n (%) | Response n (%) | PFS (mo) Median (Range) | OS (mo) Median (Range) | FU (mo) Median (Range) | Disease Status n (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Tresukosol, 1995 [34] | retrospective case report | 1992–1994 | 1 | IC 1 | S ×1, CT ×1 | paclitaxel 1(100) | PR 1 (100) | 12 | 24+ 1 | 24 1 | AWD 1 (100) 1 |
| Shavit, 2012 [35] | retrospective case report | 2006–2007 | 1 | IA | S ×2, CT ×2 | docetaxel 1 (100) | SD 1 (100) | 24 | NA | 24 | NA |
| Uygun, 2003 [36] | retrospective cohort | 1979–1999 | 4 | IIIB-IV | S 4 (100) CT 4 (100) | CC 3 (75) CAP 1 (25) | CR 2 (50) PR 2 (50) | 38 (21–73) 1 | 40.5 (33–73) 1 | 40.5 (33–73) 1 | NED 2 (50) DOD 1 (25) DOC 1 (25) |
| Pectasides, 2008 [37] | retrospective cohort | 1983–2007 | 5 | IA-IV | S 5 (100) CT 5 (100) | CP 2 (40) CVB 1 (10) 5FU 2 (40) | CR 2 (40) PR 1 (20) PD 2 (40) | 7 (0–31) 1 | 28 (4–31) 1 | NA | AWD 4 (80) DOD 1 (20) |
| van Meurs, 2014 [16] | retrospective cohort | 1968–2011 | 9 | I-IIIC | S 9 (100) RT 1 (11) AHT 1 (11) | BEP 9 (100) | CR 1 (11) PR 1 (11) SD 7 (78) | 12 (2–50) | 50 (4–165) | NA | NED 2 (22) AWD 3 (33) DOD 3 (33) DOC 1 (11) |
| Wilson, 2015 [30] | retrospective cohort | 1955–2012 | 17 2 | IA-IC | S 17 (100) CT ns, RT ns | CT 17 (100) | CR 1 (3) PR 8 (27) SD 4 (13) PD 15 (50) ×1 3 | 8.6 | NA | NA | NA |
| Brown, 2004 [11] | retrospective cohort | 1985–2002 | 21aGCT /30SCST | IA-IIIC | S 30 (100) CT 22 (73) RT 2 (7) | NPT 17 (57) PT 13 (43) | CR 3 (10) PR 7 (23) SD 7 (23) PD 12 (40) 4 | 16.8 (0–68) | NA | 100.7 (8.1–361.3) | NED 3 (10) AWD 20 (67) DOD 5 (17) DOC 2 (6) |
| Pautier, 2008 [13] | prospective cohort | 1990–2002 | 14aGCT /20GCT | I-IV | S 20 (100) CT 1 (5) | BEP 20 (100) | CR 9 (45) PR 9 (45) SD 1 (5) PD 1 (5) | 24 (4–84) | 46 | 45 (3–112) | NED 9 (45) AWD 3 (15) DOD 8 (40) |
| Burton, 2016 [31] | prospective cohort | 2000–2013 | 31 SCST | NA | S 31 (100) CT 24 (77) RT 3 (10) AHT 3 (10) IT 1 (3) | paclitaxel 31 (100) | CR 1(3) PR 8 (26) SD 15 (48) PD 6 (19) 5 | 10 | 73.6 | 67 | AWD 15 (48) DOD 16 (52) |
| Ray-Coquard, 2020 [32] | prospective RCT | 2013–2020 | 27aGCT /32 SCST | I-IV | S 32 (100) CT 32 (100) RT 4 (13) AHT 8 (25) | paclitaxel 32 (100) | CR 0 (0) PR 8 (25) SD 17 (53) PD 7 (22) | 14.7 (95% CI 11.5–18.3) | NA | 38.9 (IQR 36.4–43.8) | AWD 26 (81) DOD 6 (19) |
| Author, Year | Study Design | Study Period | Patients (n) | Stage at Diagnosis | Previous Treatment n (%) | Anti-Hormonal Therapy n (%) | Response n (%) | PFS (mo) or Median (Range) | OS (mo) Median (Range) | FU (mo) Median (Range) | Disease Status n (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fishman, 1996 [38] | retrospective cohort | 1991–1996 | 4 | NA | CT 4 (100) AHT 1 (25) | leuprolide acetate 4 (100) | PR 2 (50) SD 2 (50) | 8 (3–13+) 1 | NA | 11 | AWD 3 (75) DOD 1 (25) |
| Assi, 2017 [39] | retrospective case report | 2013–2016 | 1 | I | S ×1, CT ×2 | letrozole 1 (100) | PR 1 (100) | 35 1 | 44+ 1 | 44 1 | AWD 1 (100) |
| Hardy, 2005 [20] | retrospective case report | 1999–2004 | 1 | >II | S ×3, CT ×2 | megestrol/tamoxifen 1 (100) | CR 1 (100) | 60+ 1 | 60+ 1 | 60 1 | NED 1 (100) |
| Abdul Munem, 2012 [40] | retrospective case report | 2009–2010 | 1 | NA | S ×3, CT ×3, RT ×1 | anastrozole 1 (100) | SD 1 (100) | 20+ | 20+ | 20 | AWD 1 (100) 1 |
| AlHilli, 2012 [41] | retrospective case report | 2010 | 1 | IA | S ×7, CT ×1, RT ×4 | letrozole 1 (100) | PR 1 | 6 | NA | 6 | AWD 1 (100) 1 |
| van Meurs, 2015 [42] | retrospective cohort | 1979–2013 | 16 2 | I-III | S 16 (100) CT 8 (50) RT 7 (44) AHT 6 (38) | anastrozole 2 (9) goserelin 2 (9) letrozole 6 (27) megestrol acetate 6 (27) tamoxifen 5 (23) aromatase inhibitor 1 (5) | SD 14 (64) PD 8 (36) | 4 (2–53) 1 | NA | NA | NED 1 (6) AWD 8 (50) DOD 5 (31) DOC 2 (13) |
| Wilson, 2015 [30] | retrospective cohort | 1955–2012 | 26 3 | IA-IC | S 26 (100) CT ns, RT ns | AHT 126 (100) | CR 1 (2) PR 5 (11) SD 21 (48) PD 12 (27) 4 | 18 (6–54) | NA | NA | NA |
| Lamm, 2016 [43] | retrospective case report | 2012 | 1 | IA 1 | S ×6, AHT ×1 | letrozole 1 (100) | CR 1 (100) | 8 | NA | 12 1 | AWD 1 (100) |
| Schwartz, 2016 [44] | retrospective case report | 2008–2009 | 1 5 | IA | S ×2, CT ×1, RT ×1 | anastrozole 1 (100) | PR | 19 (8–30) | NA | 37.5 (30–45) | AWD 1 (100) |
| Yazigi, 2016 [45] | retrospective case report | 2003–2014 | 1 | NA | S ×5, CT ×2, AHT ×2 | letrozole 1 (100) | PR 1 | 11 | 31 | 31 | DOD 1 (100) |
| Tsubamoto, 2019 [46] | retrospective cohort | 2007–2015 | 3 | NA | S 3 (100) | leuprolide acetate 3 (100) | SD 3 (100) | 4 (4–22) | 27 (6–74) | 27 (6–74) | AWD 1 (33) DOD 2 (67) |
| Moon, 2021 [47] | retrospective case report | NA | 1 | NA | S ×3, CT ×3, AHT ×2, RT ×1 | megestrol acetate/tamoxifen 1 (100) | SD1 | 22 | NA | 48 1 | AWD |
| Banerjee, 2021 [33] | retrospective cohort | 2012–2017 | 41 GCT | NA | S 41 (100) CT 16 (39) RT 5 (13) ns 19 (46) | anastrozole 38 (100) | PR 1 (3) SD 29 (76) PD 8 (21) | 8.6 (95% CI 5.5–13.5) | NA | 52 | NA |
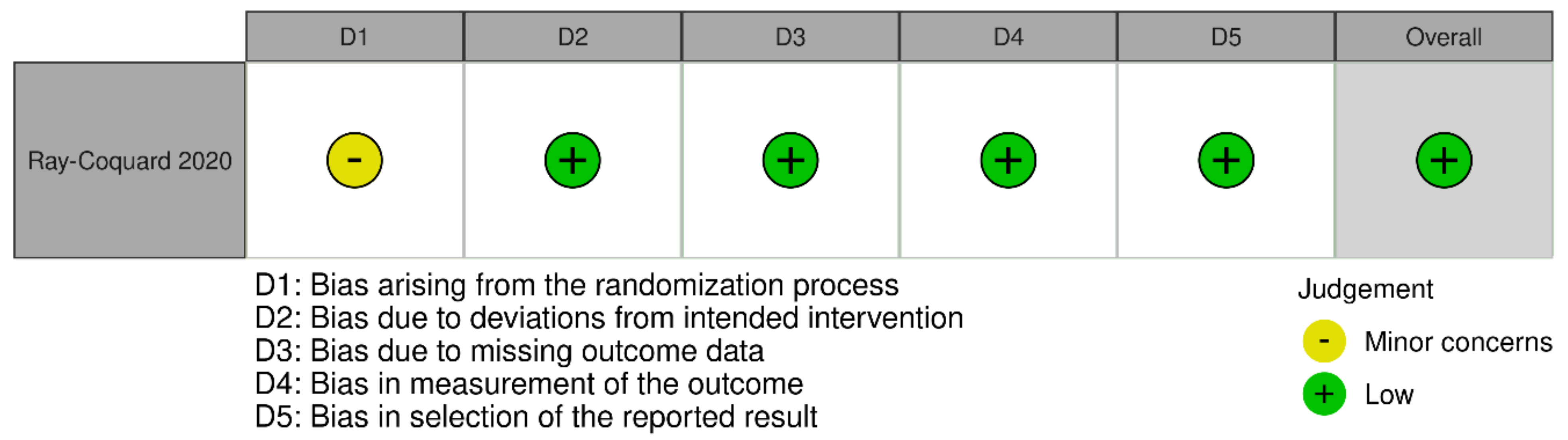
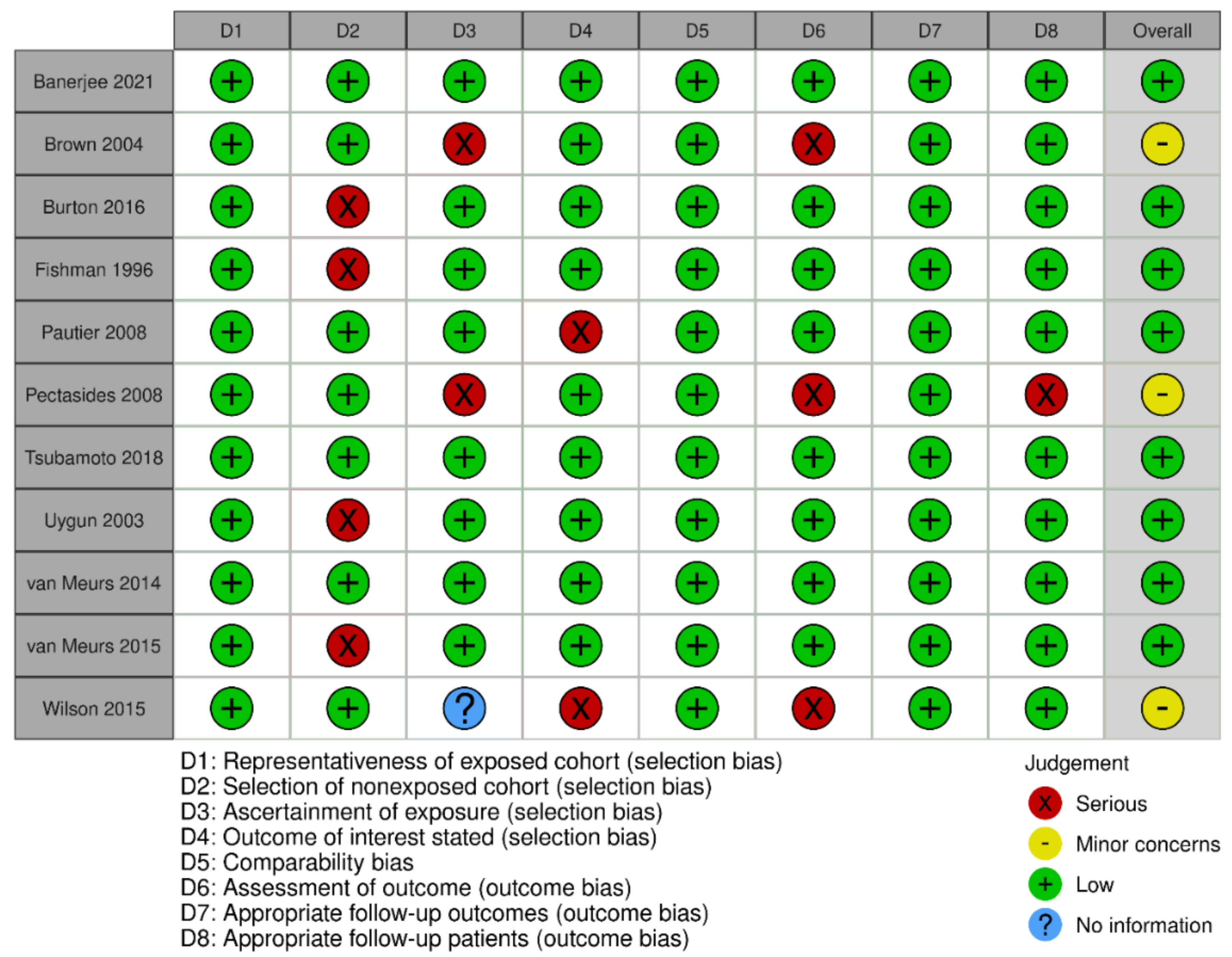
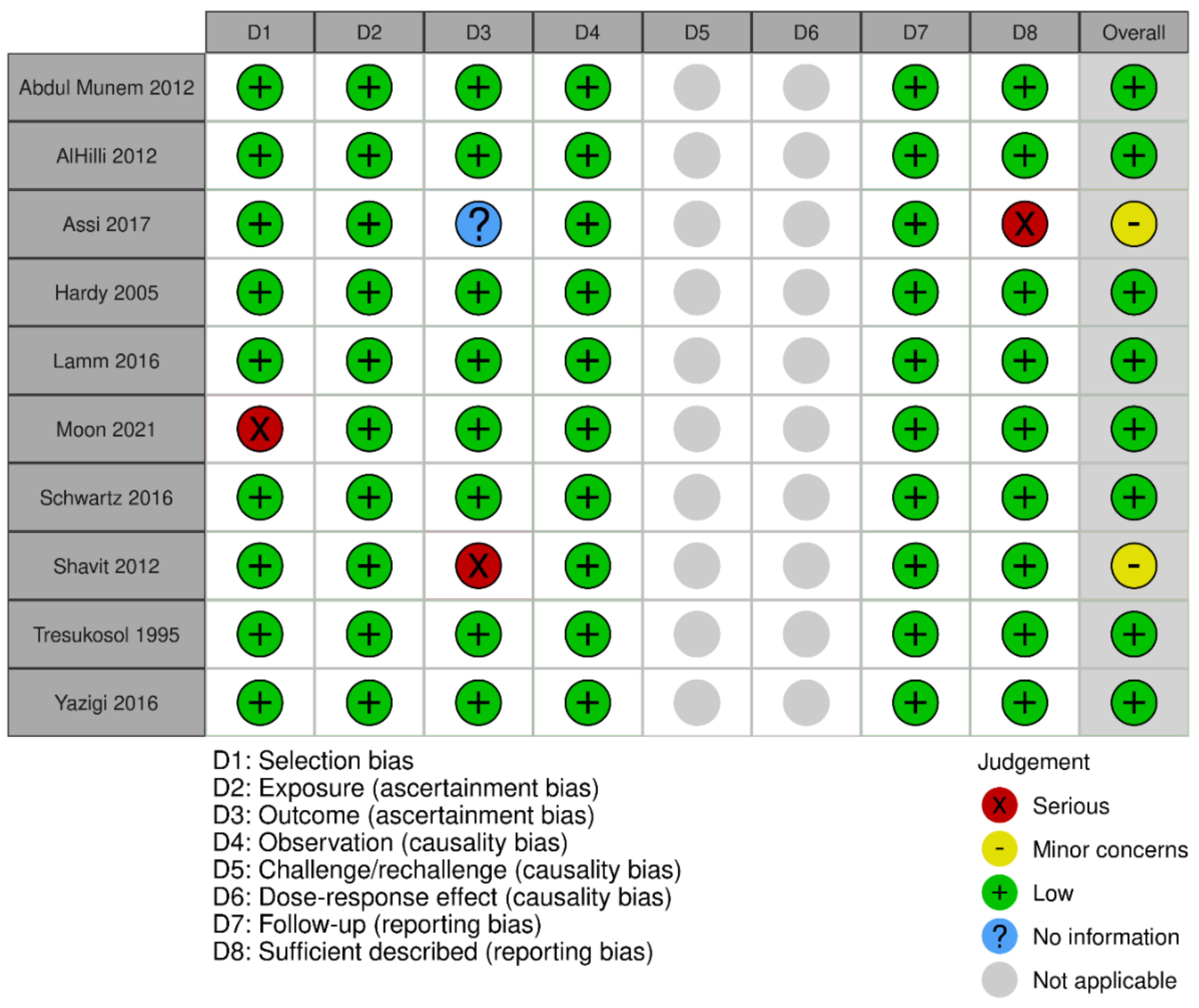
3.3. Quality Assessment
3.4. Chemotherapy
3.5. Anti-Hormonal Therapy
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A. Comprehensive Search Strategies
Appendix B. Risk of Bias Results per Type of Study
References
- Schumer, S.T.; Cannistra, S.A. Granulosa cell tumor of the ovary. J. Clin. Oncol. 2003, 21, 1180–1189. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.P.; Köbel, M.; Senz, J.; Morin, R.D.; Clarke, B.A.; Wiegand, K.C.; Leung, G.; Zayed, A.; Mehl, E.; Kalloger, S.E. Mutation of FOXL2 in granulosa-cell tumors of the ovary. N. Engl. J. Med. 2009, 360, 2719–2729. [Google Scholar] [CrossRef] [PubMed]
- Young, R.H.; Dickersin, G.R.; Scully, R.E. Juvenile granulosa cell tumor of the ovary. A clinicopathological analysis of 125 cases. Am. J. Surg. Pathol. 1984, 8, 575–596. [Google Scholar] [CrossRef] [PubMed]
- Mangili, G.; Ottolina, J.; Gadducci, A.; Giorda, G.; Breda, E.; Savarese, A.; Candiani, M.; Frigerio, L.; Scarfone, G.; Pignata, S.; et al. Long-term follow-up is crucial after treatment for granulosa cell tumours of the ovary. Br. J. Cancer 2013, 109, 29–34. [Google Scholar] [CrossRef]
- Berek, J.S.; Kehoe, S.T.; Kumar, L.; Friedlander, M. Cancer of the ovary, fallopian tube, and peritoneum. Int. J. Gynecol. Obstet. 2018, 143, 59–78. [Google Scholar] [CrossRef]
- Ray-Coquard, I.; Morice, P.; Lorusso, D.; Prat, J.; Oaknin, A.; Pautier, P.; Colombo, N. Non-epithelial ovarian cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29, iv1–iv18. [Google Scholar] [CrossRef]
- Tokunaga, H.; Mikami, M.; Nagase, S.; Kobayashi, Y.; Tabata, T.; Kaneuchi, M.; Satoh, T.; Hirashima, Y.; Matsumura, N.; Yokoyama, Y. The 2020 Japan Society of Gynecologic Oncology guidelines for the treatment of ovarian cancer, fallopian tube cancer, and primary peritoneal cancer. J. Gynecol. Oncol. 2021, 32, e49. [Google Scholar] [CrossRef]
- Brown, J.; Shvartsman, H.S.; Deavers, M.T.; Ramondetta, L.M.; Burke, T.W.; Munsell, M.F.; Gershenson, D.M. The activity of taxanes compared with bleomycin, etoposide, and cisplatin in the treatment of sex cord-stromal ovarian tumors. Gynecol. Oncol. 2005, 97, 489–496. [Google Scholar] [CrossRef]
- Gershenson, D.M.; Morris, M.; Burke, T.W.; Levenback, C.; Matthews, C.M.; Wharton, J.T. Treatment of poor-prognosis sex cord-stromal tumors of the ovary with the combination of bleomycin, etoposide, and cisplatin. Obstet. Gynecol. 1996, 87, 527–531. [Google Scholar] [CrossRef]
- Ray-Coquard, I.; Brown, J.; Harter, P.; Provencher, D.M.; Fong, P.C.; Maenpaa, J.; Ledermann, J.A.; Emons, G.; Rigaud, D.B.; Glasspool, R.M. Gynecologic Cancer InterGroup (GCIG) consensus review for ovarian sex cord stromal tumors. Int. J. Gynecol. Cancer 2014, 24, S42–S47. [Google Scholar] [CrossRef]
- Brown, J.; Shvartsman, H.S.; Deavers, M.T.; Burke, T.W.; Munsell, M.F.; Gershenson, D.M. The activity of taxanes in the treatment of sex cord-stromal ovarian tumors. J. Clin. Oncol. 2004, 22, 3517–3523. [Google Scholar] [CrossRef] [PubMed]
- Homesley, H.D.; Bundy, B.N.; Hurteau, J.A.; Roth, L.M. Bleomycin, etoposide, and cisplatin combination therapy of ovarian granulosa cell tumors and other stromal malignancies: A Gynecologic Oncology Group study. Gynecol. Oncol. 1999, 72, 131–137. [Google Scholar] [CrossRef]
- Pautier, P.; Gutierrez-Bonnaire, M.; Rey, A.; Sillet-Bach, I.; Chevreau, C.; Kerbrat, P.; Morice, P.; Duvillard, P.; Lhommé, C. Combination of bleomycin, etoposide, and cisplatin for the treatment of advanced ovarian granulosa cell tumors. Int. J. Gynecol. Cancer 2008, 18, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Colombo, N.; Sessa, C.; Landoni, F.; Sartori, E.; Pecorelli, S.; Mangioni, C. Cisplatin, vinblastine, and bleomycin combination chemotherapy in metastatic granulosa cell tumor of the ovary. Obstet. Gynecol. 1986, 67, 265–268. [Google Scholar] [CrossRef] [PubMed]
- Sigismondi, C.; Gadducci, A.; Lorusso, D.; Candiani, M.; Breda, E.; Raspagliesi, F.; Cormio, G.; Marinaccio, M.; Mangili, G. Ovarian Sertoli-Leydig cell tumors. A retrospective MITO study. Gynecol. Oncol. 2012, 125, 673–676. [Google Scholar] [CrossRef] [PubMed]
- Van Meurs, H.S.; Buist, M.R.; Westermann, A.M.; Sonke, G.S.; Kenter, G.G.; van der Velden, J. Effectiveness of chemotherapy in measurable granulosa cell tumors: A retrospective study and review of literature. Int. J. Gynecol. Cancer 2014, 24, 496–505. [Google Scholar] [CrossRef]
- Jules-Elysee, K.; White, D.A. Bleomycin-induced pulmonary toxicity. Clin. Chest Med. 1990, 11, 1–20. [Google Scholar] [CrossRef]
- Boyd, L.R.; Muggia, F.M. Carboplatin/paclitaxel induction in ovarian cancer: The finer points. Oncology 2018, 32, 418–420, 422–424. [Google Scholar]
- Steegers, E.A.; Fauser, B.C.; Hilders, C.G.; Jaddoe, V.W.; Massuger, L.F.; Schoenmakers, S.; van der Post, J.A. Textbook of Obstetrics and Gynaecology: A Life Course Approach; Bohn Stafleu van Loghum: Houten, The Netherlands, 2019. [Google Scholar]
- Hardy, R.D.; Bell, J.G.; Nicely, C.J.; Reid, G.C. Hormonal treatment of a recurrent granulosa cell tumor of the ovary: Case report and review of the literature. Gynecol. Oncol. 2005, 96, 865–869. [Google Scholar] [CrossRef]
- Farinola, M.A.; Gown, A.M.; Judson, K.; Ronnett, B.M.; Barry, T.S.; Movahedi-Lankarani, S.; Vang, R. Estrogen receptor α and progesterone receptor expression in ovarian adult granulosa cell tumors and Sertoli-Leydig cell tumors. Int. J. Gynecol. Pathol. 2007, 26, 375–382. [Google Scholar] [CrossRef]
- Van Meurs, H.S.; van Lonkhuijzen, L.R.; Limpens, J.; van der Velden, J.; Buist, M.R. Hormone therapy in ovarian granulosa cell tumors: A systematic review. Gynecol. Oncol. 2014, 134, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Booth, A.; Clarke, M.; Dooley, G.; Ghersi, D.; Moher, D.; Petticrew, M.; Stewart, L. The nuts and bolts of PROSPERO: An international prospective register of systematic reviews. Syst. Rev. 2012, 1, 2. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int. J. Surg. 2021, 88, 105906. [Google Scholar] [CrossRef] [PubMed]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—a web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Wells, G.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. Newcastle-Ottawa Quality Assessment Scale Cohort Studies; University of Ottawa: Ottawa, ON, Canada, 2014. [Google Scholar]
- Murad, M.H.; Sultan, S.; Haffar, S.; Bazerbachi, F. Methodological quality and synthesis of case series and case reports. BMJ Evid. Based Med. 2018, 23, 60–63. [Google Scholar] [CrossRef]
- Wilson, M.K.; Fong, P.; Mesnage, S.; Chrystal, K.; Shelling, A.; Payne, K.; Mackay, H.; Wang, L.; Laframboise, S.; Rouzbahman, M.; et al. Stage I granulosa cell tumours: A management conundrum? Results of long-term follow up. Gynecol. Oncol. 2015, 138, 285–291. [Google Scholar] [CrossRef]
- Burton, E.R.; Brady, M.; Homesley, H.D.; Rose, P.G.; Nakamura, T.; Kesterson, J.P.; Rotmensch, J.; Tate Thigpen, J.; Van Le, L. A phase II study of paclitaxel for the treatment of ovarian stromal tumors: An NRG Oncology/Gynecologic Oncology Group Study. Gynecol. Oncol. 2020, 140, 48–52. [Google Scholar] [CrossRef]
- Ray-Coquard, I.; Harter, P.; Lorusso, D.; Dalban, C.; Vergote, I.; Fujiwara, K.; Gladieff, L.; Lück, H.J.; Floquet, A.; Chevalier-Place, A.; et al. Effect of Weekly Paclitaxel With or Without Bevacizumab on Progression-Free Rate Among Patients With Relapsed Ovarian Sex Cord-Stromal Tumors: The ALIENOR/ENGOT-ov7 Randomized Clinical Trial. JAMA Oncol. 2020, 6, 1923–1930. [Google Scholar] [CrossRef]
- Banerjee, S.N.; Tang, M.; O’Connell, R.L.; Sjoquist, K.; Clamp, A.R.; Millan, D.; Nottley, S.; Lord, R.; Mullassery, V.M.; Hall, M.; et al. A phase 2 study of anastrozole in patients with oestrogen receptor and/progesterone receptor positive recurrent/metastatic granulosa cell tumours/sex-cord stromal tumours of the ovary: The PARAGON/ANZGOG 0903 trial. Gynecol. Oncol. 2021, 163, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Tresukosol, D.; Kudelka, A.P.; Edwards, C.L.; Charnsangavej, C.; Narboni, N.; Kavanagh, J.J. Recurrent ovarian granulosa cell tumor: A case report of a dramatic response to Taxol. Int. J. Gynecol. Cancer 1995, 5, 156–159. [Google Scholar] [CrossRef] [PubMed]
- Shavit, T.; Bruchim, I.; Ben-Harim, Z.; Fishman, A. Successful response to docetaxel treatment in recurrent ovarian granulosa cell tumor: A case report. Eur. J. Gynaecol. Oncol. 2012, 33, 419–420. [Google Scholar] [PubMed]
- Uygun, K.; Aydiner, A.; Saip, P.; Kocak, Z.; Basaran, M.; Dincer, M.; Topuz, E. Clinical parameters and treatment results in recurrent granulosa cell tumor of the ovary. Gynecol. Oncol. 2003, 88, 400–403. [Google Scholar] [CrossRef]
- Pectasides, D.; Papaxoinis, G.; Fountzilas, G.; Aravantinos, G.; Pectasides, E.; Mouratidou, D.; Economopoulos, T.; Andreadis, C. Adult granulosa cell tumors of the ovary: A clinicopathological study of 34 patients by the Hellenic Cooperative Oncology Group (HeCOG). Anticancer. Res. 2008, 28, 1421–1427. [Google Scholar]
- Fishman, A.; Kudelka, A.P.; Tresukosol, D.; Edwards, C.L.; Freedman, R.S.; Kaplan, A.L.; Girtanner, R.E.; Kavanagh, J.J. Leuprolide acetate for treating refractory or persistent ovarian granulosa cell tumor. J. Reprod Med. 1996, 41, 393–396. [Google Scholar]
- Assi, T.; Kourie, H.R.; El Rassy, E.; Moussa, T.; Kattan, J. Response of an ovarian granulosa cell tumor with everolimus and exemestane after initial response to letrozole. Anticancer. Drugs 2017, 28, 931–933. [Google Scholar] [CrossRef]
- Abdul Munem, A.; Al-Bahrani, B.; Mehdi, I.; Kamona, A.; Nadas, A.M. Aromatase inhibitors--a viable option for recurrent granulosa cell tumour of ovary: Overview and case report. J. Pak. Med. Assoc. 2012, 62, 505–507. [Google Scholar]
- Alhilli, M.M.; Long, H.J.; Podratz, K.C.; Bakkum-Gamez, J.N. Aromatase inhibitors in the treatment of recurrent ovarian granulosa cell tumors: Brief report and review of the literature. J. Obs. Gynaecol. Res. 2012, 38, 340–344. [Google Scholar] [CrossRef]
- Van Meurs, H.S.; van der Velden, J.; Buist, M.R.; van Driel, W.J.; Kenter, G.G.; van Lonkhuijzen, L.R. Evaluation of response to hormone therapy in patients with measurable adult granulosa cell tumors of the ovary. Acta Obstet. Gynecol. Scand. 2015, 94, 1269–1275. [Google Scholar] [CrossRef]
- Lamm, W.; Schiefer, A.; Nöbauer, I.M.; Horvat, R.; Speiser, P.; Köstler, W.J. Aromatase Inhibitor Therapy As Effective Rescue in a Patient With Tamoxifen-Refractory Metastatic Granulosa Cell Tumor of the Ovary. J. Clin. Oncol. 2016, 34, e31–e33. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, M.; Huang, G.S. Retreatment with aromatase inhibitor therapy in the management of granulosa cell tumor. Gynecol. Oncol. Rep. 2015, 15, 20–21. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yazigi, R.; Rodríguez, T.; Buckel, E.; Wash, A. Ovarian granulosa cell tumour and letrozole: A case report. J. Obs. Gynaecol. 2016, 36, 122–123. [Google Scholar] [CrossRef] [PubMed]
- Tsubamoto, H.; Ueda, T.; Inoue, K.; Isono-Nakata, R.; Saeki, S.; Kato, Y.; Shibahara, H. Effects of leuprorelin for the treatment of recurrent gynecological cancer by assessment including self-administered quality-of-life questionnaire. J. Obstet. Gynaecol. Res. 2018, 45, 203–209. [Google Scholar] [CrossRef]
- Moon, A.S.; Dorigo, O. Long-term efficacy of megestrol acetate and tamoxifen in a recurrent adult granulosa cell tumor of the ovary. Gynecol. Oncol. Rep. 2021, 36, 100770. [Google Scholar] [CrossRef]
- McGuinness, L.A.; Higgins, J.P. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res. Synth. Methods 2021, 12, 55–61. [Google Scholar] [CrossRef]
- Schultz, K.A.; Harris, A.K.; Schneider, D.T.; Young, R.H.; Brown, J.; Gershenson, D.M.; Dehner, L.P.; Hill, D.A.; Messinger, Y.H.; Frazier, A.L. Ovarian Sex Cord-Stromal Tumors. J. Oncol Pr. 2016, 12, 940–946. [Google Scholar] [CrossRef]
- Al Harbi, R.; McNeish, I.A.; El-Bahrawy, M. Ovarian sex cord-stromal tumors: An update on clinical features, molecular changes, and management. Int. J. Gynecol. Cancer 2021, 31, 161–168. [Google Scholar] [CrossRef]
- Woodcock, J.; LaVange, L.M. Master protocols to study multiple therapies, multiple diseases, or both. N. Engl. J. Med. 2017, 377, 62–70. [Google Scholar] [CrossRef]
- How, J.A.; Jazaeri, A.; Westin, S.N.; Sood, A.K.; Ramondetta, L.M.; Xu, M.; Abonofal, A.; Karp, D.D.; Subbiah, V.; Stephen, B. The clinical efficacy and safety of single-agent pembrolizumab in patients with recurrent granulosa cell tumors of the ovary: A case series from a phase II basket trial. Investig. New Drugs 2021, 39, 829–835. [Google Scholar] [CrossRef]
- Haltia, U.-M.; Andersson, N.; Yadav, B.; Färkkilä, A.; Kulesskiy, E.; Kankainen, M.; Tang, J.; Bützow, R.; Riska, A.; Leminen, A. Systematic drug sensitivity testing reveals synergistic growth inhibition by dasatinib or mTOR inhibitors with paclitaxel in ovarian granulosa cell tumor cells. Gynecol. Oncol. 2017, 144, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Roze, J.; Sendino Garví, E.; Stelloo, E.; Stangl, C.; Sereno, F.; Duran, K.; Groeneweg, J.; Paijens, S.; Nijman, H.; van Meurs, H. In Vitro Systematic Drug Testing Reveals Carboplatin, Paclitaxel, and Alpelisib as a Potential Novel Combination Treatment for Adult Granulosa Cell Tumors. Cancers 2021, 13, 368. [Google Scholar] [CrossRef] [PubMed]
| Regimen | Number of Regimens | CR n (%) | PR n (%) | SD n (%) | PD n (%) | Unknown n (%) |
|---|---|---|---|---|---|---|
| Platinum-based | 37 | 4 (11) | 11 (30) | 10 (27) | 10 (27) | 2 (5) 1 |
| Taxane-based | 9 | 0 (0) | 2 (22) | 3 (33) | 4 (45) | 0 (0) |
| Platinum taxane combination | 6 | 2 (33) | 0 (0) | 2 (33) | 2 (33) | 0 (0) |
| Other 2 | 6 | 0 (0) | 0 (0) | 1 (17) | 5 (83) | 0 (0) |
| Total | 58 | 6 (10) | 13 (22) | 16 (28) | 21 (36) | 2 (4) |
| Regimen | Number of Regimens | CR n (%) | PR n (%) | SD n (%) | PD n (%) | Unknown n (%) |
|---|---|---|---|---|---|---|
| Platinum-based | 37 | 4 (11) | 11 (30) | 10 (27) | 10 (27) | 2 (5) 1 |
| Taxane-based | 7 | 0 (0) | 1 (14) | 2 (29) | 4 (57) | 0 (0) |
| Platinum taxane combination | 6 | 2 (33) | 0 (0) | 2 (33) | 2 (33) | 0 (0) |
| Other 2 | 6 | 0 (0) | 0 (0) | 1 (17) | 5 (83) | 0 (0) |
| Total | 56 | 5 (9) | 12 (21) | 16 (28) | 22 (39) | 2 (3) |
| Regimen | Number of Regimens | CR n (%) | PR n (%) | SD n (%) | PD n (%) | Unknown n (%) |
|---|---|---|---|---|---|---|
| Aromatase inhibitor | 16 | 1 (6) | 5 (31) | 7 (44) | 3 (19) | 0 (0) |
| GnRH agonist | 9 | 0 (0) | 2 (22) | 6 (67) | 1 (11) | 0 (0) |
| Progestin | 6 | 0 (0) | 0 (0) | 5 (83) | 1 (17) | 0 (0) |
| SERM | 5 | 0 (0) | 0 (0) | 2 (40) | 3 (60) | 0 (0) |
| Combinations | 2 | 1 (50) | 0 (0) | 1 (50) | 0 (0) | 0 (0) |
| Type unknown | 44 | 1 (3) | 5 (11) | 21 (48) | 12 (27) | 5 (11) 1 |
| Total | 82 | 3 (4) | 12 (15) | 42 (51) | 20 (24) | 5 (6) |
| Regimen | Number of Regimens | CR n (%) | PR n (%) | SD n (%) | PD n (%) | Unknown n (%) |
|---|---|---|---|---|---|---|
| Aromatase inhibitor | 9 | 0 (0) | 0 (0) | 6 (67) | 3 (33) | 0 (0) |
| GnRH agonist | 9 | 0 (0) | 2 (22) | 6 (67) | 1 (11) | 0 (0) |
| Progestin | 6 | 0 (0) | 0 (0) | 5 (83) | 1 (17) | 0 (0) |
| SERM | 5 | 0 (0) | 0 (0) | 2 (40) | 3 (60) | 0 (0) |
| Type unknown | 44 | 1 (3) | 5 (11) | 21 (48) | 12 (27) | 5 (11) 1 |
| Total | 73 | 1 (1) | 7 (10) | 40 (55) | 20 (27) | 5 (7) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brink, G.J.; Groeneweg, J.W.; Hooft, L.; Zweemer, R.P.; Witteveen, P.O. Response to Systemic Therapies in Ovarian Adult Granulosa Cell Tumors: A Literature Review. Cancers 2022, 14, 2998. https://doi.org/10.3390/cancers14122998
Brink GJ, Groeneweg JW, Hooft L, Zweemer RP, Witteveen PO. Response to Systemic Therapies in Ovarian Adult Granulosa Cell Tumors: A Literature Review. Cancers. 2022; 14(12):2998. https://doi.org/10.3390/cancers14122998
Chicago/Turabian StyleBrink, Geertruid J., Jolijn W. Groeneweg, Lotty Hooft, Ronald P. Zweemer, and Petronella O. Witteveen. 2022. "Response to Systemic Therapies in Ovarian Adult Granulosa Cell Tumors: A Literature Review" Cancers 14, no. 12: 2998. https://doi.org/10.3390/cancers14122998
APA StyleBrink, G. J., Groeneweg, J. W., Hooft, L., Zweemer, R. P., & Witteveen, P. O. (2022). Response to Systemic Therapies in Ovarian Adult Granulosa Cell Tumors: A Literature Review. Cancers, 14(12), 2998. https://doi.org/10.3390/cancers14122998







