Low-Dose Metronomic Topotecan and Pazopanib (TOPAZ) in Children with Relapsed or Refractory Solid Tumors: A C17 Canadian Phase I Clinical Trial
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Eligibility
2.2. Drug Administration
2.3. Study Evaluations
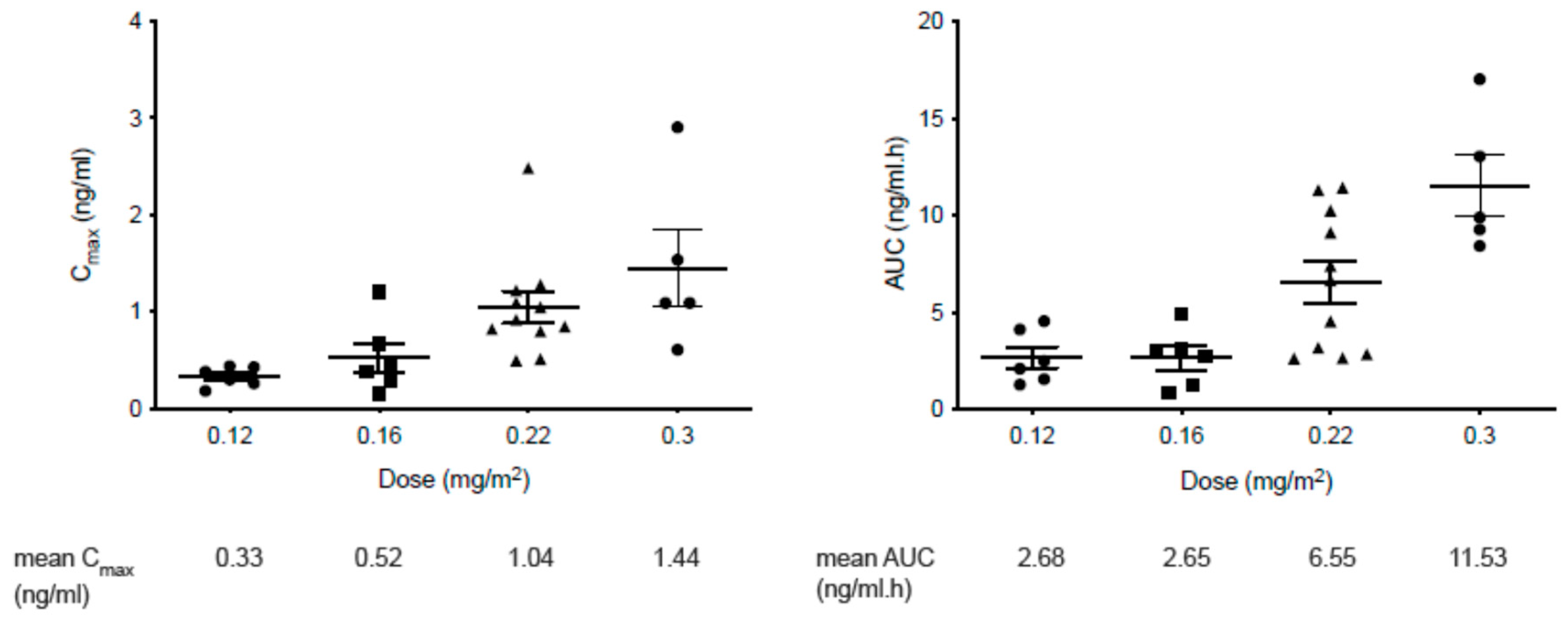
2.4. Pharmacokinetics
3. Results
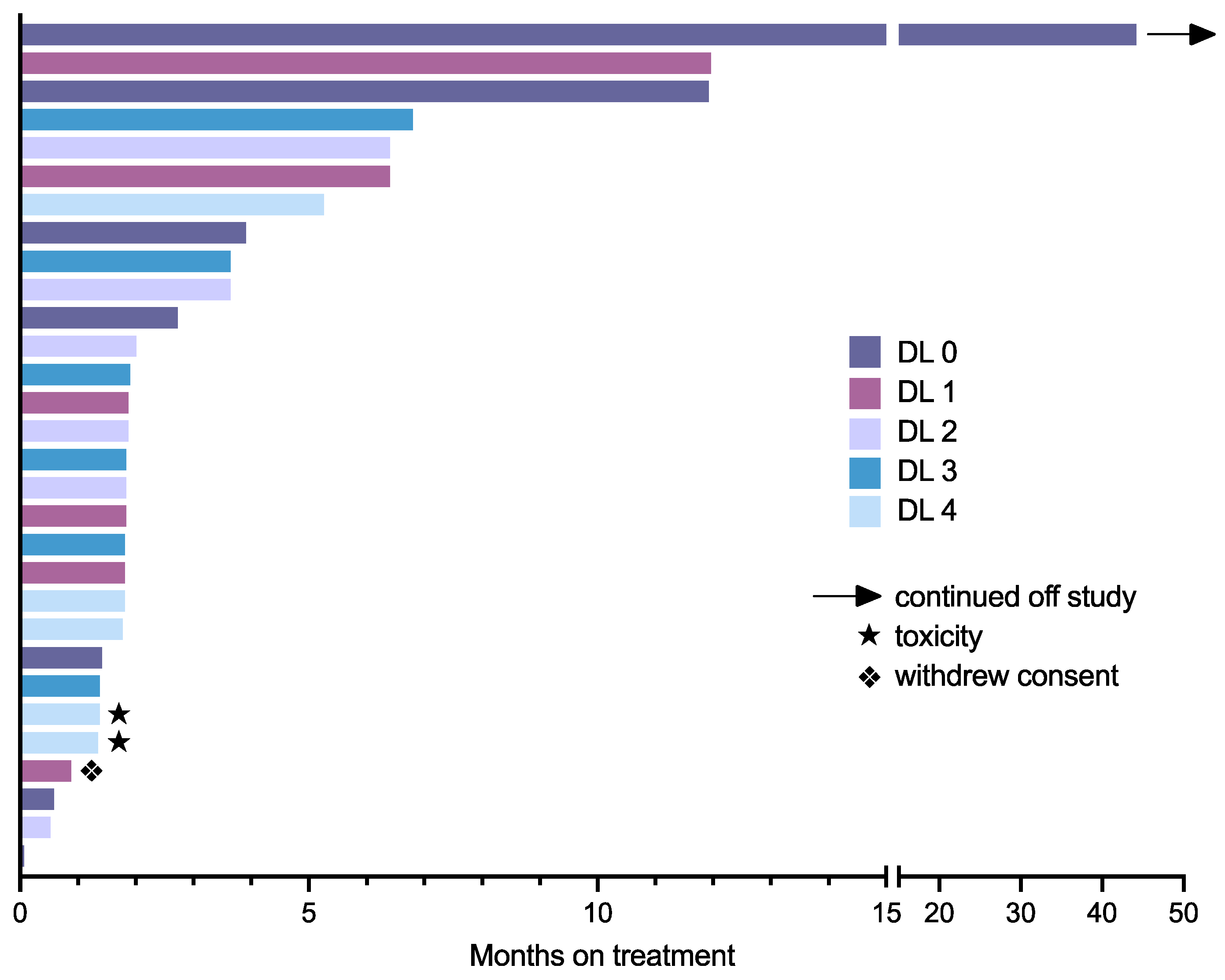
3.1. Patient Characteristics
3.2. Toxicities
3.3. PK Studies
3.4. Response Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pui, C.H.; Evans, W.E. Treatment of acute lymphoblastic leukemia. N. Engl. J. Med. 2006, 354, 166–178. [Google Scholar] [CrossRef] [PubMed]
- Yu, A.L.; Gilman, A.L.; Ozkaynak, M.F.; London, W.B.; Kreissman, S.G.; Chen, H.X.; Smith, M.; Anderson, B.; Villablanca, J.G.; Matthay, K.K.; et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N. Engl. J. Med. 2010, 363, 1324–1334. [Google Scholar] [CrossRef] [PubMed]
- Bisogno, G.; De Salvo, G.L.; Bergeron, C.; Gallego Melcon, S.; Merks, J.H.; Kelsey, A.; Martelli, H.; Minard-Colin, V.; Orbach, D.; Glosli, H.; et al. Vinorelbine and continuous low-dose cyclophosphamide as maintenance chemotherapy in patients with high-risk rhabdomyosarcoma (RMS 2005): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2019, 20, 1566–1575. [Google Scholar] [CrossRef]
- Gately, S.; Kerbel, R. Antiangiogenic scheduling of lower dose cancer chemotherapy. Cancer J. 2001, 7, 427–436. [Google Scholar]
- Browder, T.; Butterfield, C.E.; Kraling, B.M.; Shi, B.; Marshall, B.; O'Reilly, M.S.; Folkman, J. Antiangiogenic scheduling of chemotherapy improves efficacy against experimental drug-resistant cancer. Cancer Res. 2000, 60, 1878–1886. [Google Scholar]
- Pasquier, E.; Kavallaris, M.; Andre, N. Metronomic chemotherapy: New rationale for new directions. Nat. Rev. Clin. Oncol. 2010, 7, 455–465. [Google Scholar] [CrossRef]
- Peyrl, A.; Chocholous, M.; Azizi, A.; Kieran, M.W.; Nysom, K.; Sterba, J.; Sabel, M.; Czech, T.; Dieckmann, K.; Haberler, C.; et al. MEMMAT—A Phase II study of metronomic and targeted anti-angiogenesis therapy for children with recurrent/progressive medulloblastoma. Neuro Oncol. 2016, 18, i113. [Google Scholar] [CrossRef]
- Slavc, I.; Peyrl, A.; Chocholous, M.; Reisinger, D.; Mayr, L.; Azizi, A.; Dieckmann, K.; Haberler, C.; Czech, T. Response of recurrent malignant childhood CNS tumours to a MEMMAT based metronomic antiangiogenic combination therapy varies dependent on tumor type: Experience of 71 patients. Neuro Oncol. 2018, 20, i122. [Google Scholar] [CrossRef][Green Version]
- Cruz-Muñoz, W.; Di Desidero, T.; Man, S.; Xu, P.; Jaramillo, M.L.; Hashimoto, K.; Collins, C.; Banville, M.; O’Connor-Mccourt, M.D.; Kerbel, R.S. Analysis of acquired resistance to metronomic oral topotecan chemotherapy plus pazopanib after prolonged preclinical potent responsiveness in advanced ovarian cancer. Angiogenesis 2014, 17, 661–673. [Google Scholar] [CrossRef]
- Jedeszko, C.; Paez-Ribes, M.; Di Desidero, T.; Man, S.; Lee, C.R.; Xu, P.; Bjarnason, G.A.; Bocci, G.; Kerbel, R.S. Postsurgical adjuvant or metastatic renal cell carcinoma therapy models reveal potent antitumor activity of metronomic oral topotecan with pazopanib. Sci. Transl. Med. 2015, 7, 282.ra250. [Google Scholar] [CrossRef]
- Sternberg, C.N.; Davis, I.D.; Mardiak, J.; Szczylik, C.; Lee, E.; Wagstaff, J.; Barrios, C.H.; Salman, P.; Gladkov, O.A.; Kavina, A.; et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: Results of a randomized phase III trial. J. Clin. Oncol. 2010, 28, 1061–1068. [Google Scholar] [CrossRef] [PubMed]
- van der Graaf, W.T.; Blay, J.Y.; Chawla, S.P.; Kim, D.W.; Bui-Nguyen, B.; Casali, P.G.; Schoffski, P.; Aglietta, M.; Staddon, A.P.; Beppu, Y.; et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2012, 379, 1879–1886. [Google Scholar] [CrossRef]
- Glade Bender, J.L.; Lee, A.; Reid, J.M.; Baruchel, S.; Roberts, T.; Voss, S.D.; Wu, B.; Ahern, C.H.; Ingle, A.M.; Harris, P.; et al. Phase I pharmacokinetic and pharmacodynamic study of pazopanib in children with soft tissue sarcoma and other refractory solid tumors: A children's oncology group phase I consortium report. J. Clin. Oncol. 2013, 31, 3034–3043. [Google Scholar] [CrossRef]
- Weiss, A.R.; Chen, Y.L.; Scharschmidt, T.J.; Chi, Y.Y.; Tian, J.; Black, J.O.; Davis, J.L.; Fanburg-Smith, J.C.; Zambrano, E.; Anderson, J.; et al. Pathological response in children and adults with large unresected intermediate-grade or high-grade soft tissue sarcoma receiving preoperative chemoradiotherapy with or without pazopanib (ARST1321): A multicentre, randomised, open-label, phase 2 trial. Lancet Oncol. 2020, 21, 1110–1122. [Google Scholar] [CrossRef]
- Hashimoto, K.; Man, S.; Xu, P.; Cruz-Munoz, W.; Tang, T.; Kumar, R.; Kerbel, R.S. Potent Preclinical Impact of Metronomic Low-Dose Oral Topotecan Combined with the Antiangiogenic Drug Pazopanib for the Treatment of Ovarian Cancer. Mol. Cancer 2010, 9, 996–1006. [Google Scholar] [CrossRef]
- Merritt, W.M.; Nick, A.M.; Carroll, A.R.; Lu, C.; Matsuo, K.; Dumble, M.; Jennings, N.; Zhang, S.; Lin, Y.G.; Spannuth, W.A.; et al. Bridging the Gap between Cytotoxic and Biologic Therapy with Metronomic Topotecan and Pazopanib in Ovarian Cancer. Mol. Cancer Ther. 2010, 9, 985–995. [Google Scholar] [CrossRef]
- Di Desidero, T.; Xu, P.; Man, S.; Bocci, G.; Kerbel, R.S. Potent efficacy of metronomic topotecan and pazopanib combination therapy in preclinical models of primary or late stage metastatic triple-negative breast cancer. Oncotarget 2015, 6, 42396–42410. [Google Scholar] [CrossRef]
- Di Desidero, T.; Orlandi, P.; Gentile, D.; Bocci, G. Effects of Pazopanib Monotherapy vs. Pazopanib and Topotecan Combination on Anaplastic Thyroid Cancer Cells. Front. Oncol. 2019, 9, 1202. [Google Scholar] [CrossRef]
- Kumar, S.; Bayat Mokhtari, R.; Dias Oliveira, I.; Islam, S.; Toledo, S.R.C.; Yeger, H.; Baruchel, S. Tumor Dynamics in Response to Antiangiogenic Therapy with Oral Metronomic Topotecan and Pazopanib in Neuroblastoma Xenografts. Transl. Oncol. 2013, 6, 493–503. [Google Scholar] [CrossRef][Green Version]
- Kumar, S.; Mokhtari, R.B.; Sheikh, R.; Wu, B.; Zhang, L.; Xu, P.; Man, S.; Oliveira, I.D.; Yeger, H.; Kerbel, R.S.; et al. Metronomic Oral Topotecan with Pazopanib Is an Active Antiangiogenic Regimen in Mouse Models of Aggressive Pediatric Solid Tumor. Clin. Cancer Res. 2011, 17, 5656–5667. [Google Scholar] [CrossRef]
- Kerklaan, B.M.; Lolkema, M.P.J.; Devriese, L.A.; Voest, E.E.; Nol-Boekel, A.; Mergui-Roelvink, M.; Langenberg, M.; Mykulowycz, K.; Stoebenau, J.; Lane, S.; et al. Phase I and pharmacological study of pazopanib in combination with oral topotecan in patients with advanced solid tumours. Brit. J. Cancer 2015, 113, 706–715. [Google Scholar] [CrossRef] [PubMed]
- Schulte, B.; Mohindra, N.; Milhem, M.; Attia, S.; Robinson, S.; Monga, V.; Hirbe, A.C.; Oppelt, P.; Charlson, J.; Helenowski, I.; et al. Phase II study of pazopanib with oral topotecan in patients with metastatic and non-resectable soft tissue and bone sarcomas. Br. J. Cancer 2021, 125, 528–533. [Google Scholar] [CrossRef] [PubMed]
- Tillmanns, T.D.; Reed, M.E.; Privett, M.C.; Johns, A.L.; Walker, M.S.; Houts, A.C. Phase I trial of metronomic oral topotecan in combination with pazopanib utilizing a daily dosing schedule to treat recurrent or persistent gynecologic tumors. J. Clin. Oncol. 2012, 30, 5014. [Google Scholar] [CrossRef]
- Skolnik, J.M.; Barrett, J.S.; Jayaraman, B.; Patel, D.; Adamson, P.C. Shortening the timeline of pediatric phase I trials: The rolling six design. J. Clin. Oncol. 2008, 26, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Yanik, G.A.; Parisi, M.T.; Shulkin, B.L.; Naranjo, A.; Kreissman, S.G.; London, W.B.; Villablanca, J.G.; Maris, J.M.; Park, J.R.; Cohn, S.L.; et al. Semiquantitative mIBG scoring as a prognostic indicator in patients with stage 4 neuroblastoma: A report from the Children's oncology group. J. Nucl. Med. 2013, 54, 541–548. [Google Scholar] [CrossRef]
- Zhang, Y.; Huo, M.; Zhou, J.; Xie, S. PKSolver: An add-in program for pharmacokinetic and pharmacodynamic data analysis in Microsoft Excel. Comput. Methods Programs Biomed. 2010, 99, 306–314. [Google Scholar] [CrossRef]
- Hurwitz, H.I.; Dowlati, A.; Saini, S.; Savage, S.; Suttle, A.B.; Gibson, D.M.; Hodge, J.P.; Merkle, E.M.; Pandite, L. Phase I trial of pazopanib in patients with advanced cancer. Clin. Cancer Res. 2009, 15, 4220–4227. [Google Scholar] [CrossRef]
- Turner, D.C.; Tillmanns, T.D.; Harstead, K.E.; Throm, S.L.; Stewart, C.F. Combination metronomic oral topotecan and pazopanib: A pharmacokinetic study in patients with gynecological cancer. Anticancer. Res. 2013, 33, 3823–3829. [Google Scholar]
- Just, M.A.; Van Mater, D.; Wagner, L.M. Receptor tyrosine kinase inhibitors for the treatment of osteosarcoma and Ewing sarcoma. Pediatr. Blood Cancer 2021, 68, e29084. [Google Scholar] [CrossRef]
| Characteristic | Value |
|---|---|
| Age, years | |
| Median | 12 |
| Range | 3–20 |
| Sex | |
| Male | 13 |
| Female | 12 |
| Diagnosis | |
| Sarcoma | |
| Osteosarcoma | 8 |
| Rhabdomyosarcoma | 4 |
| Ewing Sarcoma/PNET | 4 |
| Synovial sarcoma | 1 |
| Neuroblastoma | 7 |
| Brain Tumor | |
| Ependymoma | 2 |
| High-grade glioma | 1 |
| Medulloblastoma | 1 |
| Adrenocortical carcinoma | 1 |
| Wilms Tumor | 1 |
| Prior chemotherapy regimens, number | |
| Median | 1.5 |
| Range | 0–10 |
| Prior radiation therapy | 22 |
| Dose Level | Topotecan Dose (mg/m2/Day) | Pazopanib Dose (mg/m2/Day) | Patients Entered | Patients Evaluable | Patients with DLT |
|---|---|---|---|---|---|
| 0 | 0.12 | 125 | 6 | 5 | 0 |
| 1 | 0.16 | 125 | 6 | 5 | 0 |
| 2 | 0.22 | 125 | 7 | 5 | 0 |
| 3 | 0.22 | 160 | 6 | 6 | 0 |
| 4 | 0.3 | 160 | 5 | 5 | 2 * |
| Number of Patients | ||||
|---|---|---|---|---|
| Maximum Grade Observed per Patient during Cycle 1 (n = 28) | Maximum Grade Observed per Patient across All Cycles (n = 28) | |||
| Toxicity Type | Any Grade | Grade 3/4 | Any Grade | Grade 3/4 |
| Hematologic | ||||
| Thrombocytopenia | 12 | 3 | 14 | 4 * |
| Neutropenia | 15 | 5 | 19 | 9 |
| Lymphopenia | 13 | 3 | 20 | 4 |
| Anemia | 16 | 2 | 20 | 5 |
| Non-hematologic † | ||||
| GI/metabolic | ||||
| Constipation | 7 | 0 | 10 | 0 |
| Diarrhea | 3 | 0 | 8 | 1 |
| Nausea | 11 | 0 | 14 | 0 |
| Vomiting | 9 | 0 | 13 | 0 |
| Anorexia | 8 | 0 | 15 | 0 |
| ALT elevation | 12 | 2 | 14 | 2 * |
| AST elevation | 13 | 2 | 18 | 3 |
| Bilirubin elevation | 5 | 0 | 8 | 0 |
| Hypoalbuminemia | 6 | 0 | 10 | 1 |
| Lipase elevation | 4 | 2 | 7 | 3 |
| Creatinine elevation | 3 | 0 | 4 | 0 |
| Hypocalcemia | 4 | 0 | 12 | 1 |
| Hyperkalemia | 3 | 0 | 9 | 0 |
| Hypokalemia | 3 | 0 | 3 | 0 |
| Hypomagnesemia | 4 | 0 | 7 | 0 |
| Hypophosphatemia | 5 | 0 | 8 | 0 |
| Cardiac/respiratory | ||||
| Sinus tachycardia | 3 | 0 | 4 | 0 |
| Cough | 5 | 0 | 9 | 0 |
| Nervous system | ||||
| Ataxia | 3 | 0 | 3 | 0 |
| Headache | 4 | 0 | 6 | 1 |
| Psychiatric | ||||
| Anxiety | 3 | 0 | 5 | 0 |
| Insomnia | 3 | 0 | 5 | 0 |
| Musculoskeletal/constitutional | ||||
| Fatigue | 11 | 0 | 17 | 0 |
| Pain | 6 | 0 | 13 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manji, A.; Samson, Y.; Deyell, R.J.; Johnston, D.L.; Lewis, V.A.; Zorzi, A.P.; Berman, J.N.; Brodeur-Robb, K.; Morrison, E.; Kee, L.; et al. Low-Dose Metronomic Topotecan and Pazopanib (TOPAZ) in Children with Relapsed or Refractory Solid Tumors: A C17 Canadian Phase I Clinical Trial. Cancers 2022, 14, 2985. https://doi.org/10.3390/cancers14122985
Manji A, Samson Y, Deyell RJ, Johnston DL, Lewis VA, Zorzi AP, Berman JN, Brodeur-Robb K, Morrison E, Kee L, et al. Low-Dose Metronomic Topotecan and Pazopanib (TOPAZ) in Children with Relapsed or Refractory Solid Tumors: A C17 Canadian Phase I Clinical Trial. Cancers. 2022; 14(12):2985. https://doi.org/10.3390/cancers14122985
Chicago/Turabian StyleManji, Arif, Yvan Samson, Rebecca J. Deyell, Donna L. Johnston, Victor A. Lewis, Alexandra P. Zorzi, Jason N. Berman, Kathy Brodeur-Robb, Ellen Morrison, Lynn Kee, and et al. 2022. "Low-Dose Metronomic Topotecan and Pazopanib (TOPAZ) in Children with Relapsed or Refractory Solid Tumors: A C17 Canadian Phase I Clinical Trial" Cancers 14, no. 12: 2985. https://doi.org/10.3390/cancers14122985
APA StyleManji, A., Samson, Y., Deyell, R. J., Johnston, D. L., Lewis, V. A., Zorzi, A. P., Berman, J. N., Brodeur-Robb, K., Morrison, E., Kee, L., Kumar, S., Baruchel, S., Whitlock, J. A., & Morgenstern, D. A. (2022). Low-Dose Metronomic Topotecan and Pazopanib (TOPAZ) in Children with Relapsed or Refractory Solid Tumors: A C17 Canadian Phase I Clinical Trial. Cancers, 14(12), 2985. https://doi.org/10.3390/cancers14122985






