Proton Therapy in the Management of Luminal Gastrointestinal Cancers: Esophagus, Stomach, and Anorectum
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
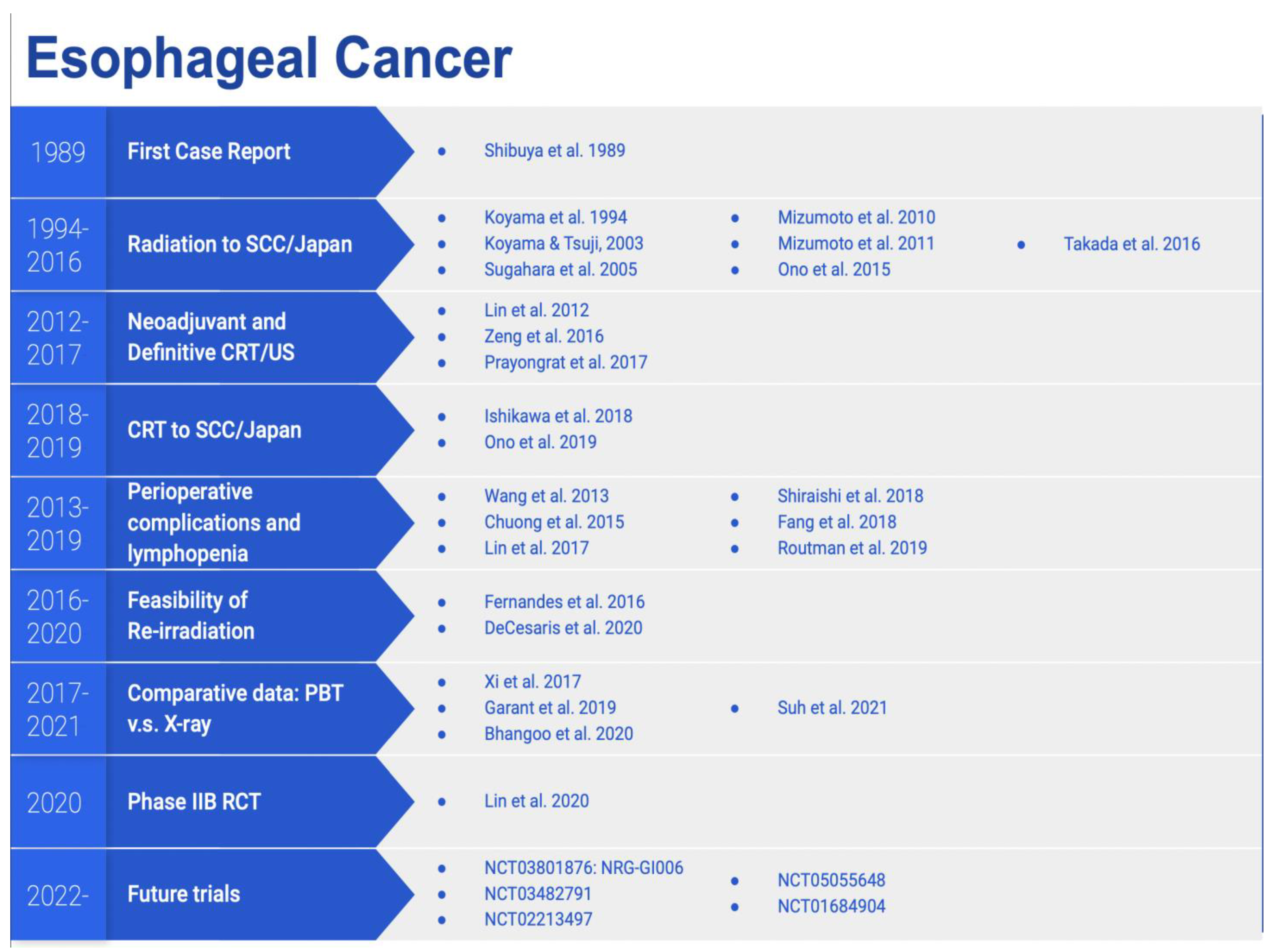
3. Esophageal Cancer
3.1. Dosimetric Data
3.2. Clinical Data
3.3. Prospective Trials
4. Gastric Cancer
4.1. Dosimetric Data
4.2. Clinical Data
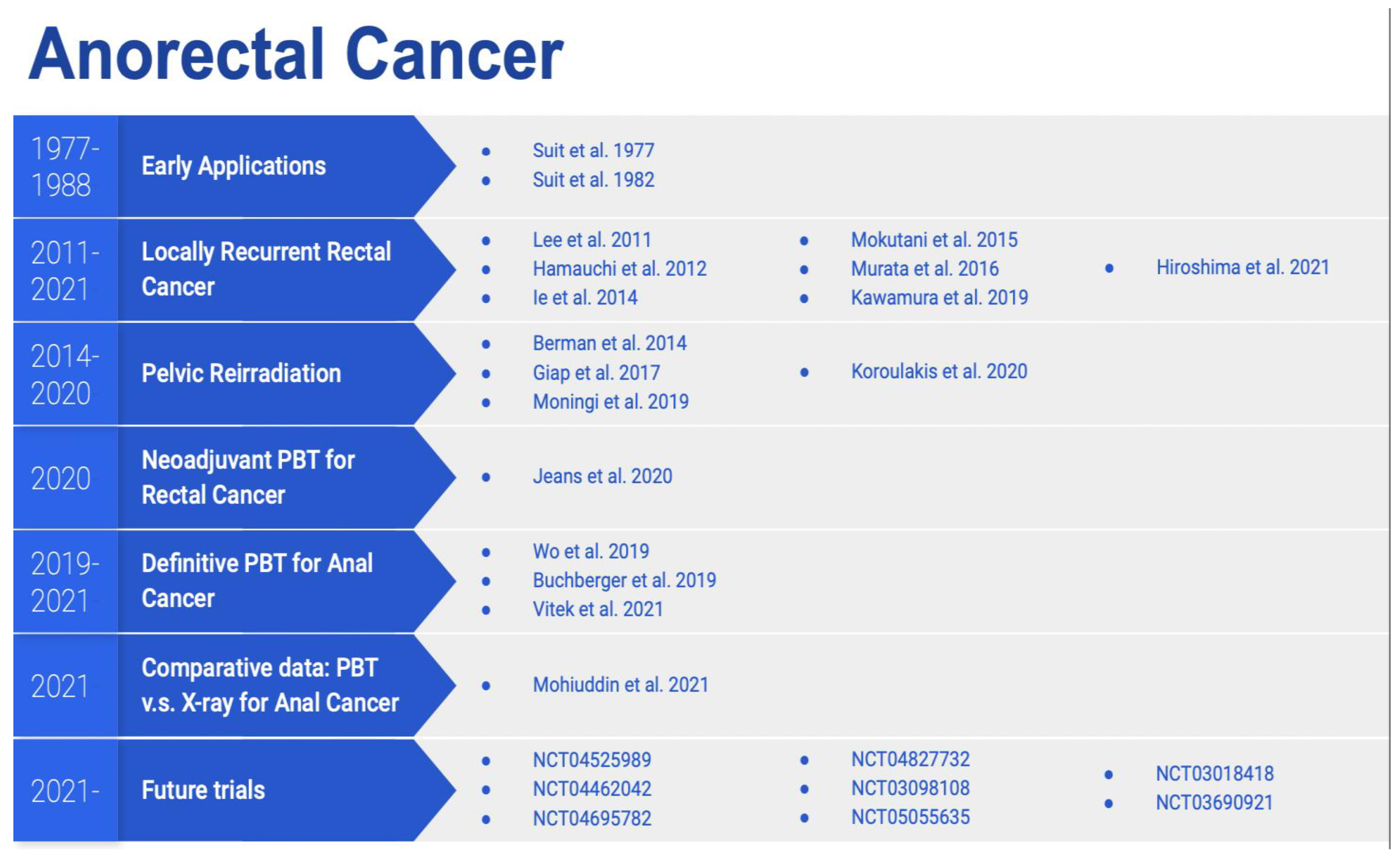
5. Anorectal Cancer
5.1. Dosimetric Data
5.2. Clinical Data
5.3. Prospective Trials
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- De Ruysscher, D.; Niedermann, G.; Burnet, N.G.; Siva, S.; Lee, A.W.M.; Hegi-Johnson, F. Author Correction: Radiotherapy toxicity. Nat. Rev. Dis. Primers 2019, 5, 15. [Google Scholar] [CrossRef] [PubMed]
- Mohan, R.; Grosshans, D. Proton Therapy—Present and Future. Adv. Drug Deliv. Rev. 2017, 109, 26–44. [Google Scholar] [CrossRef] [PubMed]
- Hong, T.; Das, P. Radiation Therapy for Gastrointestinal Cancers; Springer International Publishing: Cham, Switzerland, 2017. [Google Scholar]
- Verma, V.; Simone, C.B.; Mehta, M.P. Clinical outcomes and toxicities of proton radiotherapy for gastrointestinal neoplasms: A systematic review. J. Gastrointest. Oncol. 2016, 7, 644–664. [Google Scholar] [CrossRef] [PubMed]
- Badiyan, S.N.; Hallemeier, C.L.; Lin, S.H.; Hall, M.D.; Chuong, M.D. Proton beam therapy for gastrointestinal cancers: Past, present, and future. J. Gastrointest. Oncol. 2018, 9, 962–971. [Google Scholar] [CrossRef]
- Paganetti, H.; Beltran, C.J.; Both, S.; Dong, L.; Flanz, J.B.; Furutani, K.M.; Grassberger, C.; Grosshans, D.R.; Knopf, A.-C.; Langendijk, J.A.; et al. Roadmap: Proton therapy physics and biology. Phys. Med. Biol. 2021, 66, 5. [Google Scholar] [CrossRef]
- Cancer Statistics Center. Key Statistics for Esophageal Cancer. 2022. Available online: https://www.cancer.org/cancer/esophagus-cancer/about/key-statistics.html (accessed on 9 March 2022).
- National Comprehensive Cancer Network. Esophageal and Esophagogastric Junction Cancers. 2022. Available online: https://www.nccn.org/professionals/physician_gls/pdf/esophageal.pdf (accessed on 9 March 2022).
- Isacsson, U.; Lennernäs, B.; Grusell, E.; Jung, B.; Montelius, A.; Glimelius, B. Comparative treatment planning between proton and X-ray therapy in esophageal cancer. Int. J. Radiat. Oncol. Biol. Phys. 1998, 41, 441. [Google Scholar] [CrossRef]
- Zhang, X.; Guerrero, T.M.; McGuire, S.E.; Yaremko, B.; Komaki, R.; Cox, J.D.; Hui, Z.; Li, Y.; Newhauser, W.D.; Mohan, R.; et al. Four-Dimensional Computed Tomography–Based Treatment Planning for Intensity-Modulated Radiation Therapy and Proton Therapy for Distal Esophageal Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2008, 72, 278–287. [Google Scholar] [CrossRef]
- Ling, T.C.; Slater, J.M.; Nookala, P.; Mifflin, R.; Grove, R.; Ly, A.M.; Patyal, B.; Slater, J.D.; Yang, G.Y. Analysis of Intensity-Modulated Radiation Therapy (IMRT), Proton and 3D Conformal Radiotherapy (3D-CRT) for Reducing Perioperative Cardiopulmonary Complications in Esophageal Cancer Patients. Cancers 2014, 6, 2356–2368. [Google Scholar] [CrossRef]
- Wang, J.; Palmer, M.; Bilton, S.D.; Vu, K.N.; Greer, S.; Frame, R.; Liao, Z.; Komaki, R.; Cox, J.D.; Lin, S.H. Comparing Proton Beam to Intensity Modulated Radiation Therapy Planning in Esophageal Cancer. Int. J. Part. Ther. 2015, 1, 866–877. [Google Scholar] [CrossRef]
- Shiraishi, Y.; Xu, C.; Yang, J.; Komaki, R.; Lin, S.H. Dosimetric comparison to the heart and cardiac substructure in a large cohort of esophageal cancer patients treated with proton beam therapy or Intensity-modulated radiation therapy. Radiother. Oncol. 2017, 125, 48–54. [Google Scholar] [CrossRef]
- Lin, L.; Kang, M.; Huang, S.; Mayer, R.; Thomas, A.; Solberg, T.D.; McDonough, J.E.; Simone, C.B. Beam-specific planning target volumes incorporating 4D CT for pencil beam scanning proton therapy of thoracic tumors. J. Appl. Clin. Med. Phys. 2015, 16, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Welsh, J.; Gomez, D.; Palmer, M.B.; Riley, B.A.; Mayankkumar, A.V.; Komaki, R.; Dong, L.; Zhu, X.R.; Likhacheva, A.; Liao, Z.; et al. Intensity-Modulated Proton Therapy Further Reduces Normal Tissue Exposure During Definitive Therapy for Locally Advanced Distal Esophageal Tumors: A Dosimetric Study. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, 1336–1342. [Google Scholar] [CrossRef] [PubMed]
- Warren, S.P.; Partridge, M.P.; Bolsi, A.P.; Lomax, A.J.P.; Hurt, C.P.; Crosby, T.F.; Hawkins, M.A.F. An Analysis of Plan Robustness for Esophageal Tumors: Comparing Volumetric Modulated Arc Therapy Plans and Spot Scanning Proton Planning. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Warren, S.; Hurt, C.N.; Crosby, T.; Partridge, M.; Hawkins, M.A. Potential of Proton Therapy to Reduce Acute Hematologic Toxicity in Concurrent Chemoradiation Therapy for Esophageal Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2017, 99, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Bhangoo, R.S.; Sio, T.T.; Yu, N.Y.; Shan, J.; Chiang, J.S.; Ding, J.X.; Rule, W.G.; Korte, S.; Lara, P.; et al. Dosimetric comparison of distal esophageal carcinoma plans for patients treated with small-spot intensity-modulated proton versus volumetric-modulated arc therapies. J. Appl. Clin. Med. Phys. 2019, 20, 15–27. [Google Scholar] [CrossRef]
- Celik, E.; Baus, W.; Baues, C.; Schröder, W.; Clivio, A.; Fogliata, A.; Scorsetti, M.; Marnitz, S.; Cozzi, L. Volumetric modulated arc therapy versus intensity-modulated proton therapy in neoadjuvant irradiation of locally advanced oesophageal cancer. Radiat. Oncol. 2020, 15, 120. [Google Scholar] [CrossRef]
- Zhang, Y.; Jabbour, S.K.; Zhang, A.; Liu, B.; Yue, N.J.; Biswal, N.C. Proton beam therapy can achieve lower vertebral bone marrow dose than photon beam therapy during chemoradiation therapy of esophageal cancer. Med. Dosim. Off. J. Am. Assoc. Med. Dosim. 2021, 46, 229–235. [Google Scholar] [CrossRef]
- Hulshof, M.C.; Geijsen, D.; Rozema, T.; Oppedijk, V.; Buijsen, J.; Neelis, K.J.; Nuyttens, J.; Van Der Sangen, M.; Jeene, P.; Reinders, J.; et al. A randomized controlled phase III multicenter study on dose escalation in definitive chemoradiation for patients with locally advanced esophageal cancer: ARTDECO study. J. Clin. Oncol. 2020, 38 (Suppl. S4), 281. [Google Scholar] [CrossRef]
- Shibuya, S.; Takase, Y.; Watanabe, M.; Orii, K.; Iwasaki, Y.; Kitagawa, T. Usefulness of proton irradiation therapy as pre-operative measure for esophageal cancer. Dis. Esophagus 1989, 2, 99–104. [Google Scholar] [CrossRef]
- Koyama, S.; Tsujii, H.; Yokota, H.; Hotta, S.; Miyo, Y.; Fukutomi, H.; Osuga, T.; Tsuji, H.; Okumura, T.; Ohara, K.; et al. Proton Beam Therapy for Patients with Esophageal Carcinoma. Jpn. J. Clin. Oncol. 1994, 24, 144–153. [Google Scholar]
- Kovama, S.; Tsuiii, H. Proton Beam Therapy with High-Dose Irradiation for Superficial and Advanced Esophageal Carcinomas. Clin. Cancer Res. 2003, 9, 3571–3577. [Google Scholar]
- Sugahara, S.; Tokuuye, K.; Okumura, T.; Nakahara, A.; Saida, Y.; Kagei, K.; Ohara, K.; Hata, M.; Igaki, H.; Akine, Y. Clinical results of proton beam therapy for cancer of the esophagus. Int. J. Radiat. Oncol. Biol. Phys. 2005, 61, 76. [Google Scholar] [CrossRef] [PubMed]
- Mizumoto, M.; Sugahara, S.; Nakayama, H.; Hashii, H.; Nakahara, A.; Terashima, H.; Okumura, T.; Tsuboi, K.; Tokuuye, K.; Sakurai, H. Clinical Results of Proton-Beam Therapy for Locoregionally Advanced Esophageal Cancer. Strahlenther. Onkol. 2010, 186, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, H.; Nonaka, T.; Ohnishi, K.; Ohno, T.; Mizoguchi, N.; Murofushi, K.; Iizumi, T.; Sekino, Y.; Okumura, T.; Sakurai, H. Long-Term Follow-Up Results of Concurrent Chemo-Proton Therapy for Esophageal Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2018, 102, e31. [Google Scholar] [CrossRef]
- Ono, T.; Wada, H.; Ishikawa, H.; Tamamura, H.; Tokumaru, S. Clinical Results of Proton Beam Therapy for Esophageal Cancer: Multicenter Retrospective Study in Japan. Cancers 2019, 11, 993. [Google Scholar] [CrossRef]
- Lin, S.H.; Komaki, R.; Liao, Z.; Wei, C.; Myles, B.; Guo, X.; Palmer, M.; Mohan, R.; Swisher, S.G.; Hofstetter, W.L.; et al. Proton Beam Therapy and Concurrent Chemotherapy for Esophageal Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, e345–e351. [Google Scholar] [CrossRef]
- Zeng, Y.-C.; Vyas, S.; Dang, Q.; Schultz, L.; Bowen, S.R.; Shankaran, V.; Farjah, F.; Oelschlager, B.K.; Apisarnthanarax, S.; Zeng, J. Proton therapy posterior beam approach with pencil beam scanning for esophageal cancer: Clinical outcome, dosimetry, and feasibility. Strahlenther. Onkol. 2016, 192, 913–921. [Google Scholar] [CrossRef]
- DeCesaris, C.M.; Choi, J.I.; Carr, S.R.; Burrows, W.M.; Regine, W.F.; Simone, C.B.; Molitoris, J.K. Pathologic complete response (pCR) rates and outcomes after neoadjuvant chemoradiotherapy with proton or photon radiation for adenocarcinomas of the esophagus and gastroesophageal junction. J. Gastrointest. Oncol. 2020, 11, 663–673. [Google Scholar] [CrossRef]
- Prayongrat, A.; Xu, C.; Li, H.P.; Lin, S.H. Clinical outcomes of intensity modulated proton therapy and concurrent chemotherapy in esophageal carcinoma: A single institutional experience. Adv. Radiat. Oncol. 2017, 2, 301–307. [Google Scholar] [CrossRef]
- Wang, J.; Wei, C.P.; Tucker, S.L.P.; Myles, B.; Palmer, M.; Hofstetter, W.L.; Swisher, S.G.; Ajani, J.A.; Cox, J.D.; Komaki, R.; et al. Predictors of Postoperative Complications After Trimodality Therapy for Esophageal Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2013, 86, 885–891. [Google Scholar] [CrossRef]
- Lin, S.H.; Merrell, K.W.; Shen, J.; Verma, V.; Correa, A.M.; Wang, L.; Thall, P.F.; Bhooshan, N.; James, S.E.; Haddock, M.G.; et al. Multi-institutional analysis of radiation modality use and postoperative outcomes of neoadjuvant chemoradiation for esophageal cancer. Radiother. Oncol. 2017, 123, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Chuong, M.D.; Bhooshan, N.; Allen, P.K.; Merrell, K.W.; Mehta, M.P.; Hallemeier, C.L.; Liao, Z.; Suntharalingam, M.; Komaki, R.U.; Haddock, M.G.; et al. A Multi-institutional Analysis of Acute Toxicity After Neoadjuvant Chemoradiation Using Photons or Protons in Trimodality Esophageal Cancer Patients. Int. J. Radiat. Oncol. Biol. Phys. 2015, 93, S220. [Google Scholar] [CrossRef]
- Shiraishi, Y.; Fang, P.; Xu, C.; Song, J.; Krishnan, S.; Koay, E.J.; Mehran, R.J.; Hofstetter, W.L.; Blum-Murphy, M.; Ajani, J.A.; et al. Severe lymphopenia during neoadjuvant chemoradiation for esophageal cancer: A propensity matched analysis of the relative risk of proton versus photon-based radiation therapy. Radiother. Oncol. 2018, 128, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Fang, P.; Shiraishi, Y.; Verma, V.; Jiang, W.; Song, J.; Hobbs, B.P.; Lin, S.H. Lymphocyte-Sparing Effect of Proton Therapy in Patients with Esophageal Cancer Treated with Definitive Chemoradiation. Int. J. Part. Ther. 2018, 4, 23–32. [Google Scholar] [CrossRef]
- Routman, D.M.; Garant, A.; Lester, S.C.; Day, C.N.; Harmsen, W.S.; Sanheuza, C.T.; Yoon, H.H.; Neben-Wittich, M.A.; Martenson, J.A.; Haddock, M.G.; et al. A Comparison of Grade 4 Lymphopenia with Proton Versus Photon Radiation Therapy for Esophageal Cancer. Adv. Radiat. Oncol. 2018, 4, 63–69. [Google Scholar] [CrossRef]
- Fernandes, A.; Berman, A.T.; Mick, R.; Both, S.P.; Lelionis, K.; Lukens, J.N.; Ben-Josef, E.; Metz, J.M.; Plastaras, J.P. A Prospective Study of Proton Beam Reirradiation for Esophageal Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 483–487. [Google Scholar] [CrossRef]
- DeCesaris, C.M.; McCarroll, R.; Mishra, M.V.; Glass, E.; Greenwald, B.D.; Carr, S.; Burrows, W.; Mehra, R.; Regine, W.F.; Simone, C.B.; et al. Assessing Outcomes of Patients Treated with Re-Irradiation Utilizing Proton Pencil-Beam Scanning for Primary or Recurrent Malignancies of the Esophagus and Gastroesophageal Junction. J. Thorac. Oncol. 2020, 15, 1054–1064. [Google Scholar] [CrossRef]
- Xi, M.; Xu, C.; Liao, Z.; Chang, J.Y.; Gomez, D.R.; Jeter, M.; Cox, J.D.; Komaki, R.; Mehran, R.; Blum, M.A.; et al. Comparative Outcomes After Definitive Chemoradiotherapy Using Proton Beam Therapy Versus Intensity Modulated Radiation Therapy for Esophageal Cancer: A Retrospective, Single-Institutional Analysis. Int. J. Radiat. Oncol. Biol. Phys. 2017, 99, 667–676. [Google Scholar] [CrossRef]
- Bhangoo, R.S.; DeWees, T.A.; Yu, N.Y.; Ding, J.X.; Liu, C.; Golafshar, M.A.; Rule, W.G.; Vora, S.A.; Ross, H.J.; Ahn, D.H.; et al. Acute Toxicities and Short-Term Patient Outcomes After Intensity-Modulated Proton Beam Radiation Therapy or Intensity-Modulated Photon Radiation Therapy for Esophageal Carcinoma: A Mayo Clinic Experience. Adv. Radiat. Oncol. 2020, 5, 871–879. [Google Scholar] [CrossRef]
- Suh, Y.-G.; Bayasgalan, U.; Kim, H.T.; Lee, J.M.; Kim, M.S.; Lee, Y.; Lee, D.Y.; Lee, S.U.; Kim, T.H.; Moon, S.H. Photon Versus Proton Beam Therapy for T1–3 Squamous Cell Carcinoma of the Thoracic Esophagus Without Lymph Node Metastasis. Front. Oncol. 2021, 11, 699172. [Google Scholar] [CrossRef]
- Garant, A.; Whitaker, T.J.; Spears, G.M.; Routman, D.M.; Harmsen, W.S.; Wilhite, T.J.; Ashman, J.B.; Sio, T.T.; Rule, W.G.; Neben Wittich, M.A.; et al. A Comparison of Patient-Reported Health-Related Quality of Life During Proton Versus Photon Chemoradiation Therapy for Esophageal Cancer. Pract. Radiat. Oncol. 2019, 9, 410–417. [Google Scholar] [CrossRef]
- Lin, S.H.; Hobbs, B.P.; Verma, V.; Tidwell, R.S.; Smith, G.L.; Lei, X.; Corsini, E.M.; Mok, I.; Wei, X.; Yao, L.; et al. Randomized Phase IIB Trial of Proton Beam Therapy Versus Intensity-Modulated Radiation Therapy for Locally Advanced Esophageal Cancer. J. Clin. Oncol. 2020, 38, 1569–1579. [Google Scholar] [CrossRef] [PubMed]
- Simone, C.B., II. First Randomized Trial Supporting the Use of Proton Over Photon Chemoradiotherapy in Esophageal Cancer. J. Clin. Oncol. 2020, 38, 2952–2955. [Google Scholar] [CrossRef] [PubMed]
- N.R.G. Oncology; National Cancer Institute (NCI). Comparing Proton Therapy to Photon Radiation Therapy for Esophageal Cancer. Available online: https://ClinicalTrials.gov/show/NCT03801876 (accessed on 1 February 2022).
- University of Leeds; KU Leuven; University College, London; Aarhus University Hospital; Technische Universität Dresden; Academisch Ziekenhuis Groningen; CNAO National Center of Oncological Hadrontherapy; Agenzia Nazionale per i Servizi Sanitari Regionali; Centre Antoine Lacassagne; Centre Leon Berard; et al. PROton Versus Photon Therapy for Esophageal Cancer—A Trimodality Strategy. Available online: https://ClinicalTrials.gov/show/NCT05055648 (accessed on 1 October 2021).
- Washington University School of Medicine. Proton Beam Therapy in the Treatment of Esophageal Cancer. Available online: https://ClinicalTrials.gov/show/NCT03482791 (accessed on 1 May 2022).
- Loma Linda University. Proton Therapy for Esophageal Cancer. Available online: https://ClinicalTrials.gov/show/NCT01684904 (accessed on 1 May 2022).
- Abramson Cancer Center of the University of Pennsylvania. Dose Escalation of Neoadjuvant Proton Beam Radiotherapy With Concurrent Chemotherapy in Locally Advanced Esophageal Cancer. Available online: https://ClinicalTrials.gov/show/NCT02213497 (accessed on 1 May 2022).
- Cancer Statistics Center. Key Statistics About Stomach Cancer. 2022. Available online: https://www.cancer.org/cancer/stomach-cancer/about/key-statistics.html (accessed on 9 March 2022).
- National Cancer Institute. SEER Cancer Stat Facts: Stomach Cancer. 2022. Available online: https://seer.cancer.gov/statfacts/html/stomach.html (accessed on 9 March 2022).
- Smalley, S.R.; Benedetti, J.K.; Stemmermann, G.N.; Blanke, C.D.; Macdonald, J.S.; Haller, D.G.; Hundahl, S.A.; Estes, N.C.; Ajani, J.A.; Gunderson, L.L.; et al. Updated Analysis of SWOG-Directed Intergroup Study 0116: A Phase III Trial of Adjuvant Radiochemotherapy Versus Observation After Curative Gastric Cancer Resection. J. Clin. Oncol. 2012, 30, 2327–2333. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network. Gastric Cancer. 2022. Available online: https://www.nccn.org/professionals/physician_gls/pdf/gastric.pdf (accessed on 1 May 2022).
- Dionisi, F.; Avery, S.; Lukens, J.N.; Ding, X.; Kralik, J.; Kirk, M.; Roses, R.E.; Amichetti, M.; Metz, J.M.; Plastaras, J.P. Proton therapy in adjuvant treatment of gastric cancer: Planning comparison with advanced x-ray therapy and feasibility report. Acta Oncol. 2014, 53, 1312–1320. [Google Scholar] [CrossRef] [PubMed]
- Mondlane, G.; Gubanski, M.; Lind, P.A.; Ureba, A.; Siegbahn, A. Comparison of gastric-cancer radiotherapy performed with volumetric modulated arc therapy or single-field uniform-dose proton therapy. Acta Oncol. 2017, 56, 832–838. [Google Scholar] [CrossRef]
- Mondlane, G.A.; Ureba, A.; Gubanski, M.; Lind, P.; Siegbahn, A. EP-2006: Normal-tissue toxicity following gastric cancer radiotherapy with photon- or scanned proton beams. Radiother. Oncol. 2018, 127, S1092–S1093. [Google Scholar] [CrossRef]
- Koyama, S.; Kawanishi, N.; Fukutomi, H.; Osuga, T.; Iijima, T.; Tsujii, H.; Kitagawa, T. Advanced carcinoma of the stomach treated with definitive proton therapy. Am. J. Gastroenterol. 1990, 85, 443–447. [Google Scholar]
- Shibuya, S.; Takase, Y.; Aoyagi, H.; Orii, K.; Sharma, N.; Tsujii, H.; Tsuji, H.; Iwasaki, Y. Definitive proton beam radiation therapy for inoperable gastric cancer: A report of two cases. Radiat. Med. 1991, 9, 35. [Google Scholar]
- International Agency for Research on Cancer. Estimated Number of New Cases in 2020. 2022. Available online: https://gco.iarc.fr/today/online-analysis-pie?v=2020&mode=cancer&mode_population=continents&population=900&populations=900&key=total&sex=0&cancer=39&type=0&statistic=5&prevalence=0&population_group=0&ages_group%5B%5D=0&ages_group%5B%5D=17&nb_items=7&group_cancer=1&include_nmsc=0&include_nmsc_other=1&half_pie=0&donut=0 (accessed on 1 May 2022).
- Cancer Statistics Center. Key Statistics for Colorectal Cancer. 2022. Available online: https://www.cancer.org/cancer/colon-rectal-cancer/about/key-statistics.html (accessed on 1 May 2022).
- Cancer Statistics Center. Key Statistics for Anal Cancer. 2022. Available online: https://www.cancer.org/cancer/anal-cancer/about/what-is-key-statistics.html (accessed on 1 May 2022).
- Cancer Statistics Center. Survival Rates for Colorectal Cancer. 2022. Available online: https://www.cancer.org/cancer/colon-rectal-cancer/detection-diagnosis-staging/survival-rates.html (accessed on 1 May 2022).
- Cancer Statistics Center. Anal Cancer Survival Rates. 2022. Available online: https://www.cancer.org/cancer/anal-cancer/detection-diagnosis-staging/survival-rates.html (accessed on 1 May 2022).
- National Comprehensive Cancer Network. Rectal Cancer. 2022. Available online: https://www.nccn.org/professionals/physician_gls/pdf/rectal.pdf (accessed on 1 May 2022).
- National Comprehensive Cancer Network. Anal Carcinoma. 2022. Available online: https://www.nccn.org/professionals/physician_gls/pdf/anal.pdf (accessed on 1 May 2022).
- Tatsuzaki, H.; Urie, M.M.; Willett, C.G. 3-D comparative study of proton vs. X-ray radiation therapy for rectal cancer. Int. J. Radiat. Oncol. Biol. Phys. 1992, 22, 369–374. [Google Scholar] [CrossRef]
- Isacsson, U.; Montelius, A.; Jung, B.; Glimelius, B. Comparative treatment planning between proton and X-ray therapy in locally advanced rectal cancer. Radiother. Oncol. 1996, 41, 263. [Google Scholar] [CrossRef]
- Palmer, M.; Mok, H.; Ciura, K.; Georges, R.; Nguyen, B.; Crawford, C.; Beddar, S.; Zhu, R.; Crane, C.; Das, P. Dose Reduction to Small Bowel and Other Relevant Structures in Rectal Carcinoma with Proton Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2012, 84, S846. [Google Scholar] [CrossRef]
- Cooper, B.T.; Qu, J.; Chon, B.H.; Tsai, H.K.; Mah, D.; Du, K.L.; DeWyngaert, J.K.; Yeh, B.K. Dosimetric Comparison of Proton Therapy, Volumetric Modulated Arc Therapy, and 3-D Conformal Radiation Therapy for the Treatment of Rectal Cancer: An Early Community Experience. Int. J. Radiat. Oncol. Biol. Phys. 2015, 93, E176. [Google Scholar] [CrossRef]
- Wolff, H.A.; Wagner, D.M.; Conradi, L.-C.; Hennies, S.; Ghadimi, M.; Hess, C.F.; Christiansen, H. Irradiation with protons for the individualized treatment of patients with locally advanced rectal cancer: A planning study with clinical implications. Radiother. Oncol. 2011, 102, 30–37. [Google Scholar] [CrossRef]
- Colaco, R.J.; Nichols, R.C.; Huh, S.; Getman, N.; Ho, M.W.; Li, Z.; Morris, C.G.; Mendenhall, W.M.; Mendenhall, N.P.; Hoppe, B.S. Protons offer reduced bone marrow, small bowel, and urinary bladder exposure for patients receiving neoadjuvant radiotherapy for resectable rectal cancer. J. Gastrointest. Oncol. 2014, 5, 3–8. [Google Scholar]
- Kronborg, C.J.S.; Jørgensen, J.B.; Petersen, J.B.B.; Nyvang Jensen, L.; Iversen, L.H.; Pedersen, B.G.; Spindler, K.-L.G. Pelvic insufficiency fractures, dose volume parameters and plan optimization after radiotherapy for rectal cancer. Clin. Transl. Radiat. Oncol. 2019, 19, 72–76. [Google Scholar] [CrossRef]
- Radu, C.; Norrlid, O.; Brændengen, M.; Hansson, K.; Isacsson, U.; Glimelius, B. Integrated peripheral boost in preoperative radiotherapy for the locally most advanced non-resectable rectal cancer patients. Acta Oncol. 2013, 52, 528–537. [Google Scholar] [CrossRef]
- Rønde, H.S.; Kallehauge, J.F.; Kronborg, C.J.S.; Nyvang, L.; Rekstad, B.L.; Hanekamp, B.A.; Appelt, A.L.; Guren, M.G.; Spindler, K.L.S. Intensity modulated proton therapy planning study for organ at risk sparing in rectal cancer re-irradiation. Acta Oncol. 2021, 60, 1436–1439. [Google Scholar] [CrossRef]
- Chuter, R.; Glassborow, E.; Speight, R.; Clarke, M.; Murray, L.; Radhakrishna, G.; Lavin, V.; Aspin, L.; Aldred, M.; Gregory, S.; et al. A treatment planning comparison of photon stereotactic ablative radiotherapy and proton beam therapy for the re-irradiation of pelvic cancer recurrence. Phys. Imaging Radiat. Oncol. 2022, 21, 78–83. [Google Scholar] [CrossRef]
- Wo, J.Y.; Depauw, N.; Symonifka, J.; Kooy, H.; Hong, T.S. Dosimetric Analysis of Proton Pencil Beam Scanning Radiation Therapy Versus Dose Painted Intensity Modulated Radiation Therapy for Squamous Cell Carcinoma of the Anal Canal. Int. J. Radiat. Oncol. Biol. Phys. 2014, 90, S398. [Google Scholar] [CrossRef]
- Anand, A.; Bues, M.; Rule, W.G.; Keole, S.R.; Beltran, C.J.; Yin, J.; Haddock, M.G.; Hallemeier, C.L.; Miller, R.C.; Ashman, J.B. Scanning proton beam therapy reduces normal tissue exposure in pelvic radiotherapy for anal cancer. Radiother. Oncol. 2015, 117, 505–508. [Google Scholar] [CrossRef] [PubMed]
- Ojerholm, E.; Kirk, M.L.; Thompson, R.F.; Zhai, H.; Metz, J.M.; Both, S.; Ben-Josef, E.; Plastaras, J.P. Pencil-beam scanning proton therapy for anal cancer: A dosimetric comparison with intensity-modulated radiotherapy. Acta Oncol. 2015, 54, 1209–1217. [Google Scholar] [CrossRef] [PubMed]
- Meier, T.; Mascia, A.; Wolf, E.; Kharofa, J.R. Modeling of High-Grade Hematologic Toxicity in Anal Cancer Patients Treated with Intensity Modulated Proton Therapy (IMPT) and Volumetric Modulated Arc Therapy (VMAT). Int. J. Radiat. Oncol. Biol. Phys. 2016, 96, E145. [Google Scholar] [CrossRef]
- Meier, T.; Mascia, A.; Wolf, E.; Kharofa, J. Dosimetric Comparison of Intensity-Modulated Proton Therapy and Volumetric-Modulated Arc Therapy in Anal Cancer Patients and the Ability to Spare Bone Marrow. Int. J. Part. Ther. 2017, 4, 11–17. [Google Scholar] [CrossRef]
- Kronborg, C.; Serup-Hansen, E.; Lefevre, A.; Wilken, E.E.; Petersen, J.B.; Hansen, J.; Schouboe, A.; Nyvang, L.; Spindler, K.-L.G. Prospective evaluation of acute toxicity and patient reported outcomes in anal cancer and plan optimization. Radiother. Oncol. 2018, 128, 375–379. [Google Scholar] [CrossRef]
- Suit, H.D.; Goitein, M.; Tepper, J.E.; Verhey, L.; Koehler, A.M.; Schneider, R.; Gragoudas, E. Clinical experience and expectation with protons and heavy ions. Int. J. Radiat. Oncol. Biol. Phys. 1977, 3, 115–125. [Google Scholar] [CrossRef]
- Suit, H.; Phil, D.; Goitein, M.; Munzenrider, J.; Verhey, L.; Blitzer, P.; Gragoudas, E.; Koehler, A.M.; Urie, M.; Gentry, R.; et al. Evaluation of the clinical applicability of proton beams in definitive fractionated radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 1982, 8, 2199–2205. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, D.Y.; Kim, S.Y.; Park, J.W.; Choi, H.S.; Oh, J.H.; Chang, H.J.; Kim, T.H.; Park, S.W. Clinical outcomes of chemoradiotherapy for locally recurrent rectal cancer. Radiat. Oncol. 2011, 6, 51. [Google Scholar] [CrossRef]
- Hamauchi, S.; Yamazaki, K.; Yasui, H.; Boku, N.; Onozawa, Y.; Fukutomi, A.; Machida, N.; Yokota, T.; Todaka, A.; Taniguchi, H.; et al. Safety and Efficacy of Proton-Beam Radiation Therapy for Patients with Locally Recurrent rectal Cancer. Ann. Oncol. 2012, 23, xi160. [Google Scholar] [CrossRef]
- Hiroshima, Y.; Ishikawa, H.; Murakami, M.; Nakamura, M.; Shimizu, S.; Enomoto, T.; Oda, T.; Mizumoto, M.; Nakai, K.; Okumura, T.; et al. Proton Beam Therapy for Local Recurrence of Rectal Cancer. Anticancer. Res. 2021, 41, 3589–3595. [Google Scholar] [CrossRef]
- Simone, C.B.; Plastaras, J.P.; Jabbour, S.K.; Lee, A.; Lee, N.Y.; Choi, J.I.; Frank, S.J.; Chang, J.Y.; Bradley, J. Proton Reirradiation: Expert Recommendations for Reducing Toxicities and Offering New Chances of Cure in Patients with Challenging Recurrence Malignancies. Semin. Radiat. Oncol. 2020, 30, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Berman, A.T.; Both, S.; Sharkoski, T.; Goldrath, K.; Tochner, Z.; Apisarnthanarax, S.; Metz, J.M.; Plastaras, J.P. Proton Reirradiation of Recurrent Rectal Cancer: Dosimetric Comparison, Toxicities, and Preliminary Outcomes. Int. J. Part. Ther. 2014, 1, 2–13. [Google Scholar] [CrossRef]
- Koroulakis, A.; Molitoris, J.; Kaiser, A.; Hanna, N.; Bafford, A.; Jiang, Y.; Bentzen, S.; Regine, W.F. Reirradiation for Rectal Cancer Using Pencil Beam Scanning Proton Therapy: A Single Institutional Experience. Adv. Radiat. Oncol. 2021, 6, 100595. [Google Scholar] [CrossRef] [PubMed]
- Moningi, S.; Ludmir, E.B.; Polamraju, P.; Williamson, T.; Melkun, M.M.; Herman, J.D.; Krishnan, S.; Koay, E.J.; Koong, A.C.; Minsky, B.D.; et al. Definitive hyperfractionated, accelerated proton reirradiation for patients with pelvic malignancies. Clin. Transl. Radiat. Oncol. 2019, 19, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Jeans, E.B.; Jethwa, K.R.; Harmsen, W.S.; Neben-Wittich, M.; Ashman, J.B.; Merrell, K.W.; Giffey, B.; Ito, S.; Kazemba, B.; Beltran, C.; et al. Clinical Implementation of Preoperative Short-Course Pencil Beam Scanning Proton Therapy for Patients with Rectal Cancer. Adv. Radiat. Oncol. 2020, 5, 865–870. [Google Scholar] [CrossRef] [PubMed]
- Wo, J.Y.; Plastaras, J.P.; Metz, J.M.; Jiang, W.; Yeap, B.Y.; Drapek, L.C.; Adams, J.; Baglini, C.; Ryan, D.P.; Murphy, J.E.; et al. Pencil Beam Scanning Proton Beam Chemoradiation Therapy With 5-Fluorouracil and Mitomycin-C for Definitive Treatment of Carcinoma of the Anal Canal: A Multi-institutional Pilot Feasibility Study. Int. J. Radiat. Oncol. Biol. Phys. 2019, 105, 90–95. [Google Scholar] [CrossRef]
- Vítek, P.; Kubeš, J.; Vondráček, V.; Andrlik, M.; Navrátíl, M.; Zapletal, R.; Haas, A.; Dědečková, K.; Ondrová, B.; Grebenyuk, A.; et al. Pencil Beam Scanning (PBS) Intensity-Modulated Proton Therapy (IMPT) Chemoradiotherapy for Anal Canal Cancer-Single Institution Experience. Cancers 2021, 14, 185. [Google Scholar] [CrossRef]
- Mohiuddin, J.J.; Jethwa, K.R.; Grandhi, N.; Breen, W.G.; Wang, X.; Anvari, A.; Lin, H.; Sandhyavenu, H.; Doucette, A.; Plastaras, J.P.; et al. Multi-institutional Comparison of Intensity Modulated Photon Versus Proton Radiation Therapy in the Management of Squamous Cell Carcinoma of the Anus. Adv. Radiat. Oncol. 2021, 6, 100744. [Google Scholar] [CrossRef]
- Alexander, V. Preoperative Short-Course Radiation Therapy with PROtons Compared to Photons In High-Risk RECTal Cancer (PRORECT). Available online: https://ClinicalTrials.gov/show/NCT04525989 (accessed on 1 May 2022).
- Umeå University. Region Västerbotten. Proton Versus Photon Therapy in Anal Squamous Cell Carcinoma. Available online: https://ClinicalTrials.gov/show/NCT04462042 (accessed on 1 May 2022).
- University of Aarhus. Pencil Beam Proton Therapy for Pelvic Recurrences in Rectal Cancer Patients Previously Treated with Radiotherapy. Available online: https://ClinicalTrials.gov/show/NCT04695782 (accessed on 1 May 2022).
- University of Aarhus. Pencil Beam Proton Therapy for Recurrences in Anal Cancer Patients Previously Treated with Radiotherapy (DACG 5). Available online: https://ClinicalTrials.gov/show/NCT05055635 (accessed on 1 May 2022).
- Samsung Medical Center. Concurrent Chemo-proton Radiotherapy with or without Resection and Spacer Insertion for Loco-regional Recurrence of Previous Irradiated Rectal Cancer. Available online: https://ClinicalTrials.gov/show/NCT03098108 (accessed on 31 October 2019).
- Washington University School of Medicine. Hypofractionated Pencil-Beam Scanning Intensity-modulated Proton Therapy (IMPT) in Recurrent Rectal Cancer. Available online: https://ClinicalTrials.gov/show/NCT04827732 (accessed on 1 May 2022).
- Jordan, K. Proton Therapy in Reducing Toxicity in Anal Cancer. Available online: https://ClinicalTrials.gov/show/NCT03018418 (accessed on 1 May 2022).
- M.D. Anderson Cancer Center; National Cancer Institute (NCI). LET-IMPT and Standard Chemotherapy in Treating Patients with Newly Diagnosed Stage I-III Anal Canal Squamous Cell Cancer. Available online: https://ClinicalTrials.gov/show/NCT03690921 (accessed on 1 May 2022).
- Corrigan, K.L.; Rooney, M.K.; De, B.; Ludmir, E.D.; Das, P.; Smith, G.L.; Taniguchi, C.; Minsky, B.D.; Koay, E.J.; Koong, A.; et al. Patient-reported sexual function in long-term survivors of anal cancer treated with definitive intensity-modulated radiotherapy and concurrent chemotherapy. Pract. Radiat. Oncol. 2022, in press. [Google Scholar] [CrossRef]
- De, B.; Corrigan, K.L.; Rooney, M.K.; Ludmir, E.B.; Das, P.; Smith, G.L.; Taniguchi, C.M.; Minsky, B.D.; Koay, E.J.; Koong, A.; et al. Patient-reported bowel and urinary function in long-term survivors of squamous cell carcinoma of the anus treated with definitive intensity-modulated radiotherapy and concurrent chemotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2022, in press. [Google Scholar] [CrossRef]
- Tambas, M.; Steenbakkers, R.J.H.M.; van der Laan, H.P.; Wolters, A.M.; Kierkels, R.G.J.; Scandurra, D.; Korevaar, E.W.; Oldehinkel, E.; van Zon-Meijer, T.W.H.; Both, S.; et al. First experience with model-based selection of head and neck cancer patients for proton therapy. Radiother. Oncol. 2020, 151, 206–213. [Google Scholar] [CrossRef] [PubMed]
| Authors | Year | Study Design | n | Comparison | OS | PFS | Toxicity |
|---|---|---|---|---|---|---|---|
| Esophageal Cancer | |||||||
| Xi et al. [41] | 2017 | Retrospective | 343 | 5-yr | 5-yr | Grade 3/4 | |
| PBT (1) | 34.6% | 33.5% | 37.9% | ||||
| IMRT | 25.0% | 13.2% | 45.0% | ||||
| p-value | 0.038 | 0.005 | 0.192 | ||||
| Bhangoo et al. [42] | 2020 | Retrospective | 64 | 1-yr | 1-yr | Grade 3 | |
| IMPT | 74% | 71% | 16% | ||||
| IMRT | 71% | 45% | 9% | ||||
| p-value | 0.62 | 0.15 | 0.71 | ||||
| Lin et al. [45] | 2020 | Randomized phase IIB trial | 107 | 3-yr | 3-yr | TTB (2) | |
| PBT | 51.2% | 44.5% | |||||
| IMRT | 50.8% | 44.5% | 2.3 times higher in the IMRT arm across all patients | ||||
| p-value | 0.60 | 0.70 | |||||
| Suh et al. [43] | 2021 | Retrospective | 77 | 5-yr | 5-yr | ||
| PBT | 64.9% | 56.5% | NA | ||||
| 3D-CRT or IMRT | NA | ||||||
| p-value | 0.52 | 0.72 | |||||
| NRG-GI006 NCT03801876 [47] | 2032 (est.) (3) | Randomized phase III trial | 300 (est.) | Up to 8 years In progress | |||
| PBT | |||||||
| IMRT | |||||||
| PROTECT NCT05055648 [48] | 2029 (est.) | Randomized phase III trial | 396 (est.) | Up to 5 years In progress | |||
| Proton | |||||||
| Photon | |||||||
| Authors | Year | Study Design | n | Comparison | OS | PFS | Toxicity |
|---|---|---|---|---|---|---|---|
| Anorectal Cancers | |||||||
| Mohiuddin et al. [96] | 2021 | Retrospective | 208 (1) | 2-yr LRRFS (2) | 2-yr PFS (3) | Grade 3+ Acute | |
| IMPT | 91% | HR: 0.6 | 67% | ||||
| IMRT | 88% | 68% | |||||
| p-value | 0.49 | N.S. | 0.96 | ||||
| PRORECT NCT04525989 [97] | 2028 (est.) | Randomized phase II trial | 254 (est.) (4) | Up to 5 years In progress | |||
| Photon | |||||||
| Photon | |||||||
| SWANCA NCT04462042 [98] | 2030 (est.) | Randomized phase II trial | 100 (est.) (5) | Up to 5 years In progress | |||
| Proton | |||||||
| Photon | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kobeissi, J.M.; Simone, C.B., II; Hilal, L.; Wu, A.J.; Lin, H.; Crane, C.H.; Hajj, C. Proton Therapy in the Management of Luminal Gastrointestinal Cancers: Esophagus, Stomach, and Anorectum. Cancers 2022, 14, 2877. https://doi.org/10.3390/cancers14122877
Kobeissi JM, Simone CB II, Hilal L, Wu AJ, Lin H, Crane CH, Hajj C. Proton Therapy in the Management of Luminal Gastrointestinal Cancers: Esophagus, Stomach, and Anorectum. Cancers. 2022; 14(12):2877. https://doi.org/10.3390/cancers14122877
Chicago/Turabian StyleKobeissi, Jana M., Charles B. Simone, II, Lara Hilal, Abraham J. Wu, Haibo Lin, Christopher H. Crane, and Carla Hajj. 2022. "Proton Therapy in the Management of Luminal Gastrointestinal Cancers: Esophagus, Stomach, and Anorectum" Cancers 14, no. 12: 2877. https://doi.org/10.3390/cancers14122877
APA StyleKobeissi, J. M., Simone, C. B., II, Hilal, L., Wu, A. J., Lin, H., Crane, C. H., & Hajj, C. (2022). Proton Therapy in the Management of Luminal Gastrointestinal Cancers: Esophagus, Stomach, and Anorectum. Cancers, 14(12), 2877. https://doi.org/10.3390/cancers14122877







