Are Mutation Carrier Patients Different from Non-Carrier Patients? Genetic, Pathology, and US Features of Patients with Breast Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Study Population
2.2. Imaging Technique
2.3. Pathologic and Genetic Data
2.4. Statistical Analysis
3. Results
3.1. Associations between Clinico-Pathological Data and Mutation Status
3.2. Associations between US Features and Mutation Status
3.3. Associations between US Features and Specific Pathogenic Mutations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BC | breast cancer |
| US | ultrasound |
| BI-RADS | breast imaging and reporting data system |
| ER | estrogen receptor |
| “−” | negative |
| “+” | positive |
| PR | progesterone receptor |
| Ki67% | proliferative index |
| SE | strain elastography |
| ACR | American College of Radiology |
| VUS | variants of uncertain significance |
| BGR | blue-green-red elastography appearance |
References
- Hu, C.; Hart, S.N.; Gnanaolivu, R.; Huang, H.; Lee, K.Y.; Na, J.; Gao, C.; Lilyquist, J.; Yadav, S.; Boddicker, N.J.; et al. A Population-Based Study of Genes Previously Implicated in Breast Cancer. N. Engl. J. Med. 2021, 384, 440–451. [Google Scholar] [CrossRef] [PubMed]
- Filippini, S.E.; Vega, A. Breast cancer genes: Beyond BRCA1 and BRCA2. Front. Biosci. 2013, 1, 1358–1372. [Google Scholar]
- Iannuzzi, C.M.; Atencio, D.P.; Green, S.; Stock, R.G.; Rosenstein, B.S. ATM mutations in female breast cancer patients predict for an increase in radiation-induced late effects. Int. J. Radiat. Oncol. 2002, 52, 606–613. [Google Scholar] [CrossRef]
- Knappskog, S.; Chrisanthar, R.; Løkkevik, E.; Anker, G.; Østenstad, B.; Lundgren, S.; Risberg, T.; Mjaaland, I.; Leirvaag, B.; Miletic, H.; et al. Low expression levels of ATM may substitute for CHEK2/TP53 mutations predicting resistance towards anthracycline and mitomycin chemotherapy in breast cancer. Breast Cancer Res. 2012, 14, R47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- NCCN Guidelines Version 1.2020. Breast Cancer Risk Reduction. Available online: https://www2.tri-kobe.org/nccn/guideline/breast/english/breast_risk.pdf (accessed on 1 August 2021).
- Manahan, E.R.; Kuerer, H.M.; Sebastian, M.; Hughes, K.S.; Boughey, J.C.; Euhus, D.M.; Boolbol, S.K.; Taylor, W.A. Consensus Guidelines on Genetic’ Testing for Hereditary Breast Cancer from the American Society of Breast Surgeons. Ann. Surg. Oncol. 2019, 26, 3025–3031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Orsi, C.J.; Sickles, E.A.; Mendelson, E.B.; Morris, A.; Creech, E.W.; Butler, F.P.; Wiegmann, P.G.; Chatfield, B.M.; Meyer, W.L.; Wilcox, A.P. ACR BI-RADS Atlas, Breast Imaging Reporting and Data System, 5th ed.; American College of Radiology: Reston, VA, USA, 2013. [Google Scholar]
- Mainiero, M.B.; Lourenco, A.; Mahoney, M.C.; Newell, M.S.; Bailey, L.; Barke, L.D.; D’Orsi, C.; Harvey, J.A.; Hayes, M.K.; Huynh, P.T.; et al. ACR Appropriateness Criteria—Breast Cancer Screening. J. Am. Coll. Radiol. 2016, 13, R45–R49. [Google Scholar] [CrossRef] [PubMed]
- Schrading, S.; Kuhl, C.K. Mammographic, US, and MR imaging phenotypes of familial breast cancer. Radiology 2008, 246, 58–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veltman, J.; Mann, R.; Kok, T.; Obdeijn, I.M.; Hoogerbrugge, N.; Blickman, J.G.; Boetes, C. Breast tumor characteristics of BRCA1 and BRCA2 gene mutation carriers on MRI. Eur. Radiol. 2008, 18, 931–938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ha, S.M.; Chae, E.Y.; Cha, J.H.; Kim, H.H.; Shin, H.J.; Choi, W.J. Association of BRCA Mutation Types, Imaging Features, and Pathologic Findings in Patients With Breast Cancer With BRCA1 and BRCA2 Mutations. Am. J. Roentgenol. 2017, 209, 920–928. [Google Scholar] [CrossRef] [PubMed]
- Kuhl, C.K.; Schmutzler, R.K.; Leutner, C.C.; Kempe, A.; Wardelmann, E.; Hocke, A.; Maringa, M.; Pfeifer, U.; Krebs, D.; Schild, H.H. Breast MR Imaging Screening in 192 Women Proved or Suspected to Be Carriers of a Breast Cancer Susceptibility Gene: Preliminary Results. Radiology 2000, 215, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Marino, M.A.; Riedl, C.C.; Bernathova, M.; Bernhart, C.; Baltzer, P.A.; Helbich, T.H.; Pinker, K. Imaging Phenotypes in Women at High Risk for Breast Cancer on Mammography, Ultrasound, and Magnetic Resonance Imaging Using the Fifth Edition of the Breast Imaging Reporting and Data System. Eur. J. Radiol. 2018, 106, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Blaichman, J.; Marcus, J.C.; Alsaadi, T.; El-Khoury, M.; Meterissian, S.; Mesurolle, B. Sonographic Appearance of Invasive Ductal Carcinoma of the Breast According to Histologic Grade. Am. J. Roentgenol. 2012, 199, W402–W408. [Google Scholar] [CrossRef] [PubMed]
- Mercado, C.L.; Naidrich, S.A.; Hamele-Bena, D.; Fineberg, S.A.; Buchbinder, S.S. Pseudoangiomatous Stromal Hyperplasia of the Breast: Sonographic Features with Histopathologic Correlation. Breast J. 2004, 10, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Noh, J.M.; Han, B.-K.; Choi, D.H.; Rhee, S.J.; Cho, E.Y.; Huh, S.J.; Park, W.; Park, H.; Nam, S.J.; Lee, J.E.; et al. Association betweenBRCAMutation Status, Pathological Findings, and Magnetic Resonance Imaging Features in Patients with Breast Cancer at Risk for the Mutation. J. Breast Cancer 2013, 16, 308–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, J.; Fenghua, L.; Jing, D.; Yifen, G. Strain elastography features in invasive breast cancer: Relationship between stiffness and pathological factors. Int. J. Clin. Exp. Med. 2017, 10, 13290–13297. [Google Scholar]
- Ganau, S.; Andreu, F.J.; Escribano, F.; Martín, A.; Tortajada, L.; Villajos, M.; Baré, M.; Teixidó, M.; Ribé, J.; Sentís, M. Shear-wave elastography and immunohistochemical profiles in invasive breast cancer: Evaluation of maximum and mean elasticity values. Eur. J. Radiol. 2015, 84, 617–622. [Google Scholar] [CrossRef] [PubMed]
- Chiorean, A.; Duma, M.; Dudea, S.; Bolboaca, S.; Dumitriu, D.; Eniu, D.; Sfrangeu, S. Typical and Unusual Sonoelastographic Patterns of Breast Cystic Lesions: Impact on BI-RADS Classification. Ultraschall Med. Eur. J. Ultrasound 2010, 33, E138–E144. [Google Scholar] [CrossRef] [PubMed]
- Pintican, R.; Duma, M.; Chiorean, A.; Fetica, B.; Badan, M.; Bura, V.; Szep, M.; Feier, D.; Dudea, S. Mucinous versus medullary breast carcinoma: Mammography, ultrasound, and MRI findings. Clin. Radiol. 2020, 75, 483–496. [Google Scholar] [CrossRef] [PubMed]
- Pintican, R.; Duma, M.M.; Szep, M.; Feier, D.; Eniu, D.; Goidescu, I.; Chiorean, A. The Role of US in Depicting Axillary Metastasis in High-Risk Breast Cancer Patients. J. Pers. Med. 2021, 11, 1379. [Google Scholar] [CrossRef] [PubMed]

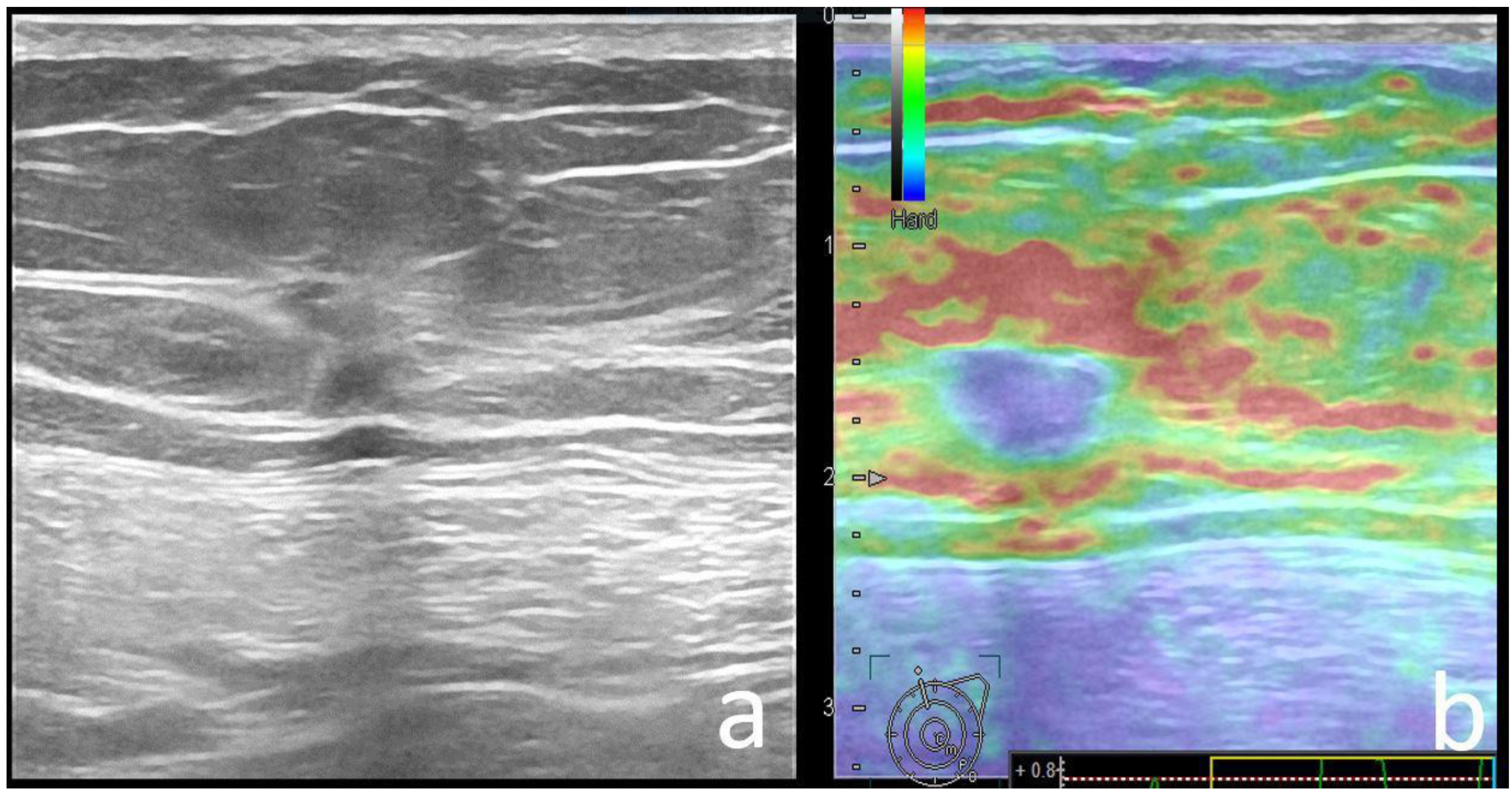
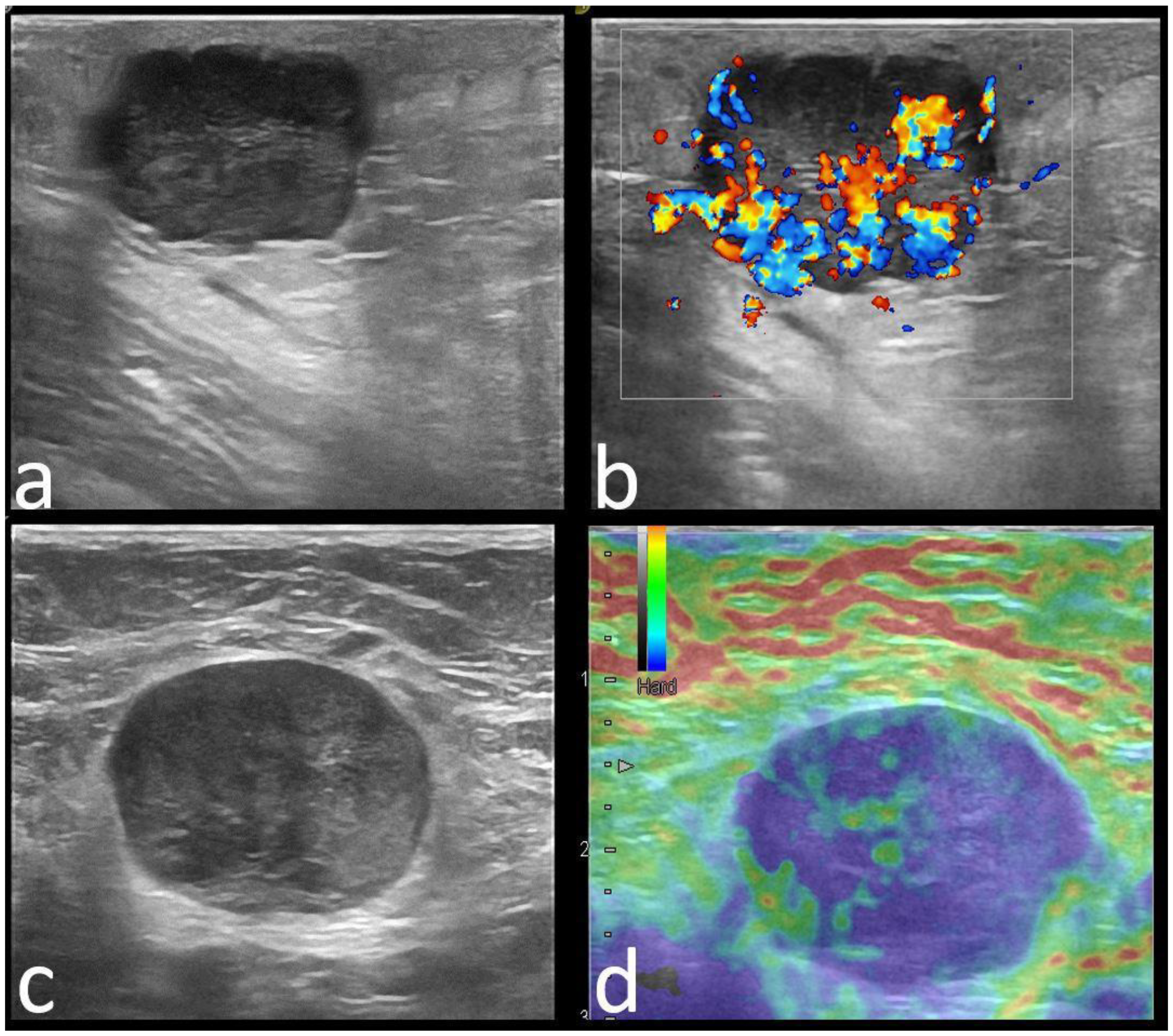


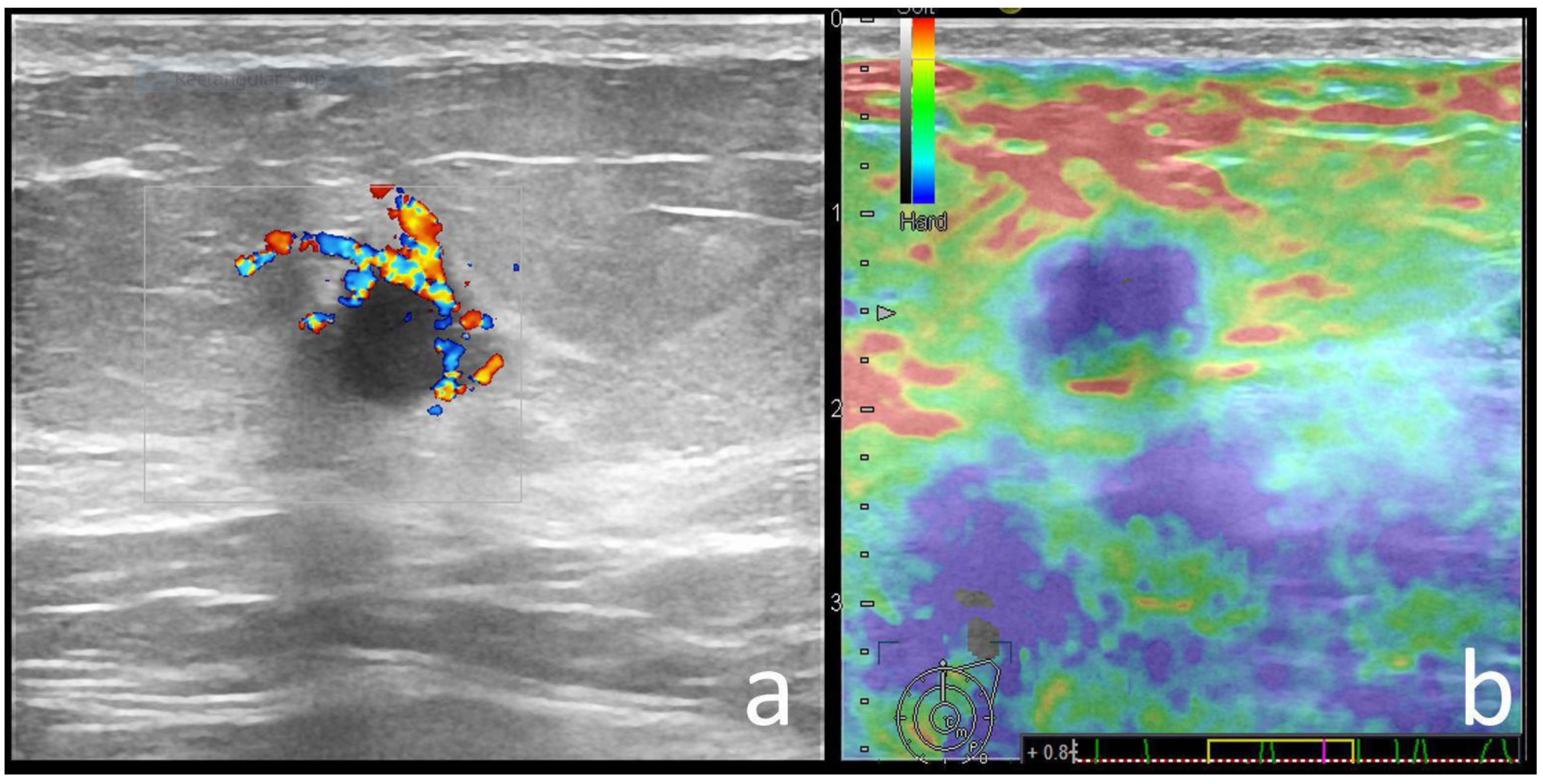
| Variable | Pathogenic Carrier Group | Mutation-Negative Group | p-Value |
|---|---|---|---|
| Patient age (y), mean (range) | 43.5 (30–67) | 44 (24–73) | 0.644 |
| Patient origin | 0.957 | ||
| Urban | 80 (81.6) | 120 (82.8) | |
| Rural | 18 (18.4) | 25 (17.2) | |
| Symptoms | 0.424 | ||
| Absent | 51 (52) | 83 (57) | |
| Present | 47 (48) | 62 (43) | |
| Breast cancer type | |||
| Invasive ductal carcinoma NST | 88 (89.8) | 120 (82.8) | 0.178 |
| Other * | 10 (10.2) | 25 (17.2) | |
| “In Situ” component | 13 (13.3) | 29 (20) | 0.234 |
| Number of tumors | 0.032 | ||
| Unifocal | 61 (62.2) | 67 (46.2) | |
| Multifocal | 14 (14.3) | 37 (25.5) | |
| Multicentric | 23 (23.5) | 41 (28.3) | |
| Tumor size (mm), mean (range) | 0.884 | ||
| <2 cm | 24 (24.5) | 29 (20) | |
| >2 cm | 74 (75.5) | 116 (80) | |
| Lymph node status | 0.329 | ||
| Negative | 45 (46.9) | 78 (54.2) | |
| Positive | 51 (53.1) | 66 (45.8) | |
| Histologic grade | 0.000 | ||
| Low | 6 (6.1) | 33 (22.8) | |
| Intermediate | 49 (50) | 80 (55.2) | |
| High | 43 (43.9) | 32 (22.1) | |
| Lympho-vascular invasion | 0.927 | ||
| Absent | 72 (73.5) | 105 (72.4) | |
| Present | 26 (26.5) | 40 (27.6) | |
| Immunohistochemistry | |||
| ER+ | 68 (69.4) | 110 (75.8) | 0.3 |
| ER− | 30 (30.6) | 35 (24.1) | |
| HER2+ | 21 (21.4) | 23 (15.8) | |
| HER2− | 77 (78.5) | 122 (84.1) | 0.3 |
| Ki-67% status | 0.001 | ||
| >20% | 77 (78.5) | 84 (60) | |
| <20% | 21 (21.4) | 61 (42) | |
| TNM Stage | 0.002 | ||
| 0 | 4 (4.1) | 3 (2.1) | |
| I | 10 (10.2) | 21 (14.5) | |
| IIA | 38 (38.7) | 59 (40.7) | |
| IIB | 22 (22.4) | 30 (20.7) | |
| IIIA | 15 (15.3) | 27 (18.6) | |
| IIIC | 8 (8.2) | 3 (2.1) | |
| IV | 1 (1) | 2 (1.4) | |
| Variants of uncertain significance (VUS) | 0.000 | ||
| Yes | 24 a (24.4) | 4 (2.7) | |
| No | 74 (75.5) | 141 (97.2) |
| US Feature | Pathogenic Carrier Group | Negative, Non-Carrier Group | p-Value |
|---|---|---|---|
| Lesion type | 0.107 | ||
| Mass | 91 (93) | 141 (97) | |
| Non-mass | 7 (7) | 4 (3) | |
| Shape | 0.391 | ||
| Round | 6 (6.1) | 14 (9.7) | |
| Oval | 27 (27.6) | 31 (21.4) | |
| Irregular | 65 (66.3) | 100 (69) | |
| Orientation | 0.861 | ||
| Parallel | 44 (44.9) | 68 (46.9) | |
| Non-parallel | 54 (55.1) | 77 (53.1) | |
| Margins | |||
| Circumscribed | 22 (22.4) | 44 (30.3) | 0.226 |
| Non-circumscribed | |||
| Spiculated | 19 (19.4) | 46 (31.7) | 0.047 |
| Indistinct | |||
| Angular | 21 (21.4) | 19 (13.1) | 0.263 |
| Microlobulated | 10 (10.2) | 8 (5.5) | |
| Echo pattern | 0.000 | ||
| Hypoechoic | 57 (58.2) | 100 (70) | |
| Heterogeneous | 36 (36.7) | 19 (13) | |
| Isoechoic | 5 (5.1) | 26 (17) | |
| Posterior features | 0.000 | ||
| None | 39 (39.8) | 71 (49) | |
| Enhancement | 27 (27.6) | 12 (8.3) | |
| Shadowing | 15 (15.3) | 54 (37.2) | |
| Combined | 17 (17.3) | 8 (5.5) | |
| Calcifications | 0.001 | ||
| Absent | 63 (64.3) | 122 (84.1) | |
| Present * | 35 (35.7) | 23 (15.9) | |
| A | 30 (30.6) | 15 (10.3) | |
| B | 3 (3.1) | 2 (2.1) | |
| D | 2 (2) | 5 (3.4) | |
| Associated features | 0.001 | ||
| None | 59 (60.2) | 115 (79.3) | |
| Hyperechoic Rim | 11 (11.2) | 8 (5.5) | |
| Duct ectasia | 10 (10.2) | 16 (11) | |
| Distortion | 6 (4.1) | 18 (18.4) | |
| Color Doppler signal | 0.696 | ||
| Absent | 9 (9.3) | 17 (11.7) | |
| Present | 88 (90.7) | 128 (88.3) | |
| Strain Elastography | 0.029 | ||
| Soft | 40 (40), 9 BGR | 30 (20.6), 1 BGR | |
| Hard | 58 (60) | 115 (79.3) | |
| BI-RADS | 0.799 | ||
| 4 | 12 (12.2) | 15 (10) | |
| 5 | 86 (87) | 130 (90) | |
| Axillary US | 0.026 | ||
| Negative | 61 (62.2) | 68 (46.9) | |
| Positive | 37 (37.8) | 77 (53.1) |
| Variable | BRCA1 | BRCA2 | CHEK2 | RAD Group * | PALB | NBN | TP 53 | ATM |
|---|---|---|---|---|---|---|---|---|
| No. of patients (%) | 29 (29.5) | 15 (15.3) | 15 (15.3) | 15 (15.3) | 7 (7.1) | 6 (6.1) | 3 (3) | 3 (3) |
| Breast cancer type | IDC-NST ** | IDC-NST | IDC-NST | IDC-NST | IDC-NST | IDC-NST | IDC-NST | IDC-NST |
| Molecular sub-type (%) | ER− (69) | ER+ (100) | ER+ (100) | ER+ (66) ER− (33) | ER+ (85) | ER+ (100) | ER− (100) | ER+ (100) |
| US features (+/No. of patients) Orientation | NP (17/29) | NP (9/15) | NP (10/15) | P (7/15) | P (4/7) | P (4/6) | P (2/3) | P (2/3) |
| Margins | C (20/29) | NC (11/15) | NC (11/15) | NC (11/15) | C (5/7) | NC (5/6) | C (3/3) | NC (3/3) |
| Echo pattern | Hypoechoic (19/29) | Hypoechoic (9/15) | Heterogeneous (5/15) | Hypoechoic (11/15) | Hypoechoic (4/7) | Heterogenous (3/3) | - | Hypoechoic (2/3) |
| Posterior features | Enhancement (16/29) | Absent (9/15) | Shadowing (6/15) | Enhancement (6/15)/Combined pattern (5/15) | No posterior (4/7) | - | Enhancement (2/3) | - |
| Associated features | Hyperechoic rim (6/29) Soft elastography (11/29) | Calcifications (7/15) Hard elastography (12/15) | Calcifications (7/15) | - | - | Calcifications (3/6) Hyperechoic rim (3/6) | - | Calcifications (2/3) Architectural distortion (2/3) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pintican, R.M.; Chiorean, A.; Duma, M.; Feier, D.; Szep, M.; Eniu, D.; Goidescu, I.; Dudea, S. Are Mutation Carrier Patients Different from Non-Carrier Patients? Genetic, Pathology, and US Features of Patients with Breast Cancer. Cancers 2022, 14, 2759. https://doi.org/10.3390/cancers14112759
Pintican RM, Chiorean A, Duma M, Feier D, Szep M, Eniu D, Goidescu I, Dudea S. Are Mutation Carrier Patients Different from Non-Carrier Patients? Genetic, Pathology, and US Features of Patients with Breast Cancer. Cancers. 2022; 14(11):2759. https://doi.org/10.3390/cancers14112759
Chicago/Turabian StylePintican, Roxana Maria, Angelica Chiorean, Magdalena Duma, Diana Feier, Madalina Szep, Dan Eniu, Iulian Goidescu, and Sorin Dudea. 2022. "Are Mutation Carrier Patients Different from Non-Carrier Patients? Genetic, Pathology, and US Features of Patients with Breast Cancer" Cancers 14, no. 11: 2759. https://doi.org/10.3390/cancers14112759
APA StylePintican, R. M., Chiorean, A., Duma, M., Feier, D., Szep, M., Eniu, D., Goidescu, I., & Dudea, S. (2022). Are Mutation Carrier Patients Different from Non-Carrier Patients? Genetic, Pathology, and US Features of Patients with Breast Cancer. Cancers, 14(11), 2759. https://doi.org/10.3390/cancers14112759







