Near-Infrared Photoimmunotherapy (NIR-PIT) in Urologic Cancers
Abstract
Simple Summary
Abstract
1. Introduction
2. NIR-PIT
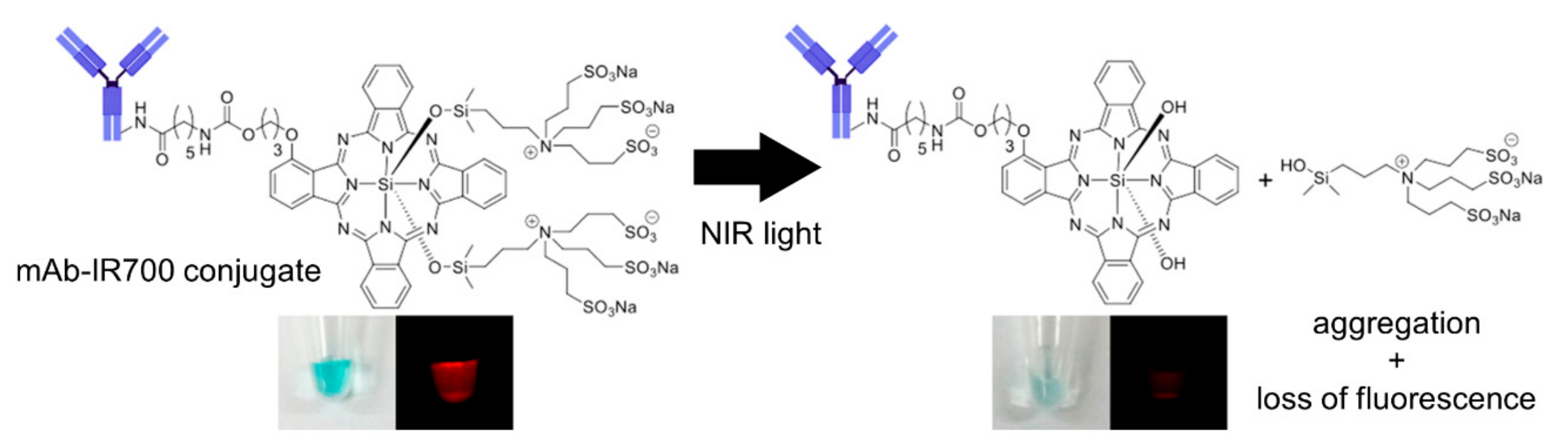
2.1. Mechanism of NIR-PIT
2.2. Activation of Anti-Cancer Immunity
2.3. Selective Depletion of Immune Suppressive Cells or Cancer-Associated Fibroblasts
2.4. Super-Enhanced Permeability and Retention (SUPR) Effects
3. NIR-PIT for Urologic Cancers
3.1. Bladder Cancer
3.1.1. Application of NIR-PIT to Bladder Cancer
3.1.2. Target Molecules for NIR-PIT in Bladder Cancer
EGFR
HER2
CD44
CD47
Tumor-Associated Calcium Signal Transducer 2 (TROP-2)
Programmed Death-Ligand 1 (PD-L1)
CTLA4 or CD25
Selection of Target Molecules for NIR-PIT According to Molecular Subtypes
3.2. Upper Tract Urothelial Cancer
3.2.1. Application of NIR-PIT to Upper Tract Urothelial Cancer
3.2.2. Target Molecules for NIR-PIT in Upper Tract Urothelial Cancer
3.3. Prostate Cancer
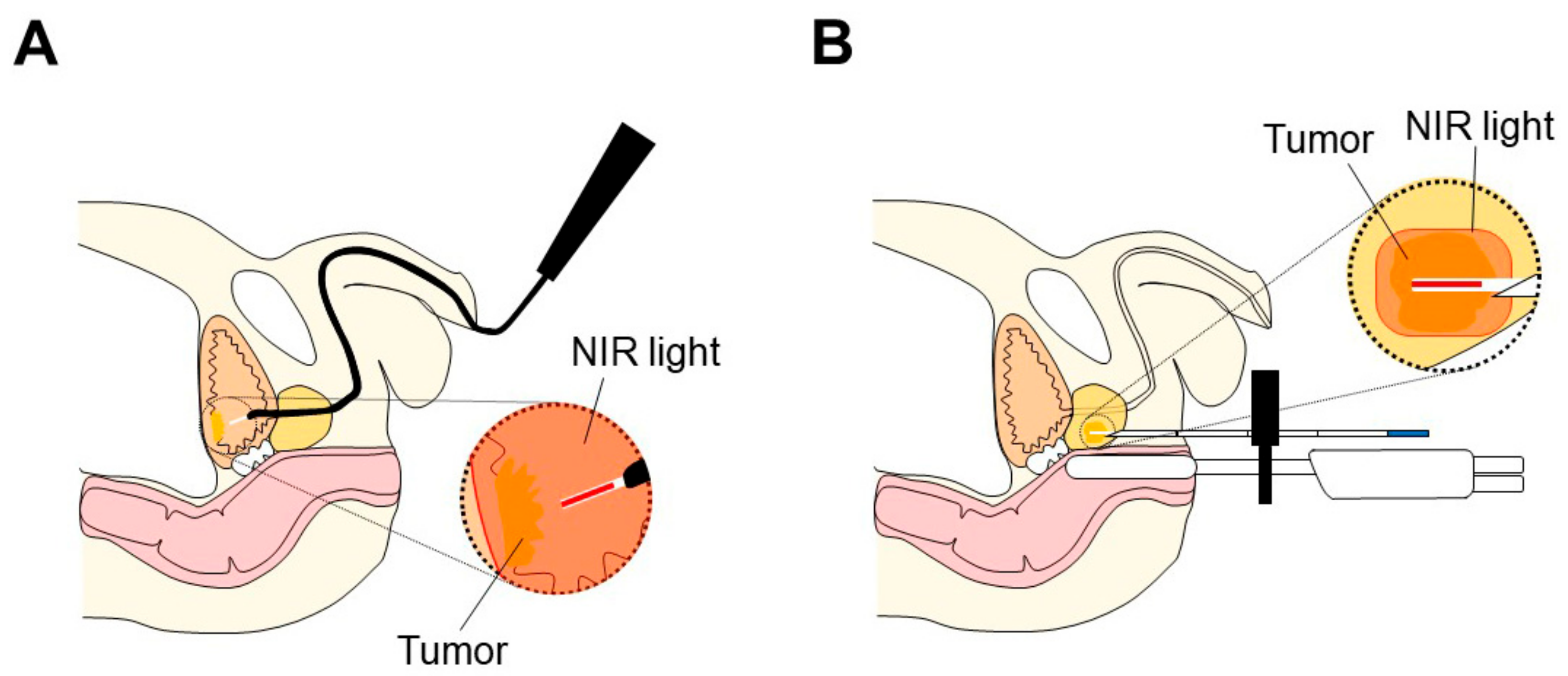
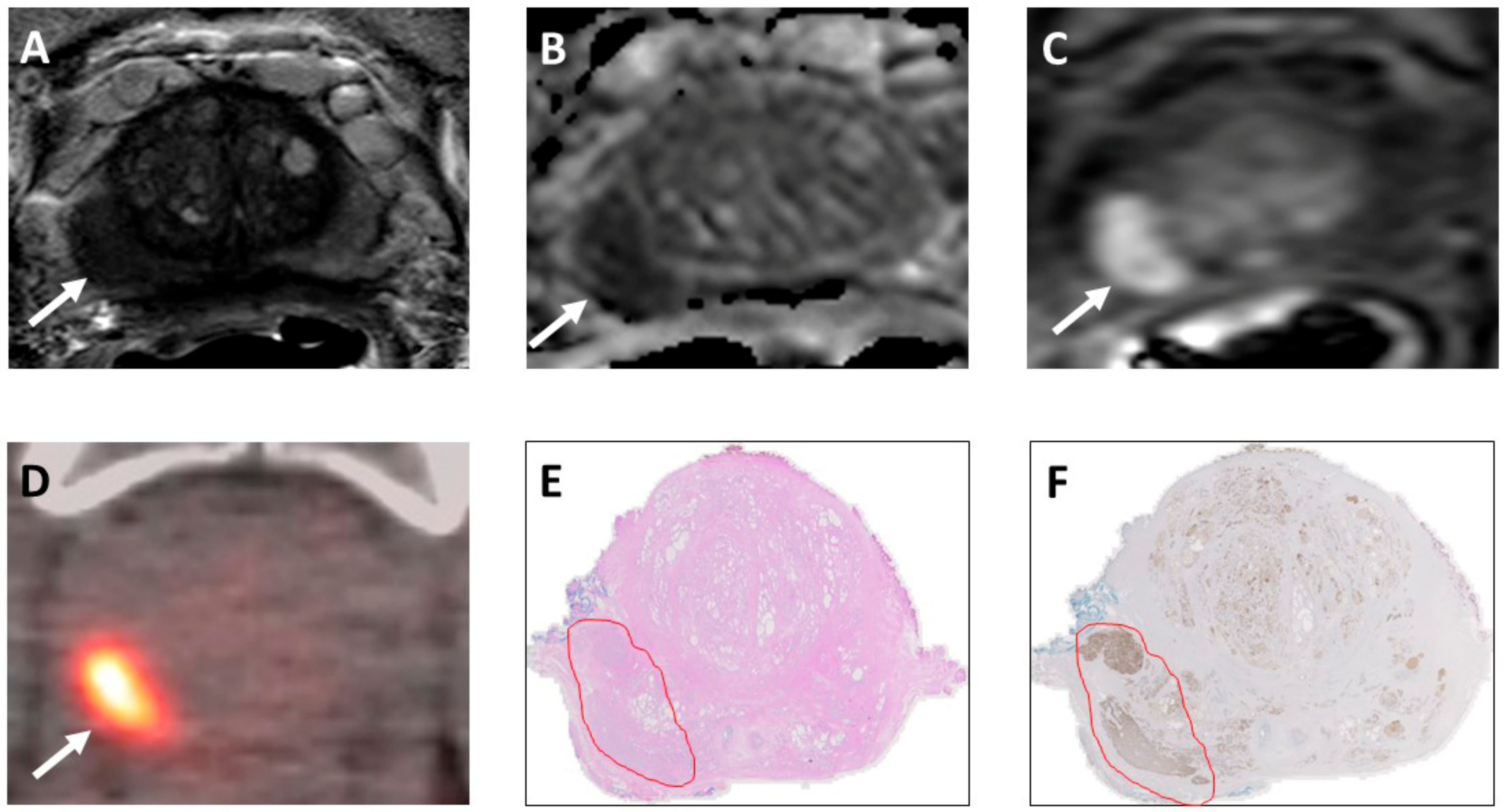
3.3.1. Application of NIR-PIT to Prostate Cancer
3.3.2. Target Molecules for NIR-PIT in Prostate Cancer
PSMA
Other Target Molecules
3.4. Renal Cell Carcinoma
3.4.1. Application of NIR-PIT to Renal Cell Carcinoma
3.4.2. Target Molecules for NIR-PIT in Renal Cell Carcinoma
EGFR
PD-L1
CTLA4 or CD25
VEGFR-2
3.5. Testicular Cancer
3.5.1. Application of NIR-PIT to Testicular Cancer
3.5.2. Target Molecules for NIR-PIT in Testicular Cancer
3.6. Penile Cancer
4. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mitsunaga, M.; Ogawa, M.; Kosaka, N.; Rosenblum, L.T.; Choyke, P.L.; Kobayashi, H. Cancer cell-selective in vivo near infrared photoimmunotherapy targeting specific membrane molecules. Nat. Med. 2011, 17, 1685–1691. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Choyke, P.L. Near-Infrared Photoimmunotherapy of Cancer. Acc. Chem. Res. 2019, 52, 2332–2339. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Griffiths, G.L.; Choyke, P.L. Near-Infrared Photoimmunotherapy: Photoactivatable Antibody-Drug Conjugates (ADCs). Bioconjug. Chem. 2020, 31, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Wakiyama, H.; Furusawa, A.; Choyke, P.L.; Kobayashi, H. Near Infrared Photoimmunotherapy; A Review of Targets for Cancer Therapy. Cancers 2021, 13, 2535. [Google Scholar] [CrossRef]
- Wakiyama, H.; Kato, T.; Furusawa, A.; Choyke, P.L.; Kobayashi, H. Near infrared photoimmunotherapy of cancer; possible clinical applications. Nanophotonics 2021, 10, 3135–3151. [Google Scholar] [CrossRef]
- Jing, H.; Weidensteiner, C.; Reichardt, W.; Gaedicke, S.; Zhu, X.; Grosu, A.L.; Kobayashi, H.; Niedermann, G. Imaging and Selective Elimination of Glioblastoma Stem Cells with Theranostic Near-Infrared-Labeled CD133-Specific Antibodies. Theranostics 2016, 6, 862–874. [Google Scholar] [CrossRef]
- Sato, K.; Ando, K.; Okuyama, S.; Moriguchi, S.; Ogura, T.; Totoki, S.; Hanaoka, H.; Nagaya, T.; Kokawa, R.; Takakura, H.; et al. Photoinduced Ligand Release from a Silicon Phthalocyanine Dye Conjugated with Monoclonal Antibodies: A Mechanism of Cancer Cell Cytotoxicity after Near-Infrared Photoimmunotherapy. ACS Cent. Sci. 2018, 4, 1559–1569. [Google Scholar] [CrossRef]
- Okada, R.; Kato, T.; Furusawa, A.; Inagaki, F.; Wakiyama, H.; Fujimura, D.; Okuyama, S.; Furumoto, H.; Fukushima, H.; Choyke, P.L.; et al. Selection of antibody and light exposure regimens alters therapeutic effects of EGFR-targeted near-infrared photoimmunotherapy. Cancer Immunol. Immunother, 2022; published online ahead of print. [Google Scholar] [CrossRef]
- Okada, R.; Furusawa, A.; Vermeer, D.W.; Inagaki, F.; Wakiyama, H.; Kato, T.; Nagaya, T.; Choyke, P.L.; Spanos, W.C.; Allen, C.T.; et al. Near-infrared photoimmunotherapy targeting human-EGFR in a mouse tumor model simulating current and future clinical trials. EBioMedicine 2021, 67, 103345. [Google Scholar] [CrossRef]
- Okada, R.; Furusawa, A.; Inagaki, F.; Wakiyama, H.; Kato, T.; Okuyama, S.; Furumoto, H.; Fukushima, H.; Choyke, P.L.; Kobayashi, H. Endoscopic Near-infrared Photoimmunotherapy in an Orthotopic Head and Neck Cancer Model. Cancer Sci. 2021, 112, 3041–3049. [Google Scholar] [CrossRef]
- Nagaya, T.; Sato, K.; Harada, T.; Nakamura, Y.; Choyke, P.L.; Kobayashi, H. Near Infrared Photoimmunotherapy Targeting EGFR Positive Triple Negative Breast Cancer: Optimizing the Conjugate-Light Regimen. PLoS ONE 2015, 10, e0136829. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Ohler, Z.W.; Householder, D.; Nagaya, T.; Sato, K.; Okuyama, S.; Ogata, F.; Daar, D.; Hoa, T.; Choyke, P.L.; et al. Near Infrared Photoimmunotherapy in a Transgenic Mouse Model of Spontaneous Epidermal Growth Factor Receptor (EGFR)-expressing Lung Cancer. Mol. Cancer Ther. 2017, 16, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Maczynska, J.; Raes, F.; Da Pieve, C.; Turnock, S.; Boult, J.K.R.; Hoebart, J.; Niedbala, M.; Robinson, S.P.; Harrington, K.J.; Kaspera, W.; et al. Triggering anti-GBM immune response with EGFR-mediated photoimmunotherapy. BMC Med. 2022, 20, 16. [Google Scholar]
- Nagaya, T.; Okuyama, S.; Ogata, F.; Maruoka, Y.; Choyke, P.L.; Kobayashi, H. Near infrared photoimmunotherapy using a fiber optic diffuser for treating peritoneal gastric cancer dissemination. Gastric Cancer 2019, 22, 463–472. [Google Scholar] [CrossRef]
- Sato, K.; Nagaya, T.; Choyke, P.L.; Kobayashi, H. Near infrared photoimmunotherapy in the treatment of pleural disseminated NSCLC: Preclinical experience. Theranostics 2015, 5, 698–709. [Google Scholar] [CrossRef]
- Sato, K.; Choyke, P.L.; Kobayashi, H. Photoimmunotherapy of gastric cancer peritoneal carcinomatosis in a mouse model. PLoS ONE 2014, 9, e113276. [Google Scholar] [CrossRef]
- Sato, K.; Hanaoka, H.; Watanabe, R.; Nakajima, T.; Choyke, P.L.; Kobayashi, H. Near infrared photoimmunotherapy in the treatment of disseminated peritoneal ovarian cancer. Mol. Cancer Ther. 2015, 14, 141–150. [Google Scholar] [CrossRef]
- Yamaguchi, H.; On, J.; Morita, T.; Suzuki, T.; Okada, Y.; Ono, J.; Evdokiou, A. Combination of Near-Infrared Photoimmunotherapy Using Trastuzumab and Small Protein Mimetic for HER2-Positive Breast Cancer. Int. J. Mol. Sci. 2021, 22, 12213. [Google Scholar] [CrossRef]
- Takahashi, K.; Taki, S.; Yasui, H.; Nishinaga, Y.; Isobe, Y.; Matsui, T.; Shimizu, M.; Koike, C.; Sato, K. HER2 targeting near-infrared photoimmunotherapy for a CDDP-resistant small-cell lung cancer. Cancer Med. 2021, 10, 8808–8819. [Google Scholar] [CrossRef]
- Cognetti, D.M.; Johnson, J.M.; Curry, J.M.; Kochuparambil, S.T.; McDonald, D.; Mott, F.; Fidler, M.J.; Stenson, K.; Vasan, N.R.; Razaq, M.A.; et al. Phase 1/2a, open-label, multicenter study of RM-1929 photoimmunotherapy in patients with locoregional, recurrent head and neck squamous cell carcinoma. Head Neck 2021, 43, 3875–3887. [Google Scholar] [CrossRef]
- Mew, D.; Wat, C.K.; Towers, G.H.N.; Levy, J.G. Photoimmunotherapy—Treatment of Animal Tumors with Tumor-Specific Monoclonal Antibody-Hematoporphyrin Conjugates. J. Immunol. 1983, 130, 1473–1477. [Google Scholar] [PubMed]
- Lin, C.W.; Amano, T.; Rutledge, A.R.; Shulok, J.R.; Prout, G.R. Photodynamic Effect in an Experimental Bladder-Tumor Treated with Intratumor Injection of Hematoporphyrin Derivative. Cancer Res. 1988, 48, 6115–6120. [Google Scholar] [PubMed]
- Sobolev, A.S.; Jans, D.A.; Rosenkranz, A.A. Targeted intracellular delivery of photosensitizers. Prog. Biophys. Mol. Bio. 2000, 73, 51–90. [Google Scholar] [CrossRef]
- Ogawa, M.; Takakura, H. Photoimmunotherapy: A new cancer treatment using photochemical reactions. Bioorg. Med. Chem. 2021, 43, 116274. [Google Scholar] [CrossRef]
- Kato, T.; Okada, R.; Goto, Y.; Furusawa, A.; Inagaki, F.; Wakiyama, H.; Furumoto, H.; Daar, D.; Turkbey, B.; Choyke, P.L.; et al. Electron Donors Rather Than Reactive Oxygen Species Needed for Therapeutic Photochemical Reaction of Near-Infrared Photoimmunotherapy. ACS Pharmacol. Transl. Sci. 2021, 4, 1689–1701. [Google Scholar] [CrossRef]
- Ogata, F.; Nagaya, T.; Okuyama, S.; Maruoka, Y.; Choyke, P.L.; Yamauchi, T.; Kobayashi, H. Dynamic changes in the cell membrane on three dimensional low coherent quantitative phase microscopy (3D LC-QPM) after treatment with the near infrared photoimmunotherapy. Oncotarget 2017, 8, 104295–104302. [Google Scholar] [CrossRef]
- Ulfo, L.; Costantini, P.E.; Di Giosia, M.; Danielli, A.; Calvaresi, M. EGFR-Targeted Photodynamic Therapy. Pharmaceutics 2022, 14, 241. [Google Scholar] [CrossRef]
- Hou, Y.J.; Yang, X.X.; Liu, R.Q.; Zhao, D.; Guo, C.X.; Zhu, A.C.; Wen, M.N.; Liu, Z.; Qu, G.F.; Meng, H.X. Pathological Mechanism of Photodynamic Therapy and Photothermal Therapy Based on Nanoparticles. Int. J. Nanomed. 2020, 15, 6827–6838. [Google Scholar] [CrossRef]
- Okamoto, I.; Okada, T.; Tokashiki, K.; Tsukahara, K. Photoimmunotherapy for Managing Recurrent Laryngeal Cancer Cervical Lesions: A Case Report. Case Rep. Oncol. 2022, 15, 34–39. [Google Scholar] [CrossRef]
- Henderson, T.A.; Morries, L.D. Near-infrared photonic energy penetration: Can infrared phototherapy effectively reach the human brain? Neuropsychiatr. Dis. Treat. 2015, 11, 2191–2208. [Google Scholar] [CrossRef]
- Sato, K.; Nagaya, T.; Mitsunaga, M.; Choyke, P.L.; Kobayashi, H. Near infrared photoimmunotherapy for lung metastases. Cancer Lett. 2015, 365, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Maruoka, Y.; Nagaya, T.; Sato, K.; Ogata, F.; Okuyama, S.; Choyke, P.L.; Kobayashi, H. Near Infrared Photoimmunotherapy with Combined Exposure of External and Interstitial Light Sources. Mol. Pharm. 2018, 15, 3634–3641. [Google Scholar] [CrossRef] [PubMed]
- Nagaya, T.; Okuyama, S.; Ogata, F.; Maruoka, Y.; Choyke, P.L.; Kobayashi, H. Endoscopic near infrared photoimmunotherapy using a fiber optic diffuser for peritoneal dissemination of gastric cancer. Cancer Sci. 2018, 109, 1902–1908. [Google Scholar] [CrossRef] [PubMed]
- Tahara, M.; Okano, S.; Enokida, T.; Ueda, Y.; Fujisawa, T.; Shinozaki, T.; Tomioka, T.; Okano, W.; Biel, M.A.; Ishida, K.; et al. A phase I, single-center, open-label study of RM-1929 photoimmunotherapy in Japanese patients with recurrent head and neck squamous cell carcinoma. Int. J. Clin. Oncol. 2021, 26, 1812–1821. [Google Scholar] [CrossRef]
- Ogawa, M.; Tomita, Y.; Nakamura, Y.; Lee, M.J.; Lee, S.; Tomita, S.; Nagaya, T.; Sato, K.; Yamauchi, T.; Iwai, H.; et al. Immunogenic cancer cell death selectively induced by near infrared photoimmunotherapy initiates host tumor immunity. Oncotarget 2017, 8, 10425–10436. [Google Scholar] [CrossRef]
- Kobayashi, H.; Furusawa, A.; Rosenberg, A.; Choyke, P.L. Near-infrared photoimmunotherapy of cancer: A new approach that kills cancer cells and enhances anti-cancer host immunity. Int. Immunol. 2021, 33, 7–15. [Google Scholar] [CrossRef]
- Ahmed, A.; Tait, S.W.G. Targeting immunogenic cell death in cancer. Mol. Oncol. 2020, 14, 2994–3006. [Google Scholar] [CrossRef]
- Kroemer, G.; Galluzzi, L.; Kepp, O.; Zitvogel, L. Immunogenic cell death in cancer therapy. Annu. Rev. Immunol. 2013, 31, 51–72. [Google Scholar] [CrossRef]
- Krysko, D.V.; Garg, A.D.; Kaczmarek, A.; Krysko, O.; Agostinis, P.; Vandenabeele, P. Immunogenic cell death and DAMPs in cancer therapy. Nat. Rev. Cancer 2012, 12, 860–875. [Google Scholar] [CrossRef]
- Hannun, Y.A. Apoptosis and the dilemma of cancer chemotherapy. Blood 1997, 89, 1845–1853. [Google Scholar] [CrossRef]
- Balcer-Kubiczek, E.K. Apoptosis in radiation therapy: A double-edged sword. Exp. Oncol. 2012, 34, 277–285. [Google Scholar] [PubMed]
- Voll, R.E.; Herrmann, M.; Roth, E.A.; Stach, C.; Kalden, J.R.; Girkontaite, I. Immunosuppressive effects of apoptotic cells. Nature 1997, 390, 350–351. [Google Scholar] [CrossRef] [PubMed]
- Newton, K.; Manning, G. Necroptosis and Inflammation. Annu. Rev. Biochem. 2016, 85, 743–763. [Google Scholar] [CrossRef]
- Nagaya, T.; Friedman, J.; Maruoka, Y.; Ogata, F.; Okuyama, S.; Clavijo, P.E.; Choyke, P.L.; Allen, C.; Kobayashi, H. Host Immunity Following Near-Infrared Photoimmunotherapy Is Enhanced with PD-1 Checkpoint Blockade to Eradicate Established Antigenic Tumors. Cancer Immunol. Res. 2019, 7, 401–413. [Google Scholar] [CrossRef] [PubMed]
- Wakiyama, H.; Furusawa, A.; Okada, R.; Inagaki, F.; Kato, T.; Maruoka, Y.; Choyke, P.L.; Kobayashi, H. Increased Immunogenicity of a Minimally Immunogenic Tumor after Cancer-Targeting Near Infrared Photoimmunotherapy. Cancers 2020, 12, 3747. [Google Scholar] [CrossRef] [PubMed]
- Maruoka, Y.; Furusawa, A.; Okada, R.; Inagaki, F.; Fujimura, D.; Wakiyama, H.; Kato, T.; Nagaya, T.; Choyke, P.L.; Kobayashi, H. Near-Infrared Photoimmunotherapy Combined with CTLA4 Checkpoint Blockade in Syngeneic Mouse Cancer Models. Vaccines 2020, 8, 528. [Google Scholar] [CrossRef] [PubMed]
- Furusawa, A.; Okada, R.; Inagaki, F.; Wakiyama, H.; Kato, T.; Furumoto, H.; Fukushima, H.; Okuyama, S.; Choyke, P.L.; Kobayashi, H. CD29 targeted near-infrared photoimmunotherapy (NIR-PIT) in the treatment of a pigmented melanoma model. Oncoimmunology 2022, 11, 2019922. [Google Scholar] [CrossRef]
- Okada, R.; Maruoka, Y.; Furusawa, A.; Inagaki, F.; Nagaya, T.; Fujimura, D.; Choyke, P.L.; Kobayashi, H. The Effect of Antibody Fragments on CD25 Targeted Regulatory T Cell Near-Infrared Photoimmunotherapy. Bioconjug. Chem. 2019, 30, 2624–2633. [Google Scholar] [CrossRef]
- Sato, K.; Sato, N.; Xu, B.; Nakamura, Y.; Nagaya, T.; Choyke, P.L.; Hasegawa, Y.; Kobayashi, H. Spatially selective depletion of tumor-associated regulatory T cells with near-infrared photoimmunotherapy. Sci. Transl. Med. 2016, 8, 352ra110. [Google Scholar] [CrossRef]
- Kato, T.; Okada, R.; Furusawa, A.; Inagaki, F.; Wakiyama, H.; Furumoto, H.; Okuyama, S.; Fukushima, H.; Choyke, P.L.; Kobayashi, H. Simultaneously Combined Cancer Cell- and CTLA4-Targeted NIR-PIT Causes a Synergistic Treatment Effect in Syngeneic Mouse Models. Mol. Cancer Ther. 2021, 20, 2262–2273. [Google Scholar] [CrossRef]
- Okada, R.; Kato, T.; Furusawa, A.; Inagaki, F.; Wakiyama, H.; Choyke, P.L.; Kobayashi, H. Local Depletion of Immune Checkpoint Ligand CTLA4 Expressing Cells in Tumor Beds Enhances Antitumor Host Immunity. Adv. Ther. 2021, 4, 2000269. [Google Scholar] [CrossRef] [PubMed]
- Katsube, R.; Noma, K.; Ohara, T.; Nishiwaki, N.; Kobayashi, T.; Komoto, S.; Sato, H.; Kashima, H.; Kato, T.; Kikuchi, S.; et al. Fibroblast activation protein targeted near infrared photoimmunotherapy (NIR PIT) overcomes therapeutic resistance in human esophageal cancer. Sci. Rep. 2021, 11, 1693. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Noma, K.; Ohara, T.; Kashima, H.; Sato, H.; Kato, T.; Urano, S.; Katsube, R.; Hashimoto, Y.; Tazawa, H.; et al. Photoimmunotherapy for cancer-associated fibroblasts targeting fibroblast activation protein in human esophageal squamous cell carcinoma. Cancer Biol. Ther. 2019, 20, 1234–1248. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, Y.; Maeda, H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986, 46, 6387–6392. [Google Scholar] [PubMed]
- Maeda, H.; Wu, J.; Sawa, T.; Matsumura, Y.; Hori, K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: A review. J. Control. Release 2000, 65, 271–284. [Google Scholar] [CrossRef]
- Sano, K.; Nakajima, T.; Choyke, P.L.; Kobayashi, H. Markedly enhanced permeability and retention effects induced by photo-immunotherapy of tumors. ACS Nano 2013, 7, 717–724. [Google Scholar] [CrossRef]
- Inagaki, F.F.; Furusawa, A.; Choyke, P.L.; Kobayashi, H. Enhanced nanodrug delivery in tumors after near-infrared photoimmunotherapy. Nanophotonics 2019, 8, 1673–1688. [Google Scholar] [CrossRef]
- Kobayashi, H.; Choyke, P.L. Super enhanced permeability and retention (SUPR) effects in tumors following near infrared photoimmunotherapy. Nanoscale 2016, 8, 12504–12509. [Google Scholar] [CrossRef]
- Nakamura, Y.; Mochida, A.; Choyke, P.L.; Kobayashi, H. Nanodrug Delivery: Is the Enhanced Permeability and Retention Effect Sufficient for Curing Cancer? Bioconjugate Chem. 2016, 27, 2225–2238. [Google Scholar] [CrossRef]
- Hanaoka, H.; Nakajima, T.; Sato, K.; Watanabe, R.; Phung, Y.; Gao, W.; Harada, T.; Kim, I.; Paik, C.H.; Choyke, P.L.; et al. Photoimmunotherapy of hepatocellular carcinoma-targeting Glypican-3 combined with nanosized albumin-bound paclitaxel. Nanomedicine 2015, 10, 1139–1147. [Google Scholar] [CrossRef]
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A.; et al. Proteomics. Tissue-based map of the human proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Babjuk, M.; Burger, M.; Capoun, O.; Cohen, D.; Comperat, E.M.; Dominguez Escrig, J.L.; Gontero, P.; Liedberg, F.; Masson-Lecomte, A.; Mostafid, A.H.; et al. European Association of Urology Guidelines on Non-muscle-invasive Bladder Cancer (Ta, T1, and Carcinoma in Situ). Eur. Urol. 2022, 81, 75–94. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, H.; Moriyama, S.; Waseda, Y.; Fukuda, S.; Uehara, S.; Tanaka, H.; Kijima, T.; Yoshida, S.; Yokoyama, M.; Ishioka, J.; et al. Significance of Bladder Neck Involvement in Risk Substratification of Intermediate-Risk Non-muscle-invasive Bladder Cancer. Eur. Urol. Focus 2021, 7, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Dovey, Z.; Pfail, J.; Martini, A.; Steineck, G.; Dey, L.; Renstrom, L.; Hosseini, A.; Sfakianos, J.P.; Wiklund, P. Bladder Cancer (NMIBC) in a population-based cohort from Stockholm County with long-term follow-up; A comparative analysis of prediction models for recurrence and progression, including external validation of the updated 2021 E.A.U. model. Urol. Oncol. 2021, 40, 106.e1–106.e10. [Google Scholar] [CrossRef] [PubMed]
- Leo, M.C.; McMullen, C.K.; O’Keeffe-Rosetti, M.; Weinmann, S.; Garg, T.; Nielsen, M.E. External validation of the EORTC and NCCN bladder cancer recurrence and progression risk calculators in a U.S. community-based health system. Urol. Oncol. 2020, 38, 39.e21–39.e27. [Google Scholar] [CrossRef] [PubMed]
- Balbay, M.D.; Cimentepe, E.; Unsal, A.; Bayrak, O.; Koc, A.; Akbulut, Z. The actual incidence of bladder perforation following transurethral bladder surgery. J. Urol. 2005, 174, 2260–2262. [Google Scholar] [CrossRef]
- Sylvester, R.J.; van der, M.A.; Lamm, D.L. Intravesical bacillus Calmette-Guerin reduces the risk of progression in patients with superficial bladder cancer: A meta-analysis of the published results of randomized clinical trials. J. Urol. 2002, 168, 1964–1970. [Google Scholar] [CrossRef]
- Sylvester, R.J.; Brausi, M.A.; Kirkels, W.J.; Hoeltl, W.; Calais Da Silva, F.; Powell, P.H.; Prescott, S.; Kirkali, Z.; van de Beek, C.; Gorlia, T.; et al. Long-term efficacy results of EORTC genito-urinary group randomized phase 3 study 30911 comparing intravesical instillations of epirubicin, bacillus Calmette-Guerin, and bacillus Calmette-Guerin plus isoniazid in patients with intermediate- and high-risk stage Ta T1 urothelial carcinoma of the bladder. Eur. Urol. 2010, 57, 766–773. [Google Scholar]
- Railkar, R.; Agarwal, P.K. Photodynamic Therapy in the Treatment of Bladder Cancer: Past Challenges and Current Innovations. Eur. Urol. Focus 2018, 4, 509–511. [Google Scholar] [CrossRef]
- Witjes, J.A.; Bruins, H.M.; Cathomas, R.; Comperat, E.M.; Cowan, N.C.; Gakis, G.; Hernandez, V.; Linares Espinos, E.; Lorch, A.; Neuzillet, Y.; et al. European Association of Urology Guidelines on Muscle-invasive and Metastatic Bladder Cancer: Summary of the 2020 Guidelines. Eur. Urol. 2021, 79, 82–104. [Google Scholar] [CrossRef] [PubMed]
- Grossman, H.B.; Natale, R.B.; Tangen, C.M.; Speights, V.O.; Vogelzang, N.J.; Trump, D.L.; deVere White, R.W.; Sarosdy, M.F.; Wood, D.P., Jr.; Raghavan, D.; et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N. Engl. J. Med. 2003, 349, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Djaladat, H.; Katebian, B.; Bazargani, S.T.; Miranda, G.; Cai, J.; Schuckman, A.K.; Daneshmand, S. 90-Day complication rate in patients undergoing radical cystectomy with enhanced recovery protocol: A prospective cohort study. World J. Urol. 2017, 35, 907–911. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.C.; Smith, Z.L.; Golan, S.; Rodriguez, J.F., III; Smith, N.D.; Steinberg, G.D. Temporal trends in perioperative morbidity for radical cystectomy using the National Surgical Quality Improvement Program database. Urol. Oncol. 2017, 35, 659.e613–659.e619. [Google Scholar] [CrossRef]
- Giacalone, N.J.; Shipley, W.U.; Clayman, R.H.; Niemierko, A.; Drumm, M.; Heney, N.M.; Michaelson, M.D.; Lee, R.J.; Saylor, P.J.; Wszolek, M.F.; et al. Long-term Outcomes After Bladder-preserving Tri-modality Therapy for Patients with Muscle-invasive Bladder Cancer: An Updated Analysis of the Massachusetts General Hospital Experience. Eur. Urol. 2017, 71, 952–960. [Google Scholar] [CrossRef]
- Tanaka, H.; Fukushima, H.; Kijima, T.; Nakamura, Y.; Yajima, S.; Uehara, S.; Yoshida, S.; Yokoyama, M.; Ishioka, J.; Matsuoka, Y.; et al. Feasibility and outcomes of selective tetramodal bladder-preservation therapy in elderly patients with muscle-invasive bladder cancer. Int. J. Urol. 2020, 27, 236–243. [Google Scholar] [CrossRef]
- Nason, G.J.; Ajib, K.; Tan, G.H.; Kulkarni, G.S. Bladder-sparing treatment options in localized muscle-invasive bladder cancer. Expert Rev. Anticancer Ther. 2020, 20, 179–188. [Google Scholar] [CrossRef]
- Roskoski, R., Jr. The ErbB/HER family of protein-tyrosine kinases and cancer. Pharmacol. Res. 2014, 79, 34–74. [Google Scholar] [CrossRef]
- Xu, M.J.; Johnson, D.E.; Grandis, J.R. EGFR-targeted therapies in the post-genomic era. Cancer Metastasis Rev. 2017, 36, 463–473. [Google Scholar] [CrossRef]
- Chow, N.H.; Chan, S.H.; Tzai, T.S.; Ho, C.L.; Liu, H.S. Expression profiles of ErbB family receptors and prognosis in primary transitional cell carcinoma of the urinary bladder. Clin. Cancer Res. 2001, 7, 1957–1962. [Google Scholar]
- Chaux, A.; Cohen, J.S.; Schultz, L.; Albadine, R.; Jadallah, S.; Murphy, K.M.; Sharma, R.; Schoenberg, M.P.; Netto, G.J. High epidermal growth factor receptor immunohistochemical expression in urothelial carcinoma of the bladder is not associated with EGFR mutations in exons 19 and 21: A study using formalin-fixed, paraffin-embedded archival tissues. Hum. Pathol. 2012, 43, 1590–1595. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, Y.; Tan, S.; Rao, Q.; Zhu, T.; Huang, G.; Li, Z.; Liu, G. Overexpression of Epidermal Growth Factor Receptor (EGFR) and HER-2 in Bladder Carcinoma and Its Association with Patients’ Clinical Features. Med. Sci. Monit. 2018, 24, 7178–7185. [Google Scholar] [CrossRef] [PubMed]
- Railkar, R.; Krane, L.S.; Li, Q.Q.; Sanford, T.; Siddiqui, M.R.; Haines, D.; Vourganti, S.; Brancato, S.J.; Choyke, P.L.; Kobayashi, H.; et al. Epidermal Growth Factor Receptor (EGFR)-targeted Photoimmunotherapy (PIT) for the Treatment of EGFR-expressing Bladder Cancer. Mol. Cancer Ther. 2017, 16, 2201–2214. [Google Scholar] [CrossRef] [PubMed]
- Nagaya, T.; Okuyama, S.; Ogata, F.; Maruoka, Y.; Knapp, D.W.; Karagiannis, S.N.; Fazekas-Singer, J.; Choyke, P.L.; LeBlanc, A.K.; Jensen-Jarolim, E.; et al. Near infrared photoimmunotherapy targeting bladder cancer with a canine anti-epidermal growth factor receptor (EGFR) antibody. Oncotarget 2018, 9, 19026–19038. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kim, D.; Kim, J.M.; Kim, J.S.; Kim, S.; Kim, K.H. Differential Expression and Clinicopathological Significance of HER2, Indoleamine 2,3-Dioxygenase and PD-L1 in Urothelial Carcinoma of the Bladder. J. Clin. Med. 2020, 9, 1265. [Google Scholar] [CrossRef]
- Inoue, M.; Koga, F.; Yoshida, S.; Tamura, T.; Fujii, Y.; Ito, E.; Kihara, K. Significance of ERBB2 Overexpression in Therapeutic Resistance and Cancer-Specific Survival in Muscle-Invasive Bladder Cancer Patients Treated with Chemoradiation-Based Selective Bladder-Sparing Approach. Int. J. Radiat. Oncol. 2014, 90, 303–311. [Google Scholar] [CrossRef]
- Siddiqui, M.R.; Railkar, R.; Sanford, T.; Crooks, D.R.; Eckhaus, M.A.; Haines, D.; Choyke, P.L.; Kobayashi, H.; Agarwal, P.K. Targeting Epidermal Growth Factor Receptor (EGFR) and Human Epidermal Growth Factor Receptor 2 (HER2) Expressing Bladder Cancer Using Combination Photoimmunotherapy (PIT). Sci. Rep. 2019, 9, 2084. [Google Scholar] [CrossRef]
- Chen, C.; Zhao, S.; Karnad, A.; Freeman, J.W. The biology and role of CD44 in cancer progression: Therapeutic implications. J. Hematol. Oncol. 2018, 11, 64. [Google Scholar] [CrossRef]
- Hagiwara, M.; Kikuchi, E.; Tanaka, N.; Kosaka, T.; Mikami, S.; Saya, H.; Oya, M. Variant isoforms of CD44 involves acquisition of chemoresistance to cisplatin and has potential as a novel indicator for identifying a cisplatin-resistant population in urothelial cancer. BMC Cancer 2018, 18, 113. [Google Scholar] [CrossRef]
- Wu, C.T.; Lin, W.Y.; Chang, Y.H.; Chen, W.C.; Chen, M.F. Impact of CD44 expression on radiation response for bladder cancer. J. Cancer 2017, 8, 1137–1144. [Google Scholar] [CrossRef]
- Wu, C.T.; Lin, W.Y.; Chen, W.C.; Chen, M.F. Predictive Value of CD44 in Muscle-Invasive Bladder Cancer and Its Relationship with IL-6 Signaling. Ann. Surg. Oncol. 2018, 25, 3518–3526. [Google Scholar] [CrossRef] [PubMed]
- Nagaya, T.; Nakamura, Y.; Okuyama, S.; Ogata, F.; Maruoka, Y.; Choyke, P.L.; Allen, C.; Kobayashi, H. Syngeneic Mouse Models of Oral Cancer Are Effectively Targeted by Anti-CD44-Based NIR-PIT. Mol. Cancer Res. 2017, 15, 1667–1677. [Google Scholar] [CrossRef] [PubMed]
- Maruoka, Y.; Furusawa, A.; Okada, R.; Inagaki, F.; Fujimura, D.; Wakiyama, H.; Kato, T.; Nagaya, T.; Choyke, P.L.; Kobayashi, H. Combined CD44- and CD25-Targeted Near-Infrared Photoimmunotherapy Selectively Kills Cancer and Regulatory T Cells in Syngeneic Mouse Cancer Models. Cancer Immunol. Res. 2020, 8, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Chao, M.P.; Weissman, I.L.; Majeti, R. The CD47-SIRPalpha pathway in cancer immune evasion and potential therapeutic implications. Curr. Opin. Immunol. 2012, 24, 225–232. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, J.; Yang, C.; Kuang, R.; Kazobinka, G.; Pang, Z.; Hou, T. Prognostic Implication of Urothelial Stem Cell Markers Differs According to Primary Tumour Location in Non-Muscle-Invasive Bladder Cancer. Cell Physiol. Biochem. 2018, 48, 2364–2373. [Google Scholar] [CrossRef]
- Kiss, B.; van den Berg, N.S.; Ertsey, R.; McKenna, K.; Mach, K.E.; Zhang, C.A.; Volkmer, J.P.; Weissman, I.L.; Rosenthal, E.L.; Liao, J.C. CD47-Targeted Near-Infrared Photoimmunotherapy for Human Bladder Cancer. Clin. Cancer Res. 2019, 25, 3561–3571. [Google Scholar] [CrossRef]
- Shvartsur, A.; Bonavida, B. Trop2 and its overexpression in cancers: Regulation and clinical/therapeutic implications. Genes Cancer 2015, 6, 84–105. [Google Scholar] [CrossRef]
- Faltas, B.; Goldenberg, D.M.; Ocean, A.J.; Govindan, S.V.; Wilhelm, F.; Sharkey, R.M.; Hajdenberg, J.; Hodes, G.; Nanus, D.M.; Tagawa, S.T. Sacituzumab Govitecan, a Novel Antibody--Drug Conjugate, in Patients With Metastatic Platinum-Resistant Urothelial Carcinoma. Clin. Genitourin. Cancer 2016, 14, e75–e79. [Google Scholar] [CrossRef]
- Nishimura, T.; Mitsunaga, M.; Sawada, R.; Saruta, M.; Kobayashi, H.; Matsumoto, N.; Kanke, T.; Yanai, H.; Nakamura, K. Photoimmunotherapy targeting biliary-pancreatic cancer with humanized anti-TROP2 antibody. Cancer Med. 2019, 8, 7781–7792. [Google Scholar] [CrossRef]
- Tagawa, S.T.; Balar, A.V.; Petrylak, D.P.; Kalebasty, A.R.; Loriot, Y.; Flechon, A.; Jain, R.K.; Agarwal, N.; Bupathi, M.; Barthelemy, P.; et al. TROPHY-U-01: A Phase II Open-Label Study of Sacituzumab Govitecan in Patients With Metastatic Urothelial Carcinoma Progressing After Platinum-Based Chemotherapy and Checkpoint Inhibitors. J. Clin. Oncol. 2021, 39, 2474–2485. [Google Scholar] [CrossRef]
- Nagaya, T.; Nakamura, Y.; Sato, K.; Harada, T.; Choyke, P.L.; Hodge, J.W.; Schlom, J.; Kobayashi, H. Near infrared photoimmunotherapy with avelumab, an anti-programmed death-ligand 1 (PD-L1) antibody. Oncotarget 2017, 8, 8807–8817. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Sivakumar, I.; Mironchik, Y.; Krishnamachary, B.; Wildes, F.; Barnett, J.D.; Hung, C.F.; Nimmagadda, S.; Kobayashi, H.; Bhujwalla, Z.M.; et al. PD-L1 near Infrared Photoimmunotherapy of Ovarian Cancer Model. Cancers 2022, 14, 619. [Google Scholar] [CrossRef] [PubMed]
- Taki, S.; Matsuoka, K.; Nishinaga, Y.; Takahashi, K.; Yasui, H.; Koike, C.; Shimizu, M.; Sato, M.; Sato, K. Spatiotemporal depletion of tumor-associated immune checkpoint PD-L1 with near-infrared photoimmunotherapy promotes antitumor immunity. J. Immunother. Cancer 2021, 9, e003036. [Google Scholar] [CrossRef] [PubMed]
- Pichler, R.; Fritz, J.; Zavadil, C.; Schafer, G.; Culig, Z.; Brunner, A. Tumor-infiltrating immune cell subpopulations influence the oncologic outcome after intravesical Bacillus Calmette-Guerin therapy in bladder cancer. Oncotarget 2016, 7, 39916–39930. [Google Scholar] [CrossRef]
- Lee, C.H.; Yelensky, R.; Jooss, K.; Chan, T.A. Update on Tumor Neoantigens and Their Utility: Why It Is Good to Be Different. Trends Immunol. 2018, 39, 536–548. [Google Scholar] [CrossRef]
- Kamoun, A.; de Reynies, A.; Allory, Y.; Sjodahl, G.; Robertson, A.G.; Seiler, R.; Hoadley, K.A.; Groeneveld, C.S.; Al-Ahmadie, H.; Choi, W.; et al. A Consensus Molecular Classification of Muscle-invasive Bladder Cancer. Eur. Urol. 2020, 77, 420–433. [Google Scholar] [CrossRef]
- Robertson, A.G.; Groeneveld, C.S.; Jordan, B.; Lin, X.; McLaughlin, K.A.; Das, A.; Fall, L.A.; Fantini, D.; Taxter, T.J.; Mogil, L.S.; et al. Identification of Differential Tumor Subtypes of T1 Bladder Cancer. Eur. Urol. 2020, 78, 533–537. [Google Scholar] [CrossRef]
- Sjodahl, G. Molecular Subtype Profiling of Urothelial Carcinoma Using a Subtype-Specific Immunohistochemistry Panel; Humana Press: New York, NY, USA, 2018; pp. 53–64. [Google Scholar]
- Sjodahl, G.; Lovgren, K.; Lauss, M.; Patschan, O.; Gudjonsson, S.; Chebil, G.; Aine, M.; Eriksson, P.; Mansson, W.; Lindgren, D.; et al. Toward a molecular pathologic classification of urothelial carcinoma. Am. J. Pathol. 2013, 183, 681–691. [Google Scholar] [CrossRef]
- Roupret, M.; Babjuk, M.; Burger, M.; Capoun, O.; Cohen, D.; Comperat, E.M.; Cowan, N.C.; Dominguez-Escrig, J.L.; Gontero, P.; Hugh Mostafid, A.; et al. European Association of Urology Guidelines on Upper Urinary Tract Urothelial Carcinoma: 2020 Update. Eur. Urol. 2021, 79, 62–79. [Google Scholar] [CrossRef]
- Fukushima, H.; Saito, K.; Ishioka, J.; Matsuoka, Y.; Numao, N.; Koga, F.; Masuda, H.; Fujii, Y.; Sakai, Y.; Arisawa, C.; et al. Equivalent survival and improved preservation of renal function after distal ureterectomy compared with nephroureterectomy in patients with urothelial carcinoma of the distal ureter: A propensity score-matched multicenter study. Int. J. Urol. 2014, 21, 1098–1104. [Google Scholar] [CrossRef]
- Petros, F.G.; Li, R.; Matin, S.F. Endoscopic Approaches to Upper Tract Urothelial Carcinoma. Urol. Clin. 2018, 45, 267–286. [Google Scholar] [CrossRef] [PubMed]
- Shenhar, C.; Veredgorn, Y.; Bulis, S.; Aviv, T.; Darawsha, A.E.; Gilad, R.; Baniel, J.; Ehrlich, Y.; Lifshitz, D. Endoscopic Management of Low-Grade Upper Tract Urothelial Carcinoma: Characterizing the Long-term Burden of Care in Comparison to Radical Nephroureterectomy. Urology 2022, 159, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Cutress, M.L.; Stewart, G.D.; Zakikhani, P.; Phipps, S.; Thomas, B.G.; Tolley, D.A. Ureteroscopic and percutaneous management of upper tract urothelial carcinoma (UTUC): Systematic review. BJU Int. 2012, 110, 614–628. [Google Scholar] [CrossRef] [PubMed]
- Long, X.; Zu, X.; Li, Y.; He, W.; Hu, X.; Tong, S.; Wang, Z.; Chen, M.; Qi, L. Epidermal Growth Factor Receptor and Ki-67 as Predictive Biomarkers Identify Patients Who Will Be More Sensitive to Intravesical Instillations for the Prevention of Bladder Cancer Recurrence after Radical Nephroureterectomy. PLoS ONE 2016, 11, e0166884. [Google Scholar] [CrossRef]
- Soria, F.; Moschini, M.; Haitel, A.; Wirth, G.J.; Karam, J.A.; Wood, C.G.; Roupret, M.; Margulis, V.; Karakiewicz, P.I.; Briganti, A.; et al. HER2 overexpression is associated with worse outcomes in patients with upper tract urothelial carcinoma (UTUC). World J. Urol. 2017, 35, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Imai, T.; Kimura, M.; Takeda, M.; Tomita, Y. Significance of Epidermal Growth-Factor Receptor and C-Erbb-2 Protein Expression in Transitional-Cell Cancer of the Upper Urinary-Tract for Tumor Recurrence at the Urinary-Bladder. Br. J. Cancer 1995, 71, 69–72. [Google Scholar] [CrossRef]
- Leibl, S.; Zigeuner, R.; Hutterer, G.; Chromecki, T.; Rehak, P.; Langner, C. EGFR expression in urothelial carcinoma of the upper urinary tract is associated with disease progression and metaplastic morphology. Apmis 2008, 116, 27–32. [Google Scholar] [CrossRef]
- Kim, G.; Chung, Y.R.; Kim, B.; Song, B.; Moon, K.C. Comparison of the FDA and ASCO/CAP Criteria for HER2 Immunohistochemistry in Upper Urinary Tract Urothelial Carcinoma. J. Pathol. Transl. Med. 2016, 50, 436–441. [Google Scholar] [CrossRef]
- Schroder, F.H.; Hugosson, J.; Roobol, M.J.; Tammela, T.L.; Zappa, M.; Nelen, V.; Kwiatkowski, M.; Lujan, M.; Maattanen, L.; Lilja, H.; et al. Screening and prostate cancer mortality: Results of the European Randomised Study of Screening for Prostate Cancer (ERSPC) at 13 years of follow-up. Lancet 2014, 384, 2027–2035. [Google Scholar] [CrossRef]
- Raldow, A.C.; Zhang, D.; Chen, M.H.; Braccioforte, M.H.; Moran, B.J.; D’Amico, A.V. Risk Group and Death From Prostate Cancer: Implications for Active Surveillance in Men With Favorable Intermediate-Risk Prostate Cancer. JAMA Oncol. 2015, 1, 334–340. [Google Scholar] [CrossRef]
- Sanda, M.G.; Dunn, R.L.; Michalski, J.; Sandler, H.M.; Northouse, L.; Hembroff, L.; Lin, X.; Greenfield, T.K.; Litwin, M.S.; Saigal, C.S.; et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. New Engl. J. Med. 2008, 358, 1250–1261. [Google Scholar] [CrossRef] [PubMed]
- Klotz, L.; Vesprini, D.; Sethukavalan, P.; Jethava, V.; Zhang, L.; Jain, S.; Yamamoto, T.; Mamedov, A.; Loblaw, A. Long-term follow-up of a large active surveillance cohort of patients with prostate cancer. J. Clin. Oncol. 2015, 33, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Ahdoot, M.; Lebastchi, A.H.; Turkbey, B.; Wood, B.; Pinto, P.A. Contemporary treatments in prostate cancer focal therapy. Curr. Opin. Oncol. 2019, 31, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Borofsky, S.; George, A.K.; Gaur, S.; Bernardo, M.; Greer, M.D.; Mertan, F.V.; Taffel, M.; Moreno, V.; Merino, M.J.; Wood, B.J.; et al. What Are We Missing? False-Negative Cancers at Multiparametric MR Imaging of the Prostate. Radiology 2018, 286, 186–195. [Google Scholar] [CrossRef]
- Mehralivand, S.; George, A.K.; Hoang, A.N.; Rais-Bahrami, S.; Rastinehad, A.R.; Lebastchi, A.H.; Ahdoot, M.; Siddiqui, M.M.; Bloom, J.; Sidana, A.; et al. MRI-guided focal laser ablation of prostate cancer: A prospective single-arm, single-center trial with 3 years of follow-up. Diagn. Interv. Radiol. 2021, 27, 394–400. [Google Scholar] [CrossRef]
- Walser, E.; Nance, A.; Ynalvez, L.; Yong, S.; Aoughsten, J.S.; Eyzaguirre, E.J.; Williams, S.B. Focal Laser Ablation of Prostate Cancer: Results in 120 Patients with Low- to Intermediate-Risk Disease. J. Vasc. Interv. Radiol. 2019, 30, 401–409. [Google Scholar] [CrossRef]
- Hopstaken, J.S.; Bomers, J.G.R.; Sedelaar, M.J.P.; Valerio, M.; Futterer, J.J.; Rovers, M.M. An Updated Systematic Review on Focal Therapy in Localized Prostate Cancer: What Has Changed over the Past 5 Years? Eur. Urol. 2022, 81, 5–33. [Google Scholar] [CrossRef]
- Lebastchi, A.H.; George, A.K.; Polascik, T.J.; Coleman, J.; de la Rosette, J.; Turkbey, B.; Wood, B.J.; Gorin, M.A.; Sidana, A.; Ghai, S.; et al. Standardized Nomenclature and Surveillance Methodologies After Focal Therapy and Partial Gland Ablation for Localized Prostate Cancer: An International Multidisciplinary Consensus. Eur. Urol. 2020, 78, 371–378. [Google Scholar] [CrossRef]
- Sugano, D.; Sidana, A.; Calio, B.; Cobb, K.; Turkbey, B.; Pinto, P.A. MRI-targeted biopsy: Is systematic biopsy obsolete? Can J. Urol. 2017, 24, 8876–8882. [Google Scholar]
- Sun, B.L. Immunotherapy in treatment of metastatic prostate cancer: An approach to circumvent immunosuppressive tumor microenvironment. Prostate 2021, 81, 1125–1134. [Google Scholar] [CrossRef]
- Haberkorn, U.; Eder, M.; Kopka, K.; Babich, J.W.; Eisenhut, M. New Strategies in Prostate Cancer: Prostate-Specific Membrane Antigen (PSMA) Ligands for Diagnosis and Therapy. Clin. Cancer Res. 2016, 22, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Perner, S.; Hofer, M.D.; Kim, R.; Shah, R.B.; Li, H.; Moller, P.; Hautmann, R.E.; Gschwend, J.E.; Kuefer, R.; Rubin, M.A. Prostate-specific membrane antigen expression as a predictor of prostate cancer progression. Hum. Pathol. 2007, 38, 696–701. [Google Scholar] [CrossRef] [PubMed]
- Mangadlao, J.D.; Wang, X.; McCleese, C.; Escamilla, M.; Ramamurthy, G.; Wang, Z.; Govande, M.; Basilion, J.P.; Burda, C. Prostate-Specific Membrane Antigen Targeted Gold Nanoparticles for Theranostics of Prostate Cancer. ACS Nano 2018, 12, 3714–3725. [Google Scholar] [CrossRef] [PubMed]
- Nagaya, T.; Nakamura, Y.; Okuyama, S.; Ogata, F.; Maruoka, Y.; Choyke, P.L.; Kobayashi, H. Near-Infrared Photoimmunotherapy Targeting Prostate Cancer with Prostate-Specific Membrane Antigen (PSMA) Antibody. Mol. Cancer Res. 2017, 15, 1153–1162. [Google Scholar] [CrossRef]
- Watanabe, R.; Hanaoka, H.; Sato, K.; Nagaya, T.; Harada, T.; Mitsunaga, M.; Kim, I.; Paik, C.H.; Wu, A.M.; Choyke, P.L.; et al. Photoimmunotherapy targeting prostate-specific membrane antigen: Are antibody fragments as effective as antibodies? J. Nucl. Med. 2015, 56, 140–144. [Google Scholar] [CrossRef]
- Nakajima, K.; Miyazaki, F.; Terada, K.; Takakura, H.; Suzuki, M.; Ogawa, M. Comparison of low-molecular-weight ligand and whole antibody in prostate-specific membrane antigen targeted near-infrared photoimmunotherapy. Int. J. Pharm. 2021, 609, 121135. [Google Scholar] [CrossRef]
- Turkbey, B.; Choyke, P.L. (18)F-fluciclovine PET or PSMA PET for prostate cancer imaging? Nat. Rev. Urol. 2020, 17, 9–10. [Google Scholar] [CrossRef]
- Gaur, S.; Mena, E.; Harmon, S.A.; Lindenberg, M.L.; Adler, S.; Ton, A.T.; Shih, J.H.; Mehralivand, S.; Merino, M.J.; Wood, B.J.; et al. Prospective Evaluation of (18)F-DCFPyL PET/CT in Detection of High-Risk Localized Prostate Cancer: Comparison With mpMRI. AJR Am. J. Roentgenol. 2020, 215, 652–659. [Google Scholar] [CrossRef]
- Draulans, C.; Pos, F.; Smeenk, R.J.; Kerkmeijer, L.; Vogel, W.V.; Nagarajah, J.; Janssen, M.; Mai, C.; Heijmink, S.; van der Leest, M.; et al. (68)Ga-PSMA-11 PET, (18)F-PSMA-1007 PET, and MRI for Gross Tumor Volume Delineation in Primary Prostate Cancer: Intermodality and Intertracer Variability. Pract. Radiat. Oncol. 2021, 11, 202–211. [Google Scholar] [CrossRef]
- Bodar, Y.; Koene, B.; Meijer, D.; van Leeuwen, P.J.; Nadorp, S.; Donswijk, M.L.; Hendrikse, N.H.; Oprea-Lager, D.E.; Vis, A.N. Determining the diagnostic value of PSMA-PET/CT imaging in patients with persistent high prostate specific antigen levels and negative prostate biopsies. Urol. Oncol. 2022, 40, 58.e51–58.e57. [Google Scholar] [CrossRef]
- Simon, R.A.; di Sant’Agnese, P.A.; Huang, L.S.; Xu, H.D.; Yao, J.L.; Yang, Q.; Liang, S.R.; Liu, J.S.; Yu, R.; Cheng, L.; et al. CD44 expression is a feature of prostatic small cell Carcinoma and Distinguishes it from its Mimickers. Hum. Pathol. 2009, 40, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Patel, G.K.; Chugh, N.; Tripathi, M. Neuroendocrine Differentiation of Prostate Cancer-An Intriguing Example of Tumor Evolution at Play. Cancers 2019, 11, 1405. [Google Scholar] [CrossRef] [PubMed]
- Quach, N.D.; Kaur, S.P.; Eggert, M.W.; Ingram, L.; Ghosh, D.; Sheth, S.; Nagy, T.; Dawson, M.R.; Arnold, R.D.; Cummings, B.S. Paradoxical Role of Glypican-1 in Prostate Cancer Cell and Tumor Growth. Sci. Rep. 2019, 9, 11478. [Google Scholar] [CrossRef]
- Polikarpov, D.M.; Campbell, D.H.; Lund, M.E.; Lu, Y.; Lu, Y.; Wu, J.; Walsh, B.J.; Zvyagin, A.V.; Gillatt, D.A. The feasibility of Miltuximab(R)-IRDye700DX-mediated photoimmunotherapy of solid tumors. Photodiagnosis Photodyn. Ther. 2020, 32, 102064. [Google Scholar] [CrossRef]
- Sankin, A.; Hakimi, A.A.; Hsieh, J.J.; Molina, A.M. Metastatic non-clear cell renal cell carcinoma: An evidence based review of current treatment strategies. Front. Oncol. 2015, 5, 67. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Leung, D.K.; Chan, E.O.; Lok, V.; Leung, S.; Wong, I.; Lao, X.Q.; Zheng, Z.J.; Chiu, P.K.; Ng, C.F.; et al. A Global Trend Analysis of Kidney Cancer Incidence and Mortality and Their Associations with Smoking, Alcohol Consumption, and Metabolic Syndrome. Eur. Urol. Focus 2021, 8, 200–209. [Google Scholar] [CrossRef]
- Campbell, S.C.; Clark, P.E.; Chang, S.S.; Karam, J.A.; Souter, L.; Uzzo, R.G. Renal Mass and Localized Renal Cancer: Evaluation, Management, and Follow-Up: AUA Guideline: Part I. J. Urol. 2021, 206, 199–208. [Google Scholar] [CrossRef]
- Tenold, M.; Ravi, P.; Kumar, M.; Bowman, A.; Hammers, H.; Choueiri, T.K.; Lara, P.N., Jr. Current Approaches to the Treatment of Advanced or Metastatic Renal Cell Carcinoma. Am. Soc. Clin. Oncol. Educ. Book 2020, 40, 1–10. [Google Scholar] [CrossRef]
- Minner, S.; Rump, D.; Tennstedt, P.; Simon, R.; Burandt, E.; Terracciano, L.; Moch, H.; Wilczak, W.; Bokemeyer, C.; Fisch, M.; et al. Epidermal growth factor receptor protein expression and genomic alterations in renal cell carcinoma. Cancer 2012, 118, 1268–1275. [Google Scholar] [CrossRef]
- Iacovelli, R.; Nole, F.; Verri, E.; Renne, G.; Paglino, C.; Santoni, M.; Cossu Rocca, M.; Giglione, P.; Aurilio, G.; Cullura, D.; et al. Prognostic Role of PD-L1 Expression in Renal Cell Carcinoma. A Systematic Review and Meta-Analysis. Target Oncol. 2016, 11, 143–148. [Google Scholar] [CrossRef]
- Motzer, R.J.; Penkov, K.; Haanen, J.; Rini, B.; Albiges, L.; Campbell, M.T.; Venugopal, B.; Kollmannsberger, C.; Negrier, S.; Uemura, M.; et al. Avelumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2019, 380, 1103–1115. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.M.; Pei, L.J.; Yin, H.B.; Lin, F.; Li, X.Y.; Zhu, X.; He, W.Y.; Gou, X. Profiles of tumor-infiltrating immune cells in renal cell carcinoma and their clinical implications. Oncol. Lett. 2019, 18, 5235–5242. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, S.A.; Frigola, X.; Bonne-Annee, S.; Mercader, M.; Kuntz, S.M.; Krambeck, A.E.; Sengupta, S.; Dong, H.; Cheville, J.C.; Lohse, C.M.; et al. Tumor-infiltrating Foxp3-CD4+CD25+ T cells predict poor survival in renal cell carcinoma. Clin. Cancer Res. 2007, 13, 2075–2081. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Tannir, N.M.; McDermott, D.F.; Aren Frontera, O.; Melichar, B.; Choueiri, T.K.; Plimack, E.R.; Barthelemy, P.; Porta, C.; George, S.; et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2018, 378, 1277–1290. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, T.; Mitsunaga, M.; Ito, K.; Kobayashi, H.; Saruta, M. Cancer neovasculature-targeted near-infrared photoimmunotherapy (NIR-PIT) for gastric cancer: Different mechanisms of phototoxicity compared to cell membrane-targeted NIR-PIT. Gastric Cancer 2020, 23, 82–94. [Google Scholar] [CrossRef]
- Miller, K.D.; Fidler-Benaoudia, M.; Keegan, T.H.; Hipp, H.S.; Jemal, A.; Siegel, R.L. Cancer statistics for adolescents and young adults, 2020. CA Cancer J. Clin. 2020, 70, 443–459. [Google Scholar] [CrossRef]
- Oldenburg, J.; Berney, D.M.; Bokemeyer, C.; Climent, M.A.; Daugaard, G.; Gietema, J.A.; De Giorgi, U.; Haugnes, H.S.; Huddart, R.A.; Leao, R.; et al. Testicular seminoma and non-seminoma: ESMO-EURACAN Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2022, 33, 362–375. [Google Scholar] [CrossRef]
- Voutsadakis, I.A. The chemosensitivity of testicular germ cell tumors. Cell Oncol. 2014, 37, 79–94. [Google Scholar] [CrossRef]
- Quintanilla, M.; Montero-Montero, L.; Renart, J.; Martin-Villar, E. Podoplanin in Inflammation and Cancer. Int. J. Mol. Sci. 2019, 20, 707. [Google Scholar] [CrossRef]
- Nishinaga, Y.; Sato, K.; Yasui, H.; Taki, S.; Takahashi, K.; Shimizu, M.; Endo, R.; Koike, C.; Kuramoto, N.; Nakamura, S.; et al. Targeted Phototherapy for Malignant Pleural Mesothelioma: Near-Infrared Photoimmunotherapy Targeting Podoplanin. Cells 2020, 9, 1019. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, S.; Maclennan, G.T.; Biermann, K.; Foster, R.S.; Beck, S.D.; Cheng, L. Epidermal growth factor receptor protein expression and gene amplification in the chemorefractory metastatic embryonal carcinoma. Mod. Pathol. 2009, 22, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Shui, L.; Liu, Y.; Zheng, M. C-Kit, a Double-Edged Sword in Liver Regeneration and Diseases. Front. Genet. 2021, 12, 598855. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Boussouar, A.; Mazelin, L.; Tauszig-Delamasure, S.; Sun, Y.; Goldschneider, D.; Paradisi, A.; Mehlen, P. The Proto-oncogene c-Kit Inhibits Tumor Growth by Behaving as a Dependence Receptor. Mol. Cell 2018, 72, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Galvez-Carvajal, L.; Sanchez-Munoz, A.; Ribelles, N.; Saez, M.; Baena, J.; Ruiz, S.; Ithurbisquy, C.; Alba, E. Targeted treatment approaches in refractory germ cell tumors. Crit. Rev. Oncol. Hematol. 2019, 143, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, S.; Muguruma, N.; Okamoto, K.; Kurihara, T.; Sato, Y.; Miyamoto, Y.; Kitamura, S.; Miyamoto, H.; Taguchi, T.; Tsuneyama, K.; et al. A Novel Theranostic Combination of Near-infrared Fluorescence Imaging and Laser Irradiation Targeting c-KIT for Gastrointestinal Stromal Tumors. Theranostics 2018, 8, 2313–2328. [Google Scholar] [CrossRef]
- Miralles-Guri, C.; Bruni, L.; Cubilla, A.L.; Castellsague, X.; Bosch, F.X.; de Sanjose, S. Human papillomavirus prevalence and type distribution in penile carcinoma. J. Clin. Pathol. 2009, 62, 870–878. [Google Scholar] [CrossRef]
- Teh, J.; O’Connor, E.; O’Brien, J.; Lim, W.M.; Taylor, M.; Heriot, A.; Ramsay, R.; Lawrentschuk, N. Future directions in advanced penile cancer—mechanisms of carcinogenesis and a search for targeted therapy. Future Oncol. 2020, 16, 2357–2369. [Google Scholar] [CrossRef]
- Carthon, B.C.; Ng, C.S.; Pettaway, C.A.; Pagliaro, L.C. Epidermal growth factor receptor-targeted therapy in locally advanced or metastatic squamous cell carcinoma of the penis. BJU Int. 2014, 113, 871–877. [Google Scholar] [CrossRef]
| Type | Target Molecules | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EGFR | HER2 | PSMA | CD44 | PDPN | CD47 | TROP2 | PD-L1 | c-KIT | GPR87 | GPC1 | Nectin4 | FGFR3 | |
| Bladder cancer | ++ | + | + | ± | + | + | + | + | + | ++ | + | ||
| Prostate cancer | ± | ++ | + | + | + | + | + | ||||||
| Renal cell carcinoma | + | ± | ± | + | |||||||||
| Upper tract urothelial cancer | + | + | + | + | + | ||||||||
| Testicular cancer | ± | ± | + | + | + | + | + | ||||||
| Penile cancer | ++ | + | + | + | |||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fukushima, H.; Turkbey, B.; Pinto, P.A.; Furusawa, A.; Choyke, P.L.; Kobayashi, H. Near-Infrared Photoimmunotherapy (NIR-PIT) in Urologic Cancers. Cancers 2022, 14, 2996. https://doi.org/10.3390/cancers14122996
Fukushima H, Turkbey B, Pinto PA, Furusawa A, Choyke PL, Kobayashi H. Near-Infrared Photoimmunotherapy (NIR-PIT) in Urologic Cancers. Cancers. 2022; 14(12):2996. https://doi.org/10.3390/cancers14122996
Chicago/Turabian StyleFukushima, Hiroshi, Baris Turkbey, Peter A. Pinto, Aki Furusawa, Peter L. Choyke, and Hisataka Kobayashi. 2022. "Near-Infrared Photoimmunotherapy (NIR-PIT) in Urologic Cancers" Cancers 14, no. 12: 2996. https://doi.org/10.3390/cancers14122996
APA StyleFukushima, H., Turkbey, B., Pinto, P. A., Furusawa, A., Choyke, P. L., & Kobayashi, H. (2022). Near-Infrared Photoimmunotherapy (NIR-PIT) in Urologic Cancers. Cancers, 14(12), 2996. https://doi.org/10.3390/cancers14122996







