ALK+ Anaplastic Large Cell Lymphoma (ALCL)-Derived Exosomes Carry ALK Signaling Proteins and Interact with Tumor Microenvironment
Abstract
:Simple Summary
Abstract
1. Introduction
2. Results and Discussion
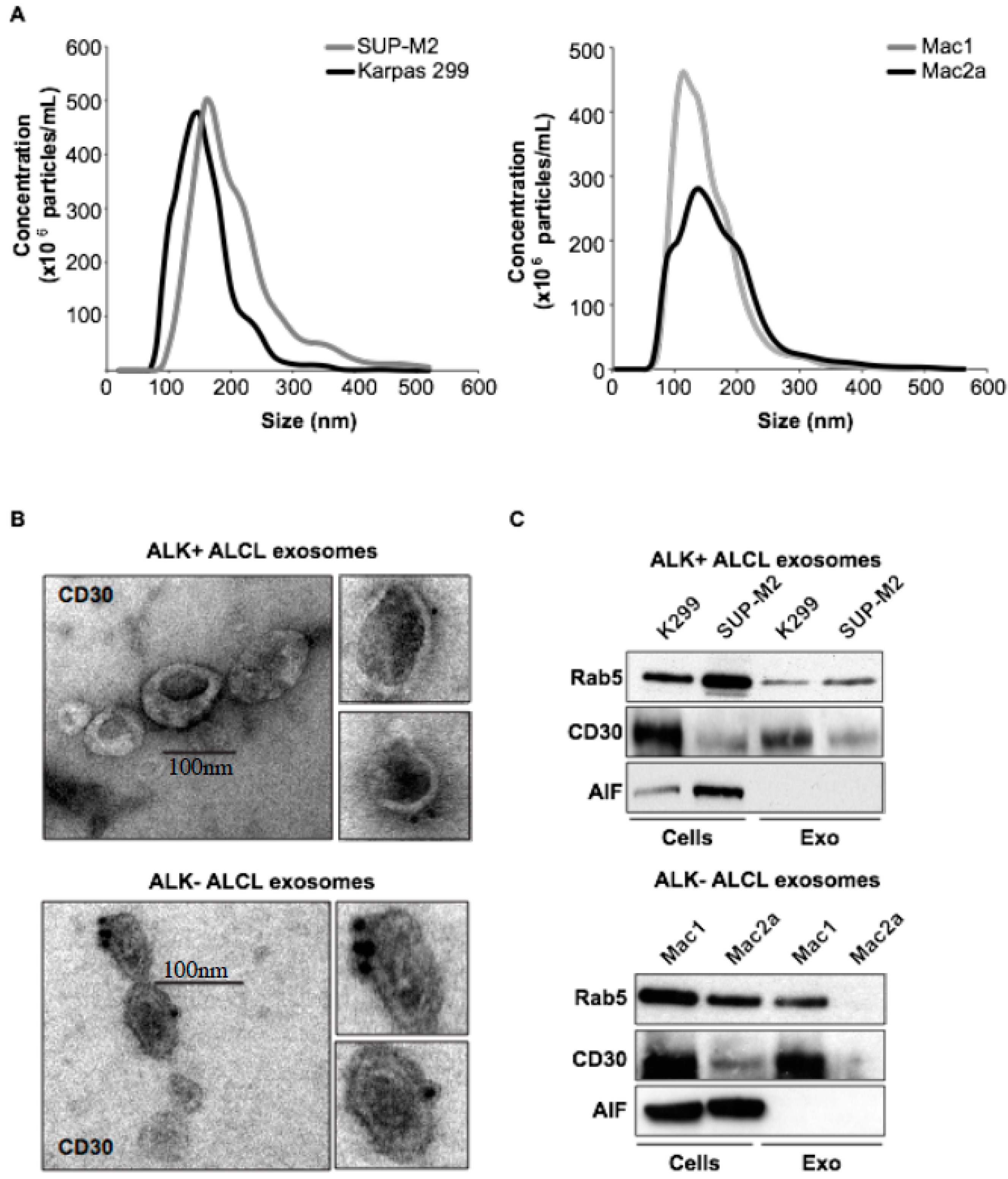
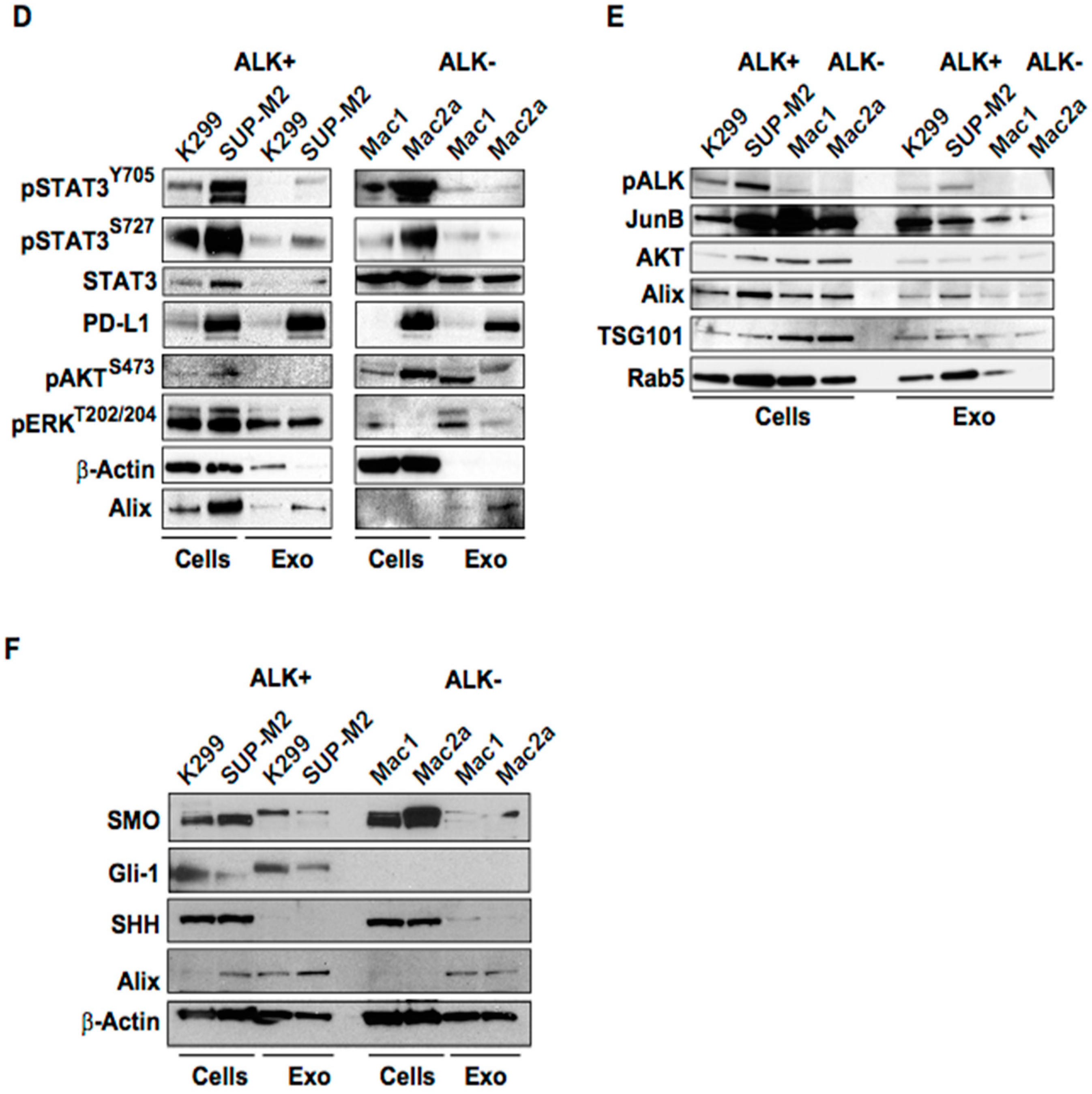
2.1. Molecular Characterization of ALK+ ALCL-Derived Exosomes
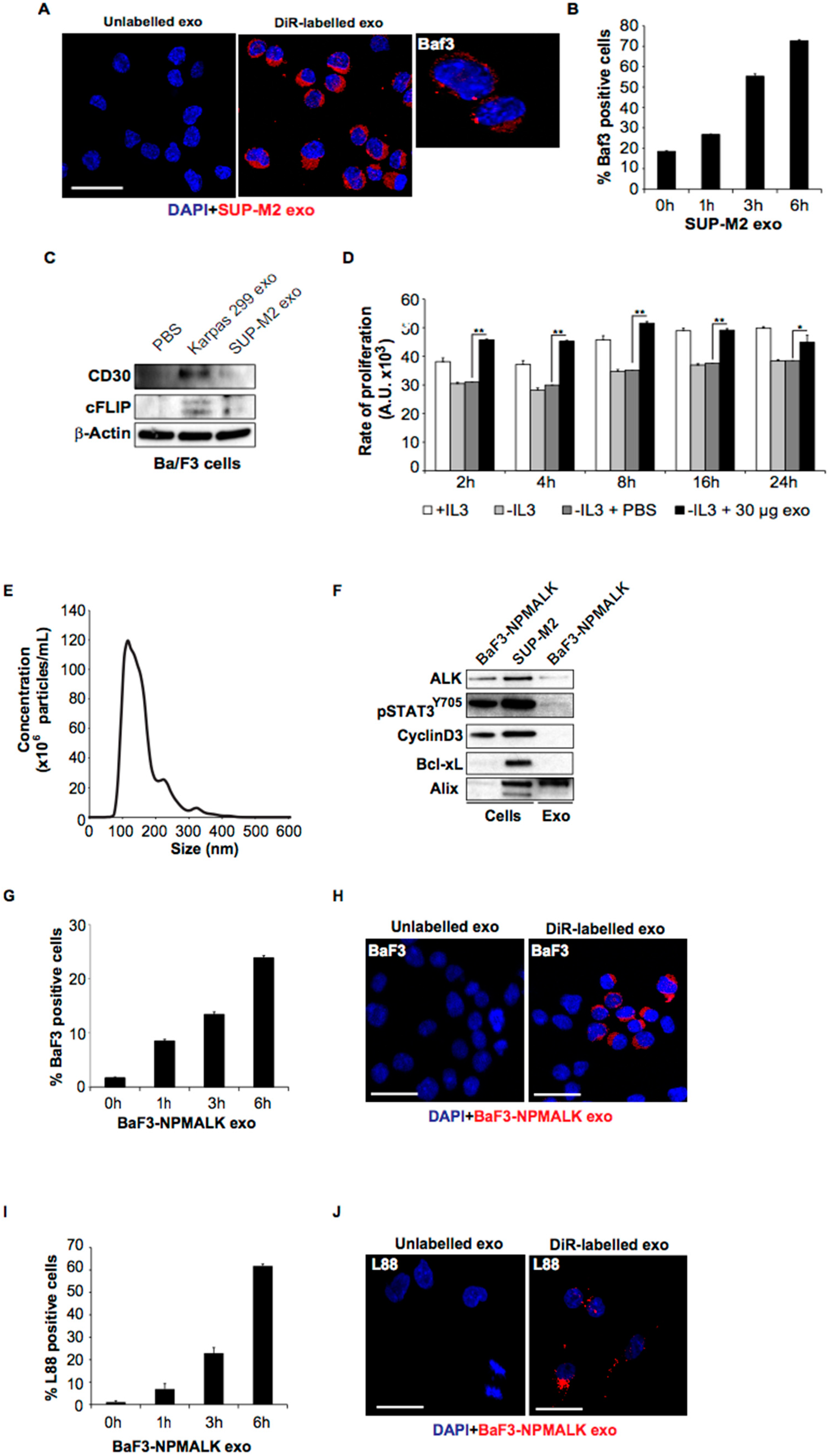
2.2. ALCL-Derived Exosomes Can Be Taken up by Lymphoid Cells
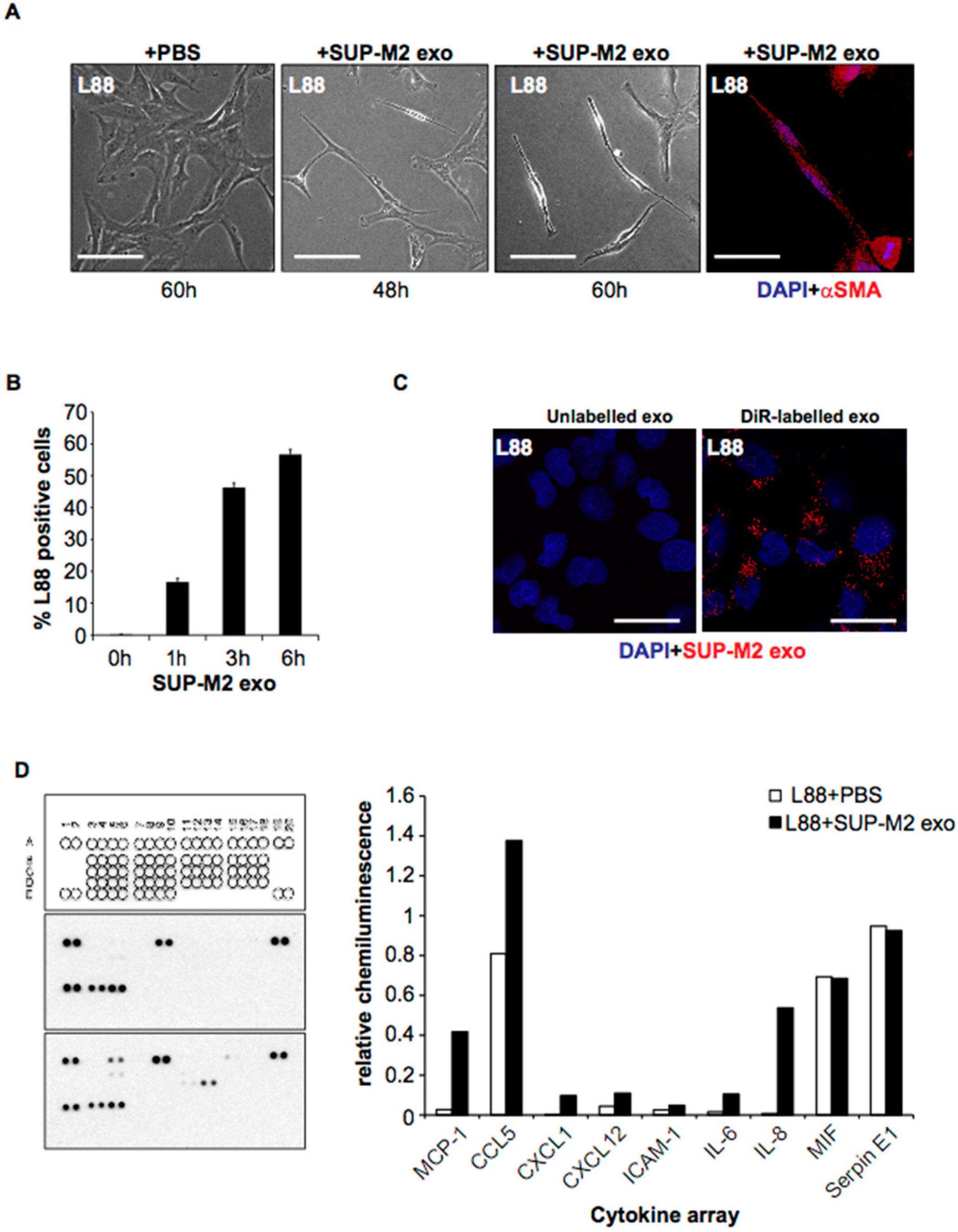
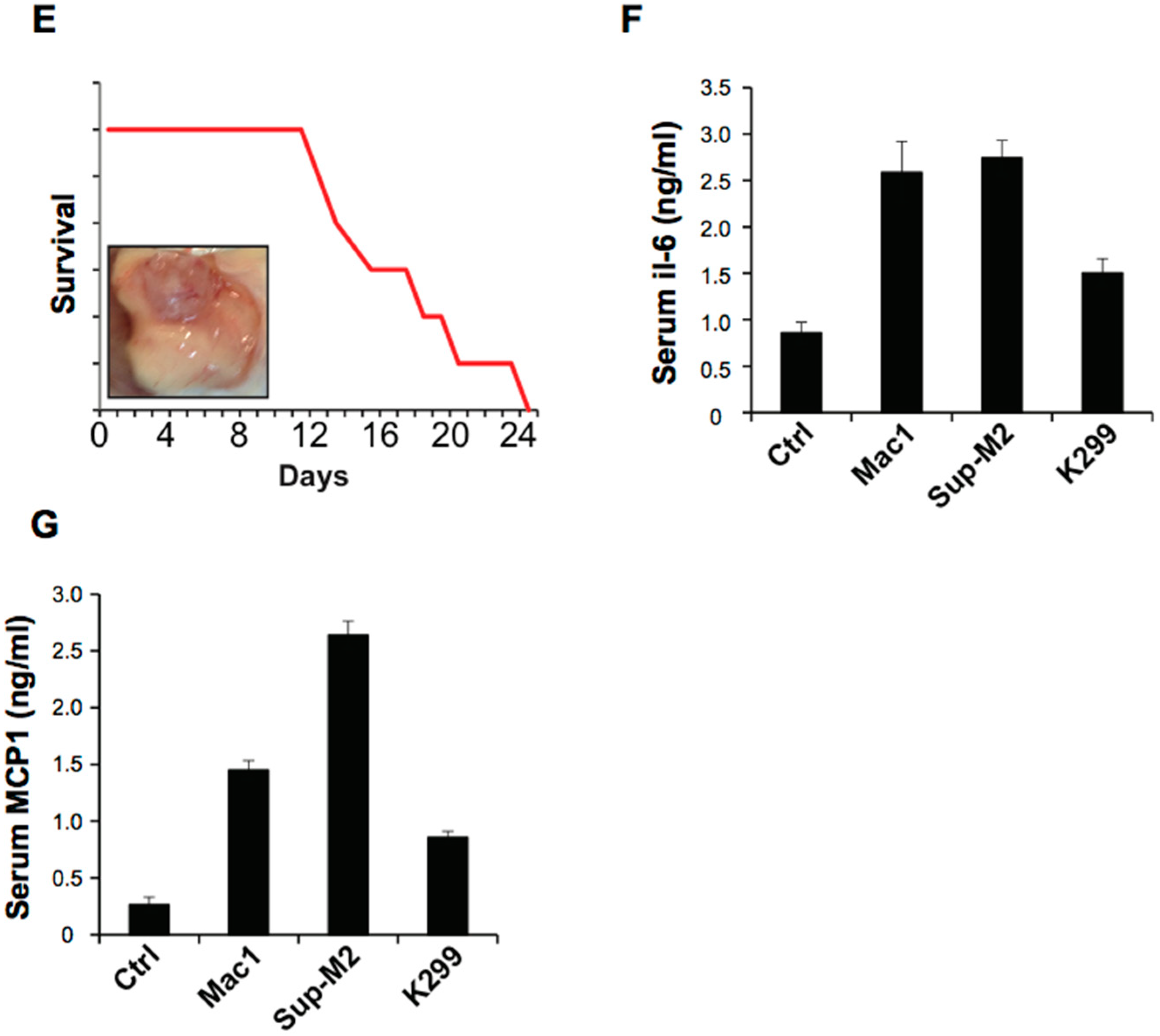
2.3. ALCL-Derived Exosomes Interact with the Tumor Microenvironment Cells
3. Materials and Methods
3.1. Cell Lines and Culturing Conditions
3.2. Exosome Isolation
3.3. Nanoparticle Tracking Analysis (NTA)
3.4. Western Blot Analysis
3.5. DiR Labelling
3.6. Exosome Uptake Assays
3.7. Cytokine Array
3.8. Cell Proliferation Assays
3.9. Electron Microscopy
3.10. Molecular Cloning of NPM1-ALK Full Length Fusion
3.11. Retroviral Construct and Infection
3.12. Human Tumor Xenografts
3.13. ELISA Assays
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Swerdlow, S.H.; Campo, E.; Pileri, S.A.; Harris, N.L.; Stein, H.; Siebert, R.; Advani, R.; Ghielmini, M.; Salles, G.A.; Zelenetz, A.D.; et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016, 127, 2375–2390. [Google Scholar] [CrossRef] [Green Version]
- Chiarle, R.; Voena, C.; Ambrogio, C.; Piva, R.; Inghirami, G. The anaplastic lymphoma kinase in the pathogenesis of cancer. Nat. Cancer 2008, 8, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Kharaziha, P.; Ceder, S.; Li, Q.; Panaretakis, T. Tumor cell-derived exosomes: A message in a bottle. Biochim. Biophys. Acta 2012, 1826, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Paggetti, J.; Haderk, F.; Seiffert, M.; Janji, B.; Distler, U.; Ammerlaan, W.; Kim, Y.J.; Adam, J.; Lichter, P.; Solary, E.; et al. Exosomes released by chronic lymphocytic leukemia cells induce the transition of stromal cells into cancer-associated fibroblasts. Blood 2015, 126, 1106–1117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hansen, H.P.; Engels, H.M.; Dams, M.; Paes Leme, A.F.; Pauletti, B.A.; Simhadri, V.L.; Dürkop, H.; Reiners, K.S.; Barnert, S.; Engert, A.; et al. Protrusion-guided extracellular vesicles mediate CD30 trans-signalling in the microenvironment of Hodgkin’s lymphoma. J. Pathol. 2014, 232, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Staber, P.B.; Vesely, P.; Haq, N.; Ott, R.G.; Funato, K.; Bambach, I.; Fuchs, C.; Schauer, S.; Linkesch, W.; Hrzenjak, A.; et al. The oncoprotein NPM-ALK of anaplastic large-cell lymphoma induces JUNB transcription via ERK1/2 and JunB translation via mTOR signaling. Blood 2007, 110, 3374–3383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atsaves, V.; Tsesmetzis, N.; Chioureas, D.; Kis, L.; Leventaki, V.; Drakos, E.; Panaretakis, T.; Grander, D.; Medeiros, L.J.; Young, K.H.; et al. PD-L1 is commonly expressed and transcriptionally regulated by STAT3 and MYC in ALK-negative anaplastic large-cell lymphoma. Leuk 2017, 31, 1633–1637. [Google Scholar] [CrossRef] [PubMed]
- Oyarzo, M.P.; Medeiros, L.J.; Atwell, C.; Feretzaki, M.; Leventaki, V.; Drakos, E.; Amin, H.M.; Rassidakis, G.Z. c-FLIP confers resistance to FAS-mediated apoptosis in anaplastic large-cell lymphoma. Blood 2006, 107, 2544–2547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koch, R.; Demant, M.; Aung, T.; Diering, N.; Cicholas, A.; Chapuy, B.; Wenzel, D.; Lahmann, M.; Güntsch, A.; Kiecke, C.; et al. Populational equilibrium through exosome-mediated Wnt signaling in tumor progression of diffuse large B-cell lymphoma. Blood 2014, 123, 2189–2198. [Google Scholar] [CrossRef] [PubMed]
- Dejean, E.; Foisseau, M.; Lagarrigue, F.; Lamant, L.; Prade, N.; Marfak, A.; Delsol, G.; Giuriato, S.; Gaits-Iacovoni, F.; Meggetto, F. ALK + ALCLs induce cutaneous, HMGB-1-dependent IL-8/CXCL8 production by keratinocytes through NF-kappaB activation. Blood 2012, 119, 4698–4707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atsaves, V.; Lekakis, L.; Drakos, E.; Leventaki, V.; Ghaderi, M.; Baltatzis, G.; Chioureas, D.; Jones, D.; Feretzaki, M.; Liakou, C.; et al. The oncogenic JUNB/CD30 axis con-tributes to cell cycle deregulation in ALK+ anaplastic large cell lymphoma. Br. J. Haematol. 2014, 167, 514–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leventaki, V.; Drakos, E.; Medeiros, L.J.; Lim, M.S.; Elenitoba-Johnson, K.S.; Claret, F.X.; Rassidakis, G.Z. NPM-ALK oncogenic kinase promotes cell-cycle progression through activation of JNK/cJun signaling in anaplastic large-cell lymphoma. Blood 2007, 110, 1621–1630. [Google Scholar] [CrossRef] [PubMed]
- Vega, F.; Medeiros, L.J.; Leventaki, V.; Atwell, C.; Cho-Vega, J.H.; Tian, L.; Claret, F.-X.; Rassidakis, G.Z. Activation of mTOR Sig-naling Pathway Contributes to Tumor Cell Survival in ALK+ Anaplastic Large Cell Lymphoma. Cancer Res. 2006, 66, 6589–6597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rassidakis, G.Z.; Thomaides, A.; Atwell, C.; Ford, R.; Jones, D.; Claret, F.-X.; Medeiros, L.J. JunB Expression is a Common Fea-ture of CD30+ Lymphomas and Lymphomatoid Papulosis. Mod Pathol. 2005, 18, 1365–1370. [Google Scholar] [CrossRef] [PubMed]
- Rassidakis, G.Z.; Feretzaki, M.; Atwell, C.; Grammatikakis, I.; Lin, Q.; Lai, R.; Claret, F.-X.; Medeiros, L.J.; Amin, H.M. Inhibition of Akt increases p27Kip1 levels and induces cell cycle arrest in anaplastic large cell lymphoma. Blood 2005, 105, 827–829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khoury, J.D.; Medeiros, L.J.; Rassidakis, G.Z.; A. Yared, M.; Tsioli, P.; Leventaki, V.; Schmitt-Graeff, A.; Herling, M.; Amin, H.M.; Lai, R. Differential expression and clinical significance of tyrosine-phosphorylated STAT3 in ALK+ and ALK- anaplastic large cell lymphoma. Clin. Cancer Res. 2003, 9, 3692–3699. [Google Scholar] [PubMed]
- Rassidakis, G.Z.; Claret, F.-X.; Lai, R.; Zhang, Q.; Sarris, A.H.; McDonnell, T.J.; Medeiros, L.J. Expression of p27kip1 and Jun ac-tivation binding protein 1 (JAB1) are inversely correlated in systemic anaplastic large cell lymphoma. Clin. Cancer Res. 2003, 9, 1121–1128. [Google Scholar] [PubMed]
- Rassidakis, G.Z.; Sarris, A.H.; Herling, M.; Ford, R.; Cabanillas, F.; McDonnell, T.J.; Medeiros, L.J. Differential expression of BCL-2 family proteins in ALK-positive and ALK-negative anaplastic large cell lymphoma of T/null-cell lineage. Am. J. Pathol. 2001, 159, 527–535. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.R.; Cho-Vega, J.H.; Davuluri, Y.; Ma, S.; Kasbidi, F.; Milito, C.; Lennon, P.A.; Drakos, E.; Medeiros, L.J.; Luthra, R.; et al. Sonic Hedgehog Signaling Pathway Is Activated in ALK-Positive Anaplastic Large Cell Lymphoma. Cancer Res. 2009, 69, 2550–2558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rassidakis, G.Z.; Drakos, E. The emerging role of CD30 and p53 as novel targets for therapy in anaplastic large cell lym-phoma. Front Biosci (Elite Ed) 2016, 8, 61–71. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chioureas, D.; Beck, J.; Baltatzis, G.; Vardaki, I.; Fonseca, P.; Tsesmetzis, N.; Vega, F.; Leventaki, V.; Eliopoulos, A.G.; Drakos, E.; et al. ALK+ Anaplastic Large Cell Lymphoma (ALCL)-Derived Exosomes Carry ALK Signaling Proteins and Interact with Tumor Microenvironment. Cancers 2022, 14, 2939. https://doi.org/10.3390/cancers14122939
Chioureas D, Beck J, Baltatzis G, Vardaki I, Fonseca P, Tsesmetzis N, Vega F, Leventaki V, Eliopoulos AG, Drakos E, et al. ALK+ Anaplastic Large Cell Lymphoma (ALCL)-Derived Exosomes Carry ALK Signaling Proteins and Interact with Tumor Microenvironment. Cancers. 2022; 14(12):2939. https://doi.org/10.3390/cancers14122939
Chicago/Turabian StyleChioureas, Dimitrios, Janina Beck, George Baltatzis, Ioulia Vardaki, Pedro Fonseca, Nikolaos Tsesmetzis, Francisco Vega, Vasiliki Leventaki, Aristides G. Eliopoulos, Elias Drakos, and et al. 2022. "ALK+ Anaplastic Large Cell Lymphoma (ALCL)-Derived Exosomes Carry ALK Signaling Proteins and Interact with Tumor Microenvironment" Cancers 14, no. 12: 2939. https://doi.org/10.3390/cancers14122939
APA StyleChioureas, D., Beck, J., Baltatzis, G., Vardaki, I., Fonseca, P., Tsesmetzis, N., Vega, F., Leventaki, V., Eliopoulos, A. G., Drakos, E., Rassidakis, G. Z., & Panaretakis, T. (2022). ALK+ Anaplastic Large Cell Lymphoma (ALCL)-Derived Exosomes Carry ALK Signaling Proteins and Interact with Tumor Microenvironment. Cancers, 14(12), 2939. https://doi.org/10.3390/cancers14122939









