Comparative Pathobiology of Canine and Human Prostate Cancer: State of the Art and Future Directions
Abstract
Simple Summary
Abstract
1. Introduction
2. History and Epidemiological Aspects of Prostate Cancer
3. Preneoplastic Prostatic Lesions
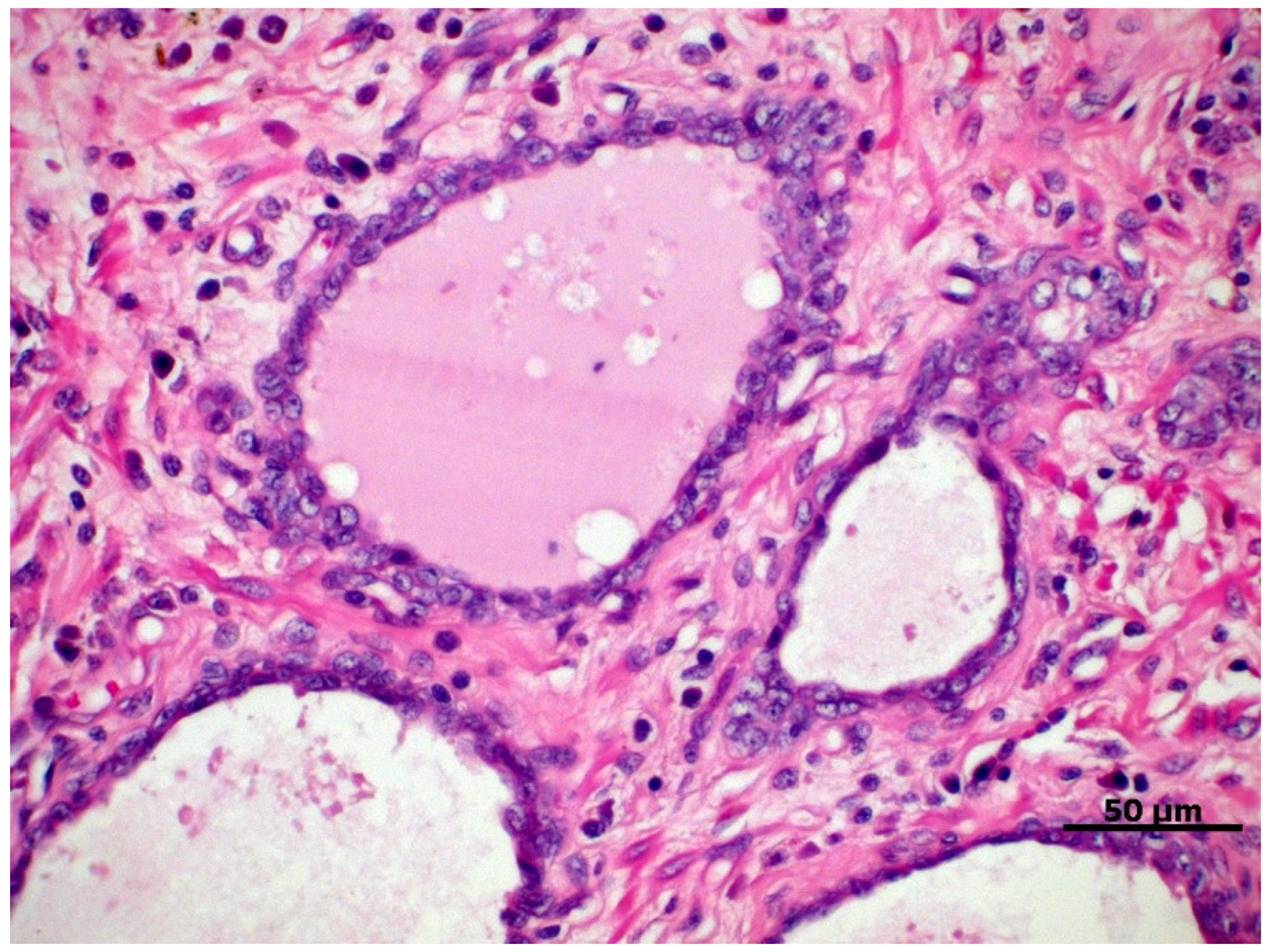
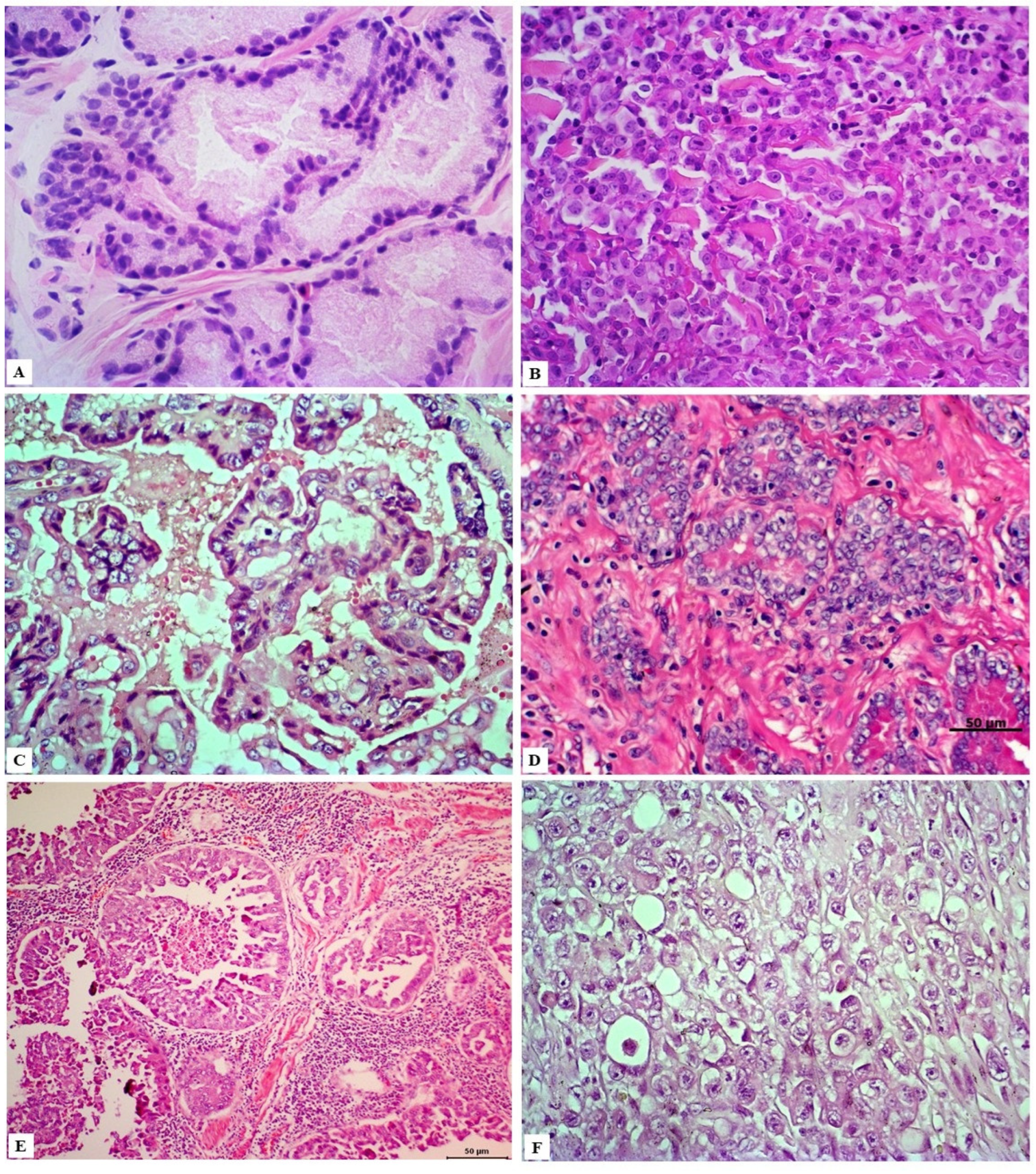
4. Classification and Histological Grading of PC
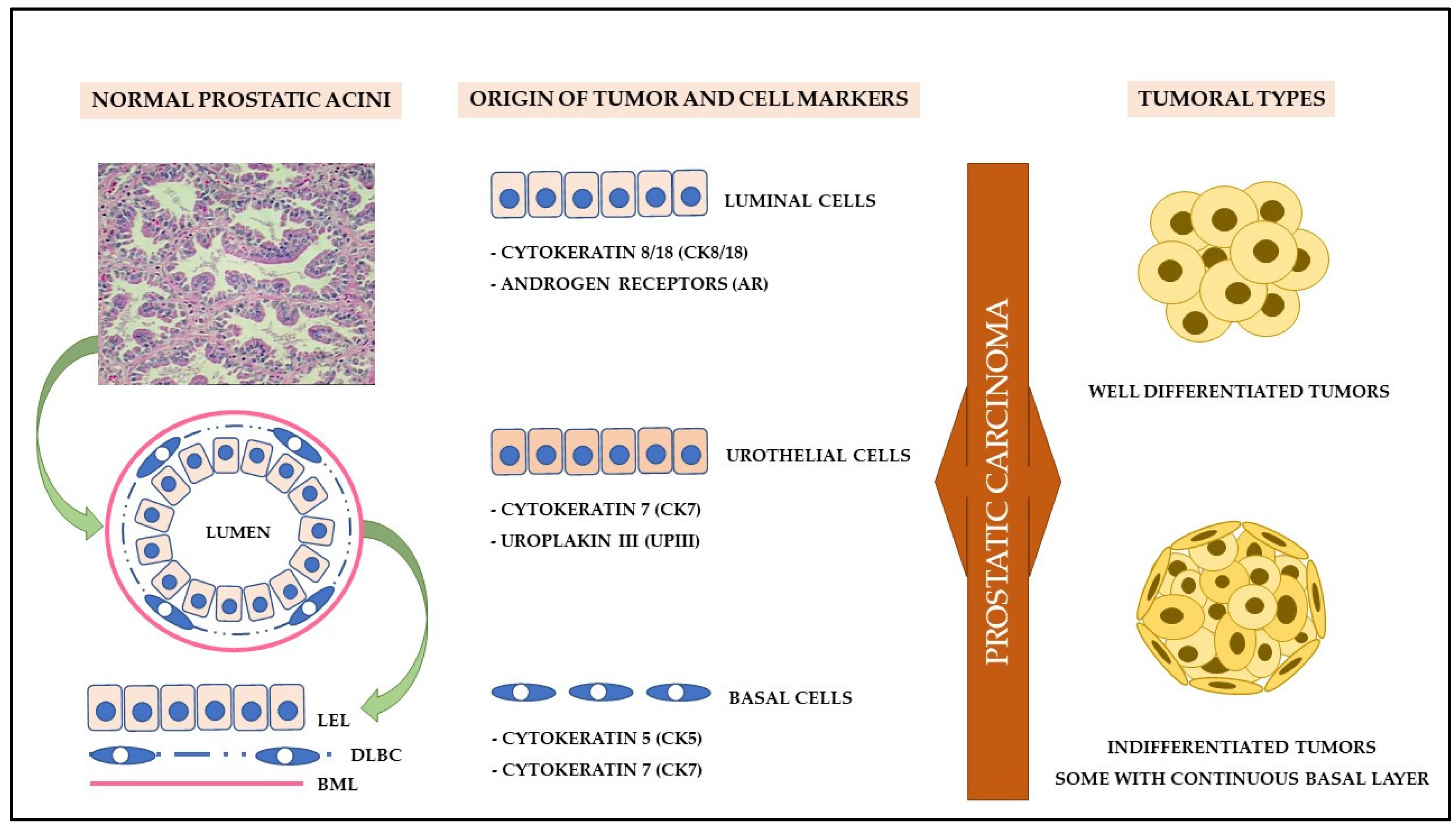
5. Cell Origin and Tumor Initiation
6. Cancer Stem Cells (CSC)
7. Molecular Biology of Prostate Cancer
7.1. Androgenic Signaling: A Key Point in Prostate Carcinogenesis?
7.2. mTOR/4E-BP1/eIF4E Cell Signaling Pathway
7.3. Ki67 Cell Proliferation Markers and Epidermal Growth Factor
7.4. Oncogenes and Tumor Suppressor Genes
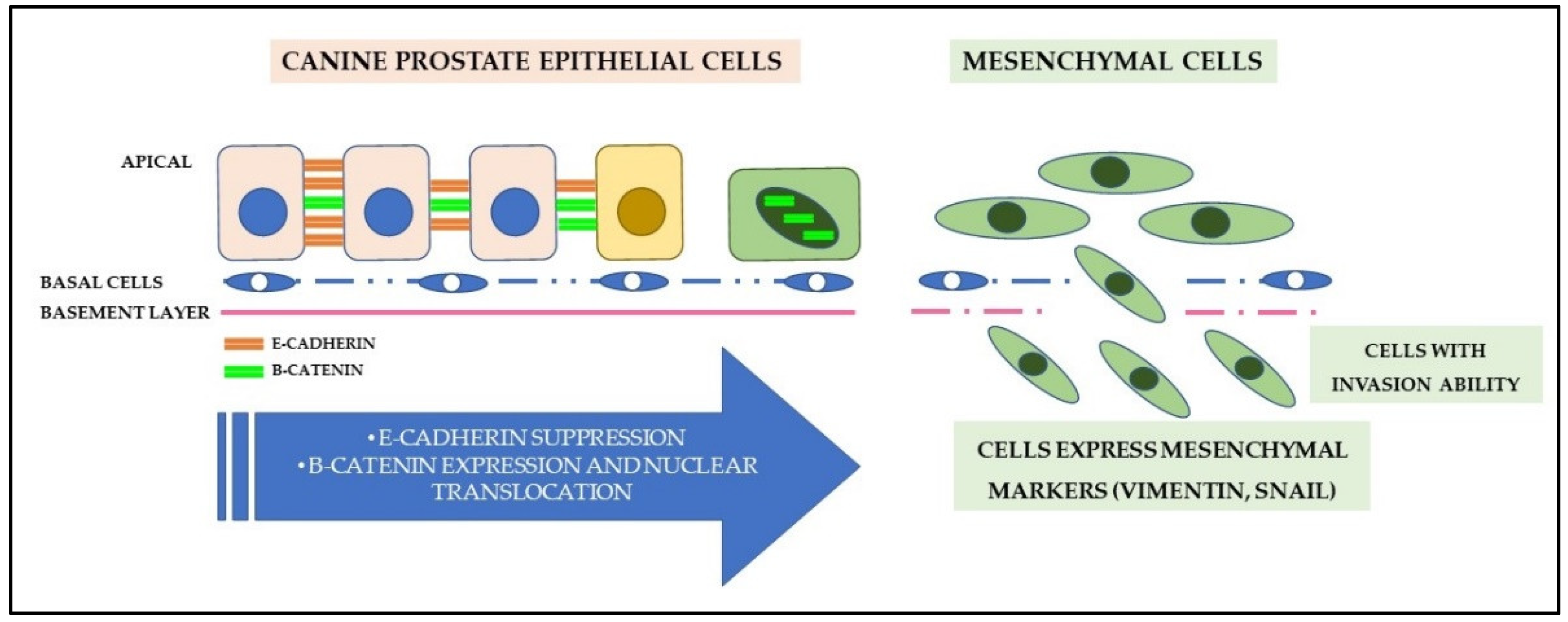
7.5. Cell Adhesion and Epithelial-Mesenchymal Transition
7.6. Angiogenesis
7.7. Tumor Microenvironment: Structural, Inflammatory, and Metabolic Aspects
8. MicroRNAs (miRs)
9. Prostate-Specific Antigen (PSA)
10. Future Directions
11. Final Considerations
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Health Estimates: Leading Causes of Death. Available online: https://www.who.int/data/gho/data/themes/mortality-and-global-health-estimates/ghe-leading-causes-of-death (accessed on 3 October 2021).
- Globocan Prostate—Global Cancer Observatory. Available online: https://gco.iarc.fr/today/data/factsheets/cancers/27-Prostate-fact-sheet.pdf (accessed on 3 October 2021).
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Laufer-Amorim, R.; Fonseca-Alves, C.E.; Villacis, R.A.R.; Linde, S.A.; Carvalho, M.; Larsen, S.J.; Marchi, F.A.; Rogatto, S.R. Comprehensive Genomic Profiling of Androgen-Receptor-Negative Canine Prostate Cancer. Int. J. Mol. Sci. 2019, 20, 1555. [Google Scholar] [CrossRef] [PubMed]
- Christensen, B.W. Canine Prostate Disease. Vet. Clin. N. Am.-Small Anim. Pract. 2018, 48, 701–719. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.; Rosenberg, A.Z.; Choi, S.M.; Fox-Talbot, K.; De Marzo, A.M.; Nonn, L.; Brennen, W.N.; Marchionni, L.; Halushka, M.K.; Lupold, S.E. Cell-type specific expression of oncogenic and tumor suppressive microRNAs in the human prostate and prostate cancer. Sci. Rep. 2018, 8, 7189. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Goltsov, A.A.; Ren, C.; Kurosaka, S.; Edamura, K.; Logothetis, R.; DeMayo, F.J.; Troncoso, P.; Blando, J.; DiGiovanni, J.; et al. Caveolin-1 upregulation contributes to c-Myc-induced high-grade prostatic intraepithelial neoplasia and prostate cancer. Mol. Cancer Res. 2012, 10, 218–229. [Google Scholar] [CrossRef]
- Langstaff, G. Cases of fungus haematodes. Med. Chir Trans. 1817, 8, 272–314. [Google Scholar]
- Adams, J. The case of scirrhous of the prostate gland with corresponding affliction of the lymphatic glands in the lumbar region and in the pelvis. Lancet 1853, 1, 393. [Google Scholar]
- Albarran, J.; Halle, N. Hypertrophie et neoplasies epitheliales de la prostate. Compt. Rend. Soc. Biol. 1898, 4, 722. [Google Scholar]
- Harrison, R. Lecture on vesical stones and prostate disorders. Lancet 1896, 2, 1660. [Google Scholar]
- Harrison, R. Case where a scirrhous cancer of the prostate was removed transurethrally. Lancet 1884, 2, 483. [Google Scholar]
- Young, H.H. Four cases of radical prostatectomy. Johns Hopkins Bull. 1905, 16, 305. [Google Scholar]
- Albarran, J. La castration a 19angio neurectomie du cordon dans l’hypertrophie de la prostate. Press. Med. Paris 1897, 11, 274. [Google Scholar]
- Pastean, O. De l’emploi du radium dans leur traitemente des cancers de la prostate. J. Urol. Med. Chir. 1913, 4, 341. [Google Scholar]
- White, W.J. Surgical of the hypertrophied prostate. Ann. Surg. 1893, 152, 20. [Google Scholar]
- Huggins, C.; Masina, M.H.; Eichelberger, L.; Wharton, J.D. Quantitative studies of prostatic secretion. 1. Characteristics of the normal Secretion; the influence of thyroid, suprarenal, and testis extirpation and androgen substitution on the prostatic output. J. Exp. Med. 1939, 70, 543. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Huggins, C.B.; Clark, P.J. Quantitative studies of prostatic secretion. 11. The effect of castration and of estrogen injection on the hyperplastic prostate glands of dogs. J. Exp. Med. 1940, 72, 747. [Google Scholar] [CrossRef]
- Rawla, P. Epidemiology of Prostate Cancer. World J. Oncol. 2019, 10, 89. [Google Scholar] [CrossRef]
- SEER Cancer Statistics Review, 1975–2013. Available online: https://seer.cancer.gov/csr/1975_2015 (accessed on 10 October 2021).
- Sticker, A. Uber den Krebs der Tiere, insbesondere uber die Empfanglichkeit der verschiedenen Haustierarten und iiber die Unterschiede des Tier- und Menschenkrebses. Arch. Klin. Chir 1902, 65, 1023–1087. [Google Scholar]
- Smith, J. Canine prostatic disease: A review of anatomy, pathology, diagnosis, and treatment. Theriogenology 2008, 70, 375–383. [Google Scholar] [CrossRef]
- Jiang, Y.; Song, Q.; Cao, D.; Guo, H.; Shuangshuang, L. The Current Status of Prostate Cancer Animal Models. J. Vet. Med. Anim. Sci. 2020, 30, 1041. [Google Scholar]
- Hensley, P.J.; Kyprianou, N. Modeling Prostate Cancer in Mice: Limitations and Opportunities. J. Androl. 2012, 33, 144. [Google Scholar] [CrossRef] [PubMed]
- Polisca, A.; Troisi, A.; Fontaine, E.; Menchetti, L.; Fontbonne, A. A retrospective study of canine prostatic diseases from 2002 to 2009 at the Alfort Veterinary College in France. Theriogenology 2016, 85, 835–840. [Google Scholar] [CrossRef] [PubMed]
- Teske, E.; Naan, E.C.; van Dijk, E.M.; Van Garderen, E.; Schalken, J.A. Canine prostate carcinoma: Epidemiological evidence of an increased risk in castrated dogs. Mol. Cell. Endocrinol. 2002, 197, 251–255. [Google Scholar] [CrossRef]
- Schrank, M.; Romagnoli, S. Prostatic Neoplasia in the Intact and Castrated Dog: How Dangerous is Castration? Animal 2020, 10, 85. [Google Scholar] [CrossRef] [PubMed]
- L’Eplattenier, H.F.; van Nimwegen, S.A.; Van Sluijs, F.J.; Kirpensteijn, J. Partial prostatectomy using Nd:YAG laser for management of canine prostate carcinoma. Vet. Surg. 2006, 35, 406–411. [Google Scholar] [CrossRef]
- Shidaifat, F.; Gharaibeh, M.; Bani-Ismail, Z. Effect of castration on extracellular matrix remodeling and angiogenesis of the prostate gland. Endocr. J. 2007, 54, 521–529. [Google Scholar] [CrossRef]
- De Marzo, A.M.; Platz, E.A.; Epstein, J.I.; Ali, T.; Billis, A.; Chan, T.Y.; Cheng, L.; Datta, M.; Egevad, L.; Ertoy-Baydar, D.; et al. A Working Group Classification of Focal Prostate Atrophy Lesions. Am. J. Surg. Pathol. 2006, 30, 1281–1291. [Google Scholar] [CrossRef]
- Toledo, D.C.; Faleiro, M.B.R.; Rodrigues, M.M.P.; Di Santis, G.W.; Amorim, R.L.; Moura, V.M.B.D. Caracterização histomorfológica da atrofia inflamatória proliferativa na próstata canina. Cienc. Rural. 2010, 40, 1372–1377. [Google Scholar] [CrossRef][Green Version]
- De Marzo, A.; Marchi, V.L.; Epstein, J.I.; Nelson, W.G. Proliferative inflammatory atrophy of the prostate, Implications for prostatic carcinogenesis. Am. J. Pathol. 1999, 55, 1985–1992. [Google Scholar] [CrossRef]
- Fernandes, G.G.; Pedrina, B.; Lainetti, P.F.; Kobayashi, P.E.; Al, E. Morphological and Molecular Characterization of Proliferative Inflammatory Atrophy in Canine Prostatic Samples. Cancers 2021, 13, 1887. [Google Scholar] [CrossRef]
- Palmieri, C.; Story, M.; Lean, F.; Akter, S.; Grieco, V.; De Marzo, A. Diagnostic Utility of Cytokeratin-5 for the Identification of Proliferative Inflammatory Atrophy in the Canine Prostate. J. Comp. Pathol. 2018, 158, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Akter, S.H.; Lean, F.Z.; Lu, J.; Grieco, V.; Palmieri, C. Different Growth Patterns of Canine Prostatic Carcinoma Suggests Different Models of Tumor-Initiating Cells. Vet. Pathol. 2015, 52, 1027–1033. [Google Scholar] [CrossRef] [PubMed]
- Faleiro, M.B.R.; Cintra, L.C.; Jesuino, R.S.A.; Damasceno, A.D.; Moura, V.M.B.D. Expression of cell cycle inhibitors in canine prostate with proliferative inflammatory atrophy and carcinoma. Arq. Bras. Med. Vet. Zootec. 2018, 70, 82–92. [Google Scholar] [CrossRef]
- Sun, C.; Dobi, A.; Mohamed, A. TMPRSS2-ERG fusion, a common genomic alteration in prostate cancer activates C-MYC and abrogates prostate epithelial differentiation. Oncogene 2008, 27, 5348–5353. [Google Scholar] [CrossRef]
- Attard, G.; Swennenhuis, J.F.; Olmos, D.; Reid, A.H.M.; Vickers, E.; A’Hern, R.; Levink, R.; Coumans, F.; Moreira, J.; Riisnaes, R.; et al. Characterization of ERG, AR and PTEN Gene Status in Circulating Tumor Cells from Patients with Castration-Resistant Prostate Cancer. Cancer Res. 2009, 69, 2912–2918. [Google Scholar] [CrossRef] [PubMed]
- Bostwick, D.G.; Cheng, L. Precursors of prostate cancer. Histopathology 2012, 60, 4–27. [Google Scholar] [CrossRef] [PubMed]
- De Marzo, A.M.; Haffner, M.C.; Lotan, T.L.; Yegnasubramanian, S.; Nelson, W.G. Premalignancy in Prostate Cancer: Rethinking What we Know. Cancer Prev. Res. 2016, 9, 648–656. [Google Scholar] [CrossRef] [PubMed]
- Croce, G.B.; Rodrigues, M.M.P.; Faleiro, M.B.R.; Moura, V.M.B.D.; Laufer Amorim, R. Óxido nítrico, GSTP-1 e p53: Qual o papel desses biomarcadores nas lesões prostáticas do cão? Arq. Bras. Med. Vet. Zootec 2011, 63, 1368–1376. [Google Scholar] [CrossRef]
- Waters, D.J.; Bostwick, D.G. Prostatic intraepithelial neoplasia occurs spontaneously in the canine prostate. J. Urol. 1997, 157, 713–716. [Google Scholar] [CrossRef]
- Waters, D.J.; Hayden, D.W.; Bell, F.W.; Klausner, J.S.; Qian, J.; Bostwick, D.G. Prostatic intraepithelial neoplasia in dogs with spontaneous prostate cancer-PubMed. Prostate 1997, 30, 92–97. [Google Scholar] [CrossRef]
- Matsuzaki, P.; Cogliati, B.; Sanches, D.; Chaible, L.; Kimura, K.; Silva, T.; Real-Lima, M.; Hernandez-Blazquez, F.; Laufer-Amorim, R.; Dagli, M. Immunohistochemical Characterization of Canine Prostatic Intraepithelial Neoplasia. J. Comp. Pathol. 2009, 142, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Toledo, D.C.; Faleiro, M.B.R.; Ferreira, H.H.; Faria, A.M.; Matos, M.P.C.; Laufer-Amorim, R.; Moura, V.M.B.D. Imunomarcação de TGF-β em próstatas caninas normais e com lesões proliferativas. Ciência Anim. Bras. 2019, 20, e-3453. [Google Scholar] [CrossRef]
- Palmieri, C.; Mancini, M.; Benazzi, C.; Della Salda, L. Heat Shock Protein 90 is Associated with Hyperplasia and Neoplastic Transformation of Canine Prostatic Epithelial Cells. J. Comp. Path. 2014, 150, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Calderwood, S.K.; Gong, J. Heat Shock Proteins Promote Cancer: It’s a Protection Racket. Trends Biochem. Sci. 2016, 41, 311–323. [Google Scholar] [CrossRef]
- Aldaoud, N.; Hallak, A.; Abdo, N.; Al Bashir, S.; Marji, N.; Graboski-Bauer, A. Interobserver Variability in the Diagnosis of High-Grade Prostatic Intraepithelial Neoplasia in a Tertiary Hospital in Northern Jordan. Clin. Pathol. 2020, 13, 1–14. [Google Scholar] [CrossRef]
- Brägelmann, J.; Klümper, N.; Offermann, A.; von Mässenhausen, A.; Böhm, D.; Deng, M.; Queisser, A.; Sanders, C.; Syring, I.; Merseburger, A.S.; et al. Pan-Cancer Analysis of the Mediator Complex Transcriptome Identifies CDK19 and CDK8 as Therapeutic Targets in Advanced Prostate Cancer. Clin. Cancer Res. 2016, 23, 1829–1840. [Google Scholar] [CrossRef]
- Offermann, A.; Joerg, V.; Hupe, M.C.; Becker, F.; Müller, M.; Brägelmann, J.; Kirfel, J.; Merseburger, A.S.; Sailer, V.; Tharun, L.; et al. CDK19 as a diagnostic marker for high-grade prostatic intraepithelial neoplasia. Hum. Pathol. 2021, 117, 60–67. [Google Scholar] [CrossRef]
- Soylu, H.; Acar, N.; Ozbey, O.; Unal, B.; Koksal, I.T.; Bassorgun, I.; Ciftcioglu, A.; Ustunel, I. Characterization of Notch Signalling Pathway Members in Normal Prostate, Prostatic Intraepithelial Neoplasia (PIN) and Prostatic Adenocarcinoma. Pathol. Oncol. Res. 2016, 22, 87–94. [Google Scholar] [CrossRef]
- Eble, J.N.; Sauter, G.; Epstein, J.I.; Sesterhenn, I.A. Pathology and Genetics of Tumours of the Urinary System and Male Genital Organs: WHO Classification of Tumours, 3rd ed.; IARC Press: Lyon, France, 2004. [Google Scholar]
- Palmieri, C.; Foster, R.A.; Grieco, V.; Fonseca-Alves, C.E.; Wood, G.A.; Culp, W.T.N.; Escobar, H.M.; De Marzo, A.M.; Laufer-Amorim, R. Histopathological Terminology Standards for the Reporting of Prostatic Epithelial Lesions in Dogs. J. Comp. Path. 2019, 171, 30–37. [Google Scholar] [CrossRef]
- Bell, F.W.; Klausner, J.S.; Hayden, D.W.; Feeny, D.A.; Johnson, S.D. Clinical and pathological features of prostatic adenocarcinoma in sexually intact and castrated dogs: 31 cases (1970–1987). J. Am. Vet. Med. Assoc. 1991, 199, 1623–1630. [Google Scholar]
- Waters, D.J.; Sakr, W.A.; Hayden, D.W.; Lang, C.M.; McKinney, L.; Murphy, G.P.; Radinsky, R.; Ramoner, R.; Richardson, R.C.; Tindall, D.J. Workgroup 4: Spontaneous prostate carcinoma in dogs and nonhuman primates. Prostate 1998, 36, 64–67. [Google Scholar] [CrossRef]
- Cornell, K.K.; Bostwick, D.G.; Cooley, D.M.; Hall, G.; Harvey, H.G.J.; Hendrick, M.J.; Pauli, J.A.; Stoica, G.; Sweet, D.C.; Waters, D.J. Clinical and pathological aspects of spontaneous canine prostate carcinoma: A retrospective analysis of 76 cases. Prostate 2000, 45, 173–183. [Google Scholar] [CrossRef]
- Lai, C.L.; van den Ham, R.; van Leenders, G.; van der Lugt, J.; Mol, J.; Teske, E. Histopathologicaland Immuno histochemical Characterization ofCanine ProstateCancer. Prostate 2008, 68, 477–488. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, C.; Lean, F.Z.; Akter, S.H.; Romussi, S.; Grieco, V. A retrospective analysis of 111 canine prostatic samples: Histopathological findings and classification. Res. Vet. Sci. 2014, 97, 568–573. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, P.A. Adenocarcinoma. In Prostate Pathology; Humphrey, P.A., Ed.; ASCP Press: Chicago, IL, USA, 2003; pp. 237–258. [Google Scholar]
- Gleason, D.F. Classification of prostatic carcinomas. Cancer Chemother 1966, 50, 125–128. [Google Scholar]
- Shah, R.B.; Zhuo, M. Contemporary approach to Gleason grading of Prostate cancer. In Prostate Biopsy Interpretation: An Illustrated Guide; Shah, R.B., Zhuo, M., Eds.; Springer: Berlin, Germany, 2012; pp. 41–107. [Google Scholar]
- Epstein, J.I.; Allsbrook, W.C.; Amin, M.B.; Egevad, L.L. The 2005 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma. Am. J. Surg. Pathol. 2005, 29, 1228–1242. [Google Scholar] [CrossRef]
- Billis, A.; Quintal, M.M.; Meirelles, L.; Freitas, L.L.; Costa, L.B.; Bonfitto, J.F.; Diniz, B.L.; Poletto, P.H.; Magna, L.A.; Ferreira, U. The value of the 2005 International Society of Urological Pathology (ISUP) modified Gleason grading system as a predictor of biochemical recurrence after radical prostatectomy. Int. Urol. Nephrol. 2014, 46, 935–940. [Google Scholar] [CrossRef]
- Brimo, F.; Montironi, R.; Egevad, L.; Erbersdobler, A.; Lin, D.W.; Nelson, J.B.; Rubin, M.A.; Kwast, T.V.D.; Amin, M.; Epstein, J.I. Contemporary grading for prostate cancer: Implications for patient care. Eur. Urol. 2013, 63, 892–901. [Google Scholar] [CrossRef]
- Epstein, J.I.; Zelefsky, M.J.; Sjoberg, D.D.; Nelson, J.B.; Egevad, L.; Magi-Galluzzi, C.; Vickers, A.J.; Parwani, A.V.; Reuter, V.E.; Fine, S.W.; et al. A Contemporary Prostate Cancer Grading System: A Validated Alternative to the Gleason Score. Eur. Urol. 2016, 69, 428–435. [Google Scholar] [CrossRef]
- Chen, N.; Zhou, Q. The evolving Gleason grading system. Chin. J. Cancer Res. 2016, 28, 58–64. [Google Scholar]
- Palmieri, C.; Grieco, V. Proposal of Gleason-like grading system of canine prostate carcinoma in veterinary pathology practice. Res. Vet. Sci. 2015, 103, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Melia, J.; Moseley, R.; Ball, R.Y.; Griffiths, D.F.; Grigor, K.; Harnden, P.; Jarmulowicz, M.; McWilliam, L.J.; Montironi, R.; Waller, M.; et al. A UK-based investigation of inter- and intra-observer reproducibility of Gleason grading of prostatic biopsies. Histopathology 2006, 48, 644–654. [Google Scholar] [CrossRef] [PubMed]
- Di Donato, G.; Laufer-Amorim, R.; Palmieri, C. Nuclear morphometry in histological specimens of canine prostate cancer: Correlation with histological subtypes, Gleason score, methods of collection and survival time. Res. Vet. Sci. 2017, 114, 212–217. [Google Scholar] [CrossRef]
- Lai, C.L.; van den Ham, R.; van Leenders, G.; van der Lugt, J.; Teske, E. Comparative characterization of the canine normal prostate in intact and castrated animals. Prostate 2008, 68, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Romanucci, M.; Frattone, L.; Ciccarelli, A.; Bongiovanni, L.; Malatesta, D.; Benazzi, C.; Brachelente, C.; Salda, L.D. Immunohistochemical expression of heat shock proteins, p63 and androgen receptor in benign prostatic hyperplasia and prostatic carcinoma in the dog. Vet. Comp. Oncol. 2014, 14, 337–349. [Google Scholar] [CrossRef]
- Fonseca-Alves, C.; Kobayashi, P.E.; Rivera Calderón, L.G.; Felisbino, S.L.; Rinaldi, J.C.; Drigo, S.A.; Rogatto, S.R.; Laufer-Amorim, R. Immunohistochemical panel to characterize canine prostate carcinomas according to aberrant p63 expression. PLoS ONE 2018, 13, e0199173. [Google Scholar] [CrossRef]
- Dabir, P.D.; Ottosen, P.; Høyer, S.; Hamilton-Dutoit, S. Comparative analysis of three- and two-antibody cocktails to AMACR and basal cell markers for the immunohistochemical diagnosis of prostate carcinoma. Diagn. Pathol. 2012, 7, 81. [Google Scholar] [CrossRef]
- Brot, S.; Lothion-Roy, J.; Grau-Roma, L.; White, E.; Guscetti, F.; Rubin, M.A.; Mongan, N.P. Histological and immunohistochemical investigation of canine prostate carcinoma with identification of common intraductal carcinoma component. Vet. Comp. Oncol. 2020, 20, 38–49. [Google Scholar] [CrossRef]
- Kleeberger, W.; Bova, G.S.; Nielsen, M.E.; Herawi, M.; Chuang, A.Y.; Epstein, J.I.; Berman, D.M. Roles for the stem cell associated intermediate filament Nestin in prostate cancer migration and metastasis. Cancer Res. 2007, 67, 9199–9206. [Google Scholar] [CrossRef]
- Prager, B.C.; Xie, Q.; Bao, S.; Rich, J.N. Cancer Stem Cells: The Architects of the Tumor Ecosystem. Cell Stem Cell 2019, 24, 41–53. [Google Scholar] [CrossRef]
- Costa, C.D.; Justo, A.A.; Kobayashi, P.E.; Story, M.M.; Palmieri, C.; Laufer-Amorim, R.; Fonseca-Alves, C.E. Characterization of OCT3/4, Nestin, NANOG, CD44 and CD24 as stem cell markers in canine prostate cancer. Int. J. Biochem. Cell Biol. 2019, 108, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Moulay, M.; Liu, W.; Willenbrock, S.; Sterenczak, A.; Carlson, R.; Ngezahayo, A.; Escobar, H.M.; Nolte, I. Evaluation of Stem Cell Marker Gene Expression in Canine Prostate Carcinoma- and Prostate Cyst-derived Cell Lines. Anticancer Res. 2013, 33, 5421–5432. [Google Scholar] [PubMed]
- Yang, L.; Shi, P.; Zhao, G.; Xu, J.; Peng, W.; Zhang, J.; Zhang, G.; Wang, X.; Dong, Z.; Chen, F.; et al. Targeting cancer stem cell pathways for cancer therapy. Nat.-Signal. Transduct. Target. Ther. 2020, 5, 1–35. [Google Scholar] [CrossRef] [PubMed]
- Patrawala, L.; Calhoun, T.; Schneider-Broussard, R.; Li, H.; Bhatia, B.; Tang, S.; Reilly, J.G.; Chandra, D.; Zhou, J.; Claypool, K.; et al. Highly purified CD44+ prostate cancer cells from xenograft human tumors are enriched in tumorigenic and metastatic progenitor cells. Oncogene 2006, 25, 1696–1708. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Cheung, B.B.; Beretov, J.; Duan, W.; Bucci, J.; Malouf, D.; Graham, P.; Li, Y. CD44 variant 6 is associated with prostate cancer growth and chemo-radiotherapy response in vivo. Exp. Cell Res. 2020, 388, 111850. [Google Scholar] [CrossRef]
- Bongiovanni, L.; Caposano, F.; Romanucci, M.; Grieco, V.; Malatesta, D.; Brachelente, C.; Massimini, M.; Benazzi, C.; Thomas, R.E.; Salda, L.D. Survivin and Sox9: Potential Stem Cell Markers in Canine Normal, Hyperplastic, and Neoplastic Canine Prostate. Vet. Pathol. 2019, 56, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Hood, G.; Laufer-Amorim, R.; Fonseca-Alves, C.E.; Palmieri, C. Overexpression of Ephrin A3 Receptor in Canine Prostatic Carcinoma. J. Comp. Path. 2016, 154, 180–185. [Google Scholar] [CrossRef]
- Janes, P.W.; Slape, C.I.; Farnsworth, R.H.; Atapattu, L.; Scott, A.M.; Vail, M.E. EphA3 biology and cancer. Growth Factors 2014, 32, 176–189. [Google Scholar] [CrossRef]
- Somarelli, J.; Gardner, H.; Cannataro, V.L.; Gunady, E.F.; Boddy, A.M.; Johnson, N.A.; Fisk, J.N.; Gaffney, S.G.; Chuang, J.H.; Li, S.; et al. Molecular Biology and Evolution of Cancer: From Discovery to Action. Mol. Biol. Evol. 2020, 37, 326. [Google Scholar] [CrossRef]
- Boss, M.K. Biology of Cancer and Cancer Genetics. In Clinical Small Animal Internal Medicine Volume II; Bruyett, D.S., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2020; pp. 1205–1211. [Google Scholar]
- Sharifi, N.; Auchus, R.J. Steroid biosynthesis and prostate cancer. Steroids 2012, 77, 719–726. [Google Scholar] [CrossRef]
- Takayama, K.; Inoue, S. Transcriptional network of androgen receptor in prostate cancer progression. Int. J. Urol. 2013, 20, 756–768. [Google Scholar] [CrossRef] [PubMed]
- Huggins, C.; Stephens, R.C.; Hodges, C.V. Studies on prostatic cancer: 2. The effects of castration on advanced carcinoma of the prostate gland. Arch. Surg. 1941, 43, 209. [Google Scholar] [CrossRef]
- Hellerstedt, B.A.; Pienta, K.J. The current state of hormonal therapy for prostate cancer. A Cancer J. Clin. 2002, 52, 154–179. [Google Scholar] [CrossRef] [PubMed]
- Heidegger, I.; Brandt, M.P.; Heck, M.M. Treatment of non-metastatic castration resistant prostate cancer in 2020: What is the best? Urol. Oncol. Semin. Orig. Investig. 2020, 38, 129–136. [Google Scholar] [CrossRef]
- Fujita, K.; Nonomura, N. Role of Androgen Receptor in Prostate Cancer: A Review. World J. Mens. Health 2018, 37, 288–295. [Google Scholar] [CrossRef]
- Robinson, D.; Van Allen, E.M.; Wu, Y.M.; Schultz, N.; Lonigro, R.J.; Mosquera, J.-M.; Montgomery, B.; Taplin, M.; Pritchard, C.C.; Attard, G.; et al. Integrative clinical genomics of advanced prostate cancer. Cell 2015, 161, 1215–1228. [Google Scholar] [CrossRef]
- Lai, C.L.; Van den Ham, R.; Mol, J.; Teske, E. Immunostaining of the androgen receptor and sequence analysis of its DNA-binding domain in canine prostate cancer. Vet. J. 2009, 181, 256–260. [Google Scholar] [CrossRef]
- Rivera-Calderón, L.G.; Fonseca-Alves, C.E.; Kobayashi, P.E.; Carvalho, M.; Drigo, S.A.; Vasconcelos, R.A.; Laufer-Amorim, R. Alterations in PTEN, MDM2, TP53 and AR protein and gene expression are associated with canine prostate carcinogenesis. Res. Vet. Sci. 2016, 106, 56–61. [Google Scholar] [CrossRef]
- Kobayashi, P.E.; Rodrigues, M.M.P.; Gartner, F.; Rema, A.; Fonseca-Alves, C.E.; Laufer-Amorim, R. Association between decreased expression of estrogen receptor alpha, androgen receptor and phosphatase and tensin homolog immunoexpression in the canine prostate. Pesq. Vet. Bras. 2019, 39, 40–46. [Google Scholar] [CrossRef]
- Kato, Y.; Ochiai, K.; Michishita, M.; Azakami, D.; Nakahira, R.; Morimatsu, M.; Ishiguro-Oonuma, T.; Yoshikawa, Y.; Kobayashi, M.; Bonkobara, M.; et al. Molecular cloning of canine co-chaperone small glutamine-rich tetratricopeptide repeat-containing protein α (SGTA) and investigation of its ability to suppress androgen receptor signalling in androgen-independent prostate cancer. Vet. J. 2015, 206, 143–148. [Google Scholar] [CrossRef]
- Azakami, D.; Nakahira, R.; Kato, Y.; Michishita, M.; Kobayashi, M.; Onozawa, E.; Bonkobara, M.; Kobayashi, M.; Takahashi, K.; Watanabe, M.; et al. The canine prostate cancer cell line CHP-1 shows over-expression of the co-chaperone small glutamine-rich tetratricopeptide repeat-containing protein α. Vet. Comp. Oncol. 2017, 15, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, G.; Need, E.F.; Barrett, J.M.; Bianco-Miotto, T.; Thompson, V.C.; Butler, L.M.; Marshall, V.R.; Tilley, W.D.; Coetzee, G.A. Corepressor effect on androgen receptor activity varies with the length of the CAG encoded polyglutamine repeat and is dependent on receptor/corepressor ratio in prostate cancer cells. Mol. Cell. Endocrinol. 2011, 342, 20–31. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Mi, J.; Olson, A.; Aldahl, J.; Hooker, E.; Yu, E.; Le, V.; Lee, D.H.; Kim, W.K.; Robins, D.M.; et al. Androgen receptor with short polyglutamine tract preferably enhances Wnt/β-catenin-mediated prostatic tumorigenesis. Oncogene 2020, 39, 3276–3291. [Google Scholar] [CrossRef] [PubMed]
- Ochiai, K.; Sutijarit, S.; Uemura, M.; Morimatsu, M.; Michishita, M.; Onozawa, E.; Maeda, M.; Sasaki, T.; Watanabe, M.; Tanaka, Y.; et al. The number of glutamines in the N-terminal of the canine androgen receptor affects signalling intensities. Vet. Comp. Oncol. 2020, 2020, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Shorning, B.Y.; Dass, M.S.; Smalley, M.J.; Pearson, H.B. The PI3K-AKT-mTOR Pathway and Prostate Cancer: at the Crossroads of AR, MAPK, and WNT Signaling. Int. J. Mol. Sci. 2020, 21, 4507. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, S.I.; Pe Benito, R.; Henshall, S.M.; Horvath, L.G.; Kench, J.G. Expression of phosphorylated-mTOR during the development of prostate cancer. Prostate 2014, 74, 1231–1239. [Google Scholar] [CrossRef]
- Qin, X.; Jiang, B.; Zhang, Y. 4E-BP1, a multifactor regulated multifunctional protein. Cell Cycle 2016, 15, 781–786. [Google Scholar] [CrossRef]
- Russo, G.I.; Hennenlotter, J.; Vogel, U.; Kühs, U.; Wurm, T.M.; Gerber, V.; Neumann, T.; Cimino, S.; Stenzl, A.; Todenhofer, T. Intratumoral Heterogeneity Determines the Expression of mTOR-pathway Proteins in Prostate Cancer. Hindawi 2019, 2019, 1296865. [Google Scholar] [CrossRef]
- Torrealba, N.; Vera, R.; Fraile, B.; Martınez-Onsurbe, P.; Paniagua, R.; Royuela, M. TGF-β/PI3K/AKT/mTOR/NF-kB pathway. Clinicopathological features in prostate cancer. Aging Male 2020, 23, 801–811. [Google Scholar] [CrossRef]
- Rivera-Caldero, L.G.; Fonseca-Alves, C.E.; Kobayashi, P.E.; Carvalho, M.; Vasconcelos, R.O.; Laufer-Amorim, R. p-mTOR, p-4EBP-1 and eIF4E expression in canine prostatic carcinoma. Res. Vet. Sci. 2019, 122, 86–92. [Google Scholar] [CrossRef]
- Laplante, M.; Sabatini, D.M. mTOR signaling in growth control and disease. Cell 2012, 149, 274–293. [Google Scholar] [CrossRef] [PubMed]
- Hammarsten, P.; Josefsson, A.; Thysell, E.; Lundholm, M.; Hägglöf, C.; Iglesias-Gato, D.; Flores-Morales, A.; Stattin, P.; Egevad, L.; Granfors, T.; et al. Immunoreactivity for prostate specific antigen and Ki67 differentiates subgroups of prostate cancer related to outcome. Mod. Pathol. 2019, 32, 1310–1319. [Google Scholar] [CrossRef] [PubMed]
- Fonseca-Alves, C.E.; Kobayashi, P.E.; Palmieri, C.; Laufer-Amorim, R. Investigation of c-KIT and Ki67 expression in normal, preneoplastic and neoplastic canine prostate. Vet. Res. 2017, 13, 380. [Google Scholar] [CrossRef] [PubMed]
- Faleiro, M.B.R.; Cintra, L.C.; Jesuino, R.S.A.; Araújo, E.G.; Rocha, R.M.; Moura, V.M.D. Expression of EGF receptors in canine prostate with proliferative inflammatory atrophy and carcinoma. Cienc. Rural. 2017, 47, e20170085. [Google Scholar] [CrossRef]
- Lin, X.; Wang, Y. Re-expression of microRNA-4319 inhibits growth of prostate cancer via Her-2 suppression. Clin. Transl. Oncol. 2018, 20, 1400–1407. [Google Scholar] [CrossRef] [PubMed]
- Sakai, K.; Shinohara, Y.; Kaji, K.; Yonezawa, T.; Momoi, Y.; Maeda, S. Human epidermal growth factor receptor 2 is overexpressed in canine prostate carcinoma. Transl. Regul. Sci. 2021, 3, 1–8. [Google Scholar] [CrossRef]
- Sun, W.; Yang, J. Functional mechanisms for human tumor suppressors. J. Cancer 2010, 1, 136–140. [Google Scholar] [CrossRef]
- Shi, X.W.; Gumerlock, P.H.; de Vere White, R.W. Molecular biology of prostate cancer. World J. Urol. 1996, 14, 318–328. [Google Scholar] [CrossRef]
- Kontomanolis, E.N.; Koutras, A.; Syllaios, A.; Schizas, D.; Mastoraki, A.; Garmpis, N.; Diakosavvas, M.; Angelou, K.; Tsatsaris, G.; Pagkalos, A.; et al. Role of Oncogenes and Tumor-suppressor Genes in Carcinogenesis: A Review. Anticancer. Res. 2020, 40, 6009–6015. [Google Scholar] [CrossRef]
- Grassinger, J.M.; Aupperle-Lellbach, H.; Erhard, H.; Merz, S.; Klopfleisch, R. Detection of BRAF mutation in canine prostatic diseases. Tierarztl Prax Ausg K Kleintiere Heimtiere 2019, 47, 313–320. [Google Scholar]
- Fonseca-Alves, C.E.; Kobayashi, P.E.; Laufer-Amorim, R. Evaluation of NKX3.1 and C-MYC expression in canine prostatic cancer. Res. Vet. Sci. 2018, 118, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Ashman, L.K. The biology of stem cell factor and its receptor C-kit. Int. J. Biochem. Cell Biol. 1999, 31, 1037–1051. [Google Scholar] [CrossRef]
- Mainetti, L.E.; Zhe, X.; Diedrich, J.; Saliganan, A.D.; Cho, W.J.; Cher, M.L.; Heath, E.; Fridman, R.; Kim, H.R.; Bonfil, R.D. Bone-induced c-kit expression in prostate cancer: A driver of intraosseous tumor growth. Int. J. Cancer 2015, 136, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Defourny, S.V.P.; Romanucci, M.; Grieco, V.; Quaglione, G.R.; Santolini, C.; Della Salda, L. Tumor–Microenvironment Interaction: Analysis of Mast Cell Populations in Normal Tissue and Proliferative Disorders of the Canine Prostate. Veterinay Sci. 2019, 6, 16. [Google Scholar] [CrossRef]
- Guo, J.H.; Zhou, J.; Zhao, Y.; Liu, P.Y.; Yao, H.J.; Da, J.; Zhang, M.; Zhou, Z.; Chen, Q.; Peng, Y.B.; et al. Normal peripheral prostate stromal cells stimulate prostate cancer development: Roles of c-kit signal. Am. J. Transl. Res. 2015, 7, 502–512. [Google Scholar] [CrossRef][Green Version]
- Gurel, B.; Iwata, T.; Koh, C.M.; Jenkins, R.B.; Lan, F.; Van Dang, C.; Hicks, J.L.; Morgan, J.; Cornish, T.C.; Sutcliffe, S.; et al. Nuclear MYC protein overexpression is an early alteration in human prostate carcinogenesis. Mod. Pathol. 2008, 21, 1156–1167. [Google Scholar] [CrossRef]
- Barfeld, S.J.; Urbanucci, A.; Itkonen, H.M.; Fazli, L.; Hicks, J.L.; Thiede, B.; Rennie, P.S.; Yegnasubramanian, S.; DeMarzo, A.M.; Mills, I.G. c-Myc Antagonises the Transcriptional Activity of the Androgen Receptor in Prostate Cancer Affecting Key Gene Networks. EBioMedicine 2017, 18, 83–93. [Google Scholar] [CrossRef]
- Thangapazham, R.; Saenz, F.; Katta, S.; Mohamed, A.A.; Tan, S.H.; Petrovics, G.; Srivastava, S.; Dobi, A. Loss of the NKX3.1 tumorsuppressor promotes the TMPRSS2-ERG fusion gene expression in prostate cancer. BMC Cancer 2014, 14, 16. [Google Scholar] [CrossRef]
- Vogelstein, B.; Kinzler, K.W. P53 function and dysfunction. Cell 1992, 70, 523–526. [Google Scholar] [CrossRef]
- Masson, G.R.; Williams, R.L. Structural Mechanisms of PTEN Regulation. Cold Spring Harb. Perspect. Med. 2020, 10, a036152. [Google Scholar] [CrossRef]
- Gericke, A.; Munson, M.; Ross, A.H. Regulation of the PTEN phosphatase. Gene 2006, 374, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Manfredi, J.J. The Mdm2-p53 relationship evolves: Mdm2 swings both ways as an oncogene and a tumor suppressor. Genes Dev. 2010, 24, 1580–1589. [Google Scholar] [CrossRef] [PubMed]
- Pant, V.; Lozano, G. Dissecting the p53-Mdm2 feedback loop in vivo: Uncoupling the role in p53 stability and activity. Oncotarget 2014, 5, 1149–1156. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pagliarone, S.; Frattone, L.; Pirocchi, V.; Salda, L.D.; Palmieri, C. Altered expression of p53, but not Rb, is involved in canine prostatic carcinogenesis. Res. Vet. Sci. 2016, 106, 195–199. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, Y.; Wei, S.; Bae, S.; Yang, W.H.; Smith, G.J.; Mohler, J.L.; Fontham, E.; Bensen, J.T.; Sonpavde, G.P.; et al. A CD24-p53 axis contributes to African American prostate cancer disparities. Prostate 2020, 80, 609–618. [Google Scholar] [CrossRef]
- Barkal, A.A.; Brewer, R.E.; Markovic, M.; Kowarsky, M.; Barkal, S.A.; Zaro, B.W.; Krishnan, V.; Hatakeyama, J.; Dorigo, O.; Barkal, L.J.; et al. CD24 signalling through macrophage Siglec-10 is a target for cancer immunotherapy. Nature 2019, 572, 392–396. [Google Scholar] [CrossRef]
- Lin, H.Y.; Palmieri, C. Is STAT3 and PTEN Expression Altered in Canine Prostate Cancer? J. Comp. Pathol. 2016, 155, 185–189. [Google Scholar] [CrossRef]
- Galoczova, M.; Coates, P.; Vojtesek, B. STAT3, stem cells, cancer stem cells and p63. Cell. Mol. Biol. Lett. 2018, 23, 12. [Google Scholar] [CrossRef]
- Mochizuki, H.; Kennedy, K.; Shapiro, S.G.; Breen, M. BRAF Mutations in Canine Cancers. PLoS ONE 2015, 10, e0129534. [Google Scholar] [CrossRef] [PubMed]
- Smiech, M.; Leszczynski, P.; Kono, H.; Wardell, C.; Taniguchi, H. Emerging BRAF Mutations in Cancer Progression and Their Possible Effects on Transcriptional Networks. Genes 2020, 11, 1342. [Google Scholar] [CrossRef]
- Elshafae, S.M.; Dirksen, W.P.; Alasonyalilar-Demirer, A.; Breitbach, J.; Yuan, S.; Kantake, N.; Supsavhad, W.; Hassan, B.B.; Attia, Z.; Alstadt, L.B.; et al. Canine prostatic cancer cell line (LuMa) with osteoblastic bone metastasis. Prostate 2020, 80, 698–714. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.K.; Mohamad, N.V.; Giaze, T.R.; Chin, K.Y.; Mohamede, N.; Ima-Nirwana, S. Prostate Cancer and Bone Metastases: The Underlying Mechanisms. Int. J. Mol. Sci. 2019, 20, 2587. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Wang, Z.; Li, F.; Yang, J.; Tang, L. miR-138 modulates prostate cancer cell invasion and migration via Wnt/β-catenin pathway. Mol. Med. Rep. 2018, 17, 3140–3145. [Google Scholar] [CrossRef] [PubMed]
- Murillo-Garzón, M.; Kypta, R. WNT signalling in prostate cancer. Nat. Rev. Urol. 2017, 14, 683. [Google Scholar] [CrossRef] [PubMed]
- Stamos, J.L.; Weis, W.I. The β-Catenin destruction complex. Cold Spring Harb. Perspect. Biol. 2013, 5, a007898. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Shukeir, N.; Potti, A.; Sircar, K.; Aprikian, A.; Goltzman, D.; Rabbani, S.A. Up-regulation of Wnt-1 and beta-catenin production in patients with advanced metastatic prostate carcinoma: Potential pathogenetic and prognostic implications. Cancer 2004, 101, 1345–1356. [Google Scholar] [CrossRef]
- Rodrigues, M.M.P.; Rema, A.; Gartner, M.F.; Laufer-Amorim, R. Role of adhesion molecules and proliferation hyperplasic, pre neoplastic and neoplastic lesions in canine prostate. Pak. J. Biol. Sci. 2013, 16, 1324–1329. [Google Scholar] [CrossRef]
- Cervantes-Arias, A.; Pang, L.Y.; Argyle, D.J. Epithelial-mesenchymal transition as a fundamental mechanism underlying the cancer phenotype. Vet. Comp. Oncol. 2013, 11, 169–184. [Google Scholar] [CrossRef]
- Scanlon, C.S.; Van Tubergen, E.A.; Inglehart, R.C.; D’Silva, N.J. Biomarkers of epithelial-mesenchymal transition in squamous cell carcinoma. J. Dent. Res. 2013, 92, 114–121. [Google Scholar] [CrossRef]
- Lean, F.Z.; Kontos, S.; Palmieri, C. Expression of β-catenin and mesenchymal markers in canine prostatic hyperplasia and carcinoma. J. Comp. Pathol. 2014, 150, 373–381. [Google Scholar] [CrossRef]
- Tsui, K.H.; Lin, Y.H.; Chung, L.C.; Chuang, S.T.; Feng, T.H.; Chiang, K.C.; Chang, P.L.; Yeh, C.J.; Juang, H.H. Prostate-derived ets factor represses tumorigenesis and modulates epithelial-to-mesenchymal transition in bladder carcinoma cells. Cancer Lett. 2016, 375, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Rebowe, R.E.; Wang, Z.; Li, Y.; Wang, Z.; DePaolo, J.S.; Guo, J.; Qian, C.; Liu, W. KIF3a Promotes Proliferation and Invasion via Wnt Signaling in Advanced Prostate Cancer. Mol. Cancer Res. 2014, 12, 491–503. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Qin, Z.; Yang, K.; Liu, R.; Xu, Y. Cripto-1 promotes epithelial-mesenchymal transition in prostate cancer via Wnt/β-catenin signaling. Oncol. Rep. 2017, 35, 1521–1528. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Pang, W.; Zhou, Z.; Chen, Y.; Mo, T.; Li, M.; Liu, C. Dickkopf 2 promotes proliferation and invasion via Wnt signaling in prostate cancer. Mol. Med. Rep. 2016, 14, 2283–2288. [Google Scholar] [CrossRef] [PubMed]
- Dong, B.; Xu, G.C.; Liu, S.T.; Geng, B. MiR-34a affects G2 arrest in prostate cancer PC3 cells via Wnt pathway and inhibits cell growth and migration. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 8349–8358. [Google Scholar]
- He, P.; Xiong, G.; Guo, W.; Jiang, G.; Li, Y.; Li, H. Long non-coding RNA CCAT2 promotes prostate cancer cell proliferation and invasion by regulating the Wnt/β-catenin signaling pathway. Oncol. Lett. 2020, 20, 97. [Google Scholar] [CrossRef]
- Li, J.B.; Liu, F.; Zhang, B.P.; Bai, W.K.; Cheng, W.; Zhang, Y.; Yu, L.J. LncRNA625 modulates prostate cancer cells proliferation and apoptosis through regulating the Wnt/β-catenin pathway by targeting miR-432. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 2586–2595. [Google Scholar]
- Yu, F.; Yu, C.; Li, F.; Zuo, Y.; Wang, Y.; Yao, L.; Wu, C.; Wang, C.; Ye, L. Wnt/β-catenin signaling in cancers and targeted therapies. Signal. Transduct. Target. Ther. 2021, 6, 307. [Google Scholar] [CrossRef]
- Fonseca-Alves, C.E.; Rodrigues, M.M.P.; Moura, V.M.B.D.; Rogatto, S.R.; Laufer-Amorim, R. Alterations of C-MYC, NKX3.1, and E-Cadherin Expression in Canine Prostate Carcinogenesis. Microsc Res. Technol. 2013, 76, 1250–1256. [Google Scholar] [CrossRef]
- Kobayashi, P.E.; Fonseca-Alves, C.E.; Rivera-Caldero, L.G.; Carvalho, M.; Kuasne, H.; Rogatto, S.R.; Laufer-Amorim, R. Deregulation of E-cadherin, β-catenin, APC and Caveolin-1 expression occurs in canine prostate cancer and metastatic processes. Res. Vet. Sci. 2018, 118, 254–261. [Google Scholar] [CrossRef]
- Fonseca-Alves, C.E.; Kobayashi, P.E.; Leis-Filho, A.F.; Lainetti, P.F.; Grieco, V.; Kuasne, L.; Rogatto, S.R.; Laufer-Amorim, R. E-Cadherin Downregulation is Mediated by Promoter Methylation in Canine Prostate Cancer. Front. Genet. 2019, 10, 1242. [Google Scholar] [CrossRef] [PubMed]
- Busch, E.L.; Hornick, J.L.; Umeton, R.; Albayrak, A.; Lindeman, N.I.; MacConaill, L.E.; Garcia, E.P.; Ducar, M.; Rebbeck, T.R. Somatic mutations in CDH1 and CTNNB1 in primary carcinomas at 13 anatomic sites. Oncotarget 2017, 8, 85680–85691. [Google Scholar] [CrossRef] [PubMed]
- Mostafavi-Pour, Z.; Kianpour, S.; Dehghani, M.; Mokarram, P.; Torabinejad, S.; Monabati, A. Methylation of Integrin α4 and E-Cadherin genes in human prostate cancer. Pathol. Oncol. Res. 2015, 21, 921–927. [Google Scholar] [CrossRef] [PubMed]
- Salda, L.D.; Massimini, M.; Romanucci, M.; Palmieri, C.; Perillo, A.; Grieco, V.; Malatesta, D.; Spinillo, M.A.; Passantino, G.; Dondi, F.; et al. Nectin-4 and p63 immunohistochemical expression in canine prostate tumourigenesis. Vet. Comp. Oncol. 2018, 17, 1–10. [Google Scholar] [CrossRef]
- Odero-Marah, V.; Hawsawi, O.; Henderson, V.; Sweeney, J. Epithelial-Mesenchymal Transition (EMT) and Prostate Cancer. Adv. Exp. Med. Biol. 2018, 1095, 101–110. [Google Scholar]
- Hu, J.; Tian, J.; Zhu, S.; Sun, L.; Yu, J.; Tian, H.; Dong, Q.; Luo, Q.; Jiang, N.; Niu, Y.; et al. Sox5 contributes to prostate cancer metastasis and is a master regulator of TGF-b-induced epithelial mesenchymal transition through controlling Twist1 expression. Br. J. Cancer 2018, 118, 88–97. [Google Scholar] [CrossRef]
- Zhang, L.; Sha, J.; Yang, G.; Huang, Y.; Bo, J.; Huang, Y. Activation of Notch pathway is linked with epithelial-mesenchymal transition in prostate cancer cells. Cell Cycle 2017, 16, 999–1007. [Google Scholar] [CrossRef]
- Fonseca-Alves, C.E.; Kobayashi, P.E.; Rivera-Caldero, L.G.; Laufer-Amorim, R. Evidence of epithelial-mesenchymal transition in canine prostate cancer metastasis. Res. Vet. Sci. 2015, 100, 176–181. [Google Scholar] [CrossRef]
- Elshafae, S.M.; Hassan, B.B.; Supsavhad, W.; Dirksen, W.P.; Camiener, R.Y.; Ding, H.; Tweedle, M.F.; Rosol, T.J. Gastrin-Releasing Peptide Receptor (GRPr) Promotes EMT, Growth, and Invasion in Canine Prostate Cancer. Prostate 2016, 76, 796–809. [Google Scholar] [CrossRef]
- Preston, S.R.; Miller, G.V.; Primrose, J.N. Bombesin-like peptides and cancer. Crit. Rev. Oncol. Hematol. 1996, 23, 225–238. [Google Scholar] [CrossRef]
- Leis-Filho, A.F.; Lainetti, P.D.; Kobayashi, P.E.; Palmieri, C.; Amorim, R.L.; Fonseca-Alves, C.E. Expression and prognostic significance of vascular endothelial growth factor-A (VEGF-A) and its receptor in canine prostate cancer. Prostate 2021, 81, 1021–1031. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Pérez, J.; Monter-Vera, M.D.R.; Barrientos-Alvarado, C.; Toscano-Garibay, J.D.; Cuesta-Mejías, T.; Flores-Estrada, J. Evaluation of VEGF and PEDF in prostate cancer: A preliminary study in serum and biopsies. Oncol. Lett. 2018, 15, 1072–1078. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, C. Immunohistochemical Expression of Angiogenic Factors by Neoplastic Epithelial Cells Is Associated with Canine Prostatic Carcinogenesis. Oncology 2015, 52, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Kano, M.R.; Morishita, Y.; Iwata, C.; Iwasaka, S.; Watabe, T.; Ouchi, Y.; Miyazono, K.; Miyazawa, K. VEGF-A and FGF-2 synergistically promote neoangiogenesis through enhancement of endogenous PDGF-B-PDGFRbeta signaling. J. Cell Sci. 2005, 118, 3759–3768. [Google Scholar] [CrossRef] [PubMed]
- Pecqueux, C.; Arslan, A.; Heller, M.; Falkenstein, M.; Kaczorowski, A.; Tolstov, Y.; Sültmann, H.; Grüllich, C.; Herpel, E.; Duensing, A.; et al. FGF-2 is a driving force for chromosomal instability and a stromal factor associated with adverse clinico-pathological features in prostate cancer. Urol. Oncol. 2018, 36, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Khodamoradi, P.; Amniattalab, A.; Alizadeh, S. Overexpression of GDNF and FGF-1 in Canine Benign Prostatic Hyperplasia: Evidence for a Pathogenetic Role of Neural Growth Factor. J. Comp. Path. 2021, 182, 43–53. [Google Scholar] [CrossRef]
- Li, L.; Ameri, A.H.; Wang, S.; Jansson, K.H.; Casey, O.M.; Yang, Q.; Beshiri, M.L.; Fang, L.; Lake, R.; Agarwal, S.; et al. EGR1 Regulates Angiogenic and Osteoclastogenic Factors in Prostate Cancer and Promotes Metastasis. Oncogene 2019, 38, 6241. [Google Scholar] [CrossRef]
- Su, Y.; Zhang, Y.; Zhao, J.; Zhou, W.; Wang, W.; Han, B.; Wang, X. FOXA1 promotes prostate cancer angiogenesis by inducing multiple pro-angiogenic factors expression. J. Cancer Res. Clin. Oncol. 2021, 147, 3225–3243. [Google Scholar] [CrossRef]
- Venkatesan, T.; Alaseem, A.; Chinnaiyan, A.; Dhandayuthapani, S.; Kanagasabai, T.; Alhazzani, K.; Dondapati, P.; Alobid, S.; Natarajan, U.; Schwartz, R.; et al. MDM2 Overexpression Modulates the Angiogenesis-Related Gene Expression Profile of Prostate Cancer Cells. Cells 2018, 7, 41. [Google Scholar] [CrossRef]
- Malik, R.; Lelkes, P.I.; Cukierman, E. Biomechanical and biochemical remodeling of stromal extracellular matrix in cancer. Trends Biotechnol. 2015, 33, 230–236. [Google Scholar] [CrossRef]
- Schoepp, M.; Ströse, A.J.; Haier, J. Dysregulation of miRNA Expression in Cancer Associated Fibroblasts (CAFs) and Its Consequences on the Tumor Microenvironment. Cancers 2017, 9, 54. [Google Scholar] [CrossRef] [PubMed]
- Penet, M.F.; Kakkad, S.; Pathak, A.P.; Krishnamachary, B.; Mironchik, Y.; Raman, V.; Solaiyappan, M.; Bhujwalla, Z.M. Structure and Function of a Prostate Cancer Dissemination-Permissive Extracellular Matrix. Clin. Cancer Res. 2017, 23, 2245–2254. [Google Scholar] [CrossRef] [PubMed]
- Rivera Calderón, L.G.; Kobayashi, P.E.; Vasconcelos, R.O.; Fonseca-Alves, C.E.; Laufer-Amorim, R. Characterization of collagens fibers (I, III, IV) and elastin in the extracellular matrix of normal and neoplastic canine prostate. Vet. Sci. 2019, 6, 22. [Google Scholar] [CrossRef] [PubMed]
- Duarte, A.H.; Colli, S.; Alves-Pereira, J.L.; Martins, M.P.; Sampaio, F.J.B.; Ramos, C.F. Collagen I and III and metalloproteinase gene and protein expression in prostate cancer in relation to Gleason score. Int. Braz. J. Urol. 2012, 38, 341–355. [Google Scholar] [CrossRef] [PubMed]
- Faleiro, M.B.R.; Croce, G.B.; Toledo, D.C.; Rodrigues, M.M.P.; Batista, A.C.; Damasceno, A.D.; Brito, L.A.B.; Laufer-Amorim, R.; Moura, V.M.D. Matrix metalloproteinases 2 and 9 expression in canine normal prostate and with proliferative disorders. Cienc. Rural 2013, 43, 1037–1043. [Google Scholar] [CrossRef]
- Palumbo, A.J.; Ferreira, L.B.; Reis de Souza, P.A.; Oliveira, F.L.; Pontes, B.; Viana, N.B.; Machado, D.E.; Palmero, C.Y.; Alves, L.M.; Gimba, E.R.P.; et al. Extracellular matrix secreted by reactive stroma is a main inducer of pro-tumorigenic features on LNCaP prostate cancer cells. Cancer Lett. 2012, 321, 55–64. [Google Scholar] [CrossRef]
- Cutruzzolà, F.; Giardina, G.; Marani, M.; Macone, A.; Paiardini, A.; Rinaldo, S.; Paone, A. Glucose Metabolism in the Progression of Prostate Cancer. Front. Physiol. 2017, 8, 97. [Google Scholar] [CrossRef]
- Burdelski, C.; Shihada, R.; Hinsch, A.; Angerer, A.; Göbel, C.; Friedrich, E.; Hube-Magg, C.; Burdak-Rothkamm, S.; Kluth, M.; Simon, R.; et al. High-Level Glyoxalase 1 (GLO1) expression is linked to poor prognosis in prostate cancer. Prostate 2017, 77, 1528–1538. [Google Scholar] [CrossRef]
- Khalighinejad, P.; Parrott, D.; Jordan, V.C.; Chirayil, S.; Preihs, C.; Rofsky, N.M.; Xi, Y.; Sherry, A.D. Magnetic Resonance Imaging Detection of Glucose-Stimulated Zinc Secretion in the Enlarged Dog Prostate as a Potential Method for Differentiating Prostate Cancer from Benign Prostatic Hyperplasia. Investig. Radiol. 2021, 56, 450–457. [Google Scholar] [CrossRef]
- Valle-Mendiola, A.; Soto-Cruz, I. Energy Metabolism in Cancer: The Roles of STAT3 and STAT5 in the Regulation of Metabolism-Related Genes. Cancers 2020, 12, 124. [Google Scholar] [CrossRef]
- Faleiro, M.B.R.; Toledo, D.C.; Rodrigues, M.M.P.; Laufer-Amorim, R.; Brito, L.A.B.; Damasceno, A.D.; Moura, V.M.D. uPAR expression in canine normal prostate and with proliferative disorders. Ciência Anim. Bras. 2013, 14, 237–244. [Google Scholar]
- Santi, A.L.; Napolitnao, F.; Montuori, N.; Ragno, P. The Urokinase Receptor: A Multifunctional Receptor in Cancer Cell Biology. Therapeutic Implications. Int. J. Mol. Sci. 2021, 22, 4111. [Google Scholar] [CrossRef] [PubMed]
- Sfanos, K.S.; Yegnasubramanian, S.; Nelson, W.G.; De Marzo, A.M. The inflammatory microenvironment and microbiome in prostate cancer development. Nat. Rev. Urol. 2018, 15, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Attwood, K.; Bshara, W.; Mohler, J.L.; Guru, K.; Xu, B.; Kalinski, P.; Chatta, G. High intratumoral CD8+T cell infiltration is associated withimproved survival in prostate cancer patients undergoingradical prostatectomy. Prostate 2020, 81, 20–28. [Google Scholar] [CrossRef]
- Flammiger, A.; Bayer, F.; Cirugeda-Kuhnert, A.; Huland, H.; Tennstedt, P.; Simon, R.; Minner, S.; Bokemeyer, C.; Sauter, G.; Schlomm, T.; et al. Intratumoral T but not B lymphocytes are related to clinical outcome in prostate cancer. APMIS 2012, 120, 901–908. [Google Scholar] [CrossRef]
- Strasner, A.; Karin, M. Immune Infiltration and Prostate Cancer. Front. Oncol. 2015, 5, 128. [Google Scholar] [CrossRef]
- Palmieri, C.; Hood, G.; Fonseca-Alves, C.E.; Laufer-Amorim, R.; Allavena, R. An immunohistochemical study of T and B lymphocyte density in prostatic hyperplasia and prostate carcinoma in dogs. Res. Vet. Sci. 2019, 122, 189–192. [Google Scholar] [CrossRef]
- Vitkin, N.; Nersesian, S.; Siemens, D.R.; Koti, M. The Tumor Immune Contexture of Prostate Cancer. Front. Immunol. 2019, 10, 603. [Google Scholar] [CrossRef]
- Xu, W.; Qian, J.; Zeng, F.; Li, S.; Guo, W.; Chen, L.; Li, G.; Zhang, Z.; Wang, Q.J.; Deng, F. Protein kinase Ds promote tumor angiogenesis through mast cell recruitment and expression of angiogenic factors in prostate cancer microenvironment. J. Exp. Clin. Cancer Res. 2019, 38, 114. [Google Scholar] [CrossRef]
- Moore, G.Y.; Pidgeon, G.P. Cross-Talk between Cancer Cells and the Tumour Microenvironment: The Role of the 5-Lipoxygenase Pathway. Int. J. Mol. Sci. 2017, 12, 1–27. [Google Scholar] [CrossRef]
- Meng, Z.; Cao, R.; Yang, Z.; Liu, T.; Wang, Y.; Wang, X. Inhibitor of 5-lipoxygenase, zileuton, suppresses prostate cancer metastasis by upregulating E-cadherin and paxillin. Urology 2013, 82, 1452.e7–1452.e14. [Google Scholar] [CrossRef] [PubMed]
- Sarveswaran, S.; Varma, N.R.S.; Morisetty, S.; Ghosh, J. Inhibition of 5-lipoxygenase downregulates stemness and kills prostate cancer stem cells by triggering apoptosis via activation of c-Jun N-terminal kinase. Oncotarget3 2019, 10, 424–436. [Google Scholar] [CrossRef] [PubMed]
- Goodman, L.A.; Jarrett, C.L.; Krunkosky, T.M.; Budsberg, S.C.; Nothrup, N.C.; Saba, C.F.; LeRoy, B.E. 5-Lipoxygenase expression in benign andmalignant canine prostate tissues. Vet. Comp. Oncol. 2010, 9, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Finotello, R.; Schiavo, L.; Ressel, L.; Frohmader, A.; Silvestrini, P.; Verin, R. Lipoxygenase-5 Expression in Canine Urinary Bladder: Normal Urothelium, Cystitis and Transitional Cell Carcinoma. J. Comp. Pathol. 2019, 170, 1–9. [Google Scholar] [CrossRef]
- L’Eplattenier, H.F.; Lai, C.L.; van den Ham, R.; Mol, J.; van Sluijs, F.; Teske, E. Regulation of COX-2 Expression in Canine Prostate Carcinoma: Increased COX-2 Expression is Not Related to Inflammation. J. Vet. Intern. Med. 2007, 21, 776–782. [Google Scholar] [CrossRef]
- Desai, S.J.; Prickril, B.; Rasooly, A. Mechanisms of phytonutrient modulation of Cyclooxygenase-2 (COX-2) and inflammation related to cancer. Nutr. Cancer 2019, 70, 350–375. [Google Scholar] [CrossRef]
- Cevik, O.; Turut, F.A.; Acidereli, H.; Yildirim, S. Cyclosporine-A induces apoptosis in human prostate cancer cells PC3 and DU145 via downregulation of COX-2 and upregulation of TGFβ. Turk. J. Biochem. 2019, 44, 47–54. [Google Scholar] [CrossRef]
- Rodrigues, M.M.P.; Di Santis, G.W.; Moura, V.M.B.D.; Laufer-Amorim, R. COX-2 and TGF-B expression in proliferative disorders of canine prostate. Braz. J. Vet. Pathol. 2010, 3, 31–36. [Google Scholar]
- Mosli, H.A.; Al-Abd, A.M.; El-Shaer, M.A.; Khedr, A.; Gazzaz, F.S.; Abdel-Naim, A.B. Local inflammation influences oestrogen metabolism in prostatic tissue. BJU Int. 2012, 110, 274–282. [Google Scholar] [CrossRef]
- Chughtai, B.; Lee, R.; Te, A.; Kaplan, S. Role of inflammation in benign prostatic hyperplasia. Rev. Urol. 2011, 13, 147–150. [Google Scholar]
- Bennett, T.C.; Matz, B.M.; Henderson, R.A.; Straw, R.C.; Liptak, J.M.; Selmic, L.E.; Collivignarelli, F.; Buracco, P. Total prostatectomy as a treatment for prostatic carcinoma in 25 dogs. Vet. Surg. 2018, 47, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Packeiser, E.M.; Hewicker-Trautwein, M.; Thiemeyer, H.; Mohr, A.; Junginger, J.; Schille, J.T.; Escobar, H.M.; Nolte, I. Characterization of six canine prostate adenocarcinoma and three transitional cell carcinoma cell lines derived from primary tumor tissues as well as metastasis. PLoS ONE 2020, 15, e0230272. [Google Scholar] [CrossRef] [PubMed]
- Lekchnov, E.A.; Amelina, E.V.; Bryzgunova, O.E.; Zaporozhchenko, I.A.; Konoshenko, M.Y.; Yarmoschuk, S.V.; Murashov, I.S.; Pashkovskaya, O.A.; Gorizkii, A.M.; Zheravin, A.A.; et al. Searching for the Novel Specific Predictors of Prostate Cancer in Urine: The Analysis of 84 miRNA Expression. Int. J. Mol. Sci. 2018, 19, 4088. [Google Scholar] [CrossRef] [PubMed]
- Pashaei, E.; Pashaei, E.; Ahmady, M.; Ozen, M.; Aydin, N. Meta-analysis of miRNA expression profiles for prostate cancer recurrence following radical prostatectomy. PLoS ONE 2017, 12, e0179543. [Google Scholar] [CrossRef]
- Cai, C.; Zhi, Y.; Wang, K.; Zhang, P.; Ji, Z.; Xie, C.; Sun, F. CircHIPK3 overexpression accelerates the proliferation and invasion of prostate cancer cells through regulating miRNA-338-3p. Oncol. Targets. Ther. 2019, 12, 3363–3372. [Google Scholar] [CrossRef]
- Fabian, M.R.; Sonenberg, N.; Filipowicz, W. Regulation of mRNA translation and stability by microRNAs. Annu. Rev. Biochem. 2010, 79, 351–379. [Google Scholar] [CrossRef]
- Schwarzenbach, H. The clinical relevance of circulating, exosomal miRNAs as biomarkers for cancer. Expert Rev. Mol. Diagn. 2015, 15, 1159–1169. [Google Scholar] [CrossRef]
- Yang, X.; Yang, Y.; Gan, R.; Zhao, L.; Li, W.; Zhou, H.; Wang, X.; Lu, J.; Meng, Q.H. Down-regulation of mir-221 and mir-222 restrain prostate cancer cell proliferation and migration that is partly mediated by activation of SIRT1. PLoS ONE 2014, 9, e98833. [Google Scholar] [CrossRef]
- Cui, M.; Wang, H.; Yao, X.; Zhang, D.; Xie, Y.; Cui, R.; Zhang, X. Circulating MicroRNAs in Cancer: Potential and Challenge. Front. Genet. 2019, 10, 626. [Google Scholar] [CrossRef]
- Kobayashi, M.; Saito, A.; Tanaka, Y.; Michishita, M.; Kobayashi, M.; Irimajiri, M.; Kaneda, T.; Ochiai, K.; Bonkobara, M.; Takahashi, K.; et al. MicroRNA expression profiling in canine prostate cancer. J. Vet. Med. Sci. 2017, 79, 719–725. [Google Scholar] [CrossRef]
- Bidarra, D.; Constâncio, V.; Barros-Silva, D.; Ramalho-Carvalho, J.; Moreira-Barbosa, C.; Antunes, L.; Maurício, J.; Oliveira, J.; Henrique, R.; Jerónimo, C. Circulating MicroRNAs as Biomarkers for Prostate Cancer Detection and Metastasis Development Prediction. Front. Oncol. 2019, 9, 900. [Google Scholar] [CrossRef] [PubMed]
- Urabe, F.; Matsuzaki, J.; Yamamoto, Y.; Kimura, T.; Hara, T.; Ichikawa, M.; Takizawa, S.; Aoki, Y.; Niida, S.; Sakamoto, H.; et al. Large-scale Circulating microRNA Profiling for the Liquid Biopsy of Prostate Cancer. Clin. Cancer Res. 2019, 25, 3016–3025. [Google Scholar] [CrossRef] [PubMed]
- Candas, B.; Cusan, L.; Gomez, J.; Diamond, P.; Suburu, R.; Lévesque, J.; Brousseau, G.; Bélanger, A.; Labrie, F. Evaluation of prostatic specific antigen and digital rectal examination as screening tests for prostate cancer. Prostate 2000, 45, 19–35. [Google Scholar] [CrossRef]
- De la Taille, A.; Olsson, C.; Buttyan, R.; Benson, M.; Bagiella, E.; Cao, Y.; Burchardt, M.; Chopin, D.; Katz, A. Blood-based reverse transcriptase polymerase chain reaction assays for prostatic specific antigen: Long term follow-up confirms the potential utility of this assay in identifying patients more likely to have biochemical recurrence (rising PSA) following r. Int. J. Cancer 1999, 84, 360–364. [Google Scholar] [CrossRef]
- Dubé, J.; Lazure, C.; Tremblay, R. Dog prostate arginine esterase is related to human prostate specific antigen. Clin. Investig. Med. 1986, 9, 51–54. [Google Scholar]
- Holst, B.; Carlin, S.; Fouriez-Lablée, V.; Hanås, S.; Ödling, S.; Langborg, L.; Ubhayasekera, S.; Bergquist, J.; Rydén, J.; Holmroos, E.; et al. Concentrations of canine prostate specific esterase, CPSE, at baseline are associated with the relative size of the prostate at three-year follow-up. BMC Vet. Res. 2021, 17, 173. [Google Scholar] [CrossRef]
- Aguiar, A.; Rodrigues, M.; Fonseca-Alves, C.E.; Santos, F.; Vilamaior, P.; Taboga, S. Female Paraurethral Prostate Gland in Bitches. Brazilian J. Vet. Pathol. 2013, 6, 106–110. [Google Scholar]
- Bryan, J.; Keeler, M.; Henry, C.; Bryan, M.; Hahn, A.; Caldwell, C. A population study of neutering status as a risk factor for canine prostate cancer. Prostate 2007, 67, 1174–1181. [Google Scholar] [CrossRef]
- Leis-Filho, A.; Lainetti, P.; Franzoni, M.; Palmieri, C.; Kobayshi, P.; Laufer-Amorim, R.; Fonseca-Alves, C. A Comparative in Silico Analysis of CD24’s Prognostic Value in Human and Canine Prostate Cancer. J. Pers Med. 2021, 11, 232. [Google Scholar] [CrossRef]
- Thiemeyer, H.; Taher, L.; Schille, J.; Packeiser, E.; Harder, L.; Hewicker-Trautwein, M.; Brenig, B.; Schütz, E.; Beck, J.; Nolte, I.; et al. An RNA-Seq-Based Framework for Characterizing Canine Prostate Cancer and Prioritizing Clinically Relevant Biomarker Candidate Genes. Int J. Mol. Sci 2021, 22, 11481. [Google Scholar] [CrossRef]
- Thiemeyer, H.; Taher, L.; Schille, J.; Harder, L.; Hungerbuehler, S.; Mischke, R.; Hewicker-Trautwein, M.; Kiełbowicz, Z.; Brenig, B.; Schütz, E.; et al. Suitability of ultrasound-guided fine-needle aspiration biopsy for transcriptome sequencing of the canine prostate. Sci. Rep. 2019, 9, 13216. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nascente, E.d.P.; Amorim, R.L.; Fonseca-Alves, C.E.; de Moura, V.M.B.D. Comparative Pathobiology of Canine and Human Prostate Cancer: State of the Art and Future Directions. Cancers 2022, 14, 2727. https://doi.org/10.3390/cancers14112727
Nascente EdP, Amorim RL, Fonseca-Alves CE, de Moura VMBD. Comparative Pathobiology of Canine and Human Prostate Cancer: State of the Art and Future Directions. Cancers. 2022; 14(11):2727. https://doi.org/10.3390/cancers14112727
Chicago/Turabian StyleNascente, Eduardo de Paula, Renée Laufer Amorim, Carlos Eduardo Fonseca-Alves, and Veridiana Maria Brianezi Dignani de Moura. 2022. "Comparative Pathobiology of Canine and Human Prostate Cancer: State of the Art and Future Directions" Cancers 14, no. 11: 2727. https://doi.org/10.3390/cancers14112727
APA StyleNascente, E. d. P., Amorim, R. L., Fonseca-Alves, C. E., & de Moura, V. M. B. D. (2022). Comparative Pathobiology of Canine and Human Prostate Cancer: State of the Art and Future Directions. Cancers, 14(11), 2727. https://doi.org/10.3390/cancers14112727







