External Validation of a Prognostic Score for Survival in Lung Carcinoids
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
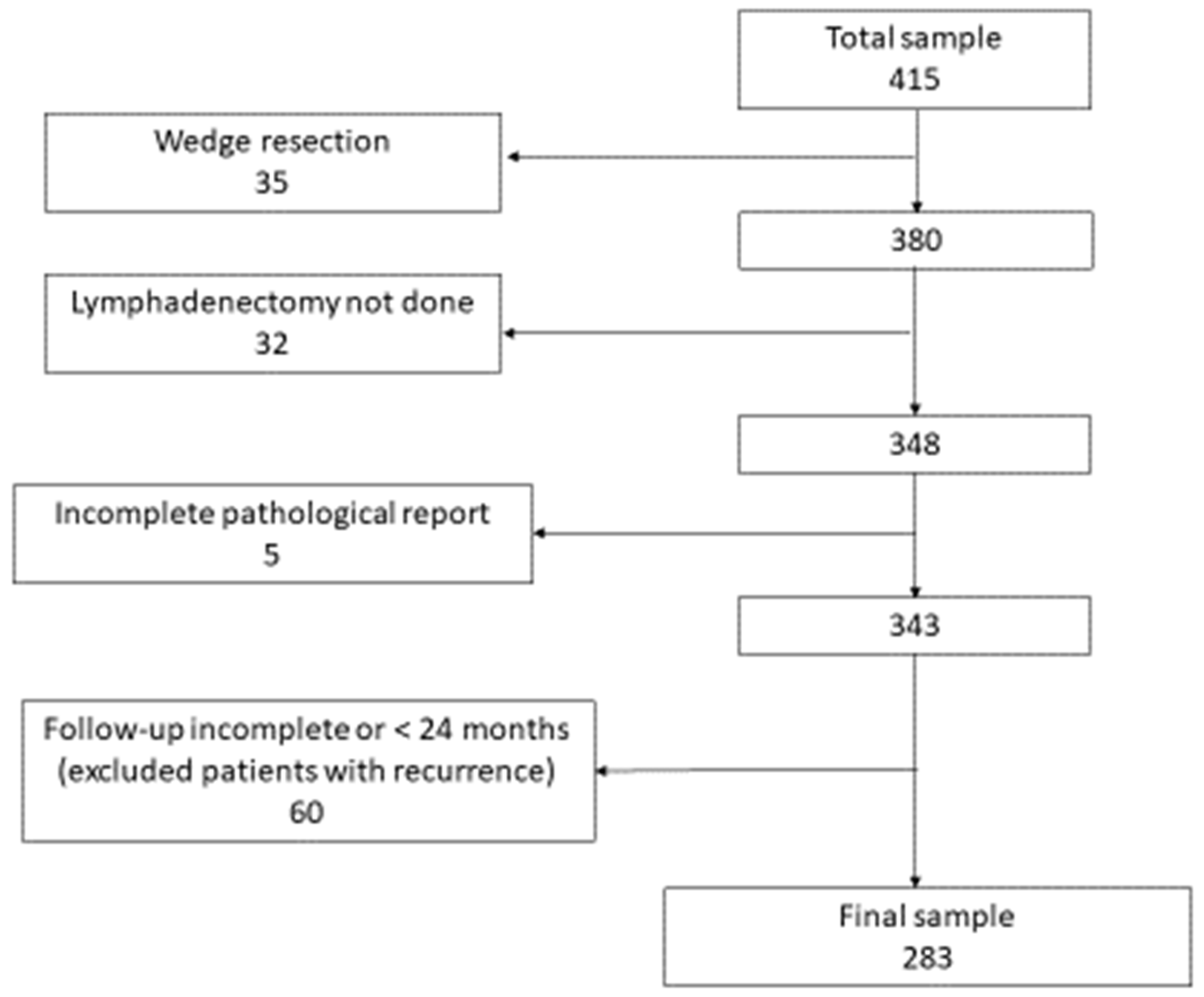
2.1. Patients
2.2. Previous Prognostic Score
2.3. Statistical Analysis
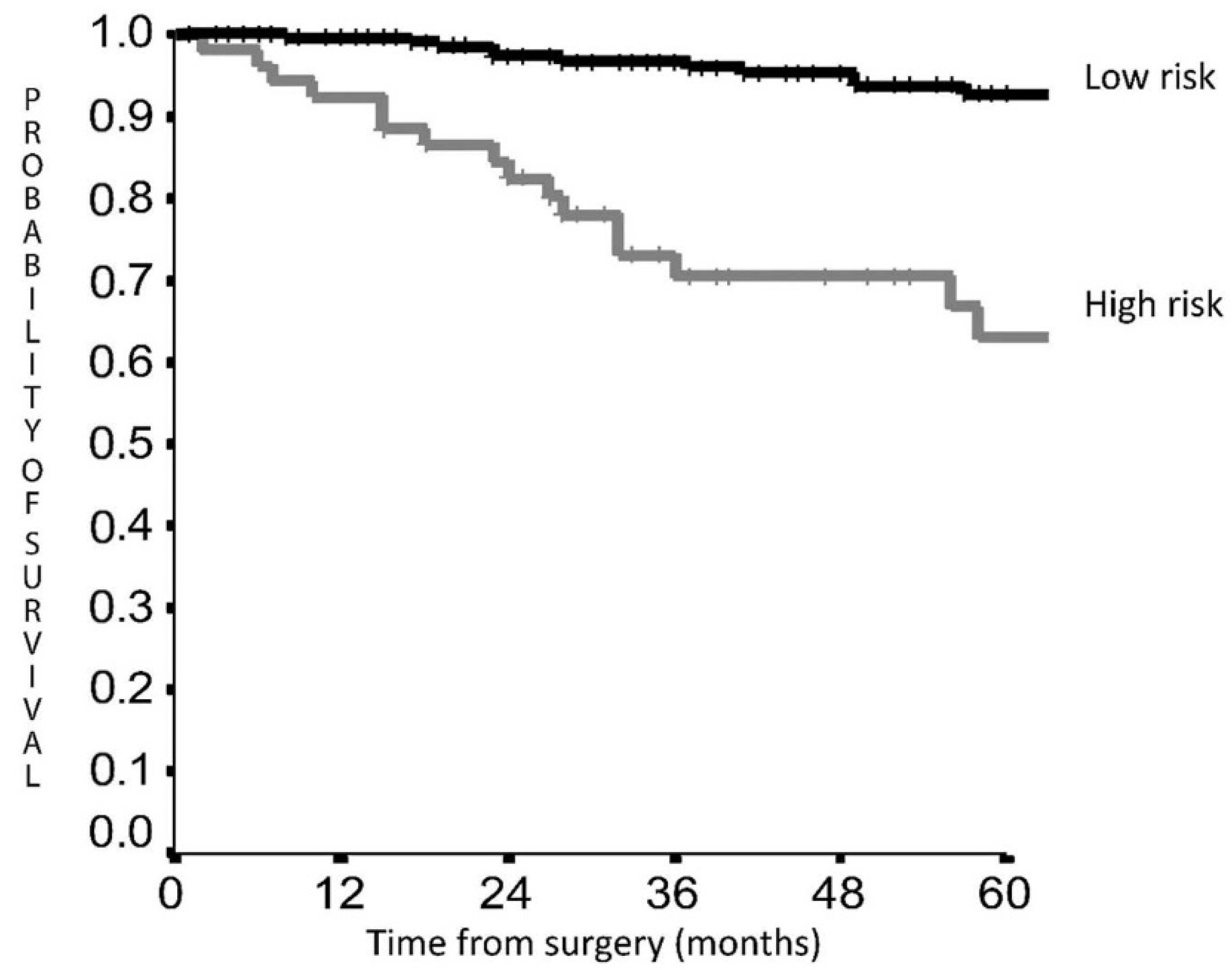
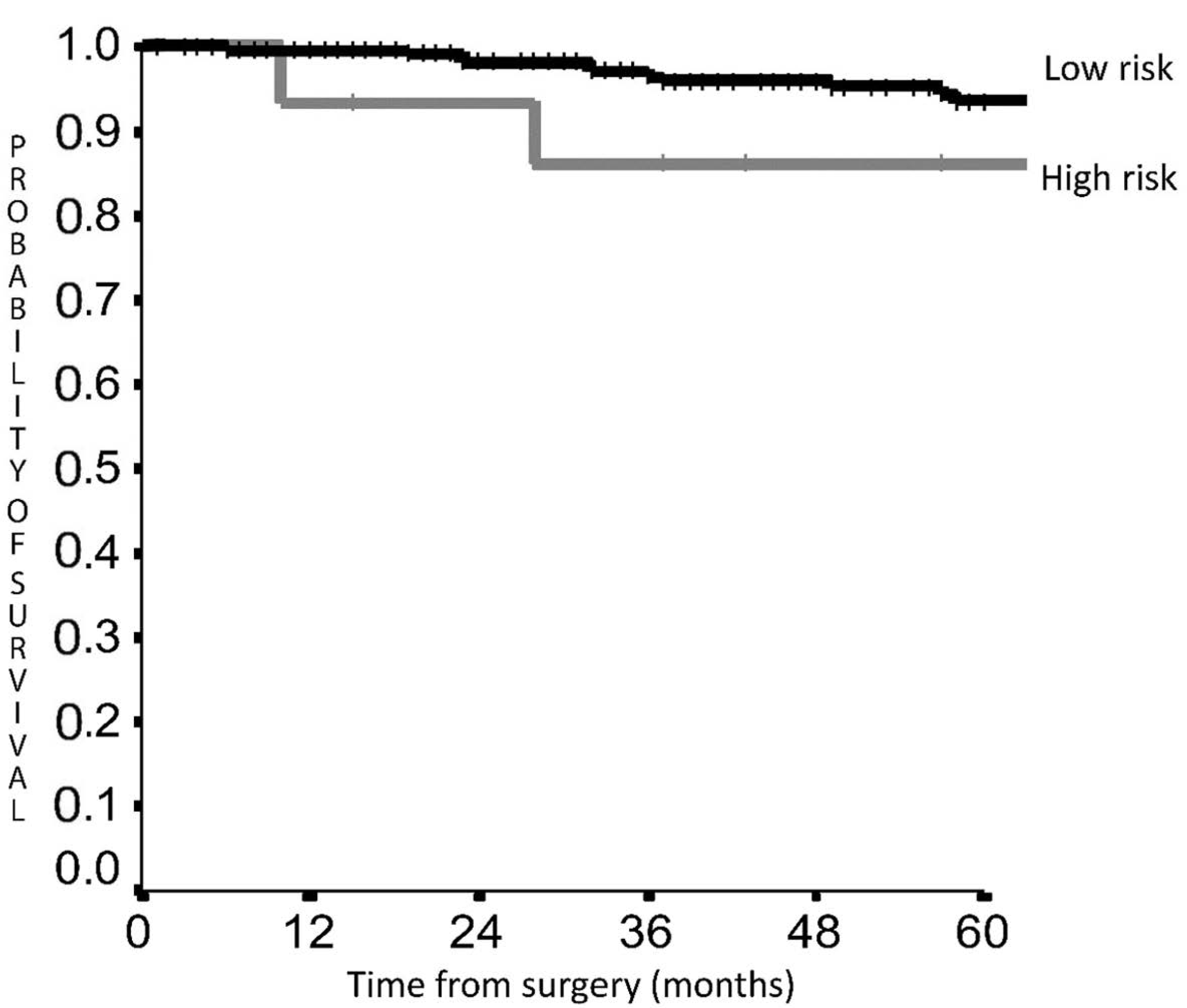
3. Results
Prognostic Score Validation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Filosso, P.L.; Falcoz, P.E.; Solidoro, P.; Pellicano, D.; Passani, S.; Guerrera, F.; Ruffini, E.; ESTS Lung Neuroendocrine Working-Group Participating Centers. The European Society of Thoracic Surgeons (ESTS) lung neuroendocrine tumors (NETs) database. J. Thorac. Dis. 2018, 10 (Suppl. 29), S3528–S3532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, J.C.; Hassan, M.; Phan, A.; Dagohoy, C.; Leary, C.; Mares, J.E.; Abdalla, E.K.; Fleming, J.B.; Vauthey, J.N.; Rashid, A.; et al. One hundred years after “Carcinoid”: Epidemiology of and prognostic factors for Neuroendocrine Tumors in 35,825 cases in the United States. J. Clin. Oncol. 2008, 26, 3063–3072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Travis, W.D.; Giroux, D.J.; Chansky, K.; Crowley, J.; Asamura, H.; Brambilla, E.; Jett, J.; Kennedy, C.; Rami-Porta, R.; Rusch, V.; et al. The IASLC Lung Cancer Staging Project: Proposals for the inclusion of bronchopulmonary carcinoid tumors in the forthcoming(seventh) edition of the TNM Classification for Lung Cancer. J. Thorac. Oncol. 2008, 3, 1213–1223. [Google Scholar] [CrossRef] [PubMed]
- Caplin, M.E.; Baudin, E.; Ferolla, P.; Filosso, P.; Garcia-Yuste, M.; Lim, E.; Oberg, K.; Pelosi, G.; Perren, A.; Rossi, R.E.; et al. Pulmonary neuroendocrine (carcinoid) tumors: European NeuroendocrineTumor Society expert consensus and recommendations or best practice for typical and atypical pulmonary carcinoids. Ann. Oncol. 2015, 26, 1604–1620. [Google Scholar] [CrossRef]
- Goldstraw, P.; Chansky, K.; Crowley, J.; Rami-Porta, R.; Asamura, H.; Eberhardt, W.E.; Nicholson, A.G.; Groome, P.; Mitchell, A.; Bolejack, V.; et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in theForthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J. Thorac. Oncol. 2016, 11, 39–51. [Google Scholar] [CrossRef] [Green Version]
- Yoon, J.Y.; Sigel, K.; Martin, J.; Jordan, R.; Beasley, M.B.; Smith, C.; Kaufman, A.; Wisnivesky, J.; Kim, M.K. Evaluation of the Prognostic Significance of TNM Staging Guidelines in Lung Carcinoid Tumors. J. Thorac. Oncol. 2019, 14, 184–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cattoni, M.; Vallières, E.; Brown, L.M.; Sarkeshik, A.A.; Margaritora, S.; Siciliani, A.; Filosso, P.L.; Guerrera, F.; Imperatori, A.; Rotolo, N.; et al. Improvement in TNM staging of pulmonary neuroendocrine tumors requires histology and regrouping of tumor size. J. Thorac. Cardiovasc. Surg. 2018, 155, 405–413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filosso, P.L.; Guerrera, F.; Evangelista, A.; Welter, S.; Thomas, P.; Casado, P.M.; Rendina, E.A.; Venuta, F.; Ampollini, L.; Brunelli, A.; et al. Prognostic model of survival for typical bronchial carcinoid tumours: Analysis of 1109 patients on behalf of the European Association of Thoracic Surgeons (ESTS) Neuroendocrine Tumours Working Group. Eur. J. Cardiothorac. Surg. 2015, 48, 441–447. [Google Scholar] [CrossRef] [Green Version]
- Chiappetta, M.; Sperduti, I.; Ciavarella, L.P.; Leuzzi, G.; Bria, E.; Mucilli, F.; Lococo, F.; Filosso, P.; Ratto, G.; Spaggiari, L.; et al. Prognostic score for survival with pulmonary carcinoids: The importance of associating clinical with pathological characteristics. Interact Cardiovasc. Thorac. Surg. 2020, 31, 315–323. [Google Scholar] [CrossRef]
- Cardillo, G.; Sera, F.; Di Martino, M.; Graziano, P.; Giunti, R.; Carbone, L.; Facciolo, F.; Martelli, M. Bronchial carcinoid tumors:nodal status and long-term survival after resection. Ann. Thorac. Surg. 2004, 77, 1781–1785. [Google Scholar] [CrossRef]
- Cusumano, G.; Fournel, L.; Strano, S.; Damotte, D.; Charpentier, M.C.; Galia, A.; Terminella, A.; Nicolosi, M.; Regnard, J.F.; Alifano, M. Surgical Resection for Pulmonary Carcinoid: Long-Term Results of Multicentric Study—The Importance of Pathological N Status, More Than We Thought. Lung 2017, 195, 789–798. [Google Scholar] [CrossRef] [PubMed]
- Kneuertz, P.J.; Kamel, M.K.; Stiles, B.M.; Lee, B.E.; Rahouma, M.; Harrison, S.W.; Altorki, N.K.; Port, J.L. Incidence and Prognostic Significance of Carcinoid Lymph Node Metastases. Ann. Thorac. Surg. 2018, 106, 981–988. [Google Scholar] [CrossRef] [PubMed]
- Westin, G.F.M.; Alsidawi, S.; Leventakos, K.; Halfdanarson, T.R.; Molina, J.R. Impact of adjuvant chemotherapy in non-metastatic node positive bronchial neuroendocrine tumors (BNET). J. Clin. Oncol. 2017, 35 (Suppl. 15), 8533. [Google Scholar] [CrossRef]
- Wegner, R.E.; Abel, S.; Hasan, S.; Horne, Z.D.; Colonias, A.; Weksler, B.; Verma, V. The role of adjuvant therapy for atypical bronchopulmonary carcinoids. Lung Cancer 2019, 131, 90–94. [Google Scholar] [CrossRef]
- Travis, W.D.; Brambilla, E.; Mueller_Hermelink, H.K.; Harris, C.C. Pathology and Genetics of Tumours of the Lung, Pleura, Thymus and Heart; WHO Classification of Tumours, 3rd ed.; IARC Press: Lyon, France, 2004; Volume 10. [Google Scholar]
- Rusch, V.W.; Asamura, H.; Watanabe, H.; Giroux, D.J.; Rami-Porta, R.; Goldstraw, P.; Members of IASLC Staging Committee. The IASLC lung cancer staging project: A proposal for a new international lymph node map in the forthcoming seventh edition of the TNM classification for lung cancer. J. Thorac. Oncol. 2009, 4, 568–577. [Google Scholar] [CrossRef] [Green Version]
- Lardinois, D.; De Leyn, P.; Van Schil, P.; Porta, R.R.; A Waller, D.; Passlick, B.; Zielinski, M.; Junker, K.; Rendina, E.A.; Ris, H.-B. ESTS guidelines for intraoperative lymph node staging in non-small cell lung cancer. Eur. J. Cardio-Thorac. Surg. 2006, 30, 787–792. [Google Scholar] [CrossRef] [Green Version]
- Gosain, R.; Mukherjee, S.; Yendamuri, S.S.; Iyer, R. Management of Typical and Atypical Pulmonary Carcinoids Based on Different Established Guidelines. Cancers 2018, 10, 510. [Google Scholar] [CrossRef] [Green Version]
- Baudin, E.; Caplin, M.; Garcia-Carbonero, R.; Fazio, N.; Ferolla, P.; Filosso, P.; Frilling, A.; de Herder, W.; Hörsch, D.; Knigge, U.; et al. Lung and thymic carcinoids: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up☆. Ann. Oncol. 2021, 32, 439–451. [Google Scholar] [CrossRef]
- Pilotto, S.; Sperduti, I.; Leuzzi, G.; Chiappetta, M.; Mucilli, F.; Ratto, G.B.; Lococo, F.; Filosso, P.L.; Spaggiari, L.; Novello, S.; et al. Prognostic Model for Resected Squamous Cell Lung Cancer: External Multicenter Validation and Propensity Score Analysis exploring the Impact of Adjuvant and Neoadjuvant Treatment. J. Thorac. Oncol. 2017, 13, 568–575. [Google Scholar] [CrossRef] [Green Version]
- Brown, L.M.; Cooke, D.T.; Jett, J.R.; David, E.A. Extent of Resection and Lymph Node Assessment for Clinical Stage T1aN0M0 Typical Carcinoid Tumors. Ann. Thorac. Surg. 2018, 105, 207–213. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Pang, Z.; Wang, Y.; Bie, F.; Zeng, Y.; Wang, G.; Du, J. The role of surgery for atypical bronchopulmonary carcinoid tumor: Development and validation of a model based on Surveillance, Epidemiology, and End Results (SEER) database. Lung Cancer 2019, 139, 94–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiappetta, M.; Lococo, F.; Sperduti, I.; Cusumano, G.; Terminella, A.; Fournel, L.; Guerrera, F.; Filosso, P.; Tabacco, D.; Nicosia, S.; et al. Lymphadenectomy for lung carcinoids: Which factors may predict nodal upstaging? A multi centric, retrospective study. J. Surg. Oncol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Robelin, P.; Hadoux, J.; Forestier, J.; Planchard, D.; Hervieu, V.; Berdelou, A.; Scoazec, J.-Y.; Valette, P.-J.; Leboulleux, S.; Ducreux, M.; et al. Characterization, Prognosis, and Treatment of Patients with Metastatic Lung Carcinoid Tumors. J. Thorac. Oncol. 2019, 14, 993–1002. [Google Scholar] [CrossRef] [PubMed]
- Mauguen, A.; Pignon, J.-P.; Burdett, S.; Domerg, C.; Fisher, D.; Paulus, R.; Mandrekar, S.J.; Belani, C.; A Shepherd, F.; Eisen, T.; et al. Surrogate endpoints for overall survival in chemotherapy and radiotherapy trials in operable and locally advanced lung cancer: A re-analysis of meta-analyses of individual patients’ data. Lancet Oncol. 2013, 14, 619–626. [Google Scholar] [CrossRef]
- Imaoka, H.; Sasaki, M.; Takahashi, H.; Hashimoto, Y.; Ohno, I.; Mitsunaga, S.; Watanabe, K.; Umemoto, K.; Kimura, G.; Suzuki, Y.; et al. Progression-free survival as a surrogate endpoint in advanced neuroendocrine neoplasms. Endocr. Relat. Cancer 2017, 24, 475–483. [Google Scholar] [CrossRef]
| Overall Survival | Score | Disease-Free Survival | Score |
|---|---|---|---|
| Age > 61 years | 1.0 | Age > 61 years | 1.4 |
| Male Sex | 1.0 | Atypical Histology | 1.0 |
| Lymph Node Ratio > 10% | 1.0 | Lymph Node Ratio > 10% | 1.5 |
| pT stage 2–3 | 1.4 | pT stage 2–3 | 1.3 |
| RISK GROUP | RISK GROUP | ||
| LOW RISK | IF SCORE ≤ 3.1 | LOW RISK | IF SCORE < 1.5 |
| HIGH RISK | IF SCORE > 3.1 | HIGH RISK | IF SCORE ≥ 1.5 |
| Male/Female | 116/167 |
|---|---|
| Age (years, median) | 62 (18–87) |
| <61 years | 135 (47.7%) |
| Histology | |
| Typical carcinoid | 239 (84.5%) |
| Atypical carcinoid | 44 (15.5%) |
| Location | |
| Central | 157 (55.2%) |
| Peripheral | 126 (44.8%) |
| Clinical Stage | |
| I | 226 (79.8%) |
| II | 41 (14.9%) |
| III | 15 (5.3%) |
| Surgery | |
| Segmentectomy | 27 (9.5%) |
| Lobectomy | 238 (84.1%) |
| Bilobectomy | 13 (4.6%) |
| Pneumonectomy | 5 (1.8%) |
| Pathological T stage | |
| T1 | 227 (80.2%) |
| T2 | 43 (15.2%) |
| T3–4 | 13 (4.6%) |
| Pathological stage | |
| I | 235 (83%) |
| II | 30 (10.6%) |
| III | 18 (6.4%) |
| NODAL CHARACTERISTICS | |
| pN | |
| 0 | 245 (86.6%) |
| 1 | 23 (8.1%) |
| 2 | 15 (5.3%) |
| N resected nodes | |
| <10 | 160 (56.5%) |
| ≥10 | 123 (43.5%) |
| N positive nodes | |
| 0 | 245 (86.6%) |
| 1 | 21 (7.4%) |
| >1 | 17 (6%) |
| N resected stations | |
| ≤3 | 90 (31.8%) |
| >3 | 193 (68.2%) |
| N positive stations | |
| 0 | 245 (86.6%) |
| 1 | 28 (9.9%) |
| >1 | 10 (3.5%) |
| Node ratio | |
| <10% | 251 (88.7%) |
| >10% | 32 (11.3%) |
| Kind of lymphadenectomy | |
| Radical node dissection | 217 (76.7%) |
| Sampling/lobe specific | 66 (23.3%) |
| Overall Survival | Disease-Free Survival | ||||
|---|---|---|---|---|---|
| Variable | # Patients (%) | Variable | # Patients (%) | ||
| LRG (#268) | HRG (#15) | LRG (#230) | HRG (#53) | ||
| Age | Age | ||||
| >61 years | 126 (47.3%) | 2 (8.3%) | >61 years | 96 (41.8%) | 41 (78%) |
| <61 years | 142 (52.7%) | 13 (91.7%) | <61 year | 134 (58.2%) | 12 (22%) |
| Sex | Histology | ||||
| Male | 101 (37.9%) | 15 (100%) | Typical | 212 (91.8%) | 32 (60%) |
| Female | 167 (62.1%) | 0 (0%) | Atypical | 18 (8.2%) | 21 (40%) |
| Lymph Node Ratio | Lymph Node Ratio | ||||
| >10% | 27 (10.1%) | 11 (72.7%) | >10% | 7 (3%) | 6 (12%) |
| <10% | 241 (89.9%) | 4 (27.3%) | <10% | 223 (97%) | 47 (88%) |
| pT stage | pT stage | ||||
| 1 | 82.2% | 0 (0%) | 1 | 209 (91%) | 38 (72%) |
| 2–3 | 17.8% | 15 (100%) | 2–3 | 21 (9%) | 15 (28%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiappetta, M.; Tabacco, D.; Sassorossi, C.; Sperduti, I.; Cusumano, G.; Terminella, A.; Fournel, L.; Alifano, M.; Guerrera, F.; Filosso, P.L.; et al. External Validation of a Prognostic Score for Survival in Lung Carcinoids. Cancers 2022, 14, 2601. https://doi.org/10.3390/cancers14112601
Chiappetta M, Tabacco D, Sassorossi C, Sperduti I, Cusumano G, Terminella A, Fournel L, Alifano M, Guerrera F, Filosso PL, et al. External Validation of a Prognostic Score for Survival in Lung Carcinoids. Cancers. 2022; 14(11):2601. https://doi.org/10.3390/cancers14112601
Chicago/Turabian StyleChiappetta, Marco, Diomira Tabacco, Carolina Sassorossi, Isabella Sperduti, Giacomo Cusumano, Alberto Terminella, Ludovic Fournel, Marco Alifano, Francesco Guerrera, Pier Luigi Filosso, and et al. 2022. "External Validation of a Prognostic Score for Survival in Lung Carcinoids" Cancers 14, no. 11: 2601. https://doi.org/10.3390/cancers14112601
APA StyleChiappetta, M., Tabacco, D., Sassorossi, C., Sperduti, I., Cusumano, G., Terminella, A., Fournel, L., Alifano, M., Guerrera, F., Filosso, P. L., Nicosia, S., Gallina, F., Facciolo, F., Margaritora, S., & Lococo, F. (2022). External Validation of a Prognostic Score for Survival in Lung Carcinoids. Cancers, 14(11), 2601. https://doi.org/10.3390/cancers14112601











