Pathological Analysis of Encased Resected Recurrent Nerves in Locally Invasive Thyroid Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, C.-W.; Dionigi, G.; Barczynski, M.; Chiang, F.-Y.; Dralle, H.; Schneider, R.; Al-Quaryshi, Z.; Angelos, P.; Brauckhoff, K.; Brooks, J.A.; et al. International neuromonitoring study group guidelines 2018: Part II: Optimal recurrent laryngeal nerve management for invasive thyroid cancer-incorporation of surgical, laryngeal, and neural electrophysiologic data. Laryngoscope 2018, 128, S18–S27. [Google Scholar] [CrossRef] [PubMed]
- Shindo, M.L.; Caruana, S.M.; Kandil, E.; McCaffrey, J.C.; Orloff, L.A.; Porterfield, J.R.; Shaha, A.; Shin, J.; Terris, D.; Randolph, G. Management of invasive well-differentiated thyroid cancer: An American head and neck society consensus statement: AHNS consensus statement. Head Neck 2014, 36, 1379–1390. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Onoda, N.; Okamoto, T. The revised clinical practice guidelines on the management of thyroid tumors by the Japan Associations of Endocrine Surgeons: Core questions and recommendations for treatments of thyroid cancer. Endocr. J. 2020, 67, 669–717. [Google Scholar] [CrossRef] [PubMed]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid Off. J. Am. Thyroid Assoc. 2016, 26, 1–133. [Google Scholar] [CrossRef]
- Amin, E.S.; Greene, F.; Byrd, D.R.; Brookland, R.K.; Washington, M.K.; Gershenwald, J.E.; Compton, C.C.; Hess, K.R.; Sullivan, D.C.; Jessup, J.M.; et al. AJCC Cancer Staging Manual, 8th ed.; Amin, E.S., Greene, F., Byrd, D.R., Brookland, R.K., Washington, M.K., Gershenwald, J.E., Compton, C.C., Hess, K.R., Sullivan, D.C., Jessup, J.M., et al., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Isshiki, N.; Morita, H.; Okamura, H.; Hiramoto, M. Thyroplasty as a New Phonosurgical Technique. Acta Oto-Laryngol. 1974, 78, 451–457. [Google Scholar] [CrossRef]
- Rosen, C.A.; Lee, A.S.; Osborne, J.; Zullo, T.; Murry, T. Development and Validation of the Voice Handicap Index-10. Laryngoscope 2004, 114, 1549–1556. [Google Scholar] [CrossRef]
- EEOfRaTo. Cancer. Available online: http://groups.eortc.be/qol/sites/default/files/img/specimen_for_printing_hn35.pdf (accessed on 1 April 2022).
- Honings, J.; Stephen, A.E.; Marres, H.A.; Gaissert, H.A. The management of thyroid carcinoma invading the larynx or trachea. Laryngoscope 2010, 120, 682–689. [Google Scholar] [CrossRef]
- Patel, K.N.; Shaha, A.R. Locally advanced thyroid cancer. Curr. Opin. Otolaryngol. Head Neck Surg. 2005, 13, 112–116. [Google Scholar] [CrossRef]
- McCaffrey, T.V.; Bergstralh, E.J.; Hay, I.D. Locally invasive papillary thyroid carcinoma: 1940–1990. Head Neck 1994, 16, 165–172. [Google Scholar] [CrossRef]
- McCaffrey, J.C. Aerodigestive Tract Invasion by Well-Differentiated Thyroid Carcinoma: Diagnosis, Management, Prognosis, and Biology. Laryngoscope 2006, 11, 1–11. [Google Scholar] [CrossRef]
- Friedman, M.; Shelton, V.K.; Berlinger, F.G.; Skolnik, E.M.; Arab, M. Laryngotracheal Invasion by Thyroid Carcinoma. Ann. Otol. Rhinol. Laryngol. 1982, 91, 363–369. [Google Scholar] [CrossRef] [PubMed]
- McConahey, W.M.; Hay, I.; Woolner, L.B.; van Heerden, J.A.; Taylor, W.F. Papillary Thyroid Cancer Treated at the Mayo Clinic, 1946 Through 1970: Initial Manifestations, Pathologic Findings, Therapy, and Outcome. Mayo Clin. Proc. 1986, 61, 978–996. [Google Scholar] [CrossRef]
- Park, C.S.; Suh, K.W.; Min, J.S. Cartilage-shaving procedure for the control of tracheal cartilage invasion by thyroid carcinoma. Head Neck 1993, 15, 289–291. [Google Scholar] [CrossRef] [PubMed]
- Sywak, M.; Pasieka, J.L.; McFadden, S.; Gelfand, G.; Terrell, J.; Dort, J. Functional results and quality of life after tracheal resection for locally invasive thyroid cancer. Am. J. Surg. 2003, 185, 462–467. [Google Scholar] [CrossRef]
- Wang, L.Y.; Nixon, I.; Patel, S.G.; Palmer, F.L.; Tuttle, R.M.; Shaha, A.; Shah, J.P.; Ganly, I. Operative management of locally advanced, differentiated thyroid cancer. Surgery 2016, 160, 738–746. [Google Scholar] [CrossRef]
- Hotomi, M.; Sugitani, I.; Toda, K.; Kawabata, K.; Fujimoto, Y. A Novel Definition of Extrathyroidal Invasion for Patients with Papillary Thyroid Carcinoma for Predicting Prognosis. World J. Surg. 2012, 36, 1231–1240. [Google Scholar] [CrossRef]
- Na, H.-S.; Kwon, H.-K.; Shin, S.-C.; Cheon, Y.-I.; Seo, M.; Lee, J.-C.; Sung, E.-S.; Lee, M.; Kim, I.-J.; Kim, B.H.; et al. Clinical outcomes of T4a papillary thyroid cancer with recurrent laryngeal nerve involvement: A retrospective analysis. Sci. Rep. 2021, 11, 6707. [Google Scholar] [CrossRef]
- Boucek, J.; Zabrodsky, M.; Kuchař, M.; Fanta, O.; Skřivan, J.; Betka, J. A New Strategy for the Surgical Management of RLN Infiltrated by Well-Differentiated Thyroid Carcinoma. BioMed Res. Int. 2014, 2014, 616521. [Google Scholar] [CrossRef]
- Eustatia-Rutten, C.F.A.; Corssmit, E.P.M.; Biermasz, N.R.; Pereira, A.M.; Romijn, J.A.; Smit, J.W. Survival and Death Causes in Differentiated Thyroid Carcinoma. J. Clin. Endocrinol. Metab. 2006, 91, 313–319. [Google Scholar] [CrossRef]
- Lang, B.H.-H.; Lo, C.-Y.; Wong, K.P.; Wan, K.Y. Should an Involved but Functioning Recurrent Laryngeal Nerve be Shaved or Resected in a Locally Advanced Papillary Thyroid Carcinoma? Ann. Surg. Oncol. 2013, 20, 2951–2957. [Google Scholar] [CrossRef]
- Tuttle, R.M.; Haugen, B.; Perrier, N.D. Updated American Joint Committee on Cancer/Tumor-Node-Metastasis Staging System for Differentiated and Anaplastic Thyroid Cancer (Eighth Edition): What Changed and Why? Thyroid Off. J. Am. Thyroid. Assoc. 2017, 27, 751–756. [Google Scholar] [CrossRef]
- Lamartina, L.; Ippolito, S.; Danis, M.; Bidault, F.; Borget, I.; Berdelou, A.; Al Ghuzlan, A.; Hartl, D.; Blanchard, P.; Terroir, M.; et al. Anti-angiogenic Tyrosine Kinase Inhibitors: Occurrence and risk factors of hemoptysis in refractory thyroid cancer. J. Clin. Endocrinol. Metab. 2016, 101, 2733–2741. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Albano, D.; Bertagna, F.; Panarotto, M.B.; Giubbini, R. Early and late adverse effects of radioiodine for pediatric differentiated thyroid cancer. Pediatr. Blood Cancer 2017, 64, e26595. [Google Scholar] [CrossRef] [PubMed]
- Parisi, M.T.; Eslamy, H.; Mankoff, D. Management of Differentiated Thyroid Cancer in Children: Focus on the American Thyroid Association Pediatric Guidelines. Semin. Nucl. Med. 2016, 46, 147–164. [Google Scholar] [CrossRef] [PubMed]
- Deandreis, D.; Al Ghuzlan, A.; Leboulleux, S.; Lacroix, L.; Garsi, J.P.; Talbot, M.; Lumbroso, J.; Baudin, E.; Caillou, B.; Bidart, J.M.; et al. Do histological, immunohistochemical, and metabolic (radioiodine and fluorodeoxyglucose uptakes) patterns of metastatic thyroid cancer correlate with patient outcome? Endocr. Relat. Cancer 2010, 18, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Robbins, R.J.; Larson, S.M. The value of positron emission tomography (PET) in the management of patients with thyroid cancer. Best Pract. Res. Clin. Endocrinol. Metab. 2008, 22, 1047–1059. [Google Scholar] [CrossRef]
- Durante, C.; Haddy, N.; Baudin, E.; Leboulleux, S.; Hartl, D.; Travagli, J.P.; Caillou, B.; Ricard, M.; Lumbroso, J.D.; De Vathaire, F.; et al. Long-Term Outcome of 444 Patients with Distant Metastases from Papillary and Follicular Thyroid Carcinoma: Benefits and Limits of Radioiodine Therapy. J. Clin. Endocrinol. Metab. 2006, 91, 2892–2899. [Google Scholar] [CrossRef]
- Reina, M.A.; Boezaart, A.P.; Tubbs, R.S.; Zasimovich, Y.; Fernández-Domínguez, M.; Fernández, P.; Sala-Blanch, X. Another (Internal) Epineurium: Beyond the Anatomical Barriers of Nerves. Clin. Anat. 2020, 33, 199–206. [Google Scholar] [CrossRef]
- Batsakis, J.G. Nerves and neurotropic carcinomas. Ann. Otol. Rhinol. Laryngol. 1985, 94, 426–427. [Google Scholar]
- Liebig, C.; Ayala, G.; Wilks, J.A.; Berger, D.H.; Albo, D. Perineural invasion in cancer. Cancer 2009, 115, 3379–3391. [Google Scholar] [CrossRef]
- Kihara, M.; Miyauchi, A.; Yabuta, T.; Higashiyama, T.; Fukushima, M.; Ito, Y.; Kobayashi, K.; Miya, A. Outcome of vocal cord function after partial layer resection of the recurrent laryngeal nerve in patients with invasive papillary thyroid cancer. Surgery 2014, 155, 184–189. [Google Scholar] [CrossRef]
- Nishida, T.; Nakao, K.; Hamaji, M.; Kamiike, W.; Kurozumi, K.; Matsuda, H. Preservation of Recurrent Laryngeal Nerve Invaded by Differentiated Thyroid Cancer. Ann. Surg. 1997, 226, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Miyamaru, S.; Murakami, D.; Nishimoto, K.; Kodama, N.; Tashiro, J.; Miyamoto, Y.; Saito, H.; Takeda, H.; Ise, M.; Orita, Y. Optimal Management of the Unilateral Recurrent Laryngeal Nerve Involvement in Patients with Thyroid Cancer. Cancers 2021, 13, 2129. [Google Scholar] [CrossRef] [PubMed]
- Moritani, S.; Takenobu, M.; Yoshioka, K.; Kawamoto, K.; Fujii, T.; Yasunaga, M.; Kitano, H. Novel surgical methods for reconstruction of the recurrent laryngeal nerve: Microscope-guided partial layer resection and intralaryngeal reconstruction of the recurrent laryngeal nerve. Surgery 2021, 169, 1124–1130. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Lei, J.; You, J.; Lei, Y.; Li, Z.; Gong, R.; Tang, H.; Zhu, J. Predictive factors and prognosis for recurrent laryngeal nerve invasion in papillary thyroid carcinoma. OncoTargets Ther. 2017, 10, 4485–4491. [Google Scholar] [CrossRef]
- Kamani, D.; Darr, E.A.; Randolph, G.W. Electrophysiologic Monitoring Characteristics of the Recurrent Laryngeal Nerve Preoperatively Paralyzed or Invaded with Malignancy. Otolaryngol. Neck Surg. Off. J. Am. Acad. Otolaryngol. Head Neck Surg. 2013, 149, 682–688. [Google Scholar] [CrossRef] [PubMed]
- Kesby, N.L.; Papachristos, A.J.; Gild, M.; Aniss, A.; Sywak, M.S.; Clifton-Bligh, R.; Sidhu, S.B.; Glover, A.R. Outcomes of Advanced Medullary Thyroid Carcinoma in the Era of Targeted Therapy. Ann. Surg. Oncol. 2022, 29, 64–71. [Google Scholar] [CrossRef]
- Crumley, R.L.; Izdebski, K. Voice Quality Following Laryngeal Reinnervation by Ansa Hypoglossi Transfer. Laryngoscope 1986, 96, 611–616. [Google Scholar] [CrossRef]
- Miyauchi, A.; Matsusaka, K.; Kihara, M.; Matsuzuka, F.; Hirai, K.; Yokozawa, T.; Kobayashi, K.; Kobayashi, A.; Kuma, K. The role of ansa-to-recurrent-laryngeal nerve anastomosis in operations for thyroid cancer. Eur. J. Surg. 1998, 164, 927–933. [Google Scholar] [CrossRef]
- Arffa, R.E.; Krishna, P.; Gartner-Schmidt, J.; Rosen, C.A. Normative Values for the Voice Handicap Index-10. J. Voice Off. J. Voice Found. 2012, 26, 462–465. [Google Scholar] [CrossRef]
- Galletti, B.; Freni, F.; Cammaroto, G.; Catalano, N.; Gangemi, G.; Galletti, F. Vocal Outcome After CO2 Laser Cordectomy Performed on Patients Affected by Early Glottic Carcinoma. J. Voice Off. J. Voice Found. 2012, 26, 801–805. [Google Scholar] [CrossRef] [PubMed]
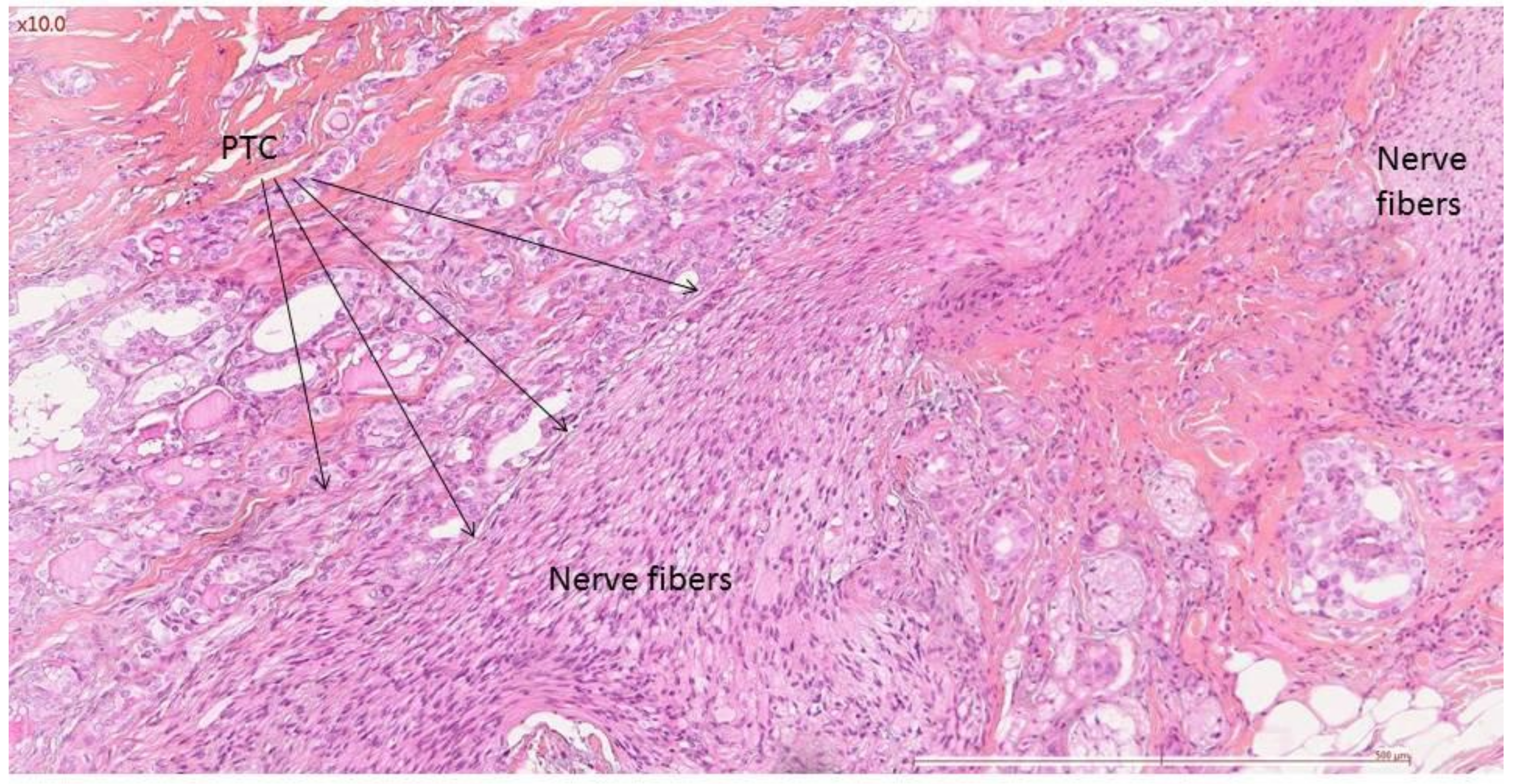
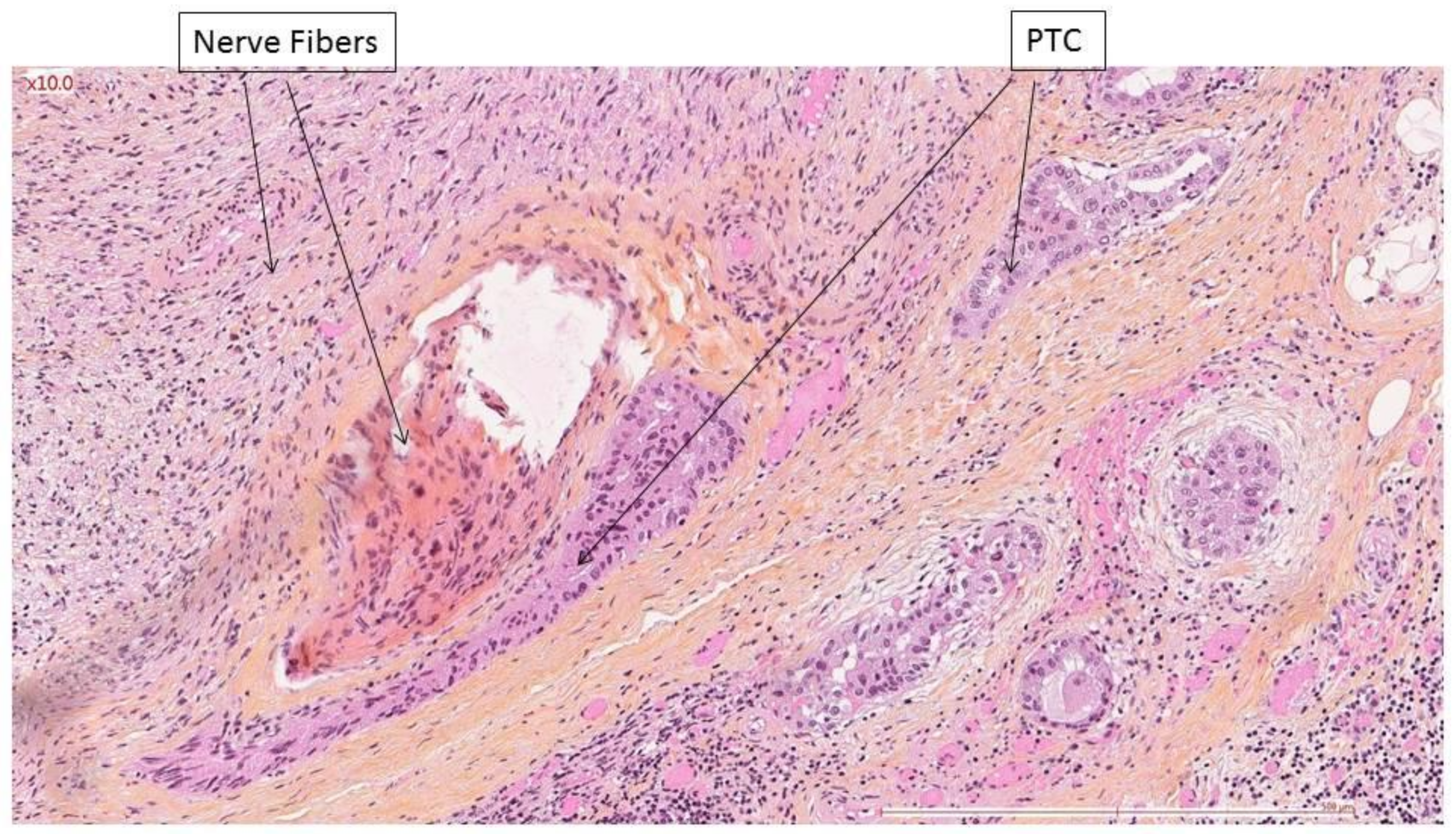
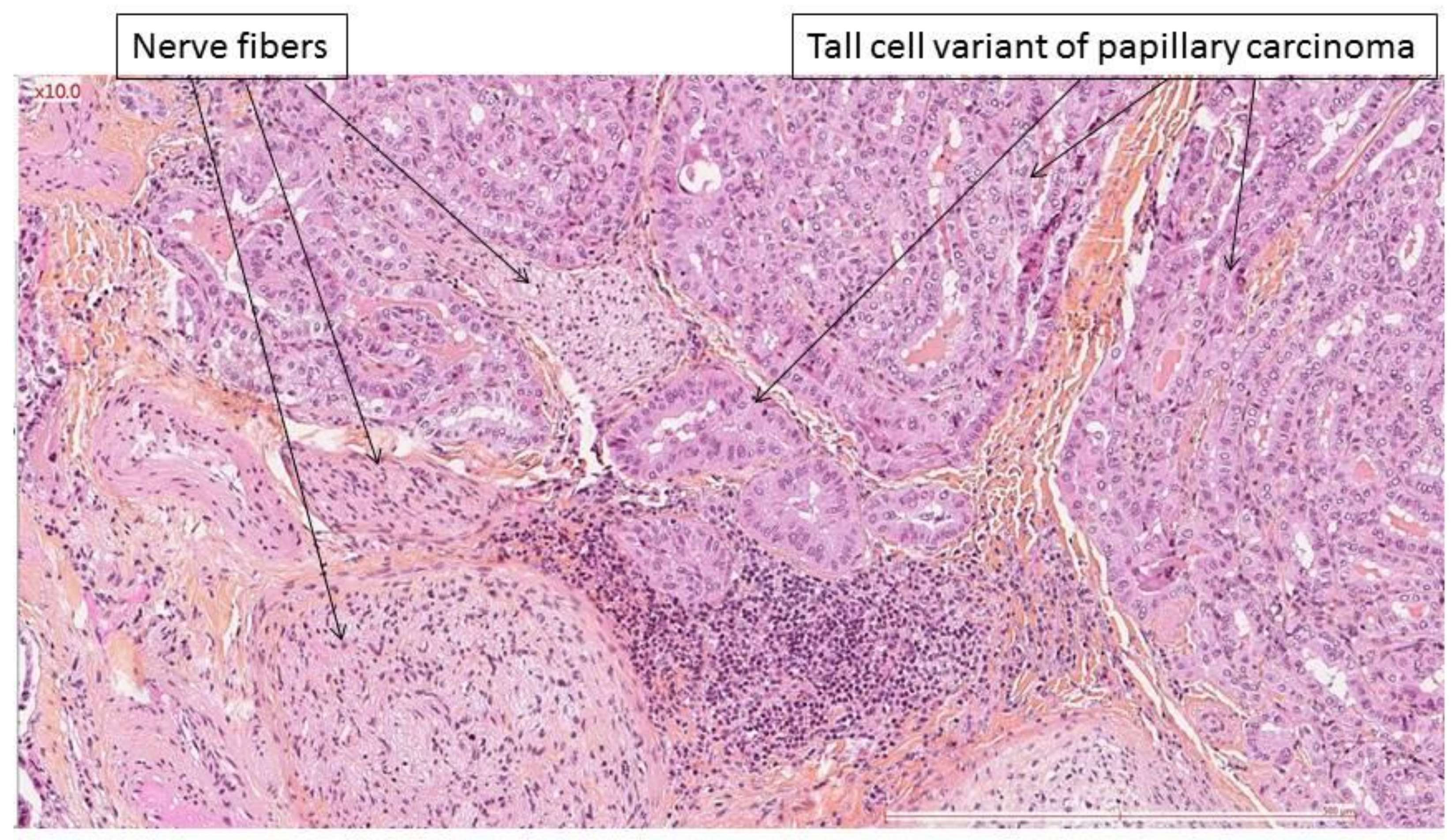
| Preoperative VF Paralysis | Presence of Nerve Invasion | Rate of Nerve Invasion | ||
|---|---|---|---|---|
| Follicular cell-derived carcinoma | Adults n = 37 | No n = 23 | 21/23 | 30/37 (81%) |
| Yes n = 14 | 9/14 | |||
| Children n = 8 | No n = 7 | 6/7 | 7/8 (88%) | |
| Yes n = 1 | 1/1 | |||
| Medullary thyroid carcinoma | Adults n = 7 | No n = 6 | 6/6 | 7/7 (100%) |
| Yes n = 1 | 1/1 |
| Patient Factors | Oncologic Factors |
|---|---|
| Age | RAI+ versus PET+ disease |
|
|
| Comorbidities Risk of aspiration pneumonia | Risk of local complications if residual disease (R2) left near the visceral axis |
| Voice and laryngeal mobility Contralateral vocal fold mobility | Complete resection possible or not |
| Quality of life choices | Aggressive histology |
| Primary or recurrent disease | |
| Distant metastases | |
| |
| External beam radiation therapy possible |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dahan, A.; Al Ghuzlan, A.; Chehab, R.; Guerlain, J.; Breuskin, I.; Garcia, C.; Lamartina, L.; Hadoux, J.; Baudin, E.; Hartl, D.M. Pathological Analysis of Encased Resected Recurrent Nerves in Locally Invasive Thyroid Cancer. Cancers 2022, 14, 2961. https://doi.org/10.3390/cancers14122961
Dahan A, Al Ghuzlan A, Chehab R, Guerlain J, Breuskin I, Garcia C, Lamartina L, Hadoux J, Baudin E, Hartl DM. Pathological Analysis of Encased Resected Recurrent Nerves in Locally Invasive Thyroid Cancer. Cancers. 2022; 14(12):2961. https://doi.org/10.3390/cancers14122961
Chicago/Turabian StyleDahan, Alexandre, Abir Al Ghuzlan, Randa Chehab, Joanne Guerlain, Ingrid Breuskin, Camilo Garcia, Livia Lamartina, Julien Hadoux, Eric Baudin, and Dana M. Hartl. 2022. "Pathological Analysis of Encased Resected Recurrent Nerves in Locally Invasive Thyroid Cancer" Cancers 14, no. 12: 2961. https://doi.org/10.3390/cancers14122961
APA StyleDahan, A., Al Ghuzlan, A., Chehab, R., Guerlain, J., Breuskin, I., Garcia, C., Lamartina, L., Hadoux, J., Baudin, E., & Hartl, D. M. (2022). Pathological Analysis of Encased Resected Recurrent Nerves in Locally Invasive Thyroid Cancer. Cancers, 14(12), 2961. https://doi.org/10.3390/cancers14122961







