LncRNA JPX Promotes Esophageal Squamous Cell Carcinoma Progression by Targeting miR-516b-5p/VEGFA Axis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Tissue Samples
2.2. Cell Culture
2.3. Small Interfering RNA (siRNA) Synthesis, Plasmid Construction, and Transfection
2.4. Subcellular Fractionation
2.5. Fluorescence In Situ Hybridization (FISH)
2.6. RNA Extraction and Quantitative Real-Time PCR (qRT-PCR)
2.7. Western Blot
2.8. Cell Proliferation Assay
2.9. 5-Ethynyl-2′-Deoxyuridine (EdU) Incorporation Assay
2.10. Transwell Migration and Invasion Assays
2.11. Cell Cycle Analysis
2.12. RNA Pull-Down Assay
2.13. RNA Immunoprecipitation (RIP) Assay
2.14. Luciferase Report Assay
2.15. Tube Formation Assay
2.16. In Vivo Experiments
2.17. Immunohistochemistry
2.18. Statistical Analysis
3. Results
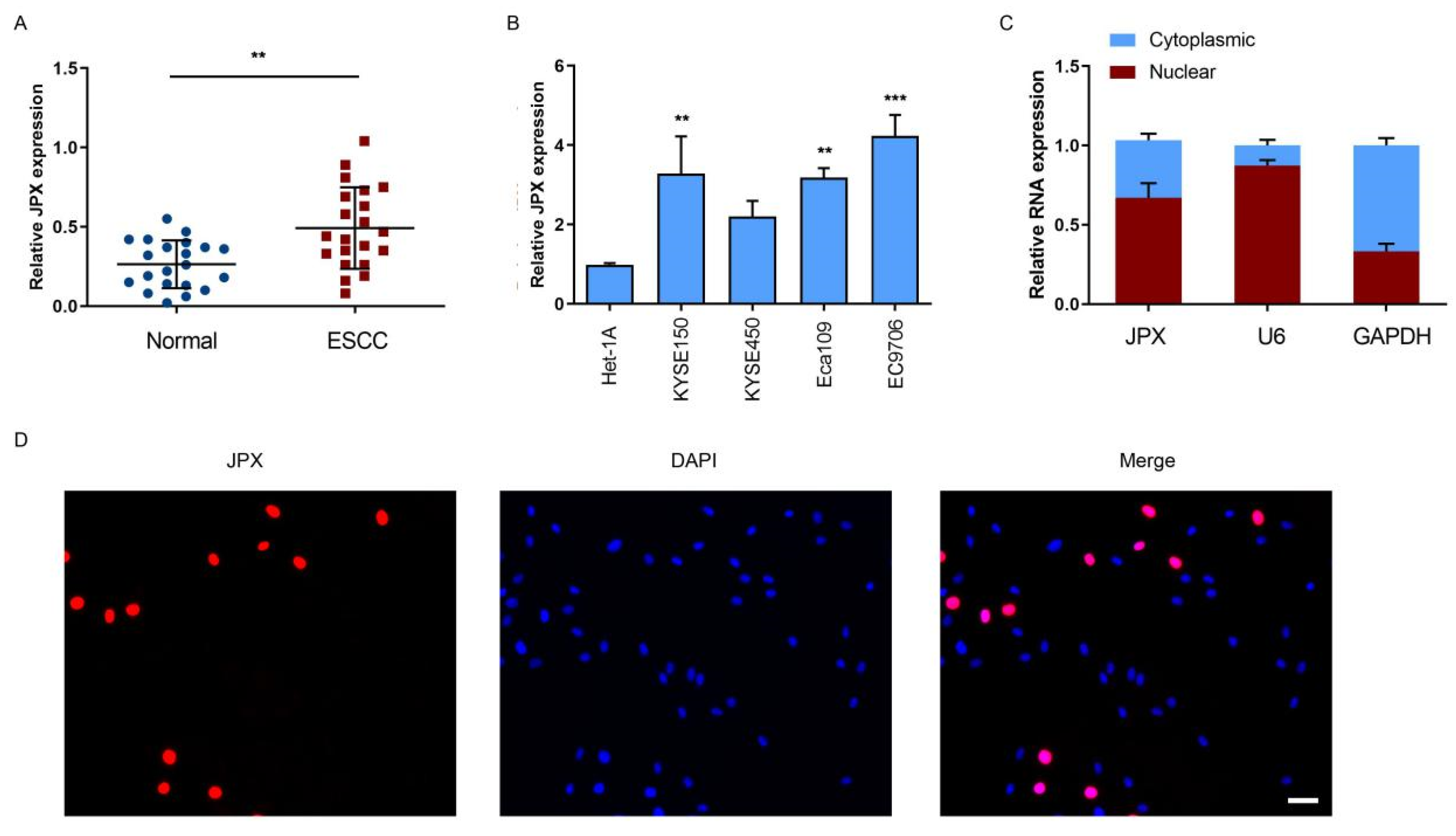
3.1. LncRNA JPX was Upregulated in ESCC
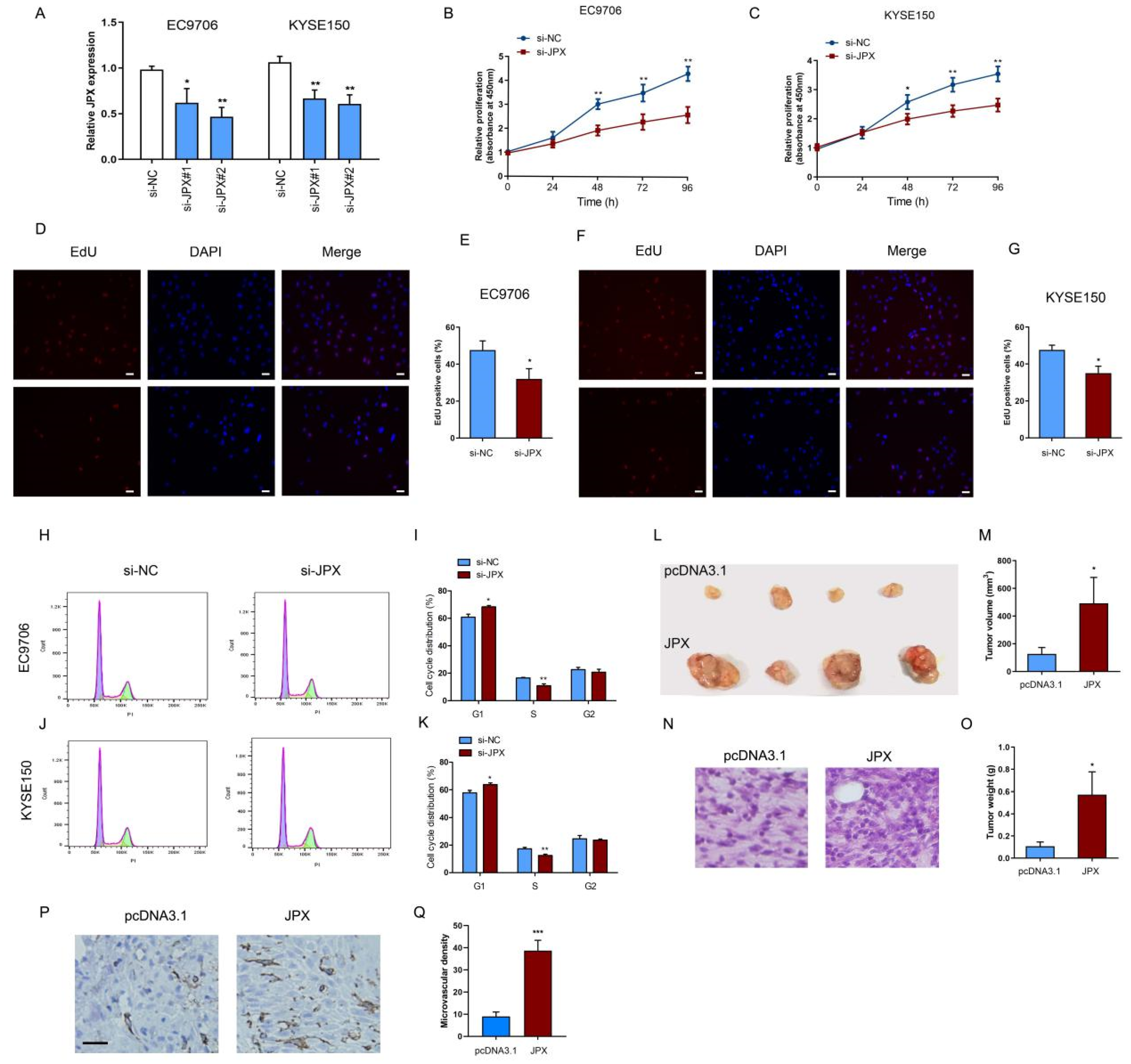
3.2. JPX Promoted Cell Proliferation and Tumor Growth in ESCC
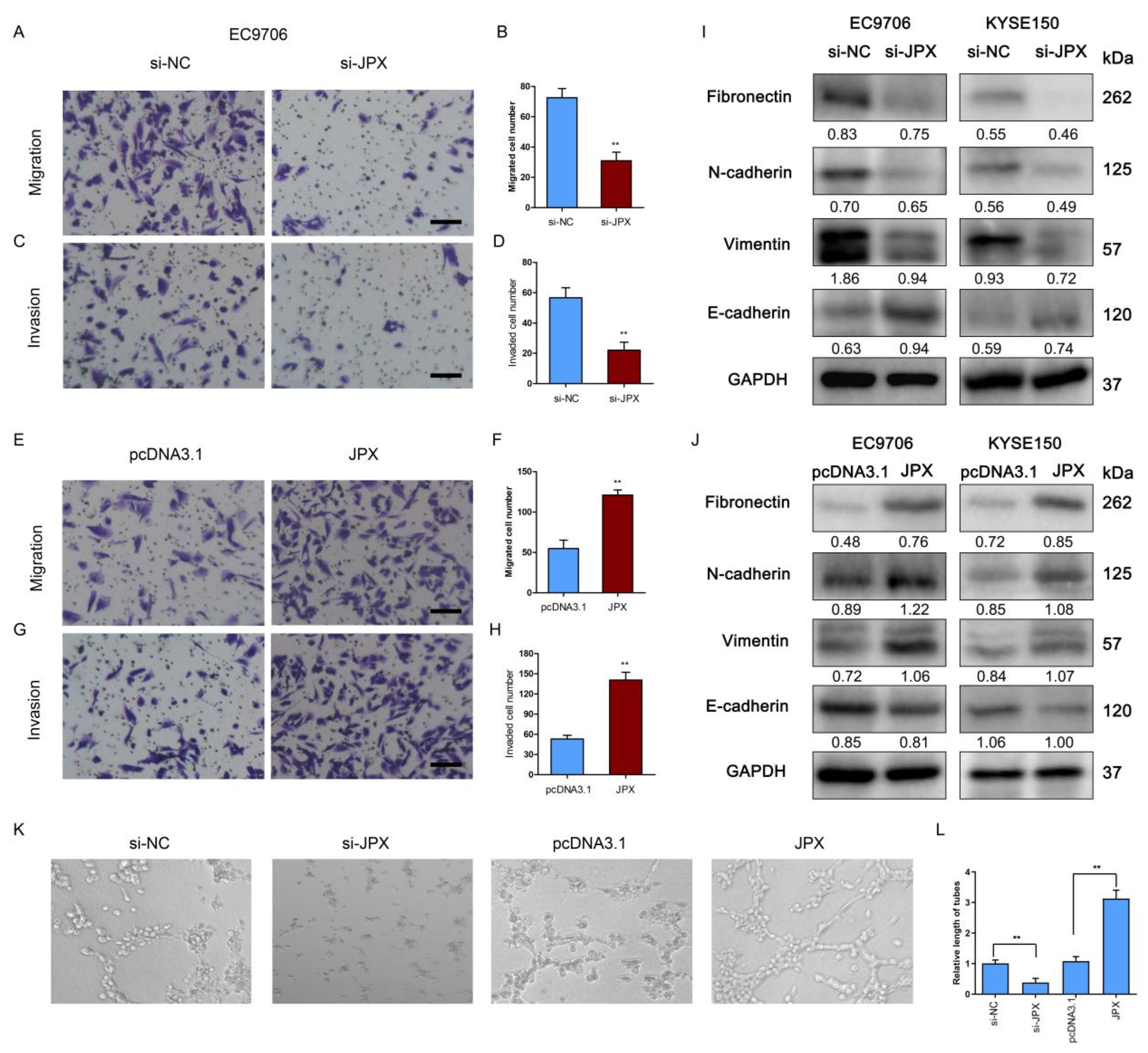
3.3. JPX Promoted ESCC Mobility In Vitro
3.4. JPX Functioned as a Sponge of miR-516b-5p in ESCC
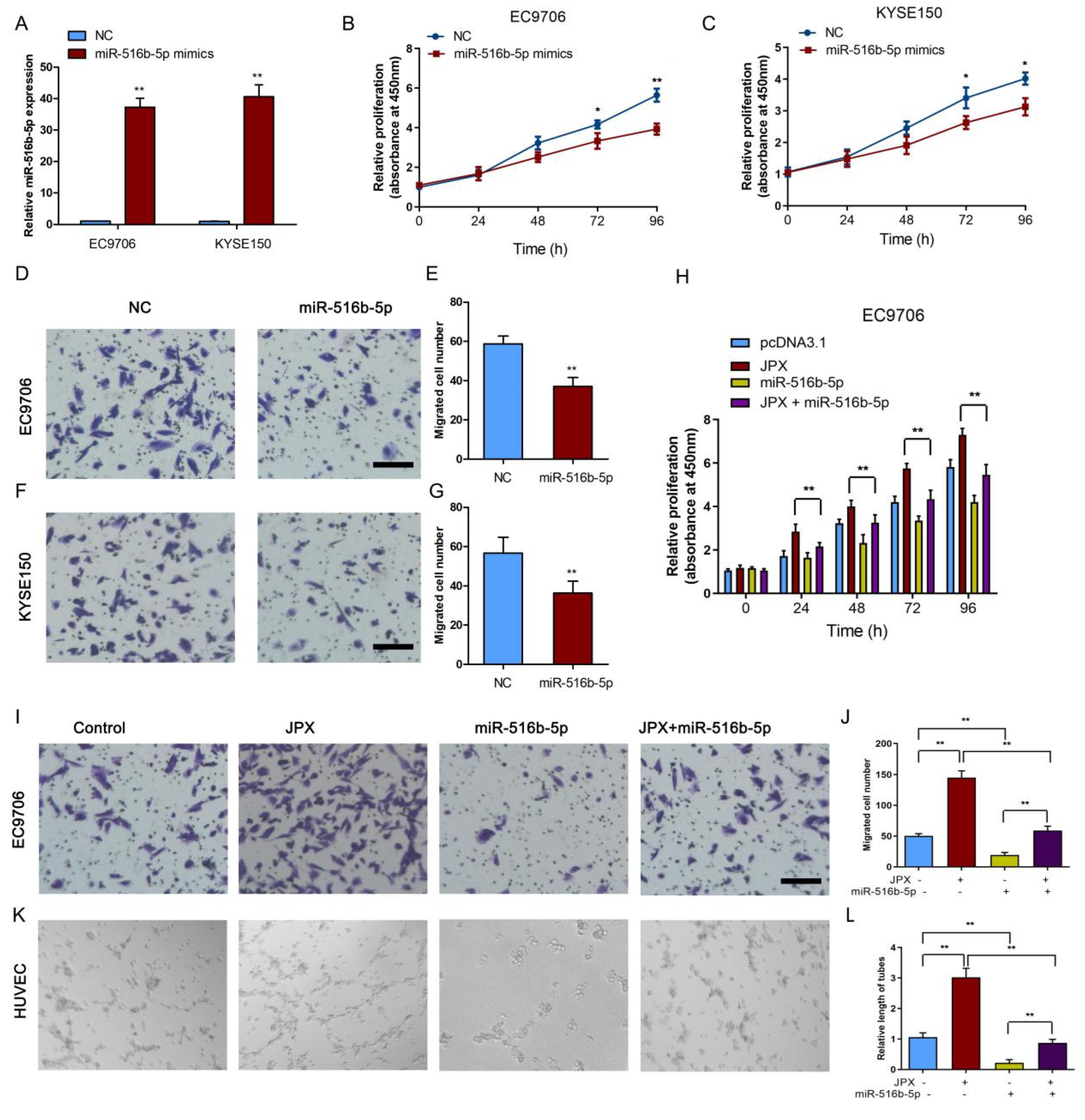
3.5. MiR-516b-5p Abolished JPX-Promoted ESCC Growth and Mobility In Vitro
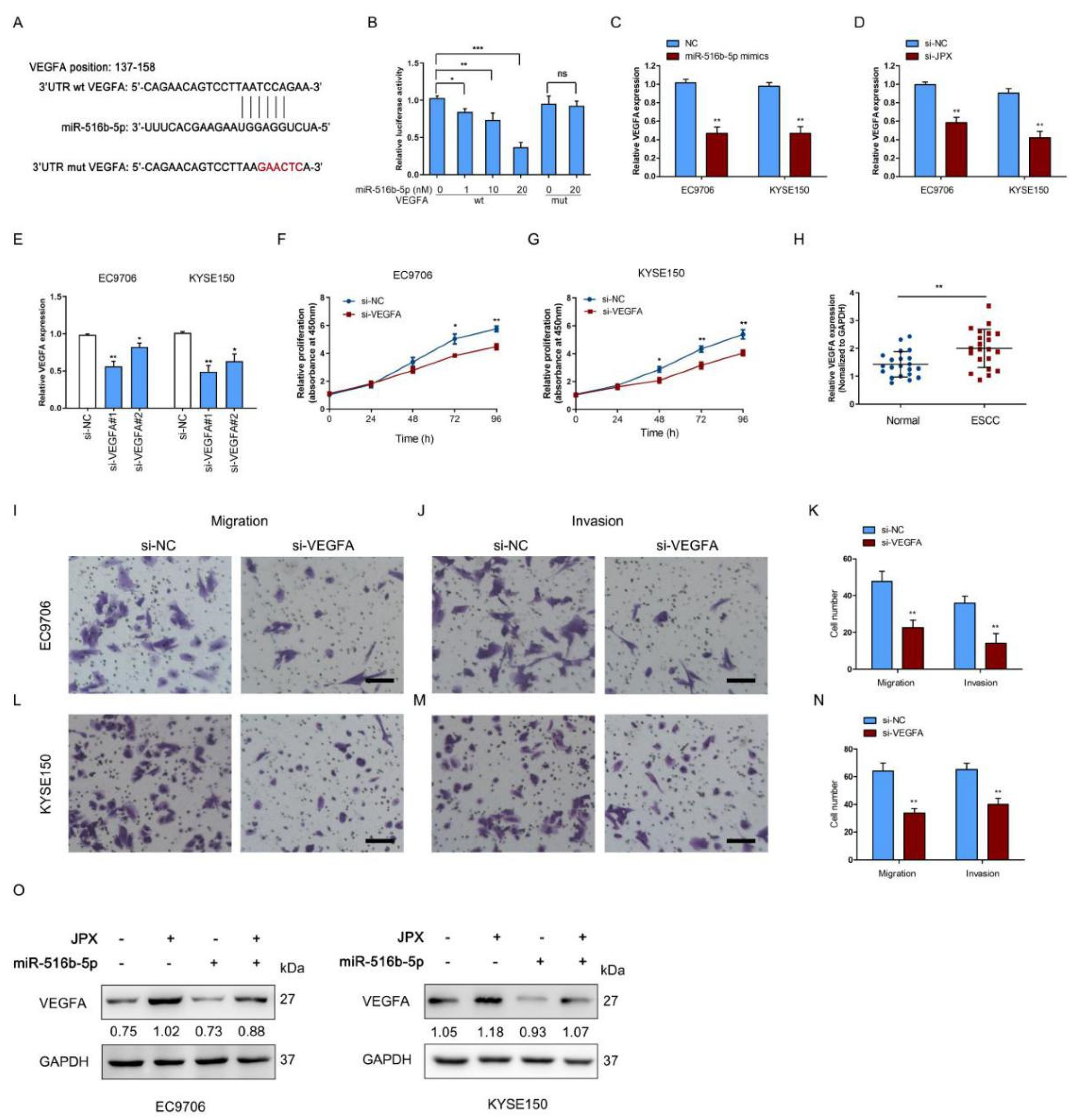
3.6. JPX Promoted Esophageal Cancer Cell Growth and Mobility via miR-516b-5p/VEGFA Signaling Pathway
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| LncRNAs | Long non-coding RNAs |
| ESCC | Esophageal squamous cell carcinoma |
| FISH | Fluorescence in situ hybridization |
| qRT-PCR | Quantitative real-time PCR |
| EdU | 5-Ethynyl-2′-Deoxyuridine |
| RIP | RNA immunoprecipitation |
| VEGFA | Vascular endothelial growth factor A |
| EAC | Esophageal adenocarcinoma |
| EZH2 | Enhancer of zeste homolog 2 |
| LAMC2 | Laminin gamma 2 |
| CBP | CREB-binding protein |
| AJCC | American Joint Committee on Cancer |
| FBS | Fetal bovine serum |
| siRNA | Small interfering RNA |
| NC | Negative control |
| CT | Cycle threshold |
| NC membranes | Nitrocellulose membranes |
| MiRNA | MicroRNA |
| Bio-labeled | Biotin-labeled |
| wt | Wild type |
| mut | Mutant type |
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.-M.; Zong, Y.-N.; Cao, S.-M.; Xu, R.-H. Current cancer situation in China: Good or bad news from the 2018 Global Cancer Statistics? Cancer Commun. 2019, 39, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, J.; Liu, Z.; Mao, X.; Tong, X.; Zhang, T.; Suo, C.; Chen, X. Global trends in the incidence and mortality of esophageal cancer from 1990 to 2017. Cancer Med. 2020, 9, 6875–6887. [Google Scholar] [CrossRef] [PubMed]
- Peters, B.A.; Wu, J.; Pei, Z.; Yang, L.; Purdue, M.P.; Freedman, N.D.; Jacobs, E.J.; Gapstur, S.M.; Hayes, R.B.; Ahn, J. Oral Microbiome Composition Reflects Prospective Risk for Esophageal Cancers. Cancer Res. 2017, 77, 6777–6787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Li, Q.; Lian, J.; Chu, Y.; Fang, K.; Xu, A.; Chen, T.; Xu, M. MLL2 promotes cancer cell lymph node metastasis by interacting with RelA and facilitating STC1 transcription. Cell. Signal. 2020, 65, 109457. [Google Scholar] [CrossRef]
- Malik, S.; Sharma, G.; Sanaka, M.R.; Thota, P.N. Role of endoscopic therapy in early esophageal cancer. World J. Gastroenterol. 2018, 24, 3965–3973. [Google Scholar] [CrossRef]
- Reichenbach, Z.W.; Murray, M.G.; Saxena, R.; Farkas, D.; Karassik, E.G.; Klochkova, A.; Patel, K.; Tice, C.; Hall, T.M.; Gang, J.; et al. Clinical and translational advances in esophageal squamous cell carcinoma. Adv. Cancer Res. 2019, 144, 95–135. [Google Scholar] [CrossRef]
- Peng, W.-X.; Koirala, P.; Mo, Y.-Y. LncRNA-mediated regulation of cell signaling in cancer. Oncogene 2017, 36, 5661–5667. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, X.; Chen, W.; Hu, X.; Li, J.; Liu, C. Regulatory roles of long noncoding RNAs implicated in cancer hallmarks. Int. J. Cancer 2020, 146, 906–916. [Google Scholar] [CrossRef]
- Fu, D.; Shi, Y.; Liu, J.-B.; Wu, T.-M.; Jia, C.-Y.; Yang, H.-Q.; Zhang, D.-D.; Yang, X.-L.; Wang, H.-M.; Ma, Y.-S. Targeting Long Non-coding RNA to Therapeutically Regulate Gene Expression in Cancer. Mol. Ther. Nucleic Acids 2020, 21, 712–724. [Google Scholar] [CrossRef]
- Hui, B.; Ji, H.; Xu, Y.; Wang, J.; Ma, Z.; Zhang, C.; Wang, K.; Zhou, Y. RREB1-induced upregulation of the lncRNA AGAP2-AS1 regulates the proliferation and migration of pancreatic cancer partly through suppressing ANKRD1 and ANGPTL4. Cell Death Dis. 2019, 10, 207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, M.; Zhong, J.; Zeng, Z.; Huang, K.; Ye, Z.; Deng, S.; Chen, H.; Xu, F.; Li, Q.; Zhao, G. Hypoxia-induced feedback of HIF-1α and lncRNA-CF129 contributes to pancreatic cancer progression through stabilization of p53 protein. Theranostics 2019, 9, 4795–4810. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yang, B.; Zhang, M.; Guo, W.; Wu, Z.; Wang, Y.; Jia, L.; Li, S.; Xie, W.; Yang, D.; et al. lncRNA Epigenetic Landscape Analysis Identifies EPIC1 as an Oncogenic lncRNA that Interacts with MYC and Promotes Cell-Cycle Progression in Cancer. Cancer Cell 2018, 33, 706–720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uroda, T.; Anastasakou, E.; Rossi, A.; Teulon, J.-M.; Pellequer, J.-L.; Annibale, P.; Pessey, O.; Inga, A.; Chillón, I.; Marcia, M. Conserved Pseudoknots in lncRNA MEG3 Are Essential for Stimulation of the p53 Pathway. Mol. Cell 2019, 75, 982–995. [Google Scholar] [CrossRef] [Green Version]
- You, B.-H.; Yoon, J.-H.; Kang, H.; Lee, E.K.; Lee, S.K.; Nam, J.-W. HERES, a lncRNA that regulates canonical and noncanonical Wnt signaling pathways via interaction with EZH2. Proc. Natl. Acad. Sci. USA 2019, 116, 24620–24629. [Google Scholar] [CrossRef] [Green Version]
- Liang, Y.; Chen, X.; Wu, Y.; Li, J.; Zhang, S.; Wang, K.; Guan, X.; Yang, K.; Bai, Y. LncRNA CASC9 promotes esophageal squamous cell carcinoma metastasis through upregulating LAMC2 expression by interacting with the CREB-binding protein. Cell Death Differ. 2018, 25, 1980–1995. [Google Scholar] [CrossRef]
- Liu, J.-Q.; Deng, M.; Xue, N.-N.; Li, T.-X.; Guo, Y.-X.; Gao, L.; Zhao, D.; Fan, R.-T. lncRNA KLF3-AS1 Suppresses Cell Migration and Invasion in ESCC by Impairing miR-185-5p-Targeted KLF3 Inhibition. Mol. Ther. Nucleic Acids 2020, 20, 231–241. [Google Scholar] [CrossRef]
- Pan, J.; Fang, S.; Tian, H.; Zhou, C.; Zhao, X.; Tian, H.; He, J.; Shen, W.; Meng, X.; Jin, X.; et al. lncRNA JPX/miR-33a-5p/Twist1 axis regulates tumorigenesis and metastasis of lung cancer by activating Wnt/β-catenin signaling. Mol. Cancer 2020, 19, 9–17. [Google Scholar] [CrossRef]
- Jin, M.; Ren, J.; Luo, M.; You, Z.; Fang, Y.; Han, Y.; Li, G.; Liu, H. Long non-coding RNA JPX correlates with poor prognosis and tumor progression in non-small-cell lung cancer by interacting with miR-145-5p and CCND2. Carcinogenesis 2020, 41, 634–645. [Google Scholar] [CrossRef]
- Li, G.; Jin, T.; Liang, H.; Tu, Y.; Zhang, W.; Gong, L.; Su, Q.; Gao, G. Skewed X-chromosome inactivation in patients with esophageal carcinoma. Diagn. Pathol. 2013, 8, 55. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Yang, J.; Wang, Y. LncRNA JPX promotes cervical cancer progression by modulating miR-25-3p/SOX4 axis. Cancer Cell Int. 2020, 20, 441. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Chen, S.; Lu, N.; Yin, Y.; Liu, Z. LncRNA JPX overexpressed in oral squamous cell carcinoma drives malignancy via miR-944/CDH2 axis. Oral Dis. 2020, 27, 924–933. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Feng, L.; Tian, C.; Tang, Y.-L.; Hu, F.-Q. Long noncoding RNA-JPX predicts the poor prognosis of ovarian cancer patients and promotes tumor cell proliferation, invasion and migration by the PI3K/Akt/mTOR signaling pathway. Eur. Rev. Med Pharmacol. Sci. 2018, 22, 8135–8144. [Google Scholar] [CrossRef] [PubMed]
- Shuai, Y.; Ma, Z.; Liu, W.; Yu, T.; Yan, C.; Jiang, H.; Tian, S.; Xu, T.; Shu, Y. TEAD4 modulated LncRNA MNX1-AS1 contributes to gastric cancer progression partly through suppressing BTG2 and activating BCL2. Mol. Cancer 2020, 19, 6. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, T.; Bao, Y.; Zhao, T.; Wang, J.; Wang, H.; Wang, A.; Gan, X.; Wu, Z.; Wang, L. CircRNA cRAPGEF5 inhibits the growth and metastasis of renal cell carcinoma via the miR-27a-3p/TXNIP pathway. Cancer Lett. 2020, 469, 68–77. [Google Scholar] [CrossRef]
- Wu, X.-G.; Zhou, C.-F.; Zhang, Y.-M.; Yan, R.-M.; Wei, W.-F.; Chen, X.-J.; Yi, H.-Y.; Liang, L.-J.; Fan, L.-S.; Liang, L.; et al. Cancer-derived exosomal miR-221-3p promotes angiogenesis by targeting THBS2 in cervical squamous cell carcinoma. Angiogenesis 2019, 22, 397–410. [Google Scholar] [CrossRef]
- Shen, W.; Yu, L.; Cong, A.; Yang, S.; Wang, P.; Han, G.; Gu, B.; Zhang, W. Silencing lncRNA AFAP1-AS1 Inhibits the Progression of Esophageal Squamous Cell Carcinoma Cells via Regulating the miR-498/VEGFA Axis. Cancer Manag. Res. 2020, 12, 6397–6409. [Google Scholar] [CrossRef]
- Wang, M.; Smith, J.S.; Wei, W. Tissue protein biomarker candidates to predict progression of esophageal squamous cell carcinoma and precancerous lesions. Ann. N. Y. Acad. Sci. 2018, 1434, 59–69. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.-F.; Yu, J.-R.; Yang, Z.; Zhu, G.-X.; Gao, P.; Wang, H.; Chen, S.-Y.; Zhang, J.; Liu, M.-Y.; Niu, Y.; et al. Promoter hypomethylation mediated upregulation of MicroRNA-10b-3p targets FOXO3 to promote the progression of esophageal squamous cell carcinoma (ESCC). J. Exp. Clin. Cancer Res. 2018, 37, 301. [Google Scholar] [CrossRef]
- Ma, T.; Zhao, Y.; Lu, Q.; Lu, Y.; Liu, Z.; Xue, T.; Shao, Y. MicroRNA-30c functions as a tumor suppressor via targeting SNAI1 in esophageal squamous cell carcinoma. Biomed. Pharmacother. 2018, 98, 680–686. [Google Scholar] [CrossRef]
- Sun, S.; Del Rosario, B.C.; Szanto, A.; Ogawa, Y.; Jeon, Y.; Lee, J.T. Jpx RNA Activates Xist by Evicting CTCF. Cell 2013, 153, 1537–1551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, W.; Wang, H.; Jing, W.; Zhou, F.; Chang, L.; Hong, Z.; Liu, H.; Liu, Z.; Yuan, Y. Downregulation of long non-coding RNAs JPX and XIST is associated with the prognosis of hepatocellular carcinoma. Clin. Res. Hepatol. Gastroenterol. 2017, 41, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Tay, Y.; Rinn, J.; Pandolfi, P.P. The multilayered complexity of ceRNA crosstalk and competition. Nature 2014, 505, 344–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, X.; Zhang, D.-H.; Wu, N.; Xiao, J.-H.; Wang, X.; Ma, W. ceRNA in cancer: Possible functions and clinical implications. J. Med. Genet. 2015, 52, 710–718. [Google Scholar] [CrossRef]
- Salmena, L.; Poliseno, L.; Tay, Y.; Kats, L.; Pandolfi, P.P. A ceRNA Hypothesis: The Rosetta Stone of a Hidden RNA Language? Cell 2011, 146, 353–358. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Sun, J.; Qi, Y.; Wang, Y.; Ding, Y.; Wang, K.; Zhou, Q.; Wang, J.; Ma, F.; Zhang, J.; et al. Long non-coding RNA TPT1-AS1 promotes angiogenesis and metastasis of colorectal cancer through TPT1-AS1/NF90/VEGFA signaling pathway. Aging 2020, 12, 6191–6205. [Google Scholar] [CrossRef]
- Liang, L.; Hui, K.; Hu, C.; Wen, Y.; Yang, S.; Zhu, P.; Wang, L.; Xia, Y.; Qiao, Y.; Sun, W.; et al. Autophagy inhibition potentiates the anti-angiogenic property of multikinase inhibitor anlotinib through JAK2/STAT3/VEGFA signaling in non-small cell lung cancer cells. J. Exp. Clin. Cancer Res. 2019, 38, 71. [Google Scholar] [CrossRef]
- Lu, Y.; Qin, T.; Li, J.; Wang, L.; Zhang, Q.; Jiang, Z.; Mao, J. MicroRNA-140-5p inhibits invasion and angiogenesis through targeting VEGF-A in breast cancer. Cancer Gene Ther. 2017, 24, 386–392. [Google Scholar] [CrossRef] [Green Version]
- Ye, J.; Zhu, J.; Chen, H.; Qian, J.; Zhang, L.; Wan, Z.; Chen, F.; Sun, S.; Li, W.; Luo, C. A novel lncRNA-LINC01116 regulates tumorigenesis of glioma by targeting VEGFA. Int. J. Cancer 2020, 146, 248–261. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Y.; Hua, R.; Yang, Y.; Li, B.; Guo, X.; Li, Z. LncRNA JPX Promotes Esophageal Squamous Cell Carcinoma Progression by Targeting miR-516b-5p/VEGFA Axis. Cancers 2022, 14, 2713. https://doi.org/10.3390/cancers14112713
He Y, Hua R, Yang Y, Li B, Guo X, Li Z. LncRNA JPX Promotes Esophageal Squamous Cell Carcinoma Progression by Targeting miR-516b-5p/VEGFA Axis. Cancers. 2022; 14(11):2713. https://doi.org/10.3390/cancers14112713
Chicago/Turabian StyleHe, Yi, Rong Hua, Yang Yang, Bin Li, Xufeng Guo, and Zhigang Li. 2022. "LncRNA JPX Promotes Esophageal Squamous Cell Carcinoma Progression by Targeting miR-516b-5p/VEGFA Axis" Cancers 14, no. 11: 2713. https://doi.org/10.3390/cancers14112713
APA StyleHe, Y., Hua, R., Yang, Y., Li, B., Guo, X., & Li, Z. (2022). LncRNA JPX Promotes Esophageal Squamous Cell Carcinoma Progression by Targeting miR-516b-5p/VEGFA Axis. Cancers, 14(11), 2713. https://doi.org/10.3390/cancers14112713





