Exploring Real World Outcomes with Nivolumab Plus Ipilimumab in Patients with Metastatic Extra-Pulmonary Neuroendocrine Carcinoma (EP-NEC)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
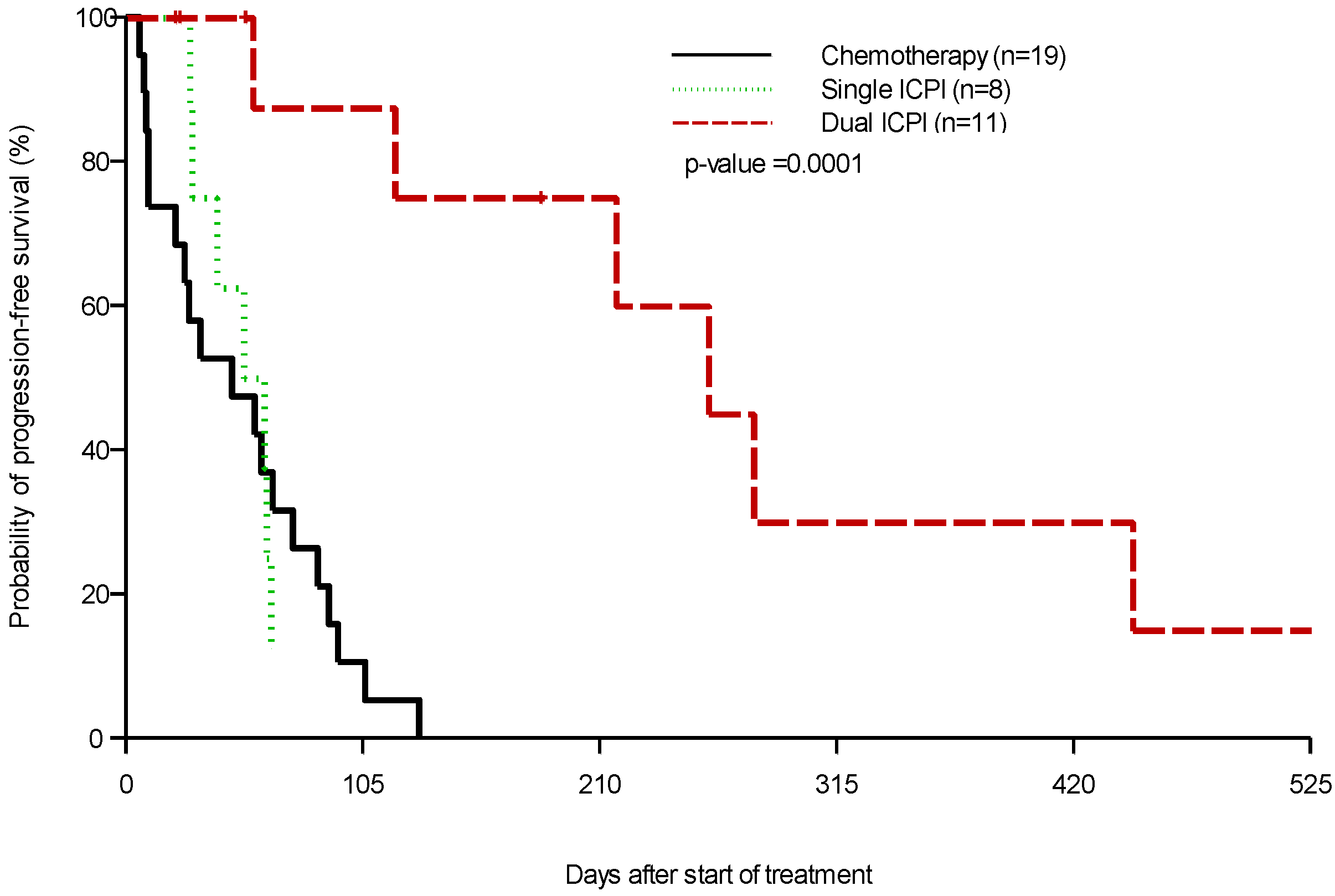
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Díez, M.; Teulé, A.; Salazar, R. Gastroenteropancreatic neuroendocrine tumors: Diagnosis and treatment. Ann. Gastroenterol. 2013, 26, 29–36. [Google Scholar] [PubMed]
- Dasari, A.; Shen, C.; Halperin, D.M.; Zhao, B.; Zhou, S.; Xu, Y.; Shih, T.; Yao, J.C. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients with Neuroendocrine Tumors in the United States. JAMA Oncol. 2017, 3, 1335–1342. [Google Scholar] [CrossRef] [PubMed]
- Strosberg, J.R.; Nasir, A.; Hodul, P.; Kvols, L. Biology and treatment of metastatic gastrointestinal neuroendocrine tumors. Gastrointest Cancer Res. 2008, 2, 113–125. [Google Scholar]
- Bongiovanni, A.; Riva, N.; Ricci, M.; Liverani, C.; La Manna, F.; De Vita, A.; Ibrahim, T.; Foca, F.; Mercatali, L.; Amadori, D.; et al. First-line chemotherapy in patients with metastatic gastroenteropancreatic neuroendocrine carcinoma. OncoTargets Ther. 2015, 8, 3613–3619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patta, A.; Fakih, M. First-line cisplatin plus etoposide in high-grade metastatic neuroendocrine tumors of colon and rectum (MCRC NET): Review of 8 cases. Anticancer Res. 2011, 31, 975–978. [Google Scholar] [PubMed]
- Mehnert, J.M.; Bergsland, E.; O’neil, B.H.; Santoro, A.; Schellens, J.H.; Cohen, R.B.; Piha-Paul, S.A. Pembrolizumab for the treatment of programmed death-ligand 1-positive advanced carcinoid or pancreatic neuroendocrine tumors: Results from the KEYNOTE-028 study. Cancer 2020, 126, 3021–3030. [Google Scholar] [CrossRef] [PubMed]
- Strosberg, J.R.; Mizuno, N.; Doi, T.; Grande, E.; Delord, J.-P.; Shapira-Frommer, R.; Bergsland, E.K.; Shah, M.H.; Fakih, M.; Takahashi, S.; et al. Efficacy and Safety of Pembrolizumab in Previously Treated Advanced Neuroendocrine Tumors: Results from the Phase II KEYNOTE-158 Study. Clin. Cancer Res. 2020, 26, 2124–2130. [Google Scholar] [CrossRef]
- Yao, J.; Strosberg, J.; Fazio, N.; Pavel, M.; Ruszniewski, P.; Bergsland, E.; Li, D.; Tafuto, S.; Raj, N.; Campana, D.; et al. Activity & safety of spartalizumab (PDR001) in patients (pts) with advanced neuroendocrine tumors (NET) of pancreatic (Pan), gastrointestinal (GI), or thoracic (T) origin, & gastroenteropancreatic neuroendocrine carcinoma (GEP NEC) who have progressed on prior treatment (Tx). Ann. Oncol. 2018, 29, viii467–viii468. [Google Scholar]
- Vijayvergia, N.; Dasari, A.; Deng, M.; Litwin, S.; Al-Toubah, T.; Alpaugh, R.K.; Strosberg, J.R. Pembrolizumab monotherapy in patients with previously treated metastatic high-grade neuroendocrine neoplasms: Joint analysis of two prospective, non-randomised trials. Br. J. Cancer 2020, 122, 1309–1314. [Google Scholar] [CrossRef]
- Patel, S.P.; Othus, M.; Chae, Y.K.; Giles, F.J.; Hansel, D.E.; Singh, P.P.; Fontaine, A.; Shah, M.H.; Kasi, A.; Al Baghdadi, T.; et al. A Phase II Basket Trial of Dual Anti–CTLA-4 and Anti–PD-1 Blockade in Rare Tumors (DART SWOG 1609) in Patients with Nonpancreatic Neuroendocrine Tumors. Clin. Cancer Res. 2020, 26, 2290–2296. [Google Scholar] [CrossRef] [Green Version]
- Klein, O.; Kee, D.; Markman, B.; Michael, M.; Underhill, C.; Carlino, M.S.; Cebon, J. Immunotherapy of Ipilimumab and Nivolumab in Patients with Advanced Neuroendocrine Tumors: A Subgroup Analysis of the CA209-538 Clinical Trial for Rare Cancers. Clin. Cancer Res. 2020, 26, 4454–4459. [Google Scholar] [CrossRef]
- Al-Toubah, T.; Halfdanarson, T.; Gile, J.; Morse, B.; Sommerer, K.; Strosberg, J. Efficacy of ipilimumab and nivolumab in patients with high-grade neuroendocrine neoplasms. ESMO Open 2021, 7, 100364. [Google Scholar] [CrossRef] [PubMed]
- Gile, J.J.; Liu, A.J.; McGarrah, P.W.; Eiring, R.A.; Hobday, T.J.; Starr, J.S.; Halfdanarson, T.R. Efficacy of Checkpoint Inhibitors in Neuroendocrine Neoplasms: Mayo Clinic Experience. Pancreas 2021, 50, 500–505. [Google Scholar] [CrossRef] [PubMed]
- Hauser, H.; Le, T.; Chou, J.; Heffernan, O.; DeMore, A.; Gonen, S.; Capanu, M.; Reidy, D.L.; Raj, N.P. Treatment response and clinical outcomes of neuroendocrine neoplasms (NENs) treated with immune checkpoint inhibitors (ICIs): A single institution experience. J. Clin. Oncol. 2022, 40, 506. [Google Scholar] [CrossRef]
- Hentic, O.; Hammel, P.; Couvelard, A.; Rebours, V.; Zappa, M.; Palazzo, M.; Ruszniewski, P. FOLFIRI regimen: An effective second-line chemotherapy after failure of etoposide-platinum combination in patients with neuroendocrine carcinomas grade 3. Endocr. Relat. Cancer 2012, 19, 751–757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walter, T.; Tougeron, D.; Baudin, E.; Le Malicot, K.; Lecomte, T.; Malka, D.; Hentic, O.; Manfredi, S.; Bonnet, I.; Guimbaud, R.; et al. Poorly differentiated gastro-entero-pancreatic neuroendocrine carcinomas: Are they really heterogeneous? Insights from the FFCD-GTE national cohort. Eur. J. Cancer 2017, 79, 158–165. [Google Scholar] [CrossRef]
- Hadoux, J.; Malka, D.; Planchard, D.; Scoazec, J.Y.; Caramella, C.; Guigay, J.; Boige, V.; Leboulleux, S.; Burtin, P.; Berdelou, A.; et al. Post-first-line FOLFOX chemotherapy for grade 3 neuroendocrine carcinoma. Endocr. Relat. Cancer 2015, 22, 289–298. [Google Scholar] [CrossRef] [Green Version]
- Olsen, I.H.; Knigge, U.; Federspiel, B.; Hansen, C.P.; Skov, A.; Kjaer, A.; Langer, S.W. Topotecan Monotherapy in Heavily Pretreated Patients with Progressive Advanced Stage Neuroendocrine Carcinomas. J. Cancer 2014, 5, 628–632. [Google Scholar] [CrossRef] [Green Version]
- Apostolidis, L.; Bergmann, F.; Jäger, D.; Winkler, E.C. Efficacy of topotecan in pretreated metastatic poorly differentiated extrapulmonary neuroendocrine carcinoma. Cancer Med. 2016, 5, 2261–2267. [Google Scholar] [CrossRef] [Green Version]
- Matos Garcia, I.; Grande, E.; Garcia-Carbonero, R.; Lopez, C.; Teule, A.; Capdevila, J. A multi-cohort phase II study of durvalumab plus tremelimumab for the treatment of patients (pts) with advanced neuroendocrine neoplasms (NENs) of gastroenteropancreatic or lung origin: The DUNE trial (GETNE 1601). Ann. Oncol. 2020, 31, 770–771. [Google Scholar]
- Naidoo, J.; Page, D.B.; Li, B.T.; Connell, L.C.; Schindler, K.; Lacouture, M.E.; Postow, M.A.; Wolchok, J.D. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann. Oncol. 2015, 26, 2375–2391. [Google Scholar] [CrossRef] [PubMed]
- Topalian, S.L.; Sznol, M.; McDermott, D.F.; Kluger, H.M.; Carvajal, R.D.; Sharfman, W.H.; Brahmer, J.R.; Lawrence, D.P.; Atkins, M.B.; Powderly, J.D.; et al. Survival, Durable Tumor Remission, and Long-Term Safety in Patients with Advanced Melanoma Receiving Nivolumab. J. Clin. Oncol. 2014, 32, 1020–1030. [Google Scholar] [CrossRef] [PubMed]
- Schrock, A.B.; Ouyang, C.; Sandhu, J.; Sokol, E.; Jin, D.; Ross, J.S.; Miller, V.A.; Lim, D.; Amanam, I.; Chao, J.; et al. Tumor mutational burden is predictive of response to immune checkpoint inhibitors in MSI-high metastatic colorectal cancer. Ann. Oncol. 2019, 30, 1096–1103. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, N.A.; Hellmann, M.D.; Snyder, A.; Kvistborg, P.; Makarov, V.; Havel, J.J.; Lee, W.; Yuan, J.; Wong, P.; Ho, T.S.; et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non–small cell lung cancer. Science 2015, 348, 124–128. [Google Scholar] [CrossRef] [Green Version]
- McGrail, D.; Pilié, P.; Rashid, N.; Voorwerk, L.; Slagter, M.; Kok, M.; Jonasch, E.; Khasraw, M.; Heimberger, A.; Lim, B.; et al. High tumor mutation burden fails to predict immune checkpoint blockade response across all cancer types. Ann. Oncol. 2021, 32, 661–672. [Google Scholar] [CrossRef]
- Das, S.; Du, L.; Lee, C.L.; Arhin, N.D.; Chan, J.A.; Kohn, E.C.; Dasari, A. Comparison of Design, Eligibility, and Outcomes of Neuroendocrine Neoplasm Trials Initiated From 2000 to 2009 vs. 2010 to 2020. JAMA Netw. Open 2021, 4, e2131744. [Google Scholar] [CrossRef]
- Zou, X.-L.; Li, X.-B.; Ke, H.; Zhang, G.-Y.; Tang, Q.; Yuan, J.; Zhou, C.-J.; Zhang, J.-L.; Zhang, R.; Chen, W.-Y. Prognostic Value of Neoantigen Load in Immune Checkpoint Inhibitor Therapy for Cancer. Front. Immunol. 2021, 12, 689076. [Google Scholar] [CrossRef]
- Zhao, X.; Pan, X.; Wang, Y.; Zhang, Y. Targeting neoantigens for cancer immunotherapy. Biomark. Res. 2021, 9, 1–12. [Google Scholar] [CrossRef]
- Takahashi, D.; Kojima, M.; Suzuki, T.; Sugimoto, M.; Kobayashi, S.; Takahashi, S.; Nagino, M. Profiling the Tumour Immune Microenvironment in Pancreatic Neuroendocrine Neoplasms with Multispectral Imaging Indicates Distinct Subpopulation Characteristics Concordant with WHO 2017 Classification. Sci. Rep. 2018, 8, 13166. [Google Scholar] [CrossRef]
- Bösch, F.; Brüwer, K.; Altendorf-Hofmann, A.; Auernhammer, C.J.; Spitzweg, C.; Westphalen, C.B.; Boeck, S.; Schubert-Fritschle, G.; Werner, J.; Heinemann, V.; et al. Immune checkpoint markers in gastroenteropancreatic neuroendocrine neoplasia. Endocr. Relat. Cancer 2019, 26, 293–301. [Google Scholar] [CrossRef]
- Mafficini, A.; Scarpa, A. Genetics and Epigenetics of Gastroenteropancreatic Neuroendocrine Neoplasms. Endocr. Rev. 2019, 40, 506–536. [Google Scholar] [CrossRef] [PubMed]
- Vijayvergia, N.; Boland, P.M.; Handorf, E.; Gustafson, K.S.; Gong, Y.; Cooper, H.S.; Sheriff, F.; Astsaturov, I.; Cohen, S.J.; Engstrom, P.F. Molecular profiling of neuroendocrine malignancies to identify prognostic and therapeutic markers: A Fox Chase Cancer Center Pilot Study. Br. J. Cancer 2016, 115, 564–570. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Characteristics | ICPIs n = 19 | Chemotherapy n = 23 | p-Value |
|---|---|---|---|
| Age (Median) | 63 | 59 | |
| Male % | 57% | 48% | |
| Primary Site (n) | |||
| Pancreatic | 7 | 4 | |
| SB | 3 | 10 | |
| Colorectal | 2 | 6 | |
| Other GI | 1 | 2 | |
| Unkown | 6 | 1 | |
| Median ki-67% | 71% | 79% | |
| Most common sites of metastases | Liver, Lymph nodes, pulmonary, and osseous metastases | Liver, Lymph nodes, and osseous metastases | |
| PFS (Days) | Dual ICPIs: 258 Single ICPI: 56.5 | 47 | 0.0001 |
| OS (Months) | Dual ICPI: NR Single ICPI: 18.7 | 10.5 | 0.004 |
| Adverse Event | Grade ½ (n = Number) | Grade ¾ (n = Number) |
|---|---|---|
| Fatigue | 11 | 1 |
| Transaminitis | 1 | 1 |
| Anorexia | 3 | 2 |
| Nausea | 3 | |
| Abdominal Pain | 2 | |
| Anorexia | 3 | 1 |
| Back Pain | 1 | |
| Diarrhea | 2 | |
| Pulmonary Embolism | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamed, A.; Vijayvergia, N.; Kurian, M.; Liu, L.; Fu, P.; Das, S. Exploring Real World Outcomes with Nivolumab Plus Ipilimumab in Patients with Metastatic Extra-Pulmonary Neuroendocrine Carcinoma (EP-NEC). Cancers 2022, 14, 2695. https://doi.org/10.3390/cancers14112695
Mohamed A, Vijayvergia N, Kurian M, Liu L, Fu P, Das S. Exploring Real World Outcomes with Nivolumab Plus Ipilimumab in Patients with Metastatic Extra-Pulmonary Neuroendocrine Carcinoma (EP-NEC). Cancers. 2022; 14(11):2695. https://doi.org/10.3390/cancers14112695
Chicago/Turabian StyleMohamed, Amr, Namrata Vijayvergia, Matthew Kurian, Lisa Liu, Pingfu Fu, and Satya Das. 2022. "Exploring Real World Outcomes with Nivolumab Plus Ipilimumab in Patients with Metastatic Extra-Pulmonary Neuroendocrine Carcinoma (EP-NEC)" Cancers 14, no. 11: 2695. https://doi.org/10.3390/cancers14112695
APA StyleMohamed, A., Vijayvergia, N., Kurian, M., Liu, L., Fu, P., & Das, S. (2022). Exploring Real World Outcomes with Nivolumab Plus Ipilimumab in Patients with Metastatic Extra-Pulmonary Neuroendocrine Carcinoma (EP-NEC). Cancers, 14(11), 2695. https://doi.org/10.3390/cancers14112695






