Simple Summary
Immune checkpoint blockade (ICB) with antibodies targeting CTLA-4 (Cytotoxic Lymphocyte Antigen 4) and/or programmed death-1 protein (PD-1)/programmed death ligand-1 (PD-L1) has significantly modified the therapeutic landscape of a broad range of human tumor types, including advanced non-small-cell lung cancer (NSCLC). Despite great advances of checkpoint immunotherapies, a minority of NSCLC patients (<20%) respond and/or experience long-term clinical benefits from these treatments. Limited response rates of T cell–based checkpoint immunotherapies suggest the presence of other checkpoints able to inhibit effective anti-tumor immune responses. Natural Killer (NK) cells represent a promising target for tumor immunotherapies, particularly against tumors that escape T-cell-mediated control. Like T cell function, NK cell function is also regulated by inhibitory immune-checkpoint molecules. In this review, we will provide an overview of the rationale, mechanisms of action, and clinical efficacy of these NK cell-based checkpoint therapy approaches. Finally, the future directions and current enhancements planned will be discussed.
Abstract
Immune checkpoint inhibitors (ICIs) immunotherapy has represented a breakthrough in cancer treatment. Clinical use of ICIs has shown an acceptable safety profile and promising antitumor activity. Nevertheless, some patients do not obtain clinical benefits after ICIs therapy. In order to improve and cure an increasing number of patients, the field has moved toward the discovery of new ICIs expressed by cells of innate immunity with an elevated inherent antitumor activity, such as natural killer cells. This review will focus on the recent findings concerning the role of classical and non-classical immune checkpoint molecules and receptors that regulate natural killer cell function, as potential targets, and their future clinical application.
1. Introduction
To date, cancer immunotherapy with immune checkpoint inhibitors (ICIs) has been a cornerstone of the treatment for several solid and hematologic malignancies; among these, non-small cell lung cancer (NSCLC) treatment has gained significant benefits from the introduction of ICIs, as such agents have been approved for use at virtually any disease stage, from resectable to advanced disease, both as a single-agent approach and as part of combination regimens with chemotherapy. Notably, the currently approved agents are directed on programmed death 1 (PD-1) protein or its ligand (PD-L1), as well as cytotoxic lymphocyte antigen 4 (CTLA-4), which mostly involve T cell lymphocytes. With reference to advanced NSCLC, single-agent PD-1 is the current standard of care for non-oncogene addicted NSCLC with high PD-L1 expression, while combinations involving ICIs and chemotherapy are employed with absent or low PD-L1 expression [1]. While the contribution of ICIs to NSCLC management is extremely relevant, there is still space for further development; indeed, it is known that the complexity of the immune system allows to consider other mechanisms. On one hand, the effect of PD-1/PD-L1 or CTLA-4 blockade on the antineoplastic activity of other immune cells, such as Natural Killer (NK) lymphocytes, still needs to be elucidated. Along this line, additional immune checkpoints (ICs) can be exploited with therapeutic intent, including specific NK-related ICs (including lirilumab and monalizumab), as well as other novel therapeutic immune targets. From a clinical perspective, the development of novel ICI-based regimens [2] might lead to the improvement of current practice; indeed, a current priority in the management of non-oncogene-addicted NSCLC is represented by the ability to overcome the resistance to established ICIs, which in turn is expected to result in improved response and survival [3]. This review aims to summarize the current state of knowledge of the effects elicited by ICIs on NK cells, as well as the novel immunotherapeutic agents likely to be soon introduced in clinical practice.
2. NK Cell Subsets Diversity
Traditionally, two main circulating NK cell populations have been defined based on their differential expression of CD56 and CD16 markers, termed CD56bright and CD56dim NK cells, which do not overlap for a number of phenotypic and functional properties [4,5]. These include the expression of a high level of perforins and granzymes, as well as, MHC class I-specific inhibitory Killer Ig-like Receptors (KIRs), which are restricted to CD56dim NK cells and license this subset to mediate strong cytotoxic response upon the engagement of activating receptors (i.e., NCR, NKG2D, and DNAM-1). Moreover, the high expression of the Fcγ receptor CD16 provides CD56dim NK cells with the capacity to exert antibody-dependent cell-mediated cytotoxicity (ADCC). Conversely, CD56bright NK cells are considered efficient cytokine producers endowed with immunoregulatory properties, but poorly cytotoxic, unless appropriately activated. A recent series of observations have greatly expanded on NK cell lineage diversity by showing that circulating NK cell subsets actually represent only a minor part of total NK cells in our body, as peripheral tissues harbor a relevant amount of “unconventional” subsets of NK cells that apparently do not recirculate in the blood or lymphatics, and possess distinct phenotypic profiles [6,7]. Tissue residency has now been described as a feature for several subsets of lymphocytes, such as NK cells, “helper”-like innate lymphoid cells (ILCs), and T cell subsets [8]. As for other lymphocytes, NK cells that reside in tissues display markers, such as CD69, CD103 (Integrin alpha E), CD49a (i.e., the α1 subunit of α1β1 integrin), and CXCR6, which are all involved in their retention within tissues and, practically, allow for their identification and isolation [9]. To date, discrete subsets of TR-NK cells have been identified in normal human districts such as the uterus (both at steady-state and during pregnancy), bone marrow (BM), secondary lymphoid organs (SLO), liver, and lung [6,7]. Recent advances have characterized several novel human (h)-NK subsets. Among them, adaptive NK cells demonstrate an intriguing, specialized antibody (Ab)-dependent response and several adaptive immune features [10]. Most adaptive NK cells express a high level of NKG2C and lack NKG2A, express KIRs, Leukocyte Ig-like Receptor B1 (LILR-B1/ILT2), low levels of natural cytotoxic receptors (NCRs) (i.e., NKp46 and NKp30), CD161, and T-cell immunoglobulin and mucin-containing domain (TIM-3). In addition to peripheral blood (PB) NK cells, adaptive NK cells can be detected the lymph nodes (LNs) tonsils, liver, pleural fluid, and other sites. Adaptive NK cells are often associated with prior human Cytomegalovirus (HCMV) infection [11]. Interestingly, adaptive NK cells can also infiltrate into tumor tissues, such as NSCLC [12]. Functionally they are able to mount ADCC, thus adaptive NK cells may play a part in tumor eradication by specific targeted Ab-based cancer therapy. In the tumor microenvironment (TME), a large number of immune-suppressive pathways are combined to inhibit NK cell function. In addition, the continuous exposure to tumor cells and the microenvironment contributes to the exhaustion of the immune effector cells. Exhausted Tumor-associated (TA)-NK cells exhibit downregulation of effector cytokines, decreased degranulation potential, downregulation of activating receptors (such as NKG2D), upregulation of inhibitory receptors such as PD-1, TIM-3, T cell immunoreceptor with Ig, and Immunoreceptor Tyrosine-based inhibition Motif (ITIM) domains (TIGIT) and NKG2A and decreased expression of Eomesodermin and T-bet transcription factors (TFs) [13,14]. Expression of the latter markers on NK cells correlates with decreased NK cell functionality and blockade of these receptors can increase NK cell cytotoxicity and function [14].
3. Expression of MHC Class I- Specific Inhibitory Receptors by NK Cells (Classical ICs)
NK cell functions depend on the balance between inhibitory and activating signals mediated by cell surface receptors.
Classical inhibitory receptors expressed by NK cells are mainly human leukocyte antigen (HLA) class I- binding molecules and include KIRs, CD94/NKG2A, and LILR-B1/ILT2. [15]. The family of inhibitory KIRs (iKIRs) includes the two domain KIR2DL1 and KIR2DL2/3 molecules and the three domain KIR3DL1 and KIR3DL2 receptors that recognize epitopes shared by different groups of classical HLA class I molecules. CD94/NKG2A is specific for the non-classical HLA-E molecules in complex with several peptides derived from the leader sequence of HLA-A, -B, or -C molecules [16,17] or from CMV [18,19]. LILR-B1/ILT2 displays a promiscuous recognition of many classical and non-classical HLA class I molecules as well as of the CMV-derived class I-like molecules UL18 [20].
4. Expression of Non-Classical ICs by NK Cells
NK cells also express other immunoregulatory receptors that function as non-classical IC. These include PD-1, TIGIT, Tactile/CD96, TIM-3, CD161, and Lymphocyte-activation gene 3 (LAG3) [21]. Resting NK cells usually do not express these molecules, while they can be often found on “stressed”/activated NK cells during infection or tumors.
PD-1 is an inhibitory receptor of cellular immune response expressed in different immune cell populations. PD-1 can bind some specific ligands, i.e., PD-L1 (B7-H1, CD274), or PD-L2 (B7-DC, CD273) [22].
These inhibitory receptors are required for peripheral tolerance generation and for the inhibition of damage during inflammation in peripheral tissues. While PD-L2 expression is mostly limited to dendritic cells (DC), macrophages, and lung cells, PD-L1 is most widely expressed and can be overexpressed on several tumor cells [23,24] thus, probably, favoring escaping of tumor cells from immune surveillance. PD-1 expression on NK cells was shown in patients with ovarian carcinoma, Kaposi sarcoma myeloma, and gastrointestinal cancers [25,26,27]. It is conceivable that the TME, through signals delivered by soluble factors and/or cells, can induce PD-1 expression [28]. Moreover, trogocytosis can act as a mechanism by which PD-1 is transferred from tumor cells to NK and T cells. PD-1 trogocytosis sharply inhibits the potential of NK cells to eradicate tumors in vivo [29]. Recently, it has been also reported that PD-1 is stored in cytotoxic granules and its surface expression increased following recognition of tumor cells, concurrent with CD107a surface mobilization [30] on NK cells. TIGIT is an inhibitory IC expressed by resting T and NK cells, upregulated along with activation [31] and often overexpressed in a variety of cancers [32]. TIGIT competes with CD226 to bind to PVR (CD155) and Nectin-2 (CD112) two molecules often up-regulated on tumor cells thus playing an important role in the NK-cell activity inhibition in part counterbalanced by the co-stimulatory activity exert by DNAM-1(CD226) receptor [33,34]. Tactile/CD96 is another important IC for NK cell effector functions, which shares with TIGIT the ligands. CD96+ NK cells are significantly increased in the intra-tumor tissues of hepatocellular carcinoma (HCC) [35,36]. CD96+ NK cells are functionally impaired with reduced capacity to release IFN-γ and TNF-α, and low gene expression levels of Tbx21, Prf1, and GzmB. TIM-3 is expressed in both adaptive and innate immune cells, but human NK cells transcribe the highest amounts of TIM-3 among lymphocytes [37].
So far, galectin-9, phosphatidylserine, and High Mobility Group Box (HMGB)1 have been described as TIM-3 ligands. To date, many aspects of the biology of TIM-3 have not yet been completely elucidated. Thus, while it has been reported that, to exert its function, TIM-3 should interact with Carcinoembryonic antigen related cell adhesion molecule (CEACAM)-1 (in cis and/or trans) [38], a recent study does not provide any evidence for an interaction between these receptors, suggesting that the inhibitory signaling in effector cells is mediated by TIM-3 cytoplasmic sequences [39]. LAG-3 is another inhibitory IC that can be found on activated NK cells [40]. In T cells, LAG-3 co-localizes with CD4 molecules in endosomes, secretory lysosomes, and MicroTubule Organizing center (MTOC) [41]. LAG-3 main ligands are MHC class II molecules, and the fibrinogen family protein 1 (FGL1) [42]. In humans, FGL1 is usually overexpressed by several tumors. In melanoma and lung cancer patients, elevated FGL1 plasma levels are correlated with resistance to immunotherapy with anti-PD-1 mAbs and poor outcome. These data indicate that the FGL1/LAG-3 interaction may aid tumor immune escape. Along this line, evidence that a combined blockade of FGL1 and PD-1 has a synergistic effect has been obtained in animal models [42]. Finally, CD161, belonging to the C-type lectin superfamily, is an inhibitory receptor that recognize Lectin-Like Transcript 1(LLT1), a ligand expressed by several tumors such as non-Hodgkin’s lymphoma (NHL) [43].
5. NK Cell Targeting to Improve Anti-Tumor Response
5.1. Harnessing NK Cells: Immune Checkpoint Inhibitors (ICIs)
To challenge infections, immune innate cells exploit their effector by a variety of activating receptors (aRs) including NCRs, NKG2D, and DNAM-1. These aRs sense pathogen-associated or endogenous molecules that are up-regulated or are expressed de novo at the cell surface under pathological conditions such as infections and/or tumors. Both events result in cytolytic activity and/or production of effector cytokines. Since during inflammation immune-mediated responses might also exert damage to self-tissues, sophisticated control mechanisms to avoid unwanted responses are needed.
These functions are mediated by a number of inhibitory receptors (iRs) called inhibitory ICs, which limit the threshold for effector cell activation and control homeostasis, resolution of inflammation, and self-tolerance. Tumors hijack inhibitory ICs to escape immune eradication. ICIs therapy was confirmed a powerful approach to cancer immunotherapy. In particular, antibodies (Abs) able to abrogate PD-1/PD-L1 interaction have demonstrated extraordinary activity in several types of cancers including metastatic melanoma and NSCLC. Monoclonal Abs (mAbs) targeting PD axis are able to prompt an effective antitumor response mainly through reinvigoration of exhausted PD-1+ CD8+ effectors T cells at the tumor site. However, resistance to PD-1/PD-L1 axis blockade remains a challenge for many patients. Besides specific T lymphocytes, also NK cells play an important role in anti-tumor immunity. Indeed, NK cells are potentially able to recognize and eliminate tumors that elude CD8+ T cell-mediated control by reducing HLA class I expression on their surface. Along this line, in long survey subjects, a lower degree of NK cytolytic potential has been correlated with cancer incidence [44]. Furthermore, several studies have provided evidence that in various solid malignancies the presence of TA-NK cells is associated with a better patient outcome [45].
The function of human NK cells is primarily regulated by classical ICs (including KIRs and NKG2A) specific for HLA-class I molecules which counteract the function of aRs. The fully anti-tumor potential of NK cells could be hindered by other non-classical ICs, recognizing ligands other than HLA class I molecules (PD-1, TIGIT, CD96, TIM-3, and LAG-3) (see above section). Of note, in hematological malignancies, a high expression of ligands for IC correlates with poor patients’ prognosis [46]. In TA-NK cells, the expression of some of these ICs can be modulated by signals present in the TME, thus inhibiting NK cell functions.
Thus, since their blocking may restore NK-cell responses against tumor cells, all these ICs may represent therapeutic targets. Due to the impressive inhibitory effect exerted by human mAbs blocking classical ICs on NK cells, they were the first to enter clinical phase. These include lirilumab (1-7F9, IPH2101) targeting KIR2DL1, KIR2DL2, and KIR2DL3 in patients with AML, myeloma, or solid tumors in AML [47,48] and IPH4102, a first-in-class monoclonal antibody targeting KIR3DL2 in patients with cutaneous T-cell lymphoma, predominantly those with Sézary syndrome. Unfortunately, only IPH4102 has shown, so far, promising clinical activity [49]. Dual ICIs therapy with nivolumab plus lirinumab in patients with recurrent resectable squamous cell carcinoma of the head and neck (SCCHN) is also being evaluated (NCT03341936).
An important improvement supporting the use in the clinic of mAbs recognizing NK cell-“specific” ICs has been obtained by Andrè and collaborators who analyzed the potential of an NKG2A blocking mAb (used either alone or combined other therapeutic mAbs) to unleash NK cell effector functions against HLA-E+ tumor cells [50].
Notably, the non-classical HLA class I molecule HLA-E is expressed by various human malignancies (such as lung, colon, pancreas, stomach, head and neck, and liver tumors), and NKG2A+ NK cells can be found in the tumor nest.
Monalizumab is an anti-NKG2A blocking mAb that not only boosts NKG2A+NK cell responses against HLA-E+ tumor cells but also promotes the effectiveness of durvalumab (a mAb that blocks PD-L1) by increasing the functional activity of NKG2A+PD-1+ NK cells against HLA-E+PD-L1+ target cells. Currently, clinical trials testing monalizumab either alone or combined with other mAbs (including anti-EGFR or anti-PD-L1) are active (see next section).
Several studies in humans have shown that NK cells from cancer patients express PD-1, which correlates with a lower anti-tumor activity [23,25,51,52]. Recently, it has been described that the therapeutic effect of PD-1 and PD-L1 blockade may rely also on the antitumor activity of NK cells [53]. Using several cancer mice models, Hsu and coworkers found that activated NK cells express PD-1 and that PD-1 engagement by PD-L1+ tumor cells potently suppress NK cell–mediated immunity to tumors [53]. Thus, the blockade of PD-1 or PD-L1, is able to activate an NK response that could be crucial for the full effect of PD-1/PD-L1 blockade. Our recently published results [54] correlate higher absolute numbers of circulating NK cells with longer overall survival (OS) in NSCLC patients treated with Nivolumab. These data are in line with what is already documented by [55], thus suggesting that the exact impact of NK cells on the response to nivolumab is an aspect that needs further studies.
TIGIT blockade can reverse the exhausted status of TA-NK cells [56], thus representing a potential new strategy to explore in immunotherapy. Since TIGIT could act as a negative regulator of NK cell functions, it represents an ideal molecule that can be targeted in checkpoint blockade strategies to boost NK tumor-immunity against CD155/PVR expressing cancers. Indeed, TIGIT has recently entered the spotlight as a promising IC target in cancer immunotherapy [57,58]. It is worth noting that IL-15 increased both DNAM-1/CD226 and TIGIT expression by TA-NK cells, thus in the presence of TIGIT-blocking mAbs, IL-15-activated NK cells can be triggered via DNAM-1/CD226. These alterations in both activating (CD226, the good one) and inhibitory (TIGIT, the bad one) receptors levels, together with TIGIT -targeted therapy may tip the balance in the net activating signaling output. It should also be stressed that translating TIGIT blockade into the clinic would be safer than the PD-1 or CTLA-4 blockade. Indeed, mice deficient for TIGIT do not show any sign of spontaneous autoimmunity or any defects in hematopoiesis. Thus far, the therapeutic role of mAbs blocking TIGIT (utilized alone or combined with anti-PD-1/PD-L1 mAbs) is being investigated in trials of patients with metastatic solid tumors (see below section). It has been described that in lung carcinoma [59] and gastric cancer patients [60] TIM-3 upregulation on peripheral blood NK cells is associated with reduced OS and advanced tumor stage, respectively. In patients suffering from esophageal tumors, NK cells expressing TIM-3 display an exhausted phenotype. Along this line, in these patients, high levels of TIM-3 on NK cells infiltrating the tumor nest are associated with tumor progression [61]. This clinical evidence suggests that in metastatic patients TIM-3 can act as a marker of exhaustion in NK cells, thus supporting the role of TIM-3 blocking mAbs in reinvigorating anti-tumor immunity. Further supporting these data, it has been described that in melanoma patients TIM-3-targeted therapy was able to restore NK cell function [62]. On the other hand, some studies described that TIM-3 can act as a stimulatory molecule able to promote T cell activation and differentiation [63,64]. Thus, since TIM-3 can also display a triggering function, in clinical use, anti-TIM- 3 blocking mAbs should be employed with care.
Lastly, whereas in mouse models it has been demonstrated that LAG-3 is able to impair NK cell activity against metastases [65], limited information exists on human LAG-3+ NK cells. In different tumor cell types, clinical trials investigating Relatlimab (a LAG-3 blocking mAb), used either alone or combined with other IC blocking mAbs, are active (see below section) (Figure 1).
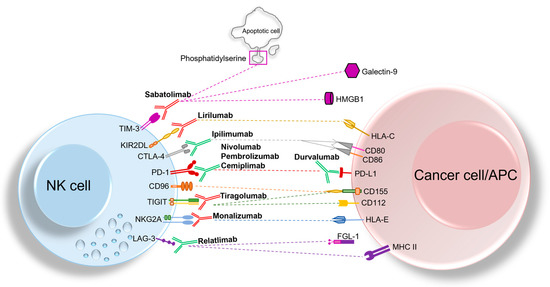
Figure 1.
Overview of classical and non-classical ICs expressed by NK cells and their cognate ligands expressed either on cancer cells or antigen-presenting cells (APCs). The blocking mAbs that are currently being investigated in clinical trials, including anti- TIM-3, anti-NKG2A, anti-KIR, and anti-TIGIT mAbs are depicted in red, while blocking mAbs that are approved by FDA and currently used in clinic (such as anti-PD-1, anti-CTLA-4, and anti-LAG-3 mAbs) are represented in green. Blockade of ICs could recover NK cell anti-tumor activity, thereby representing a promising approach for immunotherapy.
5.2. Clinical Data on Therapeutic Approaches in Solid Tumors Involving Both Classical and Emerging/Non-Classical Immune Checkpoints
Monalizumab is an ICI active on NKG2A and thus able to activate anti-tumor activity of NK cells. This agent was evaluated in combination with cetuximab and durvalumab in a non-randomized, single-arm phase II trial involving treatment-naïve patients with recurrent or metastatic SCCHN (NCT02643550); in this study, the combination including monalizumab was characterized by acceptable safety profile and promising antitumor activity [66]. With regards to NSCLC, the activity of monalizumab was recently explored in the COAST trial (NCT03822351), an open-label phase II, randomized study in which patients who had been treated with chemo-radiation for inoperable stage III NSCLC were randomized to receive maintenance with durvalumab alone, the current standard of care, or durvalumab in combination with either oleclumab (an anti-CD73 agent) or monalizumab. With regards to the arm containing durvalumab plus monalizumab, the objective response rate (ORR) was 35.5%, while the median progression-free survival (mPFS) was 15.1 months, while ORR and mPFS for durvalumab alone were 17.9% and 6.3 months, respectively; additionally, the combination was generally well tolerated [67]. Currently, other clinical trials are exploring the role of anti-NKG2A treatment. Among these, the ongoing Precision Immuno-Oncology for Advanced Non-small Cell Lung Cancer Patients With PD-1 ICI Resistance (PIONeeR) trial is extremely promising, as it aims to enroll patients with NSCLC who had previously received ICIs and who will be treated with a combination of durvalumab and other agents, including monalizumab, in order to overcome acquired resistance to single-agent ICIs (NCT03833440) [https://clinicaltrials.gov/ct2/show/NCT03833440 (accessed on 22 September 2022)].
Lirilumab is another agent active on NK cells, as it masks KIR2D receptors, hence enhancing cytotoxicity of NK cells. After showing a manageable safety profile in dose-finding, phase I studies, both alone and in combination with an anti-PD-1 agent [47,68], the activity of lirilumab in combination with nivolumab in the neoadjuvant and adjuvant setting for squamous cell cancer of head and neck was explored in a single-arm phase II trial published by Hannah et al. In this study, the combination was generally well tolerated and achieved good outcomes in terms of 1-year disease-free survival (DFS) and 1-year OS, respectively, 55.2% and 85.7% [69]. Furthermore, lirilumab was employed in combination with epacadostat (an Indoleamine 2,3-dioxygenase 1, IDO-1, inhibitor) and nivolumab in the ECHO-208 trial; recently, the enrollment for this study was stopped, but its results are still not available (NCT 03347123) [https://clinicaltrials.gov/ct2/show/results/NCT03347123 (accessed on 22 September 2022)]. The relevant clinical data of these agents are summarized in Table 1.

Table 1.
Relevant clinical data of immune checkpoint inhibitors specifically designed to enhance anti-neoplastic NK cell activity. Legend -> DCR: disease control rate; DLT: dose-limiting toxicity; DFS: disease-free survival; NSCLC: non-small cell lung cancer; SCCHN: squamous cell carcinoma of head and neck; OS: overall survival; PFS: progression-free survival; TRAE: treatment-related adverse event. * in the COAST trial, durvalumab was employed alone or in combination with oleclumab or monalizumab. In this table, we are considering the outcomes of the regimen containing durvalumab and monalizumab.
Another novel drug class with potential activity on NK is represented by the inhibitors of TIGIT; indeed, TIGIT blockade is associated with prevention of NK exhaustion and enhancement of NK anti-tumor immunity [56]. Tiragolumab is a novel immune checkpoint inhibitor designed to target TIGIT, with promising activity in solid malignancies and especially in NSCLC. Indeed, in the phase II trial CITYSCAPE, 135 patients affected by advanced NSCLC with PD-L1 expression ≥1% were randomized (1:1) to receive atezolizumab (anti-PD-L1) plus tiragolumab or atezolizumab alone. At the interim analysis, the combination regimen achieved a statistically significant advantage in terms of PFS and numerical, albeit non-statistically significant, advantage in terms of OS over single-agent PD-L1 inhibitor; notably, in the sub-group analysis, the advantage achieved by the combination was pronounced among patients whose tumor harbored high PD-L1 expression (≥50%), but not among patients with PD-L1 between 1–49%. To date, the median OS has not been reported yet [70]. Currently, the role of tiragolumab is being explored in other settings for the management of NSCLC, such as maintenance after chemo-radiation for locally advanced disease (NCT04513). Another promising immune-related molecule is LAG-3, which has been firstly found on activated NK cells. Knockout LAG-3 mice have a decreased natural killer activity. Notably, it has been observed that LAG-3 plays a critical role in NKT cell function, and its expression results in decreased proliferation and functions of NKT cells, i.e., a cell subset that expresses both NK receptors and T cell receptors [71]. Relatlimab (anti-LAG-3) was employed in combination with nivolumab in a population of patients with advanced, pre-treated melanoma, resulting substantially safe and active [72]. Based on these results, the combination of relatlimab plus nivolumab was evaluated in a placebo-controlled, randomized phase II/III trial (RELATIVITY-047) designed to include patients with advanced melanoma. In this study, mPFS, the primary endpoint, was significantly improved in the experimental arm (relatlimab plus nivolumab) compared to placebo-nivolumab; hence, the combination of LAG-3 and PD-1 inhibition seems promising from a clinical perspective [73].
Notably, a co-formulation including nivolumab/relatlimab was approved for the use of metastatic melanoma by the American Food and Drug Administration (FDA) in March 2022 [https://www.fda.gov/drugs/resources–information–approved–drugs/fda–approves–opdualag–unresectable–or–metastatic–melanoma (accessed on 22 September 2022)] and by the European Medicine Agency (EMA) in July 2022 [https://www.ema.europa.eu/en/medicines/human/EPAR/opdualag (accessed on 22 September 2022)].
Finally, another molecule TIM-3 represents a promising target as its expression is down-regulated in activated NK cells [74]. The safety and activity of sabatolimab, an anti-TIM-3 antibody, administered alone or in combination with spartalizumab (an anti-PD-1 antibody) were explored in a phase I/II trial designed to enroll patients with solid tumors; the safety profile of the combination was generally manageable at the recommended phase II dose identified in the dose escalation; indeed, the maximum tolerated dose was not reached. With regards to activity, initial responses were observed, thus leading to further clinical development of this combination [75]. The relevant clinical data of the aforementioned agents are summarized in Table 2.

Table 2.
Relevant clinical data of immunotherapy agents with potential effect on NK cells. Legend -> AE: adverse event; CI: confidence interval; CBR: clinical benefit rate; HR: hazard ratio; ITT: intent-to-treat; MTD: maximum-tolerated dose; NR: not reached; NSCLC: non-small cell lung cancer; ORR: objective response rate; RCC: renal cell carcinoma; SAE: severe adverse events. * p values were not reported in the interim analysis.
In addition to the agents specifically designed to act on IC expressed by NK cells, there are other relevant drugs, active on novel, emerging immune checkpoints; such checkpoints are acknowledged to influence, among other immune cells, also NK. Elotuzumab, a mAb directed on signaling lymphocytic activation molecule F7 (SLAM7), has shown the ability to induce NK cell infiltration and cytotoxicity, albeit this activity was specifically observed in multiple myeloma pre-clinical models [80]. In a single-arm, phase II trial, patients with multiple myeloma received elotuzumab plus pomalidomide, carfilzomib, and low-dose dexamethasone, with good tolerability and promising activity [76]. To date, knowledge on the potential role of elotuzumab in solid tumors is still limited.
Among the molecule of interest for immunotherapeutic agents, IDO-1 has emerged as a potential novel target for immune checkpoint blockade (ICB), as it is known to inhibit proliferation and activity of cytotoxic T cells and NK cells [81]. The IDO1 inhibitor epacadostat has been evaluated in several trials designed to assess its tolerability and activity. In the phase I ECHO-110 trial, epacadostat in combination with atezolizumab showed a globally manageable safety profile, as well as some level of antineoplastic activity, thus leading to further trial development [77]. Similarly, in a phase I/II trial, ECHO-202/KEYNOTE-037, epacadostat in combination with pembrolizumab was generally manageable and active, especially among patients with melanoma and NSCLC [78]. Based on these results, the efficacy of epacadostat plus pembrolizumab was evaluated in a phase III, placebo-controlled trial involving 706 patients affected by advanced melanoma and naïve from ICIs; notably, the trial did not meet any of its co-primary endpoints, PFS and OS, and the author concluded that the clinical role of IDO-1 inhibitors remains uncertain [79].
6. Role of NK Cells in Creating a More Inflamed Environment (to Prepare the Ground for ICI)
Several studies have implicated an important role of NK cells in tumor immune surveillance. Many results were derived from mouse models, which were either depleted of NK cells or impaired in conventional NK cell activities. Remarkably, these studies also demonstrated an exceptional capacity of NK cells to resist the hematogenous spread of experimental and spontaneous tumor metastases [82]. These preclinical data were further supported by observational studies in humans, even evaluating large cohorts, where NK cell deficiencies [83], as well as lower NK cell activity in peripheral blood [44], could be associated to a higher risk of developing various types of cancer. Regarding lung cancers, the prognostic value of infiltrating NK cells in resected tumors still needs to be defined. Primarily, this is due to the limited number of studies performed and the small size of cohorts analyzed in each study. Moreover, it should be noted that these analyses were performed using markers (i.e., CD57 and/or CD56) not exclusive of NK cells, but potentially expressed by other immune/non-immune cell types. As such, some initial studies indicated that the presence of NK cells in the immune infiltrate was associated with a lower risk of relapse and/or longer survival [84,85] while subsequent studies, performed using the more specific marker NKp46, failed to find an impact of high number of intra-tumoral NK cells (at early stages of disease) on OS [86]. However, recent reports showed that the number of infiltrating CD56+CD16+ NK cells in lung cancer tissue positively correlated to patient survival [87]. Moreover, an “immune cluster” with a signature of NK cells and/or plasma cells was discovered in a limited number of the analyzed NSCLC cases (5%) and was associated with improved survival. Remarkably, this subgroup showed a favorable prognosis despite the lack of markers for T cells or T-cell activation [88].
The low number of infiltrating NK cells has raised questions about the actual function of this cell population at the tumor site. However, even if generally underrepresented in solid tumors, these cells could contribute to anti-tumor immune responses by means of their cytolytic activity or their remarkable capacity to produce cytokines and chemokines that recruit and activate (or potentially suppress) other hematopoietic cells [89]. Several reports have found that cytotoxic CD56dim Perforinhigh NK cells are quite excluded from tumor tissue. Conversely, CD56bright Perforinlow NK cells represent the main NK cell population infiltrating human cancer tissues, and at least for some tumor types, such as NSCLC and breast cancer, the ratio of cytotoxic CD56dim Perforinhigh to non-cytotoxic CD56bright Perforinlow is completely inverted when compared to the matched normal tissues. Interestingly, the relative accumulation of CD56bright Perforinlow was associated with a switch in chemokine expression patterns of tissues upon the neoplastic transformation [90]. Thus far, this phenomenon remains an interesting and poorly explored aspect in the field. Whether this may represent a specific strategy used by the immune system to control tumor growth or rather a mechanism of tumor immune evasion is yet to be determined. As such, the presence of non-cytotoxic CD56bright Perforinlow NK cells, which are devoid of CD16 and represent the dominant NK cell subset infiltrating several human solid cancers, could limit the response of agents aimed at boosting NK-mediated ADCC. Overall, defining how NK cell functional diversity integrates into innate and adaptive immune responses to cancer represents a critical challenge. DCs, the most efficient APCs of the immune system, are now established as a critical immune effector based on their ability to induce anti-tumor T cell immunity and response to immunotherapies. Among different tumor-infiltrating DC phenotypes found across solid human cancers, conventional type 1 DCs (cDC1s) are specialized in antigen cross-presentation and CD8+ T cell activation. Accordingly, in human tumors, gene expression signatures related to cDC1s have been correlated with better clinical prognosis and response to ICB [91,92]. Therefore, the possibility of recruiting cDC1 into tumors, as well as improving their functionality, could prove to be useful strategies for increasing antitumor immunity and response to immunotherapies. Remarkably, an additional role of NK cells in the immune response to cancer has been demonstrated by recent publications that showed NK cells controlling the levels of intratumoral cross-presenting cDC1s, by expression of FLT3 Ligand [92] and chemokines, such as CCL5 (RANTES) and XCL-1/XCL-2 [91,93]. In patients with melanoma, levels of NK cells and intratumoral cDC1s even positively correlated with increased survival and predicted response to anti-PD-1 therapy [92]. In this respect, NK cells have been suggested as a “spark” that ignites immune cell infiltration and inflammation in the tumor [94]. It is noteworthy that unique clusters of NK cells characterized by high expression of XCL1/2 transcripts were also identified among total NK cells isolated from melanoma metastasis [95]. DCs and NK cells can reciprocally engage in a bi-directional activation that can influence the outcome of adaptive immunity, by influencing the development of T helper-1 (TH-1) cells and cytotoxic T lymphocytes (CTLs), both essential for an effective anti-tumor immune response. Granulocyte-macrophage-colony stimulating factor (GM-CSF) is a potent cytokine promoting the differentiation of myeloid cells and is essential for the differentiation of dendritic cells, which are responsible for processing and presenting tumor antigens for the priming of CTLs [96]. Interestingly, NK cells may potentially be a major source of Granulocyte-Macrophage Colony-Stimulating Factor (GM–CSF) in tumors, especially CD56bright NK cells, which are enriched in neoplastic tissues [90] and represent the NK cell subset producing the higher levels of this cytokine [4].
Overall, recruitment and modulation of APCs at the tumor site could have a great impact on cancer immune surveillance, given the positive association of CD8+ T cell infiltration with longer survival in NSCLC patients [97,98]. Interestingly, in a mouse model of lung adenocarcinoma, stimulation of tumor-infiltrating NK cells by a conditional expression of activating NK cell ligands led to an increase of tumor-specific T cells. Mechanistically, the accumulation of adaptive immune cells was not due to overt signs of cytotoxicity in tumors against tumor cells but, rather, to the direct production of chemokines, such as CCL5, or indirectly, to the stimulation of APCs, as suggested by the authors [99]. Finally, Zemek RM et al. demonstrated, in both an animal tumor model of mesothelioma and datasets from patients, that the presence of activated NK cells in the TME and expression of immune response-related genes characterized by Signal transducer and activator of transcription 1 (STAT1) activation can correlate with the clinical response to ICI [100].
7. Conclusions
Although ICI immunotherapy is well positioned as a safe anti-tumor therapy, important questions remain open. Elucidating the key parameters that unleash not only the activity and reinvigoration of T cells but also NK cell potential will be important as the field progresses into developing approaches to address challenges specific to each different neoplastic disease indication.
The relief from NK-specific IC implies potential therapeutic advantages related to the quite modest autoimmune burden and significant anti-tumor activity of NK cells. However, for the emerging NK cell therapy programs, decisions depend on still open issues, such as the altered responsiveness of NK cells in the patients and the limited persistency of NK cell activation in vivo or in adoptively transferred NK cells. The generation of engineered molecules, combining arms specific for different NK receptors and targeting tumor epitopes, represents an important element for the assembly of new therapeutic strategies. These multivalent molecules (bi- tri- or tetra-specific engagers) [101,102,103,104] may combine multiple therapeutic effects, depending on the assembled specificities, stimulating triggering NK receptors and cytokine receptors, blocking ICs, and targeting tumor cells. Another important approach deals with the use of combined cytokine cocktails (IL-12, IL-18, IL-15) to get the so-called Cytokine-Induced Memory-Like (CIML) NK cells. These cells are able to “remember” the initial cytokine boost and maintain their increased responsiveness and even persist in the patients after adoptive transfer [105]. Finally, engineered NK cell products expressing chimeric Antigen Receptors (NK-CAR) can be obtained from fresh NK cells or from precursor cells (including induced-Pluripotent Stem Cells—iPSC) [106].
Most of these tools, including the ICI, are being studied in clinics and hold promise, but the real frontier in the field is the search for appropriate therapeutic combination to maximize the anti-tumor power of NK cells in the different specific pathologic conditions [107].
Author Contributions
Conceptualization, writing original draft preparation, review, editing and preparing figure: M.G. Conceptualization, writing original draft preparation, review: P.C. Supervision and review: L.Z. (Lodovica Zullo), C.D., G.R., F.P., G.B., L.Z. (Linda Zinoli), S.C., A.A., S.M., M.P., P.O. and S.B. Supervision, review, editing, and funding acquisition M.V. Editing and funding acquisition. U.P. and M.C.M. Conceptualization, supervision, writing original draft review, and funding acquisition: G.P. and C.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by: Italian Ministry of Health, project code:RF-2016-02362288. (to G.P.); Italian Ministry of Health, project code: RF-2018-12366714 (to M.V.); Associazione Italiana per la Ricerca su Cancro (AIRC) Investigator Grant project code: AIRC IG 25023 (to M.V.); 5permille 2022-24 IRCCS Ospedale Policinico San Martino (to G.P.); 5permille 2018-19 IRCCS Ospedale Policlinico San Martino (to C.G.); Ricerca Corrente 2022-24 IRCCS Ospedale Policlinico San Martino (to G.P.); AIRC 5permille 2018-ID.21073 program-P.I. Maio Michele (to U.P.). M.G. is the recipient of a fellowship awarded by the Italian Ministry of Health (RF-2016-02362288.); S.B. is the recipient of a fellowship awarded by AIRC IG 25023.
Data Availability Statement
Not applicable.
Conflicts of Interest
G.R. received honoraria from Bristol-Myers-Squibb; Merck, Sharp & Dohme; Roche. CD received honoraria from Astra Zeneca; Bristol-Myers-Squibb; Merck, Sharp & Dohme; Roche. GB received honoraria from Astra Zeneca; Pierre Fabre; Roche. CG received honoraria from Amgen; Astra Zeneca; Bristol-Myers-Squibb; Eli Lilly; Merck, Sharp & Dohme; Novartis; Roche; Sanofi; Takeda; ThermoFisher. The other authors declare no conflict of interest.
References
- Cella, E.; Zullo, L.; Marconi, S.; Rossi, G.; Coco, S.; Dellepiane, C.; Alama, A.; Rozeboom, L.; Bennicelli, E.; Parisi, F.; et al. Immunotherapy-chemotherapy combinations for non-small cell lung cancer: Current trends and future perspectives. Expert Opin. Biol. 2022, 22, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Pandey, P.; Mishra, R.; Arif, M.; Kumar, A.; Jafri, A.; Mazumder, R. Elucidation of S-Allylcysteine Role in Inducing Apoptosis by Inhibiting PD-L1 Expression in Human Lung Cancer Cells. Anticancer Agents Med. Chem. 2021, 21, 532–541. [Google Scholar] [CrossRef] [PubMed]
- Pandey, P.; Khan, F.; Qari, H.A.; Upadhyay, T.K.; Alkhateeb, A.F.; Oves, M. Revolutionization in Cancer Therapeutics via Targeting Major Immune Checkpoints PD-1, PD-L1 and CTLA-4. Pharmaceuticals 2022, 15, 335. [Google Scholar] [CrossRef]
- Cooper, M.A.; Fehniger, T.A.; Caligiuri, M.A. The biology of human natural killer-cell subsets. Trends Immunol. 2001, 22, 633–640. [Google Scholar] [CrossRef]
- Wu, S.Y.; Fu, T.; Jiang, Y.Z.; Shao, Z.M. Natural killer cells in cancer biology and therapy. Mol. Cancer 2020, 19, 120. [Google Scholar] [CrossRef] [PubMed]
- Bjorkstrom, N.K.; Ljunggren, H.G.; Michaelsson, J. Emerging insights into natural killer cells in human peripheral tissues. Nat. Rev. Immunol. 2016, 16, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Dogra, P.; Rancan, C.; Ma, W.; Toth, M.; Senda, T.; Carpenter, D.J.; Kubota, M.; Matsumoto, R.; Thapa, P.; Szabo, P.A.; et al. Tissue Determinants of Human NK Cell Development, Function, and Residence. Cell 2020, 180, 749–763.e713. [Google Scholar] [CrossRef]
- Mackay, L.K.; Minnich, M.; Kragten, N.A.; Liao, Y.; Nota, B.; Seillet, C.; Zaid, A.; Man, K.; Preston, S.; Freestone, D.; et al. Hobit and Blimp1 instruct a universal transcriptional program of tissue residency in lymphocytes. Science 2016, 352, 459–463. [Google Scholar] [CrossRef]
- Melsen, J.E.; Lugthart, G.; Lankester, A.C.; Schilham, M.W. Human Circulating and Tissue-Resident CD56(bright) Natural Killer Cell Populations. Front. Immunol. 2016, 7, 262. [Google Scholar] [CrossRef]
- Gao, F.; Zhou, Z.; Lin, Y.; Shu, G.; Yin, G.; Zhang, T. Biology and Clinical Relevance of HCMV-Associated Adaptive NK Cells. Front. Immunol. 2022, 13, 830396. [Google Scholar] [CrossRef]
- Mujal, A.M.; Delconte, R.B.; Sun, J.C. Natural Killer Cells: From Innate to Adaptive Features. Annu. Rev. Immunol. 2021, 39, 417–447. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Yu, H.T.; Hwang, I.; Park, S.; Park, S.H.; Kim, S.; Shin, E.C. Phenotypic and Functional Analysis of Human NK Cell Subpopulations According to the Expression of FcepsilonRIgamma and NKG2C. Front. Immunol. 2019, 10, 2865. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.; Vasey, A.E.; De Souza, A.; Baker, J.; Smith, A.T.; Kohrt, H.E.; Florek, M.; Gibbs, K.D., Jr.; Tate, K.; Ritchie, D.S.; et al. Rapid development of exhaustion and down-regulation of eomesodermin limit the antitumor activity of adoptively transferred murine natural killer cells. Blood 2012, 119, 5758–5768. [Google Scholar] [CrossRef] [PubMed]
- Ben-Shmuel, A.; Biber, G.; Barda-Saad, M. Unleashing Natural Killer Cells in the Tumor Microenvironment-The Next Generation of Immunotherapy? Front. Immunol. 2020, 11, 275. [Google Scholar] [CrossRef]
- Moretta, A.; Bottino, C.; Vitale, M.; Pende, D.; Biassoni, R.; Mingari, M.C.; Moretta, L. Receptors for HLA class-I molecules in human natural killer cells. Annu. Rev. Immunol. 1996, 14, 619–648. [Google Scholar] [CrossRef]
- Lee, N.; Llano, M.; Carretero, M.; Ishitani, A.; Navarro, F.; Lopez-Botet, M.; Geraghty, D.E. HLA-E is a major ligand for the natural killer inhibitory receptor CD94/NKG2A. Proc. Natl. Acad. Sci. USA 1998, 95, 5199–5204. [Google Scholar] [CrossRef] [PubMed]
- Braud, V.M.; Allan, D.S.; O’Callaghan, C.A.; Soderstrom, K.; D’Andrea, A.; Ogg, G.S.; Lazetic, S.; Young, N.T.; Bell, J.I.; Phillips, J.H.; et al. HLA-E binds to natural killer cell receptors CD94/NKG2A, B and C. Nature 1998, 391, 795–799. [Google Scholar] [CrossRef] [PubMed]
- Ulbrecht, M.; Martinozzi, S.; Grzeschik, M.; Hengel, H.; Ellwart, J.W.; Pla, M.; Weiss, E.H. Cutting edge: The human cytomegalovirus UL40 gene product contains a ligand for HLA-E and prevents NK cell-mediated lysis. J. Immunol. 2000, 164, 5019–5022. [Google Scholar] [CrossRef]
- Hammer, Q.; Ruckert, T.; Borst, E.M.; Dunst, J.; Haubner, A.; Durek, P.; Heinrich, F.; Gasparoni, G.; Babic, M.; Tomic, A.; et al. Peptide-specific recognition of human cytomegalovirus strains controls adaptive natural killer cells. Nat. Immunol. 2018, 19, 453–463. [Google Scholar] [CrossRef]
- Chapman, T.L.; Heikeman, A.P.; Bjorkman, P.J. The inhibitory receptor LIR-1 uses a common binding interaction to recognize class I MHC molecules and the viral homolog UL18. Immunity 1999, 11, 603–613. [Google Scholar] [CrossRef]
- Sivori, S.; Pende, D.; Quatrini, L.; Pietra, G.; Della Chiesa, M.; Vacca, P.; Tumino, N.; Moretta, F.; Mingari, M.C.; Locatelli, F.; et al. NK cells and ILCs in tumor immunotherapy. Mol. Asp. Med. 2021, 80, 100870. [Google Scholar] [CrossRef] [PubMed]
- Keir, M.E.; Butte, M.J.; Freeman, G.J.; Sharpe, A.H. PD-1 and its ligands in tolerance and immunity. Annu. Rev. Immunol. 2008, 26, 677–704. [Google Scholar] [CrossRef] [PubMed]
- Pesce, S.; Greppi, M.; Grossi, F.; Del Zotto, G.; Moretta, L.; Sivori, S.; Genova, C.; Marcenaro, E. PD/1-PD-Ls Checkpoint: Insight on the Potential Role of NK Cells. Front. Immunol. 2019, 10, 1242. [Google Scholar] [CrossRef]
- Wang, X.; Teng, F.; Kong, L.; Yu, J. PD-L1 expression in human cancers and its association with clinical outcomes. Onco. Targets 2016, 9, 5023–5039. [Google Scholar] [CrossRef]
- Beldi-Ferchiou, A.; Lambert, M.; Dogniaux, S.; Vely, F.; Vivier, E.; Olive, D.; Dupuy, S.; Levasseur, F.; Zucman, D.; Lebbe, C.; et al. PD-1 mediates functional exhaustion of activated NK cells in patients with Kaposi sarcoma. Oncotarget 2016, 7, 72961–72977. [Google Scholar] [CrossRef]
- Pesce, S.; Greppi, M.; Tabellini, G.; Rampinelli, F.; Parolini, S.; Olive, D.; Moretta, L.; Moretta, A.; Marcenaro, E. Identification of a subset of human natural killer cells expressing high levels of programmed death 1: A phenotypic and functional characterization. J. Allergy Clin. Immunol. 2017, 139, 335–346.e333. [Google Scholar] [CrossRef]
- Vari, F.; Arpon, D.; Keane, C.; Hertzberg, M.S.; Talaulikar, D.; Jain, S.; Cui, Q.; Han, E.; Tobin, J.; Bird, R.; et al. Immune evasion via PD-1/PD-L1 on NK cells and monocyte/macrophages is more prominent in Hodgkin lymphoma than DLBCL. Blood 2018, 131, 1809–1819. [Google Scholar] [CrossRef]
- Quatrini, L.; Mariotti, F.R.; Munari, E.; Tumino, N.; Vacca, P.; Moretta, L. The Immune Checkpoint PD-1 in Natural Killer Cells: Expression, Function and Targeting in Tumour Immunotherapy. Cancers 2020, 12, 3285. [Google Scholar] [CrossRef]
- Hasim, M.S.; Marotel, M.; Hodgins, J.J.; Vulpis, E.; Makinson, O.J.; Asif, S.; Shih, H.Y.; Scheer, A.K.; MacMillan, O.; Alonso, F.G.; et al. When killers become thieves: Trogocytosed PD-1 inhibits NK cells in cancer. Sci. Adv. 2022, 8, eabj3286. [Google Scholar] [CrossRef]
- Pesini, C.; Hidalgo, S.; Arias, M.A.; Santiago, L.; Calvo, C.; Ocariz-Diez, M.; Isla, D.; Lanuza, P.M.; Agustin, M.J.; Galvez, E.M.; et al. PD-1 is expressed in cytotoxic granules of NK cells and rapidly mobilized to the cell membrane following recognition of tumor cells. Oncoimmunology 2022, 11, 2096359. [Google Scholar] [CrossRef]
- Yu, X.; Harden, K.; Gonzalez, L.C.; Francesco, M.; Chiang, E.; Irving, B.; Tom, I.; Ivelja, S.; Refino, C.J.; Clark, H.; et al. The surface protein TIGIT suppresses T cell activation by promoting the generation of mature immunoregulatory dendritic cells. Nat. Immunol. 2009, 10, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Mao, X.; Cheng, Q.; Liu, Z.; Liu, F. A pan-cancer analysis revealing the role of TIGIT in tumor microenvironment. Sci. Rep. 2021, 11, 22502. [Google Scholar] [CrossRef] [PubMed]
- Dougall, W.C.; Kurtulus, S.; Smyth, M.J.; Anderson, A.C. TIGIT and CD96: New checkpoint receptor targets for cancer immunotherapy. Immunol. Rev. 2017, 276, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.M.; Li, W.Q.; Wu, Y.H.; Han, L.; Cao, X.G.; Yang, X.M.; Wang, H.F.; Zhao, W.S.; Zhai, W.J.; Qi, Y.M.; et al. Intrinsic Expression of Immune Checkpoint Molecule TIGIT Could Help Tumor Growth in vivo by Suppressing the Function of NK and CD8(+) T Cells. Front. Immunol. 2018, 9, 2821. [Google Scholar] [CrossRef]
- Sun, H.; Huang, Q.; Huang, M.; Wen, H.; Lin, R.; Zheng, M.; Qu, K.; Li, K.; Wei, H.; Xiao, W.; et al. Human CD96 Correlates to Natural Killer Cell Exhaustion and Predicts the Prognosis of Human Hepatocellular Carcinoma. Hepatology 2019, 70, 168–183. [Google Scholar] [CrossRef]
- Lu, Y.; Ma, S.; Ding, W.; Sun, P.; Zhou, Q.; Duan, Y.; Sartorius, K. Resident Immune Cells of the Liver in the Tumor Microenvironment. Front. Oncol. 2022, 12, 931995. [Google Scholar] [CrossRef]
- Ndhlovu, L.C.; Lopez-Verges, S.; Barbour, J.D.; Jones, R.B.; Jha, A.R.; Long, B.R.; Schoeffler, E.C.; Fujita, T.; Nixon, D.F.; Lanier, L.L. Tim-3 marks human natural killer cell maturation and suppresses cell-mediated cytotoxicity. Blood 2012, 119, 3734–3743. [Google Scholar] [CrossRef]
- Huang, Y.H.; Zhu, C.; Kondo, Y.; Anderson, A.C.; Gandhi, A.; Russell, A.; Dougan, S.K.; Petersen, B.S.; Melum, E.; Pertel, T.; et al. CEACAM1 regulates TIM-3-mediated tolerance and exhaustion. Nature 2015, 517, 386–390. [Google Scholar] [CrossRef]
- De Sousa Linhares, A.; Kellner, F.; Jutz, S.; Zlabinger, G.J.; Gabius, H.J.; Huppa, J.B.; Leitner, J.; Steinberger, P. TIM-3 and CEACAM1 do not interact in cis and in trans. Eur. J. Immunol. 2020, 50, 1126–1141. [Google Scholar] [CrossRef]
- Triebel, F.; Jitsukawa, S.; Baixeras, E.; Roman-Roman, S.; Genevee, C.; Viegas-Pequignot, E.; Hercend, T. LAG-3, a novel lymphocyte activation gene closely related to CD4. J. Exp. Med. 1990, 171, 1393–1405. [Google Scholar] [CrossRef]
- Woo, S.R.; Li, N.; Bruno, T.C.; Forbes, K.; Brown, S.; Workman, C.; Drake, C.G.; Vignali, D.A. Differential subcellular localization of the regulatory T-cell protein LAG-3 and the coreceptor CD4. Eur. J. Immunol. 2010, 40, 1768–1777. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sanmamed, M.F.; Datar, I.; Su, T.T.; Ji, L.; Sun, J.; Chen, L.; Chen, Y.; Zhu, G.; Yin, W.; et al. Fibrinogen-like Protein 1 Is a Major Immune Inhibitory Ligand of LAG-3. Cell 2019, 176, 334–347.e312. [Google Scholar] [CrossRef] [PubMed]
- Bialoszewska, A.; Malejczyk, J. Biological and Clinical Significance of Human NKRP1A/LLT1 Receptor/Ligand Interactions. Crit Rev. Immunol. 2018, 38, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Imai, K.; Matsuyama, S.; Miyake, S.; Suga, K.; Nakachi, K. Natural cytotoxic activity of peripheral-blood lymphocytes and cancer incidence: An 11-year follow-up study of a general population. Lancet 2000, 356, 1795–1799. [Google Scholar] [CrossRef]
- Nersesian, S.; Schwartz, S.L.; Grantham, S.R.; MacLean, L.K.; Lee, S.N.; Pugh-Toole, M.; Boudreau, J.E. NK cell infiltration is associated with improved overall survival in solid cancers: A systematic review and meta-analysis. Transl. Oncol. 2021, 14, 100930. [Google Scholar] [CrossRef]
- Guan, J.; Wang, R.; Hasan, S.; Tao, L.; Wazir, M.; Jain, A.G.; Zhu, X.; Perkins, S.; Mohamed, S.; Chang, C.C.; et al. Prognostic Significance of the Dynamic Change of Programmed Death-ligand 1 Expression in Patients with Multiple Myeloma. Cureus 2019, 11, e4401. [Google Scholar] [CrossRef]
- Vey, N.; Karlin, L.; Sadot-Lebouvier, S.; Broussais, F.; Berton-Rigaud, D.; Rey, J.; Charbonnier, A.; Marie, D.; Andre, P.; Paturel, C.; et al. A phase 1 study of lirilumab (antibody against killer immunoglobulin-like receptor antibody KIR2D.; IPH2102) in patients with solid tumors and hematologic malignancies. Oncotarget 2018, 9, 17675–17688. [Google Scholar] [CrossRef]
- Carlsten, M.; Korde, N.; Kotecha, R.; Reger, R.; Bor, S.; Kazandjian, D.; Landgren, O.; Childs, R.W. Checkpoint Inhibition of KIR2D with the Monoclonal Antibody IPH2101 Induces Contraction and Hyporesponsiveness of NK Cells in Patients with Myeloma. Clin. Cancer Res. 2016, 22, 5211–5222. [Google Scholar] [CrossRef]
- Bagot, M.; Porcu, P.; Marie-Cardine, A.; Battistella, M.; William, B.M.; Vermeer, M.; Whittaker, S.; Rotolo, F.; Ram-Wolff, C.; Khodadoust, M.S.; et al. IPH4102, a first-in-class anti-KIR3DL2 monoclonal antibody, in patients with relapsed or refractory cutaneous T-cell lymphoma: An international, first-in-human, open-label, phase 1 trial. Lancet Oncol. 2019, 20, 1160–1170. [Google Scholar] [CrossRef]
- Andre, P.; Denis, C.; Soulas, C.; Bourbon-Caillet, C.; Lopez, J.; Arnoux, T.; Blery, M.; Bonnafous, C.; Gauthier, L.; Morel, A.; et al. Anti-NKG2A mAb Is a Checkpoint Inhibitor that Promotes Anti-tumor Immunity by Unleashing Both T and NK Cells. Cell 2018, 175, 1731–1743.e1713. [Google Scholar] [CrossRef]
- Liu, Y.; Cheng, Y.; Xu, Y.; Wang, Z.; Du, X.; Li, C.; Peng, J.; Gao, L.; Liang, X.; Ma, C. Increased expression of programmed cell death protein 1 on NK cells inhibits NK-cell-mediated anti-tumor function and indicates poor prognosis in digestive cancers. Oncogene 2017, 36, 6143–6153. [Google Scholar] [CrossRef]
- Benson, D.M., Jr.; Bakan, C.E.; Mishra, A.; Hofmeister, C.C.; Efebera, Y.; Becknell, B.; Baiocchi, R.A.; Zhang, J.; Yu, J.; Smith, M.K.; et al. The PD-1/PD-L1 axis modulates the natural killer cell versus multiple myeloma effect: A therapeutic target for CT-011, a novel monoclonal anti-PD-1 antibody. Blood 2010, 116, 2286–2294. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.; Hodgins, J.J.; Marathe, M.; Nicolai, C.J.; Bourgeois-Daigneault, M.C.; Trevino, T.N.; Azimi, C.S.; Scheer, A.K.; Randolph, H.E.; Thompson, T.W.; et al. Contribution of NK cells to immunotherapy mediated by PD-1/PD-L1 blockade. J. Clin. Investig. 2018, 128, 4654–4668. [Google Scholar] [CrossRef] [PubMed]
- Ottonello, S.; Genova, C.; Cossu, I.; Fontana, V.; Rijavec, E.; Rossi, G.; Biello, F.; Dal Bello, M.G.; Tagliamento, M.; Alama, A.; et al. Association Between Response to Nivolumab Treatment and Peripheral Blood Lymphocyte Subsets in Patients With Non-small Cell Lung Cancer. Front. Immunol. 2020, 11, 125. [Google Scholar] [CrossRef] [PubMed]
- Mazzaschi, G.; Facchinetti, F.; Missale, G.; Canetti, D.; Madeddu, D.; Zecca, A.; Veneziani, M.; Gelsomino, F.; Goldoni, M.; Buti, S.; et al. The circulating pool of functionally competent NK and CD8+ cells predicts the outcome of anti-PD1 treatment in advanced NSCLC. Lung Cancer 2019, 127, 153–163. [Google Scholar] [CrossRef]
- Zhang, Q.; Bi, J.; Zheng, X.; Chen, Y.; Wang, H.; Wu, W.; Wang, Z.; Wu, Q.; Peng, H.; Wei, H.; et al. Blockade of the checkpoint receptor TIGIT prevents NK cell exhaustion and elicits potent anti-tumor immunity. Nat. Immunol. 2018, 19, 723–732. [Google Scholar] [CrossRef]
- Chauvin, J.M.; Ka, M.; Pagliano, O.; Menna, C.; Ding, Q.; DeBlasio, R.; Sanders, C.; Hou, J.; Li, X.Y.; Ferrone, S.; et al. IL15 Stimulation with TIGIT Blockade Reverses CD155-mediated NK-Cell Dysfunction in Melanoma. Clin. Cancer Res. 2020, 26, 5520–5533. [Google Scholar] [CrossRef]
- Pietra, G.; Mingari, M.C.; Moretta, L. TIGIT Blockade and IL15 in Tumor Immunotherapy: Together is Better. Clin. Cancer Res. 2020, 26, 5274–5275. [Google Scholar] [CrossRef]
- Xu, L.; Huang, Y.; Tan, L.; Yu, W.; Chen, D.; Lu, C.; He, J.; Wu, G.; Liu, X.; Zhang, Y. Increased Tim-3 expression in peripheral NK cells predicts a poorer prognosis and Tim-3 blockade improves NK cell-mediated cytotoxicity in human lung adenocarcinoma. Int. Immunopharmacol. 2015, 29, 635–641. [Google Scholar] [CrossRef]
- Wang, Z.; Zhu, J.; Gu, H.; Yuan, Y.; Zhang, B.; Zhu, D.; Zhou, J.; Zhu, Y.; Chen, W. The Clinical Significance of Abnormal Tim-3 Expression on NK Cells from Patients with Gastric Cancer. Immunol. Investig. 2015, 44, 578–589. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, Y.; Lian, J.; Yang, H.; Li, F.; Zhao, S.; Qi, Y.; Zhang, Y.; Huang, L. TNF-alpha-induced Tim-3 expression marks the dysfunction of infiltrating natural killer cells in human esophageal cancer. J. Transl. Med. 2019, 17, 165. [Google Scholar] [CrossRef] [PubMed]
- da Silva, I.P.; Gallois, A.; Jimenez-Baranda, S.; Khan, S.; Anderson, A.C.; Kuchroo, V.K.; Osman, I.; Bhardwaj, N. Reversal of NK-cell exhaustion in advanced melanoma by Tim-3 blockade. Cancer Immunol. Res. 2014, 2, 410–422. [Google Scholar] [CrossRef] [PubMed]
- De Sousa Linhares, A.; Leitner, J.; Grabmeier-Pfistershammer, K.; Steinberger, P. Not All Immune Checkpoints Are Created Equal. Front. Immunol. 2018, 9, 1909. [Google Scholar] [CrossRef] [PubMed]
- Sabins, N.C.; Chornoguz, O.; Leander, K.; Kaplan, F.; Carter, R.; Kinder, M.; Bachman, K.; Verona, R.; Shen, S.; Bhargava, V.; et al. TIM-3 Engagement Promotes Effector Memory T Cell Differentiation of Human Antigen-Specific CD8 T Cells by Activating mTORC1. J. Immunol. 2017, 199, 4091–4102. [Google Scholar] [CrossRef] [PubMed]
- Ohs, I.; Ducimetiere, L.; Marinho, J.; Kulig, P.; Becher, B.; Tugues, S. Restoration of Natural Killer Cell Antimetastatic Activity by IL12 and Checkpoint Blockade. Cancer Res. 2017, 77, 7059–7071. [Google Scholar] [CrossRef] [PubMed]
- Colevas, D.A.; Misiukiewicz, K.; Pearson, A.T.; Fayette, J.; Bauman, J.R.; Cupissol, D.; Saada-Bouzid, E.; Adkins, D.R.; Marie, D.B.; Cornen, S.L.; et al. Monalizumab, cetuximab and durvalumab in first-line treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (R/M SCCHN): A phase II trial. Ann. Oncol. 2021, 32, S1432. [Google Scholar] [CrossRef]
- Martinez-Marti, A.; Majem, M.; Barlesi, F.; Costa, E.C.; Chu, Q.; Monnet, I.; Sanchez, A.; Dahkil, S.; Camidge, D.R.; He, P.; et al. COAST: An open-label, randomised, phase II platform study of durvalumab alone or in combination with novel agents in patients with locally advanced, unresectable, stage III NSCLC. Ann. Oncol. 2021, 32, S1320. [Google Scholar] [CrossRef]
- Armand, P.; Lesokhin, A.; Borrello, I.; Timmerman, J.; Gutierrez, M.; Zhu, L.; Popa McKiver, M.; Ansell, S.M. A phase 1b study of dual PD-1 and CTLA-4 or KIR blockade in patients with relapsed/refractory lymphoid malignancies. Leukemia 2021, 35, 777–786. [Google Scholar] [CrossRef]
- Hanna, G.J.; O’Neill, A.; Shin, K.Y.; Wong, K.; Jo, V.Y.; Quinn, C.T.; Cutler, J.M.; Flynn, M.; Lizotte, P.H.; Annino, D.J., Jr.; et al. Neoadjuvant and Adjuvant Nivolumab and Lirilumab in Patients with Recurrent, Resectable Squamous Cell Carcinoma of the Head and Neck. Clin. Cancer Res. 2022, 28, 468–478. [Google Scholar] [CrossRef]
- Cho, B.C.; Rodriguez-Abreu, D.; Hussein, M.; Cobo, M.; Patel, A.; Secen, N.; Gerstner, G.; Kim, D.W.; Lee, Y.G.; Su, W.C.; et al. Updated analysis and patient-reported outcomes (PROs) from CITYSCAPE: A randomised, double-blind, phase II study of the anti-TIGIT antibody tiragolumab plus atezolizumab (TA) versus placebo plus atezolizumab (PA) as first-line treatment for PD-L1+NSCLC. Ann. Oncol. 2021, 32, S1428. [Google Scholar] [CrossRef]
- Long, L.; Zhang, X.; Chen, F.; Pan, Q.; Phiphatwatchara, P.; Zeng, Y.; Chen, H. The promising immune checkpoint LAG-3: From tumor microenvironment to cancer immunotherapy. Genes Cancer 2018, 9, 176–189. [Google Scholar] [CrossRef] [PubMed]
- Ascierto, P.A.; Melero, I.; Bhatia, S.; Bono, P.; Sanborn, R.E.; Lipson, E.J.; Callahan, M.K.; Gajewski, T.; Gomez-Roca, C.A.; Hodi, F.S.; et al. Initial efficacy of anti-lymphocyte activation gene-3 (anti-LAG-3; BMS-986016) in combination with nivolumab (nivo) in pts with melanoma (MEL) previously treated with anti-PD-1/PD-L1 therapy. J. Clin. Oncol. 2017, 35, 9520. [Google Scholar] [CrossRef]
- Tawbi, H.A.; Schadendorf, D.; Lipson, E.J.; Ascierto, P.A.; Matamala, L.; Castillo Gutierrez, E.; Rutkowski, P.; Gogas, H.J.; Lao, C.D.; De Menezes, J.J.; et al. Relatlimab and Nivolumab versus Nivolumab in Untreated Advanced Melanoma. N. Engl. J. Med. 2022, 386, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Dao, T.N.; Utturkar, S.; Atallah Lanman, N.; Matosevic, S. TIM-3 Expression Is Downregulated on Human NK Cells in Response to Cancer Targets in Synergy with Activation. Cancers 2020, 12, 2417. [Google Scholar] [CrossRef]
- Curigliano, G.; Gelderblom, H.; Mach, N.; Doi, T.; Tai, D.; Forde, P.M.; Sarantopoulos, J.; Bedard, P.L.; Lin, C.C.; Hodi, F.S.; et al. Phase I/Ib Clinical Trial of Sabatolimab, an Anti-TIM-3 Antibody, Alone and in Combination with Spartalizumab, an Anti-PD-1 Antibody, in Advanced Solid Tumors. Clin. Cancer Res. 2021, 27, 3620–3629. [Google Scholar] [CrossRef] [PubMed]
- Yashar, D.; Spektor, T.M.; Martinez, D.; Ghermezi, M.; Swift, R.A.; Eades, B.; Schwartz, G.; Eshaghian, S.; Lim, S.; Vescio, R.; et al. A phase 2 trial of the efficacy and safety of elotuzumab in combination with pomalidomide, carfilzomib and dexamethasone for high-risk relapsed/refractory multiple myeloma. Leuk. Lymphoma 2022, 63, 975–983. [Google Scholar] [CrossRef]
- Hellmann, M.D.; Gettinger, S.; Chow, L.Q.M.; Gordon, M.; Awad, M.M.; Cha, E.; Gong, X.; Zhou, G.; Walker, C.; Leopold, L.; et al. Phase 1 study of epacadostat in combination with atezolizumab for patients with previously treated advanced nonsmall cell lung cancer. Int. J. Cancer 2020, 147, 1963–1969. [Google Scholar] [CrossRef]
- Mitchell, T.C.; Hamid, O.; Smith, D.C.; Bauer, T.M.; Wasser, J.S.; Olszanski, A.J.; Luke, J.J.; Balmanoukian, A.S.; Schmidt, E.V.; Zhao, Y.; et al. Epacadostat Plus Pembrolizumab in Patients With Advanced Solid Tumors: Phase I Results From a Multicenter, Open-Label Phase I/II Trial (ECHO-202/KEYNOTE-037). J. Clin. Oncol. 2018, 36, 3223–3230. [Google Scholar] [CrossRef]
- Long, G.V.; Dummer, R.; Hamid, O.; Gajewski, T.F.; Caglevic, C.; Dalle, S.; Arance, A.; Carlino, M.S.; Grob, J.J.; Kim, T.M.; et al. Epacadostat plus pembrolizumab versus placebo plus pembrolizumab in patients with unresectable or metastatic melanoma (ECHO-301/KEYNOTE-252): A phase 3, randomised, double-blind study. Lancet Oncol. 2019, 20, 1083–1097. [Google Scholar] [CrossRef]
- Collins, S.M.; Bakan, C.E.; Swartzel, G.D.; Hofmeister, C.C.; Efebera, Y.A.; Kwon, H.; Starling, G.C.; Ciarlariello, D.; Bhaskar, S.; Briercheck, E.L.; et al. Elotuzumab directly enhances NK cell cytotoxicity against myeloma via CS1 ligation: Evidence for augmented NK cell function complementing ADCC. Cancer Immunol. Immunother. 2013, 62, 1841–1849. [Google Scholar] [CrossRef]
- Tang, K.; Wu, Y.H.; Song, Y.; Yu, B. Indoleamine 2,3-dioxygenase 1 (IDO1) inhibitors in clinical trials for cancer immunotherapy. J. Hematol. Oncol. 2021, 14, 68. [Google Scholar] [CrossRef] [PubMed]
- Chockley, P.J.; Chen, J.; Chen, G.; Beer, D.G.; Standiford, T.J.; Keshamouni, V.G. Epithelial-mesenchymal transition leads to NK cell-mediated metastasis-specific immunosurveillance in lung cancer. J. Clin. Investig. 2018, 128, 1384–1396. [Google Scholar] [CrossRef] [PubMed]
- Mace, E.M.; Orange, J.S. Emerging insights into human health and NK cell biology from the study of NK cell deficiencies. Immunol. Rev. 2019, 287, 202–225. [Google Scholar] [CrossRef]
- Takanami, I.; Takeuchi, K.; Giga, M. The prognostic value of natural killer cell infiltration in resected pulmonary adenocarcinoma. J. Thorac. Cardiovasc. Surg. 2001, 121, 1058–1063. [Google Scholar] [CrossRef] [PubMed]
- Villegas, F.R.; Coca, S.; Villarrubia, V.G.; Jimenez, R.; Chillon, M.J.; Jareno, J.; Zuil, M.; Callol, L. Prognostic significance of tumor infiltrating natural killer cells subset CD57 in patients with squamous cell lung cancer. Lung Cancer 2002, 35, 23–28. [Google Scholar] [CrossRef]
- Platonova, S.; Cherfils-Vicini, J.; Damotte, D.; Crozet, L.; Vieillard, V.; Validire, P.; Andre, P.; Dieu-Nosjean, M.C.; Alifano, M.; Regnard, J.F.; et al. Profound coordinated alterations of intratumoral NK cell phenotype and function in lung carcinoma. Cancer Res. 2011, 71, 5412–5422. [Google Scholar] [CrossRef]
- Jin, S.; Deng, Y.; Hao, J.W.; Li, Y.; Liu, B.; Yu, Y.; Shi, F.D.; Zhou, Q.H. NK cell phenotypic modulation in lung cancer environment. PLoS ONE 2014, 9, e109976. [Google Scholar] [CrossRef]
- Backman, M.; La Fleur, L.; Kurppa, P.; Djureinovic, D.; Elfving, H.; Brunnstrom, H.; Mattsson, J.S.M.; Lindberg, A.; Ponten, V.; Eltahir, M.; et al. Infiltration of NK and plasma cells is associated with a distinct immune subset in non-small cell lung cancer. J. Pathol. 2021, 255, 243–256. [Google Scholar] [CrossRef]
- Di Santo, J.P. Natural killer cell developmental pathways: A question of balance. Annu. Rev. Immunol. 2006, 24, 257–286. [Google Scholar] [CrossRef]
- Carrega, P.; Bonaccorsi, I.; Di Carlo, E.; Morandi, B.; Paul, P.; Rizzello, V.; Cipollone, G.; Navarra, G.; Mingari, M.C.; Moretta, L.; et al. CD56(bright)perforin(low) noncytotoxic human NK cells are abundant in both healthy and neoplastic solid tissues and recirculate to secondary lymphoid organs via afferent lymph. J. Immunol. 2014, 192, 3805–3815. [Google Scholar] [CrossRef]
- Bottcher, J.P.; Bonavita, E.; Chakravarty, P.; Blees, H.; Cabeza-Cabrerizo, M.; Sammicheli, S.; Rogers, N.C.; Sahai, E.; Zelenay, S.; Reis e Sousa, C. NK Cells Stimulate Recruitment of cDC1 into the Tumor Microenvironment Promoting Cancer Immune Control. Cell 2018, 172, 1022–1037.e1014. [Google Scholar] [CrossRef] [PubMed]
- Barry, K.C.; Hsu, J.; Broz, M.L.; Cueto, F.J.; Binnewies, M.; Combes, A.J.; Nelson, A.E.; Loo, K.; Kumar, R.; Rosenblum, M.D.; et al. A natural killer-dendritic cell axis defines checkpoint therapy-responsive tumor microenvironments. Nat. Med. 2018, 24, 1178–1191. [Google Scholar] [CrossRef] [PubMed]
- Kirchhammer, N.; Trefny, M.P.; Natoli, M.; Brucher, D.; Smith, S.N.; Werner, F.; Koch, V.; Schreiner, D.; Bartoszek, E.; Buchi, M.; et al. NK cells with tissue-resident traits shape response to immunotherapy by inducing adaptive antitumor immunity. Sci. Transl. Med. 2022, 14, eabm9043. [Google Scholar] [CrossRef] [PubMed]
- Souza-Fonseca-Guimaraes, F.; Cursons, J.; Huntington, N.D. The Emergence of Natural Killer Cells as a Major Target in Cancer Immunotherapy. Trends Immunol. 2019, 40, 142–158. [Google Scholar] [CrossRef]
- de Andrade, L.F.; Lu, Y.; Luoma, A.; Ito, Y.; Pan, D.; Pyrdol, J.W.; Yoon, C.H.; Yuan, G.C.; Wucherpfennig, K.W. Discovery of specialized NK cell populations infiltrating human melanoma metastases. JCI Insight 2019, 4, e133103. [Google Scholar] [CrossRef]
- Yan, W.L.; Shen, K.Y.; Tien, C.Y.; Chen, Y.A.; Liu, S.J. Recent progress in GM-CSF-based cancer immunotherapy. Immunotherapy 2017, 9, 347–360. [Google Scholar] [CrossRef]
- Ganesan, A.P.; Clarke, J.; Wood, O.; Garrido-Martin, E.M.; Chee, S.J.; Mellows, T.; Samaniego-Castruita, D.; Singh, D.; Seumois, G.; Alzetani, A.; et al. Tissue-resident memory features are linked to the magnitude of cytotoxic T cell responses in human lung cancer. Nat. Immunol. 2017, 18, 940–950. [Google Scholar] [CrossRef]
- Amsen, D.; Hombrink, P.; van Lier, R.A.W. Tumor immunity requires border patrol to fight the enemy within. Nat. Immunol. 2017, 18, 870–872. [Google Scholar] [CrossRef]
- Schmidt, L.; Eskiocak, B.; Kohn, R.; Dang, C.; Joshi, N.S.; DuPage, M.; Lee, D.Y.; Jacks, T. Enhanced adaptive immune responses in lung adenocarcinoma through natural killer cell stimulation. Proc. Natl. Acad. Sci. USA 2019, 116, 17460–17469. [Google Scholar] [CrossRef]
- Zemek, R.M.; De Jong, E.; Chin, W.L.; Schuster, I.S.; Fear, V.S.; Casey, T.H.; Forbes, C.; Dart, S.J.; Leslie, C.; Zaitouny, A.; et al. Sensitization to immune checkpoint blockade through activation of a STAT1/NK axis in the tumor microenvironment. Sci. Transl. Med. 2019, 11, eaav7816. [Google Scholar] [CrossRef]
- Demaria, O.; Gauthier, L.; Debroas, G.; Vivier, E. Natural killer cell engagers in cancer immunotherapy: Next generation of immuno-oncology treatments. Eur J. Immunol. 2021, 51, 1934–1942. [Google Scholar] [CrossRef] [PubMed]
- Yanakieva, D.; Pekar, L.; Evers, A.; Fleischer, M.; Keller, S.; Mueller-Pompalla, D.; Toleikis, L.; Kolmar, H.; Zielonka, S.; Krah, S. Beyond bispecificity: Controlled Fab arm exchange for the generation of antibodies with multiple specificities. MAbs 2022, 14, 2018960. [Google Scholar] [CrossRef] [PubMed]
- Schmohl, J.U.; Felices, M.; Todhunter, D.; Taras, E.; Miller, J.S.; Vallera, D.A. Tetraspecific scFv construct provides NK cell mediated ADCC and self-sustaining stimuli via insertion of IL-15 as a cross-linker. Oncotarget 2016, 7, 73830–73844. [Google Scholar] [CrossRef] [PubMed]
- Colomar-Carando, N.; Gauthier, L.; Merli, P.; Loiacono, F.; Canevali, P.; Falco, M.; Galaverna, F.; Rossi, B.; Bosco, F.; Caratini, M.; et al. Exploiting Natural Killer Cell Engagers to Control Pediatric B-cell Precursor Acute Lymphoblastic Leukemia. Cancer Immunol. Res. 2022, 10, 291–302. [Google Scholar] [CrossRef]
- Berrien-Elliott, M.M.; Cashen, A.F.; Cubitt, C.C.; Neal, C.C.; Wong, P.; Wagner, J.A.; Foster, M.; Schappe, T.; Desai, S.; McClain, E.; et al. Multidimensional Analyses of Donor Memory-Like NK Cells Reveal New Associations with Response after Adoptive Immunotherapy for Leukemia. Cancer Discov. 2020, 10, 1854–1871. [Google Scholar] [CrossRef]
- Karagiannis, P.; Kim, S.I. iPSC-Derived Natural Killer Cells for Cancer Immunotherapy. Mol. Cells 2021, 44, 541–548. [Google Scholar] [CrossRef]
- Dong, H.; Ham, J.D.; Hu, G.; Xie, G.; Vergara, J.; Liang, Y.; Ali, A.; Tarannum, M.; Donner, H.; Baginska, J.; et al. Memory-like NK cells armed with a neoepitope-specific CAR exhibit potent activity against NPM1 mutated acute myeloid leukemia. Proc. Natl. Acad. Sci. USA 2022, 119, e2122379119. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).