High Levels of MFG-E8 Confer a Good Prognosis in Prostate and Renal Cancer Patients
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Tissue Microarrays (TMAs)
2.2. Immunofluorescence (IF)
2.3. Statistical Analysis
3. Results
3.1. Localization of MFG-E8 Expression in Malignant Areas of Prostate and Renal Cancer Tissues
3.2. MFG-E8 Expression Differs According to the Grade of Cancer
3.3. CD206+ Cells Are Differentially Distributed in Renal Cancers
3.4. MFG-E8 Expression Correlates with Patient Outcome
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, J.; Choi, B.I.; Park, S.Y.; An, S.Y.; Han, J.; Kim, J.H. Fetal hematopoietic stem cells express MFG-E8 during mouse embryogenesis. Exp. Mol. Med. 2015, 47, e174. [Google Scholar] [CrossRef]
- Brissette, M.J.; Lepage, S.; Lamonde, A.S.; Sirois, I.; Groleau, J.; Laurin, L.P.; Cailhier, J.F. MFG-E8 released by apoptotic endothelial cells triggers anti-inflammatory macrophage reprogramming. PLoS ONE 2012, 7, e36368. [Google Scholar] [CrossRef] [Green Version]
- Raymond, A.; Ensslin, M.A.; Shur, B.D. SED1/MFG-E8: A bi-motif protein that orchestrates diverse cellular interactions. J. Cell. Biochem. 2009, 106, 957–966. [Google Scholar] [CrossRef] [Green Version]
- Oshima, K.; Aoki, N.; Kato, T.; Kitajima, K.; Matsuda, T. Secretion of a peripheral membrane protein, MFG-E8, as a complex with membrane vesicles. Eur. J. Biochem. 2002, 269, 1209–1218. [Google Scholar] [CrossRef]
- Webber, J.; Stone, T.C.; Katilius, E.; Smith, B.C.; Gordon, B.; Mason, M.D.; Tabi, Z.; Brewis, I.A.; Clayton, A. Proteomics analysis of cancer exosomes using a novel modified aptamer-based array (SOMAscan) platform. Mol. Cell. Proteom. 2014, 13, 1050–1064. [Google Scholar] [CrossRef] [Green Version]
- Miyasaka, K.; Hanayama, R.; Tanaka, M.; Nagata, S. Expression of milk fat globule epidermal growth factor 8 in immature dendritic cells for engulfment of apoptotic cells. Eur. J. Immunol. 2004, 34, 1414–1422. [Google Scholar] [CrossRef]
- Carrascosa, C.; Obula, R.G.; Missiaglia, E.; Lehr, H.A.; Delorenzi, M.; Frattini, M.; Ruegg, C.; Mariotti, A. MFG-E8/lactadherin regulates cyclins D1/D3 expression and enhances the tumorigenic potential of mammary epithelial cells. Oncogene 2012, 31, 1521–1532. [Google Scholar] [CrossRef] [Green Version]
- Silvestre, J.S.; Thery, C.; Hamard, G.; Boddaert, J.; Aguilar, B.; Delcayre, A.; Houbron, C.; Tamarat, R.; Blanc-Brude, O.; Heeneman, S.; et al. Lactadherin promotes VEGF-dependent neovascularization. Nat. Med. 2005, 11, 499–506. [Google Scholar] [CrossRef]
- Yamada, K.; Uchiyama, A.; Uehara, A.; Perera, B.; Ogino, S.; Yokoyama, Y.; Takeuchi, Y.; Udey, M.C.; Ishikawa, O.; Motegi, S. MFG-E8 Drives Melanoma Growth by Stimulating Mesenchymal Stromal Cell-Induced Angiogenesis and M2 Polarization of Tumor-Associated Macrophages. Cancer Res. 2016, 76, 4283–4292. [Google Scholar] [CrossRef] [Green Version]
- Ait-Oufella, H.; Kinugawa, K.; Zoll, J.; Simon, T.; Boddaert, J.; Heeneman, S.; Blanc-Brude, O.; Barateau, V.; Potteaux, S.; Merval, R.; et al. Lactadherin deficiency leads to apoptotic cell accumulation and accelerated atherosclerosis in mice. Circulation 2007, 115, 2168–2177. [Google Scholar] [CrossRef] [Green Version]
- Boddaert, J.; Kinugawa, K.; Lambert, J.C.; Boukhtouche, F.; Zoll, J.; Merval, R.; Blanc-Brude, O.; Mann, D.; Berr, C.; Vilar, J.; et al. Evidence of a role for lactadherin in Alzheimer’s disease. Am. J. Pathol. 2007, 170, 921–929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brissette, M.J.; Laplante, P.; Qi, S.; Latour, M.; Cailhier, J.F. Milk fat globule epidermal growth factor-8 limits tissue damage through inflammasome modulation during renal injury. J. Leukoc. Biol. 2016, 100, 1135–1146. [Google Scholar] [CrossRef] [PubMed]
- Hanayama, R.; Tanaka, M.; Miyasaka, K.; Aozasa, K.; Koike, M.; Uchiyama, Y.; Nagata, S. Autoimmune disease and impaired uptake of apoptotic cells in MFG-E8-deficient mice. Science 2004, 304, 1147–1150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laplante, P.; Brillant-Marquis, F.; Brissette, M.J.; Joannette-Pilon, B.; Cayrol, R.; Kokta, V.; Cailhier, J.F. MFG-E8 Reprogramming of Macrophages Promotes Wound Healing by Increased bFGF Production and Fibroblast Functions. J. Investig. Dermatol. 2017, 137, 2005–2013. [Google Scholar] [CrossRef] [Green Version]
- Miksa, M.; Wu, R.; Dong, W.; Komura, H.; Amin, D.; Ji, Y.; Wang, Z.; Wang, H.; Ravikumar, T.S.; Tracey, K.J.; et al. Immature dendritic cell-derived exosomes rescue septic animals via milk fat globule epidermal growth factor-factor VIII [corrected]. J. Immunol. 2009, 183, 5983–5990. [Google Scholar] [CrossRef]
- Uchiyama, A.; Yamada, K.; Ogino, S.; Yokoyama, Y.; Takeuchi, Y.; Udey, M.C.; Ishikawa, O.; Motegi, S. MFG-E8 regulates angiogenesis in cutaneous wound healing. Am. J. Pathol. 2014, 184, 1981–1990. [Google Scholar] [CrossRef] [Green Version]
- Jinushi, M.; Nakazaki, Y.; Carrasco, D.R.; Draganov, D.; Souders, N.; Johnson, M.; Mihm, M.C.; Dranoff, G. Milk fat globule EGF-8 promotes melanoma progression through coordinated Akt and twist signaling in the tumor microenvironment. Cancer Res. 2008, 68, 8889–8898. [Google Scholar] [CrossRef] [Green Version]
- Yamazaki, M.; Maruyama, S.; Abe, T.; Essa, A.; Babkair, H.; Cheng, J.; Saku, T. MFG-E8 expression for progression of oral squamous cell carcinoma and for self-clearance of apoptotic cells. Lab. Investig. 2014, 94, 1260–1272. [Google Scholar] [CrossRef] [Green Version]
- Ellert-Miklaszewska, A.; Wisniewski, P.; Kijewska, M.; Gajdanowicz, P.; Pszczolkowska, D.; Przanowski, P.; Dabrowski, M.; Maleszewska, M.; Kaminska, B. Tumour-processed osteopontin and lactadherin drive the protumorigenic reprogramming of microglia and glioma progression. Oncogene 2016, 35, 6366–6377. [Google Scholar] [CrossRef]
- Yu, L.; Zhao, L.; Jia, Z.; Bi, J.; Wei, Q.; Song, X.; Jiang, L.; Lin, S.; Wei, M. MFG-E8 overexpression is associated with poor prognosis in breast cancer patients. Pathol. Res. Pract. 2019, 215, 490–498. [Google Scholar] [CrossRef]
- Zhao, Q.; Xu, L.; Sun, X.; Zhang, K.; Shen, H.; Tian, Y.; Sun, F.; Li, Y. MFG-E8 overexpression promotes colorectal cancer progression via AKT/MMPs signalling. Tumour Biol. 2017, 39, 1010428317707881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanemura, T.; Miyata, H.; Makino, T.; Tanaka, K.; Sugimura, K.; Hamada-Uematsu, M.; Mizote, Y.; Uchida, H.; Miyazaki, Y.; Takahashi, T.; et al. Immunoregulatory influence of abundant MFG-E8 expression by esophageal cancer treated with chemotherapy. Cancer Sci. 2018, 109, 3393–3402. [Google Scholar] [CrossRef] [PubMed]
- Tibaldi, L.; Leyman, S.; Nicolas, A.; Notebaert, S.; Dewulf, M.; Ngo, T.H.; Zuany-Amorim, C.; Amzallag, N.; Bernard-Pierrot, I.; Sastre-Garau, X.; et al. New blocking antibodies impede adhesion, migration and survival of ovarian cancer cells, highlighting MFGE8 as a potential therapeutic target of human ovarian carcinoma. PLoS ONE 2013, 8, e72708. [Google Scholar] [CrossRef] [PubMed]
- Neutzner, M.; Lopez, T.; Feng, X.; Bergmann-Leitner, E.S.; Leitner, W.W.; Udey, M.C. MFG-E8/lactadherin promotes tumor growth in an angiogenesis-dependent transgenic mouse model of multistage carcinogenesis. Cancer Res. 2007, 67, 6777–6785. [Google Scholar] [CrossRef] [Green Version]
- Sugano, G.; Bernard-Pierrot, I.; Lae, M.; Battail, C.; Allory, Y.; Stransky, N.; Krumeich, S.; Lepage, M.L.; Maille, P.; Donnadieu, M.H.; et al. Milk fat globule--epidermal growth factor--factor VIII (MFGE8)/lactadherin promotes bladder tumor development. Oncogene 2011, 30, 642–653. [Google Scholar] [CrossRef] [Green Version]
- Soki, F.N.; Koh, A.J.; Jones, J.D.; Kim, Y.W.; Dai, J.; Keller, E.T.; Pienta, K.J.; Atabai, K.; Roca, H.; McCauley, L.K. Polarization of prostate cancer-associated macrophages is induced by milk fat globule-EGF factor 8 (MFG-E8)-mediated efferocytosis. J. Biol. Chem. 2014, 289, 24560–24572. [Google Scholar] [CrossRef] [Green Version]
- Mantovani, A.; Sozzani, S.; Locati, M.; Allavena, P.; Sica, A. Macrophage polarization: Tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002, 23, 549–555. [Google Scholar] [CrossRef]
- Lewis, C.E.; Pollard, J.W. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006, 66, 605–612. [Google Scholar] [CrossRef] [Green Version]
- Sica, A.; Schioppa, T.; Mantovani, A.; Allavena, P. Tumour-associated macrophages are a distinct M2 polarised population promoting tumour progression: Potential targets of anti-cancer therapy. Eur. J. Cancer 2006, 42, 717–727. [Google Scholar] [CrossRef]
- Cailhier, J.F.; Laplante, P.; Hebert, M.J. Endothelial apoptosis and chronic transplant vasculopathy: Recent results, novel mechanisms. Am. J. Transplant. 2006, 6, 247–253. [Google Scholar] [CrossRef]
- Cailhier, J.F.; Partolina, M.; Vuthoori, S.; Wu, S.; Ko, K.; Watson, S.; Savill, J.; Hughes, J.; Lang, R.A. Conditional macrophage ablation demonstrates that resident macrophages initiate acute peritoneal inflammation. J. Immunol. 2005, 174, 2336–2342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cailhier, J.F.; Sawatzky, D.A.; Kipari, T.; Houlberg, K.; Walbaum, D.; Watson, S.; Lang, R.A.; Clay, S.; Kluth, D.; Savill, J.; et al. Resident pleural macrophages are key orchestrators of neutrophil recruitment in pleural inflammation. Am. J. Respir. Crit. Care Med. 2006, 173, 540–547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kluth, D.C.; Erwig, L.P.; Rees, A.J. Multiple facets of macrophages in renal injury. Kidney Int. 2004, 66, 542–557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, S.L.; Castano, A.P.; Nowlin, B.T.; Lupher, M.L., Jr.; Duffield, J.S. Bone marrow Ly6Chigh monocytes are selectively recruited to injured kidney and differentiate into functionally distinct populations. J. Immunol. 2009, 183, 6733–6743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atri, C.; Guerfali, F.Z.; Laouini, D. Role of Human Macrophage Polarization in Inflammation during Infectious Diseases. Int. J. Mol. Sci. 2018, 19, 1801. [Google Scholar] [CrossRef] [Green Version]
- Mosser, D.M. The many faces of macrophage activation. J. Leukoc. Biol. 2003, 73, 209–212. [Google Scholar] [CrossRef]
- Sica, A.; Bronte, V. Altered macrophage differentiation and immune dysfunction in tumor development. J. Clin. Investig. 2007, 117, 1155–1166. [Google Scholar] [CrossRef]
- Rizzo, A.; Mollica, V.; Santoni, M.; Ricci, A.D.; Rosellini, M.; Marchetti, A.; Montironi, R.; Ardizzoni, A.; Massari, F. Impact of Clinicopathological Features on Survival in Patients Treated with First-line Immune Checkpoint Inhibitors Plus Tyrosine Kinase Inhibitors for Renal Cell Carcinoma: A Meta-analysis of Randomized Clinical Trials. Eur. Urol. Focus 2021, in press. [Google Scholar] [CrossRef]
- Rizzo, A.; Mollica, V.; Cimadamore, A.; Santoni, M.; Scarpelli, M.; Giunchi, F.; Cheng, L.; Lopez-Beltran, A.; Fiorentino, M.; Montironi, R.; et al. Is There a Role for Immunotherapy in Prostate Cancer? Cells 2020, 9, 2051. [Google Scholar] [CrossRef]
- Sharma, N.V.; Pellegrini, K.L.; Ouellet, V.; Giuste, F.O.; Ramalingam, S.; Watanabe, K.; Adam-Granger, E.; Fossouo, L.; You, S.; Freeman, M.R.; et al. Identification of the Transcription Factor Relationships Associated with Androgen Deprivation Therapy Response and Metastatic Progression in Prostate Cancer. Cancers 2018, 10, 379. [Google Scholar] [CrossRef] [Green Version]
- Cen, C.; Aziz, M.; Yang, W.L.; Zhou, M.; Nicastro, J.M.; Coppa, G.F.; Wang, P. Milk fat globule-epidermal growth factor-factor VIII attenuates sepsis-induced acute kidney injury. J. Surg. Res. 2017, 213, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, B.Y.; Li, X.L.; Cheng, M.; Yu, F.; Lu, W.D.; Cai, Q.; Wang, J.F.; Zhou, R.H.; Gao, H.Q.; et al. Proteomic analysis of kidney and protective effects of grape seed procyanidin B2 in db/db mice indicate MFG-E8 as a key molecule in the development of diabetic nephropathy. Biochim. Biophys. Acta 2013, 1832, 805–816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colotta, F.; Allavena, P.; Sica, A.; Garlanda, C.; Mantovani, A. Cancer-related inflammation, the seventh hallmark of cancer: Links to genetic instability. Carcinogenesis 2009, 30, 1073–1081. [Google Scholar] [CrossRef] [Green Version]
- Murdoch, C.; Muthana, M.; Coffelt, S.B.; Lewis, C.E. The role of myeloid cells in the promotion of tumour angiogenesis. Nat. Rev. Cancer 2008, 8, 618–631. [Google Scholar] [CrossRef] [PubMed]
- Pollard, J.W. Tumour-educated macrophages promote tumour progression and metastasis. Nat. Rev. Cancer 2004, 4, 71–78. [Google Scholar] [CrossRef]
- Gregory, C.D.; Pound, J.D. Cell death in the neighbourhood: Direct microenvironmental effects of apoptosis in normal and neoplastic tissues. J. Pathol. 2011, 223, 177–194. [Google Scholar] [CrossRef]
- Borges, E.; Jan, Y.; Ruoslahti, E. Platelet-derived growth factor receptor beta and vascular endothelial growth factor receptor 2 bind to the beta 3 integrin through its extracellular domain. J. Biol. Chem. 2000, 275, 39867–39873. [Google Scholar] [CrossRef] [Green Version]
- Jia, M.; Yao, H.; Chen, C.; Wang, Y.; Wang, H.; Cui, T.; Zhu, J. Prognostic Correlation Between MFG-E8 Expression Level and Colorectal Cancer. Arch. Med. Res. 2017, 48, 270–275. [Google Scholar] [CrossRef]
- Oba, J.; Moroi, Y.; Nakahara, T.; Abe, T.; Hagihara, A.; Furue, M. Expression of milk fat globule epidermal growth factor-VIII may be an indicator of poor prognosis in malignant melanoma. Br. J. Dermatol. 2011, 165, 506–512. [Google Scholar] [CrossRef]
- Yang, C.; Hayashida, T.; Forster, N.; Li, C.; Shen, D.; Maheswaran, S.; Chen, L.; Anderson, K.S.; Ellisen, L.W.; Sgroi, D.; et al. The integrin alpha(v)beta(3-5) ligand MFG-E8 is a p63/p73 target gene in triple-negative breast cancers but exhibits suppressive functions in ER(+) and erbB2(+) breast cancers. Cancer Res. 2011, 71, 937–945. [Google Scholar] [CrossRef] [Green Version]
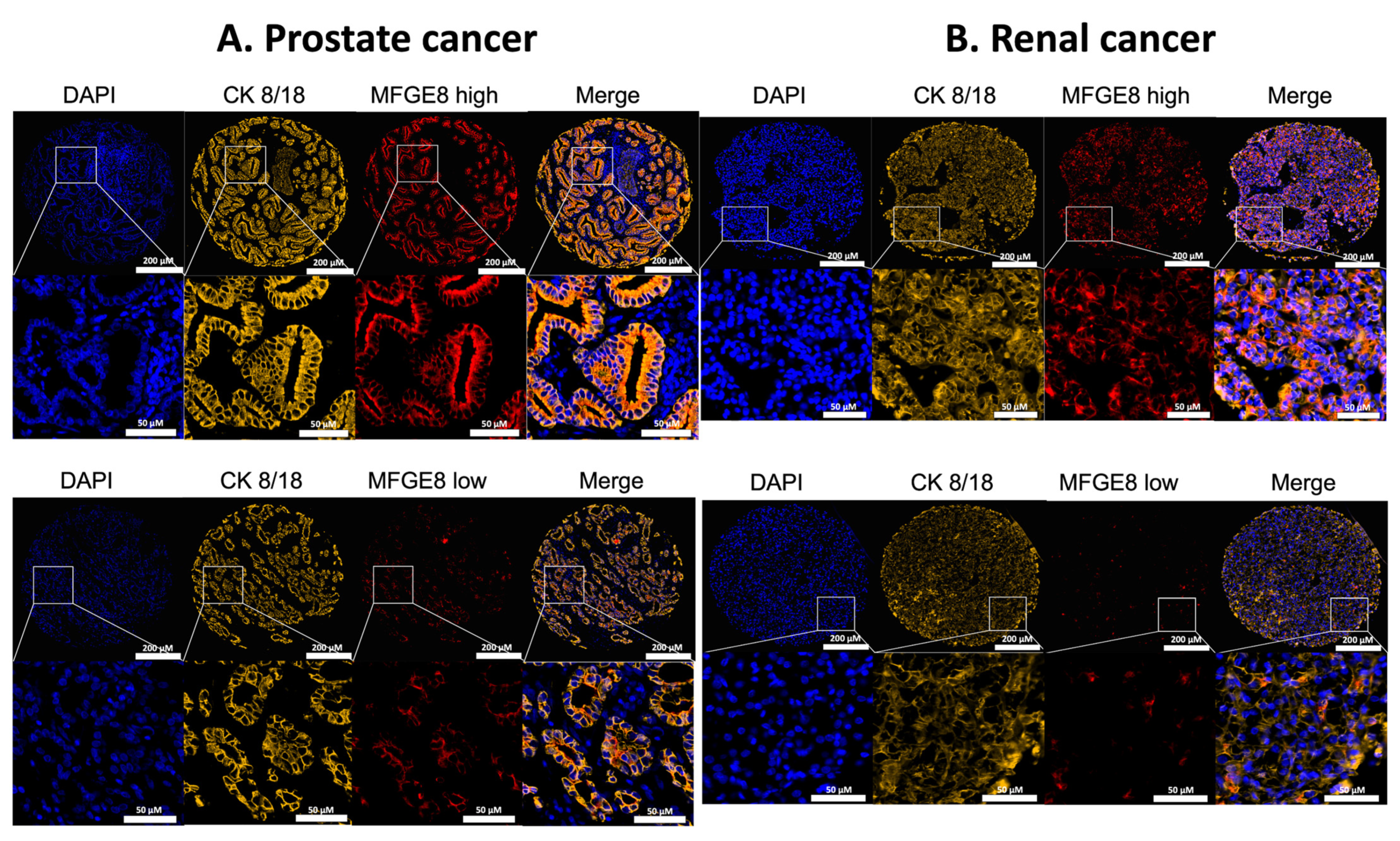

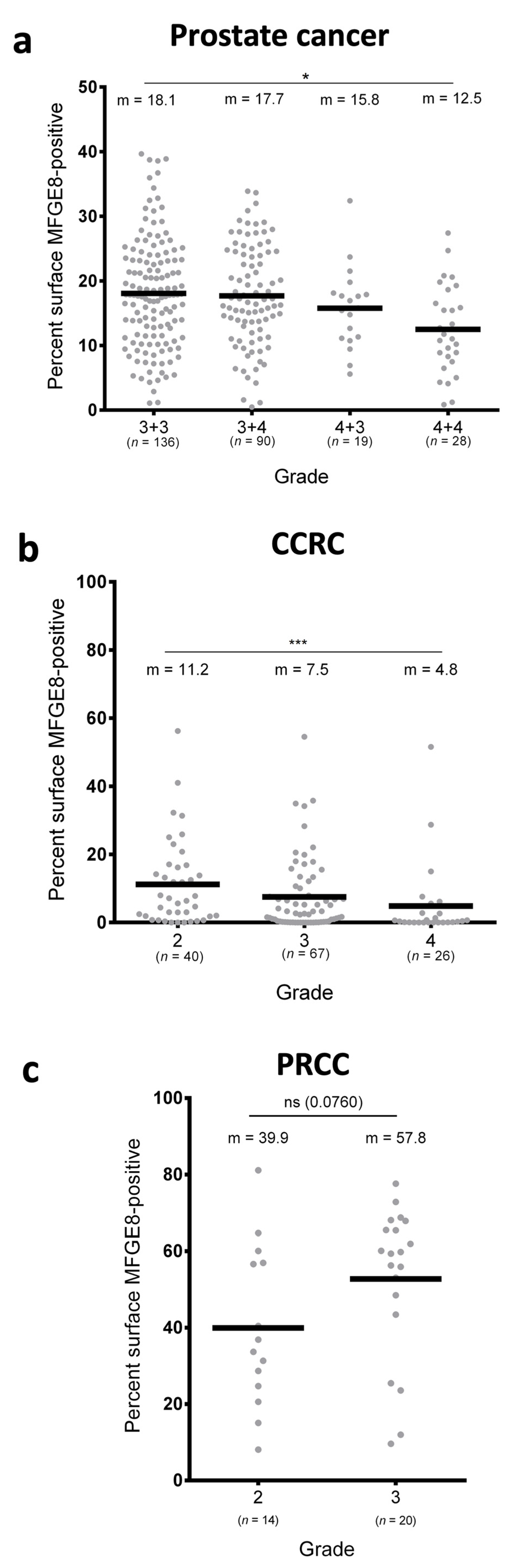
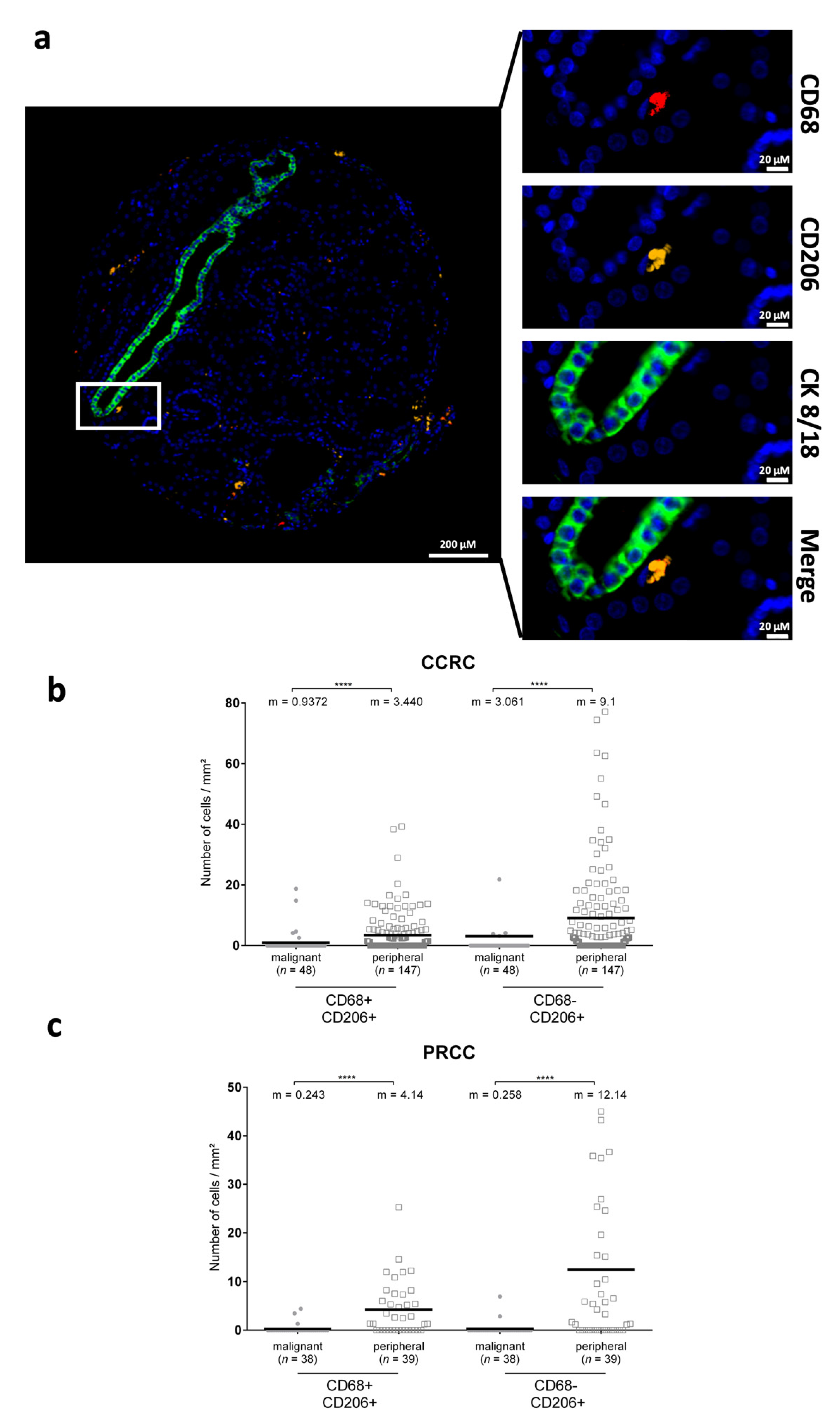
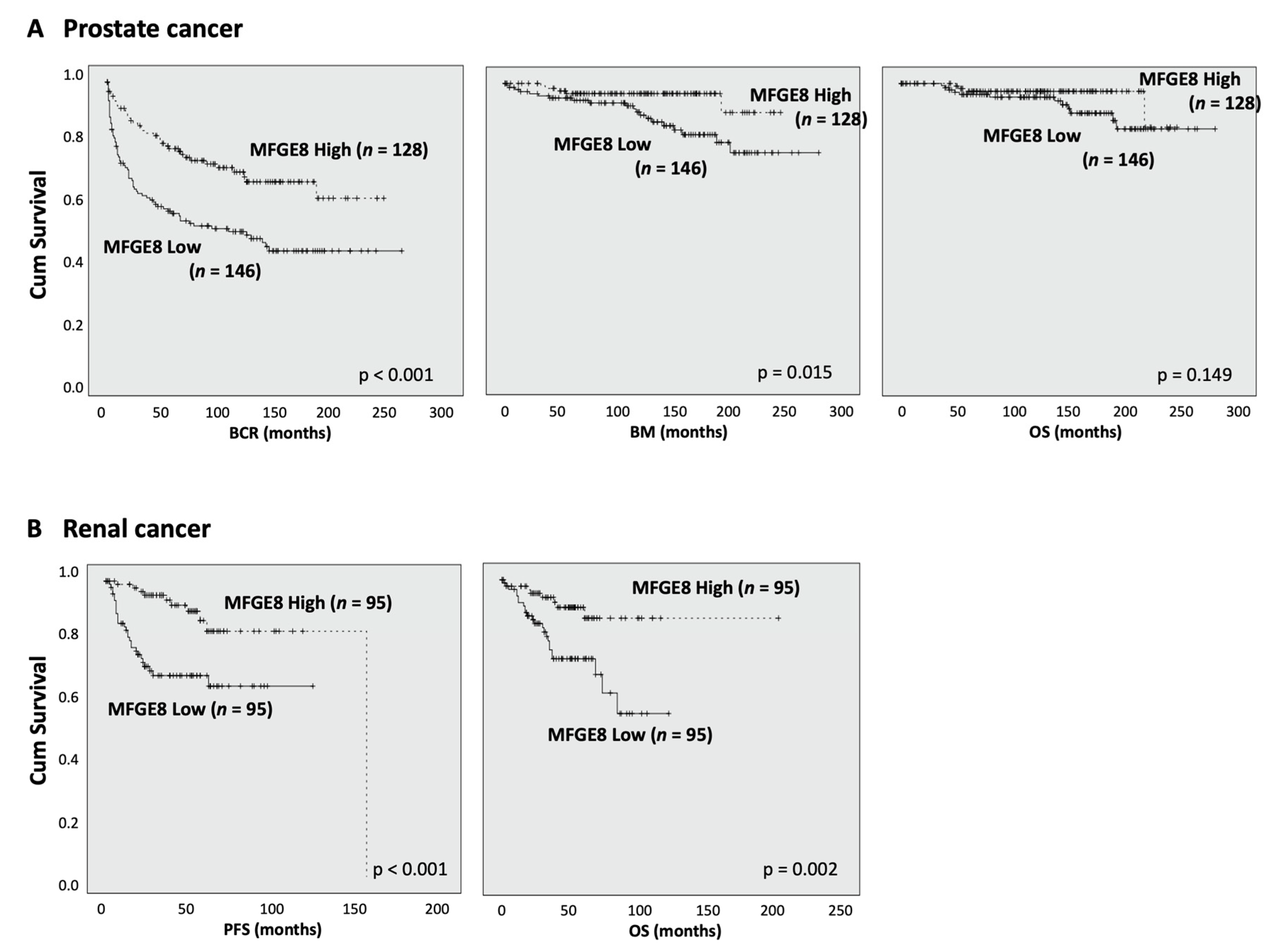

| Prostate Cancer Cohort | Characteristics | MFG-E8 Low | MFG-E8 High |
|---|---|---|---|
| Number of patients | 146 | 128 | |
| Diagnosis | Median age (years) | 63 (51–73) | 63 (47–74) |
| PSA pre-surgery | Median rate (ng/mL) | 7.1 (1.6–37) | 6.9 (2.4–40.5) |
| Margin | 54 | 38 | |
| Gleason score | 3 + 3 | 62 | 71 |
| 3 + 4 | 48 | 42 | |
| 4 + 3 | 11 | 8 | |
| 4 + 4 | 21 | 7 | |
| cTNM | 1 | 31 | 21 |
| 2 | 115 | 107 | |
| pTNM | 2 | 97 | 95 |
| 3 | 44 | 29 | |
| 4 | 5 | 4 | |
| Follow-up | 0–280 | 0–246 | |
| Progression time | BCR—Median (months) | 74 (55) | 37 (87) |
| BM—Median (months) | 20 (133) | 5 (122) | |
| Death rate | From prostatic cancer | 12 | 4 |
| From all causes | 26 | 20 | |
| Other | LNI | 5 | 4 |
| SVI | 8 | 8 | |
| Extra-prostatic extension | 48 | 30 | |
| CRPC | 21 | 4 |
| Renal Cancer Cohort | Characteristics | MFG-E8-Low | MFG-E8-High |
|---|---|---|---|
| Number of patients | 95 | 95 | |
| Sex | Women | 28 | 24 |
| Men | 67 | 71 | |
| Antecedents | Cancer in family | 16 | 14 |
| Renal cancer | 2 | 0 | |
| Laterality | Left | 47 | 42 |
| Right | 48 | 53 | |
| Histopathology | CCRC | 78 | 64 |
| PRCC | 10 | 24 | |
| CRCC | 7 | 7 | |
| Stages | 1 | 0 | 1 |
| 2 | 26 | 27 | |
| 3 | 45 | 42 | |
| 4 | 17 | 15 | |
| Follow-up | 0–123 | 0–204 | |
| Surgery | Complete | 51 | 44 |
| Partial | 39 | 50 | |
| Progression | Median (months) | 33 | 39 |
| Survival | OS—Median (months) | 42 | 42 |
| Other | Capsular invasion | 12 | 37 |
| Regional nodes | 0 | 9 | |
| Necrosis | 28 | 66 | |
| Pre-operative chemotherapy | 4 | 7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geoffroy, K.; Laplante, P.; Clairefond, S.; Azzi, F.; Trudel, D.; Lattouf, J.-B.; Stagg, J.; Saad, F.; Mes-Masson, A.-M.; Bourgeois-Daigneault, M.-C.; et al. High Levels of MFG-E8 Confer a Good Prognosis in Prostate and Renal Cancer Patients. Cancers 2022, 14, 2790. https://doi.org/10.3390/cancers14112790
Geoffroy K, Laplante P, Clairefond S, Azzi F, Trudel D, Lattouf J-B, Stagg J, Saad F, Mes-Masson A-M, Bourgeois-Daigneault M-C, et al. High Levels of MFG-E8 Confer a Good Prognosis in Prostate and Renal Cancer Patients. Cancers. 2022; 14(11):2790. https://doi.org/10.3390/cancers14112790
Chicago/Turabian StyleGeoffroy, Karen, Patrick Laplante, Sylvie Clairefond, Feryel Azzi, Dominique Trudel, Jean-Baptiste Lattouf, John Stagg, Fred Saad, Anne-Marie Mes-Masson, Marie-Claude Bourgeois-Daigneault, and et al. 2022. "High Levels of MFG-E8 Confer a Good Prognosis in Prostate and Renal Cancer Patients" Cancers 14, no. 11: 2790. https://doi.org/10.3390/cancers14112790
APA StyleGeoffroy, K., Laplante, P., Clairefond, S., Azzi, F., Trudel, D., Lattouf, J.-B., Stagg, J., Saad, F., Mes-Masson, A.-M., Bourgeois-Daigneault, M.-C., & Cailhier, J.-F. (2022). High Levels of MFG-E8 Confer a Good Prognosis in Prostate and Renal Cancer Patients. Cancers, 14(11), 2790. https://doi.org/10.3390/cancers14112790








