Daratumumab Improves Bone Turnover in Relapsed/Refractory Multiple Myeloma; Phase 2 Study “REBUILD”
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patient Selection
2.3. Study Endpoints
2.4. Evaluation of Bone Remodeling
2.5. Statistical Analysis
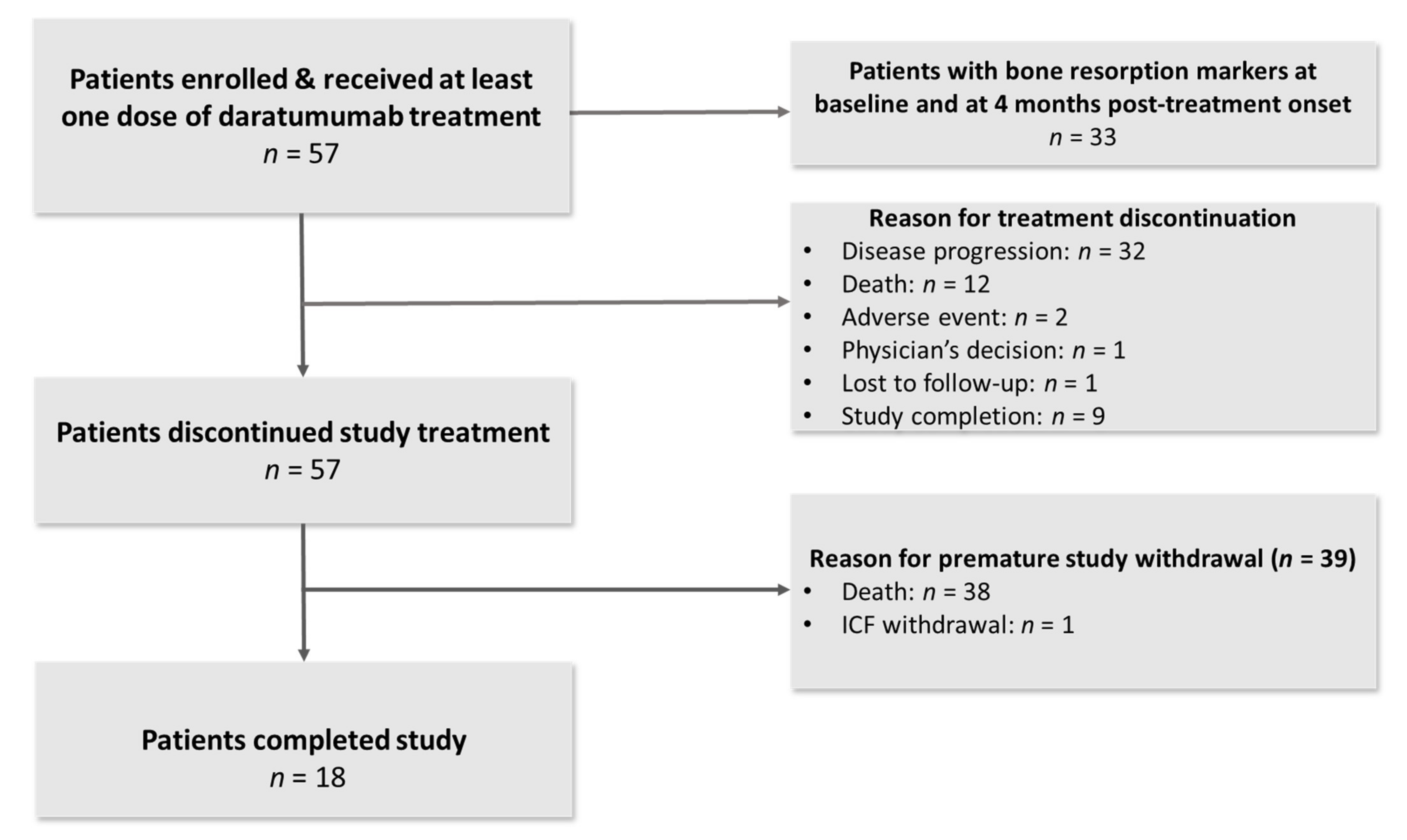
3. Results
3.1. Patient Characteristics
3.2. Impact of Daratumumab on Bone Metabolism
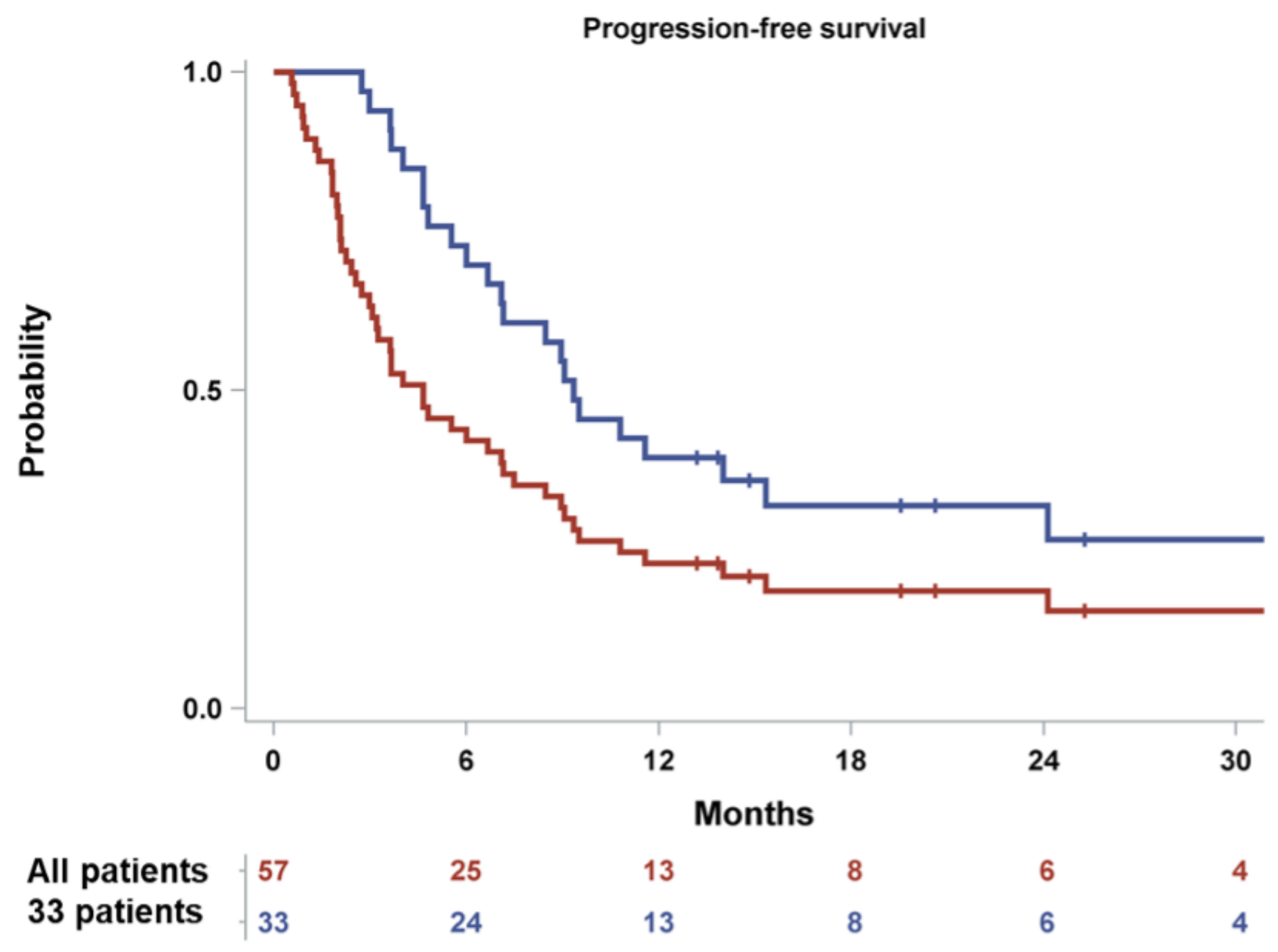
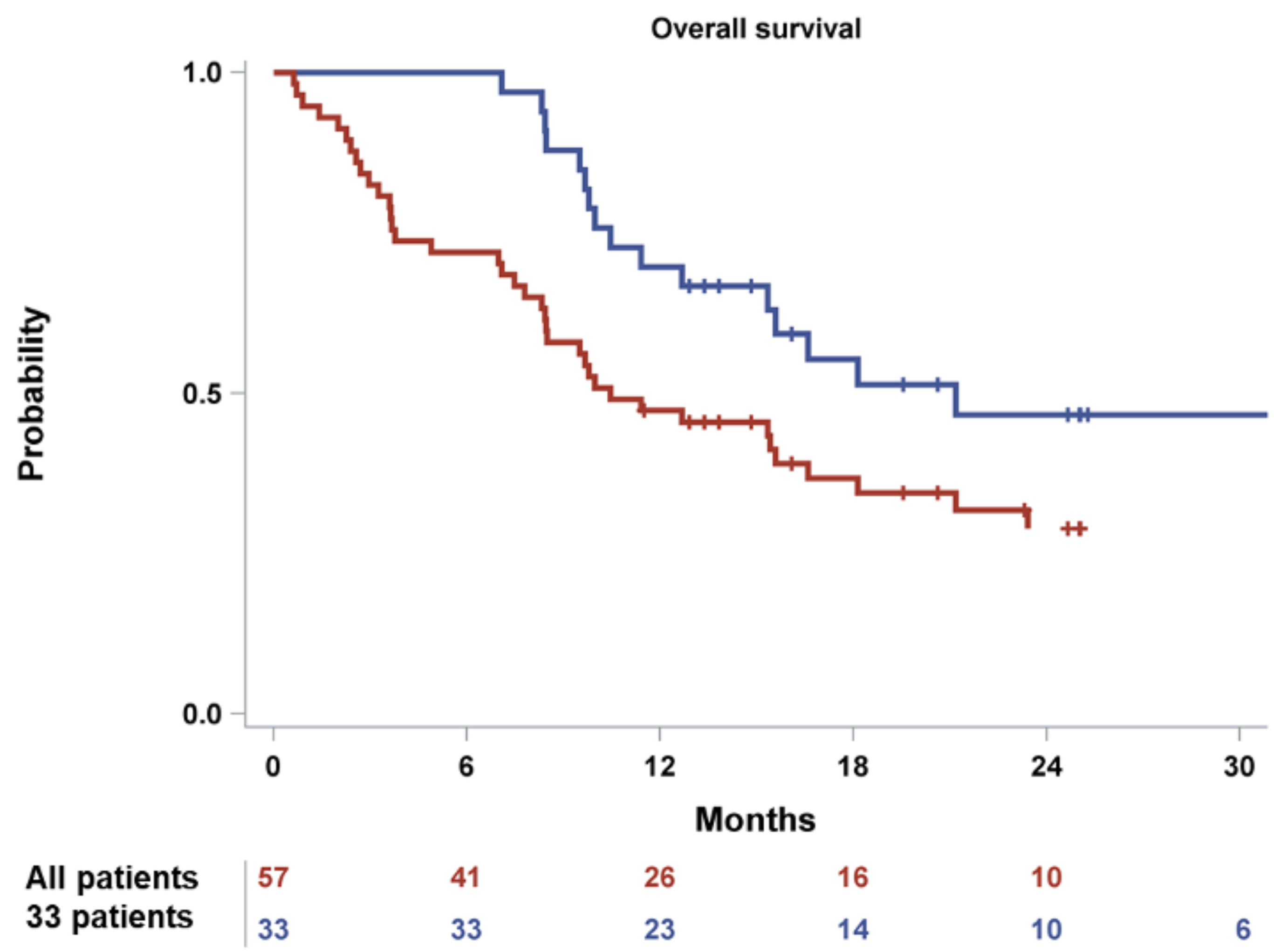
3.3. Efficacy and Survival
3.4. Safety
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Verelst, S.G.R.; Blommestein, H.M.; De Groot, S.; Gonzalez-McQuire, S.; DeCosta, L.; de Raad, J.B.; Uyl-de Groot, C.A.; Sonneveld, P. Long-term Outcomes in Patients With Multiple Myeloma: A Retrospective Analysis of the Dutch Population-based HAematological Registry for Observational Studies (PHAROS). Hemasphere 2018, 2, e45. [Google Scholar] [CrossRef] [PubMed]
- Terpos, E.; Ntanasis-Stathopoulos, I.; Dimopoulos, M.A. Myeloma bone disease: From biology findings to treatment approaches. Blood 2019, 133, 1534–1539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terpos, E.; Zamagni, E.; Lentzsch, S.; Drake, M.T.; Garcia-Sanz, R.; Abildgaard, N.; Ntanasis-Stathopoulos, I.; Schjesvold, F.; de la Rubia, J.; Kyriakou, C.; et al. Treatment of multiple myeloma-related bone disease: Recommendations from the Bone Working Group of the International Myeloma Working Group. Lancet Oncol. 2021, 22, e119–e130. [Google Scholar] [CrossRef]
- Mohty, M.; Malard, F.; Mohty, B.; Savani, B.; Moreau, P.; Terpos, E. The effects of bortezomib on bone disease in patients with multiple myeloma. Cancer 2014, 120, 618–623. [Google Scholar] [CrossRef] [Green Version]
- Terpos, E.; Ntanasis-Stathopoulos, I.; Katodritou, E.; Kyrtsonis, M.C.; Douka, V.; Spanoudakis, E.; Papatheodorou, A.; Eleutherakis-Papaiakovou, E.; Kanellias, N.; Gavriatopoulou, M.; et al. Carfilzomib Improves Bone Metabolism in Patients with Advanced Relapsed/Refractory Multiple Myeloma: Results of the CarMMa Study. Cancers 2021, 13, 1257. [Google Scholar] [CrossRef]
- Terpos, E.; Katodritou, E.; Symeonidis, A.; Zagouri, F.; Gerofotis, A.; Christopoulou, G.; Gavriatopoulou, M.; Christoulas, D.; Ntanasis-Stathopoulos, I.; Kourakli, A.; et al. Effect of induction therapy with lenalidomide, doxorubicin and dexamethasone on bone remodeling and angiogenesis in newly diagnosed multiple myeloma. Int. J. Cancer 2019, 145, 559–568. [Google Scholar] [CrossRef]
- Terpos, E.; Kastritis, E.; Ntanasis-Stathopoulos, I.; Christoulas, D.; Papatheodorou, A.; Eleutherakis-Papaiakovou, E.; Kanellias, N.; Fotiou, D.; Ziogas, D.C.; Migkou, M.; et al. Consolidation therapy with the combination of bortezomib and lenalidomide (VR) without dexamethasone in multiple myeloma patients after transplant: Effects on survival and bone outcomes in the absence of bisphosphonates. Am. J. Hematol. 2019, 94, 400–407. [Google Scholar] [CrossRef]
- Gavriatopoulou, M.; Terpos, E.; Ntanasis-Stathopoulos, I.; Malandrakis, P.; Eleutherakis-Papaiakovou, E.; Papatheodorou, A.; Kanellias, N.; Migkou, M.; Fotiou, D.; Dialoupi, I.; et al. Consolidation with carfilzomib, lenalidomide, and dexamethasone (KRd) following ASCT results in high rates of minimal residual disease negativity and improves bone metabolism, in the absence of bisphosphonates, among newly diagnosed patients with multiple myeloma. Blood Cancer J. 2020, 10, 25. [Google Scholar] [CrossRef]
- Bolzoni, M.; Toscani, D.; Storti, P.; Marchica, V.; Costa, F.; Giuliani, N. Possible targets to treat myeloma-related osteoclastogenesis. Expert Rev. Hematol. 2018, 11, 325–336. [Google Scholar] [CrossRef]
- Terpos, E.; Ntanasis-Stathopoulos, I. Controversies in the use of new bone-modifying therapies in multiple myeloma. Br. J. Haematol. 2021, 193, 1034–1043. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Moreau, P.; Terpos, E.; Mateos, M.V.; Zweegman, S.; Cook, G.; Delforge, M.; Hajek, R.; Schjesvold, F.; Cavo, M.; et al. Multiple Myeloma: EHA-ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-up. Hemasphere 2021, 5, e528. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Iqbal, J.; Dolgilevich, S.; Yuen, T.; Wu, X.B.; Moonga, B.S.; Adebanjo, O.A.; Bevis, P.J.; Lund, F.; Huang, C.L.; et al. Disordered osteoclast formation and function in a CD38 (ADP-ribosyl cyclase)-deficient mouse establishes an essential role for CD38 in bone resorption. FASEB J. 2003, 17, 369–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, F.; Toscani, D.; Chillemi, A.; Quarona, V.; Bolzoni, M.; Marchica, V.; Vescovini, R.; Mancini, C.; Martella, E.; Campanini, N.; et al. Expression of CD38 in myeloma bone niche: A rational basis for the use of anti-CD38 immunotherapy to inhibit osteoclast formation. Oncotarget 2017, 8, 56598–56611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajkumar, S.V.; Dimopoulos, M.A.; Palumbo, A.; Blade, J.; Merlini, G.; Mateos, M.V.; Kumar, S.; Hillengass, J.; Kastritis, E.; Richardson, P.; et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014, 15, e538–e548. [Google Scholar] [CrossRef]
- Kumar, S.; Paiva, B.; Anderson, K.C.; Durie, B.; Landgren, O.; Moreau, P.; Munshi, N.; Lonial, S.; Blade, J.; Mateos, M.V.; et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016, 17, e328–e346. [Google Scholar] [CrossRef]
- Krejcik, J.; Casneuf, T.; Nijhof, I.S.; Verbist, B.; Bald, J.; Plesner, T.; Syed, K.; Liu, K.; van de Donk, N.W.; Weiss, B.M.; et al. Daratumumab depletes CD38+ immune regulatory cells, promotes T-cell expansion, and skews T-cell repertoire in multiple myeloma. Blood 2016, 128, 384–394. [Google Scholar] [CrossRef] [Green Version]
- Van de Donk, N.; Richardson, P.G.; Malavasi, F. CD38 antibodies in multiple myeloma: Back to the future. Blood 2018, 131, 13–29. [Google Scholar] [CrossRef]
- Malavasi, F.; Deaglio, S.; Funaro, A.; Ferrero, E.; Horenstein, A.L.; Ortolan, E.; Vaisitti, T.; Aydin, S. Evolution and function of the ADP ribosyl cyclase/CD38 gene family in physiology and pathology. Physiol. Rev. 2008, 88, 841–886. [Google Scholar] [CrossRef] [Green Version]
- De Weers, M.; Tai, Y.T.; van der Veer, M.S.; Bakker, J.M.; Vink, T.; Jacobs, D.C.; Oomen, L.A.; Peipp, M.; Valerius, T.; Slootstra, J.W.; et al. Daratumumab, a novel therapeutic human CD38 monoclonal antibody, induces killing of multiple myeloma and other hematological tumors. J. Immunol. 2011, 186, 1840–1848. [Google Scholar] [CrossRef]
- Overdijk, M.B.; Verploegen, S.; Bogels, M.; van Egmond, M.; Lammerts van Bueren, J.J.; Mutis, T.; Groen, R.W.; Breij, E.; Martens, A.C.; Bleeker, W.K.; et al. Antibody-mediated phagocytosis contributes to the anti-tumor activity of the therapeutic antibody daratumumab in lymphoma and multiple myeloma. MAbs 2015, 7, 311–321. [Google Scholar] [CrossRef]
- Storti, P.; Vescovini, R.; Costa, F.; Marchica, V.; Toscani, D.; Dalla Palma, B.; Craviotto, L.; Malavasi, F.; Giuliani, N. CD14(+) CD16(+) monocytes are involved in daratumumab-mediated myeloma cells killing and in anti-CD47 therapeutic strategy. Br. J. Haematol. 2020, 190, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Terpos, E.; Szydlo, R.; Apperley, J.F.; Hatjiharissi, E.; Politou, M.; Meletis, J.; Viniou, N.; Yataganas, X.; Goldman, J.M.; Rahemtulla, A. Soluble receptor activator of nuclear factor kappaB ligand-osteoprotegerin ratio predicts survival in multiple myeloma: Proposal for a novel prognostic index. Blood 2003, 102, 1064–1069. [Google Scholar] [CrossRef] [PubMed]
- An, G.; Acharya, C.; Feng, X.; Wen, K.; Zhong, M.; Zhang, L.; Munshi, N.C.; Qiu, L.; Tai, Y.T.; Anderson, K.C. Osteoclasts promote immune suppressive microenvironment in multiple myeloma: Therapeutic implication. Blood 2016, 128, 1590–1603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hurchla, M.A.; Garcia-Gomez, A.; Hornick, M.C.; Ocio, E.M.; Li, A.; Blanco, J.F.; Collins, L.; Kirk, C.J.; Piwnica-Worms, D.; Vij, R.; et al. The epoxyketone-based proteasome inhibitors carfilzomib and orally bioavailable oprozomib have anti-resorptive and bone-anabolic activity in addition to anti-myeloma effects. Leukemia 2013, 27, 430–440. [Google Scholar] [CrossRef] [Green Version]
- Terpos, E.; Ntanasis-Stathopoulos, I.; Gavriatopoulou, M.; Dimopoulos, M.A. Pathogenesis of bone disease in multiple myeloma: From bench to bedside. Blood Cancer J. 2018, 8, 7. [Google Scholar] [CrossRef] [Green Version]
- Datta, H.K.; Ng, W.F.; Walker, J.A.; Tuck, S.P.; Varanasi, S.S. The cell biology of bone metabolism. J. Clin. Pathol. 2008, 61, 577–587. [Google Scholar] [CrossRef]
- Krishnan, V.; Bryant, H.U.; Macdougald, O.A. Regulation of bone mass by Wnt signaling. J. Clin. Investig. 2006, 116, 1202–1209. [Google Scholar] [CrossRef]
- Tian, E.; Zhan, F.; Walker, R.; Rasmussen, E.; Ma, Y.; Barlogie, B.; Shaughnessy, J.D., Jr. The role of the Wnt-signaling antagonist DKK1 in the development of osteolytic lesions in multiple myeloma. N. Engl. J. Med. 2003, 349, 2483–2494. [Google Scholar] [CrossRef]
- Politou, M.C.; Heath, D.J.; Rahemtulla, A.; Szydlo, R.; Anagnostopoulos, A.; Dimopoulos, M.A.; Croucher, P.I.; Terpos, E. Serum concentrations of Dickkopf-1 protein are increased in patients with multiple myeloma and reduced after autologous stem cell transplantation. Int. J. Cancer 2006, 119, 1728–1731. [Google Scholar] [CrossRef]
- Eda, H.; Santo, L.; Wein, M.N.; Hu, D.Z.; Cirstea, D.D.; Nemani, N.; Tai, Y.T.; Raines, S.E.; Kuhstoss, S.A.; Munshi, N.C.; et al. Regulation of Sclerostin Expression in Multiple Myeloma by Dkk-1: A Potential Therapeutic Strategy for Myeloma Bone Disease. J. Bone Miner. Res. 2016, 31, 1225–1234. [Google Scholar] [CrossRef] [Green Version]
- Brunetti, G.; Oranger, A.; Mori, G.; Specchia, G.; Rinaldi, E.; Curci, P.; Zallone, A.; Rizzi, R.; Grano, M.; Colucci, S. Sclerostin is overexpressed by plasma cells from multiple myeloma patients. Ann. N. Y. Acad. Sci. 2011, 1237, 19–23. [Google Scholar] [CrossRef]
- Delgado-Calle, J.; Sato, A.Y.; Bellido, T. Role and mechanism of action of sclerostin in bone. Bone 2017, 96, 29–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colucci, S.; Brunetti, G.; Oranger, A.; Mori, G.; Sardone, F.; Specchia, G.; Rinaldi, E.; Curci, P.; Liso, V.; Passeri, G.; et al. Myeloma cells suppress osteoblasts through sclerostin secretion. Blood Cancer J. 2011, 1, e27. [Google Scholar] [CrossRef] [PubMed]
- Terpos, E.; Christoulas, D.; Katodritou, E.; Bratengeier, C.; Gkotzamanidou, M.; Michalis, E.; Delimpasi, S.; Pouli, A.; Meletis, J.; Kastritis, E.; et al. Elevated circulating sclerostin correlates with advanced disease features and abnormal bone remodeling in symptomatic myeloma: Reduction post-bortezomib monotherapy. Int. J. Cancer 2012, 131, 1466–1471. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.T.; He, Y.C.; Zhou, S.Y.; Jiang, J.Z.; Huang, Y.M.; Liang, Y.Z.; Lai, Y.R. Bone marrow plasma macrophage inflammatory protein protein-1 alpha(MIP-1 alpha) and sclerostin in multiple myeloma: Relationship with bone disease and clinical characteristics. Leuk. Res. 2014, 38, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Ntanasis-Stathopoulos, I.; Fotiou, D.; Terpos, E. CCL3 Signaling in the Tumor Microenvironment. Adv. Exp. Med. Biol. 2020, 1231, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Terpos, E.; Politou, M.; Szydlo, R.; Goldman, J.M.; Apperley, J.F.; Rahemtulla, A. Serum levels of macrophage inflammatory protein-1 alpha (MIP-1alpha) correlate with the extent of bone disease and survival in patients with multiple myeloma. Br. J. Haematol. 2003, 123, 106–109. [Google Scholar] [CrossRef]
- Roussou, M.; Tasidou, A.; Dimopoulos, M.A.; Kastritis, E.; Migkou, M.; Christoulas, D.; Gavriatopoulou, M.; Zagouri, F.; Matsouka, C.; Anagnostou, D.; et al. Increased expression of macrophage inflammatory protein-1alpha on trephine biopsies correlates with extensive bone disease, increased angiogenesis and advanced stage in newly diagnosed patients with multiple myeloma. Leukemia 2009, 23, 2177–2181. [Google Scholar] [CrossRef] [Green Version]
- Vallet, S.; Pozzi, S.; Patel, K.; Vaghela, N.; Fulciniti, M.T.; Veiby, P.; Hideshima, T.; Santo, L.; Cirstea, D.; Scadden, D.T.; et al. A novel role for CCL3 (MIP-1alpha) in myeloma-induced bone disease via osteocalcin downregulation and inhibition of osteoblast function. Leukemia 2011, 25, 1174–1181. [Google Scholar] [CrossRef]
- Black, D.M.; Bauer, D.C.; Vittinghoff, E.; Lui, L.Y.; Grauer, A.; Marin, F.; Khosla, S.; de Papp, A.; Mitlak, B.; Cauley, J.A.; et al. Treatment-related changes in bone mineral density as a surrogate biomarker for fracture risk reduction: Meta-regression analyses of individual patient data from multiple randomised controlled trials. Lancet Diabetes Endocrinol. 2020, 8, 672–682. [Google Scholar] [CrossRef]
- Raje, N.; Terpos, E.; Willenbacher, W.; Shimizu, K.; Garcia-Sanz, R.; Durie, B.; Legiec, W.; Krejci, M.; Laribi, K.; Zhu, L.; et al. Denosumab versus zoledronic acid in bone disease treatment of newly diagnosed multiple myeloma: An international, double-blind, double-dummy, randomised, controlled, phase 3 study. Lancet Oncol. 2018, 19, 370–381. [Google Scholar] [CrossRef]
- Kim, C.; Bhatta, S.; Cyprien, L.; Fonseca, R.; Hernandez, R.K. Incidence of skeletal-related events among multiple myeloma patients in the United States at oncology clinics: Observations from real-world data. J. Bone Oncol. 2019, 14, 100215. [Google Scholar] [CrossRef]
- Jiang, Z.; Tang, E.T.; Li, C.; Zhu, L.; Zhang, B.; Glennane, T.; Zhang, L. What is the relationship between bone turnover markers and skeletal-related events in patients with bone metastases from solid tumors and in patients with multiple myeloma? A systematic review and meta-regression analysis. Bone Rep. 2020, 12, 100272. [Google Scholar] [CrossRef]
- Usmani, S.Z.; Weiss, B.M.; Plesner, T.; Bahlis, N.J.; Belch, A.; Lonial, S.; Lokhorst, H.M.; Voorhees, P.M.; Richardson, P.G.; Chari, A.; et al. Clinical efficacy of daratumumab monotherapy in patients with heavily pretreated relapsed or refractory multiple myeloma. Blood 2016, 128, 37–44. [Google Scholar] [CrossRef]
- Lonial, S.; Weiss, B.M.; Usmani, S.Z.; Singhal, S.; Chari, A.; Bahlis, N.J.; Belch, A.; Krishnan, A.; Vescio, R.A.; Mateos, M.V.; et al. Daratumumab monotherapy in patients with treatment-refractory multiple myeloma (SIRIUS): An open-label, randomised, phase 2 trial. Lancet 2016, 387, 1551–1560. [Google Scholar] [CrossRef]
- Usmani, S.Z.; Nahi, H.; Plesner, T.; Weiss, B.M.; Bahlis, N.J.; Belch, A.; Voorhees, P.M.; Laubach, J.P.; van de Donk, N.; Ahmadi, T.; et al. Daratumumab monotherapy in patients with heavily pretreated relapsed or refractory multiple myeloma: Final results from the phase 2 GEN501 and SIRIUS trials. Lancet Haematol. 2020, 7, e447–e455. [Google Scholar] [CrossRef]
- Lokhorst, H.M.; Plesner, T.; Laubach, J.P.; Nahi, H.; Gimsing, P.; Hansson, M.; Minnema, M.C.; Lassen, U.; Krejcik, J.; Palumbo, A.; et al. Targeting CD38 with Daratumumab Monotherapy in Multiple Myeloma. N. Engl. J. Med. 2015, 373, 1207–1219. [Google Scholar] [CrossRef] [PubMed]
| Variable | Patient Disposition |
|---|---|
| Male sex, n (%) | 26 (45.6%) |
| Karnofsky Performance Status, median (range) | 90 (70–100) |
| Caucasian race, n (%) | 57 (100%) |
| Age at enrolment, median (range), years | 73 (52–87) |
| Age at diagnosis, median (range), years | 68 (44–83) |
| Time from diagnosis to enrolment, median (Q1–Q3), years | 4.6 (2.9–7.7) |
| Number of prior lines of therapy, median (range) | 3 (2–9) |
| Prior ASCT, n (%) | 7 (12.3%) |
| Refractory to the last line of therapy, n (%) | 41 (71.9%) |
| Refractory to PI, n (%) | 37 (64.9%) |
| Refractory to IMiD, n (%) | 47 (82.5%) |
| Refractory to both PI and IMiD, n (%) | 36 (63.2%) |
| No Lytic Bone Lesions, n (%) | 12 (21.1%) |
| 1–3 Lytic Bone Lesions, n (%) | 6 (10.5%) |
| 4–10 Lytic Bone Lesions, n (%) | 9 (15.8%) |
| More than 10 Lytic Bone Lesions, n (%) | 30 (52.6%) |
| ISS stage I, n (%) | 13 (22.8%) |
| ISS stage II, n (%) | 24 (42.1%) |
| ISS stage III, n (%) | 20 (35.1%) |
| IgG Myeloma, n (%) | 29 (50.9%) |
| IgA Myeloma, n (%) | 9 (15.8%) |
| Kappa Light Chain Myeloma, n (%) | 8 (14.0%) |
| Lamda Light Chain Myeloma, n (%) | 8 (14.0%) |
| IgD Myeloma, n (%) | 1 (1.8%) |
| Biclonal Myeloma, n (%) | 1 (1.8%) |
| CTX (ng/mL), median (Q1, Q3) | 1.0 (0.4, 2.0) |
| TRACP-5B (U/L), median (Q1, Q3) | 1.9 (1.1, 2.8) |
| RANKL (pmol/L), median (Q1, Q3) | 0.1 (0.0, 0.2) |
| bALP (μg/L), median (Q1, Q3) | 8.5 (6.6, 11.2) |
| OC (ng/mL), median (Q1, Q3) | 2.7 (1.2, 6.3) |
| PINP (pg/mL), median (Q1, Q3) | 521.0 (307.1, 1071.2) |
| SOST (pmol/L), median (Q1, Q3) | 34.2 (21.0, 64.3) |
| Dkk1 (pmol/L), median (Q1, Q3) | 58.1 (36.0, 100.6) |
| CCL3 (ng/mL), median (Q1, Q3) | 35.4 (19.2, 57.5) |
| Baseline | 2 Months | 4 Months | 6 Months | 8 Months | 10 Months | 12 Months | |
|---|---|---|---|---|---|---|---|
| bALP (μg/L) | |||||||
| n | 56 | 43 | 33 | 24 | 18 | 14 | 14 |
| Median absolute change from baseline (Q1, Q3) | 2.1 (−0.7, 5.1) | 1.4 (−0.8, 3.6) | 1.7 (−1.8, 4.3) | 1.8 (−1.5, 5.2) | 2.0 (−0.5, 6.3) | 2.0 (−0.2, 4.8) | |
| p-value for absolute change a | 0.005 | 0.045 | 0.129 | 0.129 | 0.055 | 0.215 | |
| Osteocalcin (ng/mL) | |||||||
| n | 56 | 43 | 33 | 24 | 18 | 14 | 14 |
| Median absolute change from baseline (Q1, Q3) | 2.0 (−1.2, 6.7) | 1.5 (−1.3, 6.7) | 1.1 (−3.2, 6.3) | 4.2 (1.2, 8.7) | 3.1 (−0.1, 7.5) | 4.4 (0.3, 9.7) | |
| p-value for absolute change a | 0.023 | 0.061 | 0.039 | 0.001 | 0.014 | 0.008 | |
| PINP (pg/mL) | |||||||
| n | 56 | 43 | 33 | 24 | 18 | 14 | 14 |
| Median absolute change from baseline (Q1, Q3) | 35.2 (−167.3, 198.1) | 34.4 (−155.5, 225.8) | 82.7 (−98.8, 266.0) | 375.7 (36.0, 1785.0) | 149.5 (−103.8, 1880.4) | 376.5 (−80.7, 1539.3) | |
| p-value for absolute change a | 0.686 | 0.348 | 0.810 | 0.010 | 0.074 | 0.085 | |
| CTX (ng/mL) | |||||||
| n | 56 | 43 | 33 | 24 | 18 | 14 | 14 |
| Median absolute change from baseline (Q1, Q3) | −0.0 (−0.2, 0.3) | 0.0 (−0.2, 0.3) | 0.1 (−0.0, 0.5) | 0.0 (−0.3, 0.7) | −0.1 (−0.6, 0.2) | −0.1 (−1.1, 0.0) | |
| p-value for absolute change a | 0.728 | 0.695 | 0.990 | 0.522 | 0.179 | 0.060 | |
| TRACP-5B (U/L) | |||||||
| n | 56 | 43 | 33 | 24 | 18 | 14 | 14 |
| Median absolute change from baseline (Q1, Q3) | −0.2 (−0.7, 0.7) | −0.1 (−0.5, 0.7) | −0.1 (−0.9, 0.5) | 0.2 (−0.5, 1.0) | 0.3 (−0.6, 0.9) | 0.1 (−0.4, 0.6) | |
| p-value for absolute change a | 0.753 | 0.273 | 0.720 | 0.277 | 0.780 | 0.969 | |
| RANKL (pmol/L) | |||||||
| n | 56 | 43 | 33 | 24 | 18 | 14 | 14 |
| Median absolute change from baseline (Q1, Q3) | 0.0 (−0.0, 0.1) | 0.0 (−0.0, 0.1) | 0.0 (−0.0, 0.1) | 0.0 (−0.0, 0.2) | 0.0 (0.0, 0.1) | 0.1 (0.0, 0.2) | |
| p-value for absolute change a | 0.089 | 0.149 | 0.268 | 0.111 | 0.201 | 0.028 | |
| RANKL/OPG ratio | |||||||
| n | 56 | 43 | 33 | 24 | 18 | 14 | 14 |
| Median absolute change from baseline (Q1, Q3) | 0.0 (−0.0, 0.0) | 0.0 (−0.0, 0.0) | 0.0 (−0.0, 0.0) | 0.0 (−0.0, 0.0) | 0.0 (−0.0, 0.0) | 0.0 (−0.0, 0.0) | |
| p-value for absolute change a | 0.112 | 0.269 | 0.275 | 0.327 | 0.204 | 0.085 | |
| SOST (pmol/L) | |||||||
| n | 56 | 43 | 33 | 24 | 18 | 14 | 14 |
| Median absolute change from baseline (Q1, Q3) | −1.2 (−12.8, 6.0) | 2.8 (−13.9, 16.5) | −6.3 (−26.5, 8.0) | −4.1 (−42.1, 1.5) | 0.5 (−40.4, 19.5) | −4.0 (−33.4, 52.0) | |
| p-value for absolute change a | 0.971 | 0.363 | 0.878 | 0.836 | 0.877 | 0.589 | |
| DKK1 (pmol/L) | |||||||
| n | 56 | 43 | 33 | 24 | 18 | 14 | 14 |
| Median absolute change from baseline (Q1, Q3) | −7.9 (−21.2, −1.5) | −8.7 (−29.2, −2.1) | −11.9 (−24.4, −2.4) | −13.7 (−25.7, −6.5) | −16.2 (−36.8, −9.7) | −17.0 (−33.6, −11.1) | |
| p-value for absolute change a | 0.079 | 0.191 | 0.042 | 0.049 | 0.006 | 0.002 | |
| CCL3 (ng/mL) | |||||||
| n | 56 | 43 | 33 | 24 | 18 | 14 | 14 |
| Median absolute change from baseline (Q1, Q3) | −2.4 (−6.0, 9.3) | −3.6 (−13.7, 1.7) | −5.7 (−28.2, 0.8) | −7.8 (−32.7, 5.3) | −10.4 (−25.9, 3.0) | −8.3 (−24.8, −0.7) | |
| p-value for absolute change a | 0.366 | 0.068 | 0.021 | 0.007 | 0.008 | 0.006 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terpos, E.; Ntanasis-Stathopoulos, I.; Kastritis, E.; Hatjiharissi, E.; Katodritou, E.; Eleutherakis-Papaiakovou, E.; Verrou, E.; Gavriatopoulou, M.; Leonidakis, A.; Manousou, K.; et al. Daratumumab Improves Bone Turnover in Relapsed/Refractory Multiple Myeloma; Phase 2 Study “REBUILD”. Cancers 2022, 14, 2768. https://doi.org/10.3390/cancers14112768
Terpos E, Ntanasis-Stathopoulos I, Kastritis E, Hatjiharissi E, Katodritou E, Eleutherakis-Papaiakovou E, Verrou E, Gavriatopoulou M, Leonidakis A, Manousou K, et al. Daratumumab Improves Bone Turnover in Relapsed/Refractory Multiple Myeloma; Phase 2 Study “REBUILD”. Cancers. 2022; 14(11):2768. https://doi.org/10.3390/cancers14112768
Chicago/Turabian StyleTerpos, Evangelos, Ioannis Ntanasis-Stathopoulos, Efstathios Kastritis, Evdoxia Hatjiharissi, Eirini Katodritou, Evangelos Eleutherakis-Papaiakovou, Evgenia Verrou, Maria Gavriatopoulou, Alexandros Leonidakis, Kyriaki Manousou, and et al. 2022. "Daratumumab Improves Bone Turnover in Relapsed/Refractory Multiple Myeloma; Phase 2 Study “REBUILD”" Cancers 14, no. 11: 2768. https://doi.org/10.3390/cancers14112768
APA StyleTerpos, E., Ntanasis-Stathopoulos, I., Kastritis, E., Hatjiharissi, E., Katodritou, E., Eleutherakis-Papaiakovou, E., Verrou, E., Gavriatopoulou, M., Leonidakis, A., Manousou, K., Delimpasi, S., Malandrakis, P., Kyrtsonis, M.-C., Papaioannou, M., Symeonidis, A., & Dimopoulos, M.-A. (2022). Daratumumab Improves Bone Turnover in Relapsed/Refractory Multiple Myeloma; Phase 2 Study “REBUILD”. Cancers, 14(11), 2768. https://doi.org/10.3390/cancers14112768









