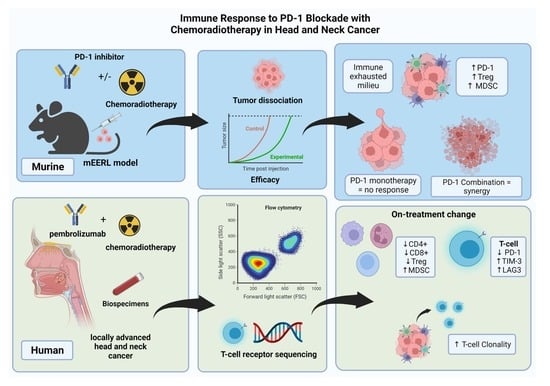
Characterization of the Immune Response to PD-1 Blockade during Chemoradiotherapy for Head and Neck Squamous Cell Carcinoma
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Studies
2.2. Human Subjects
2.3. Human Sample Collection and Processing
2.4. Multicolor Flow Cytometry
2.5. T-Cell Clonality
2.6. Statistical Analysis
3. Results
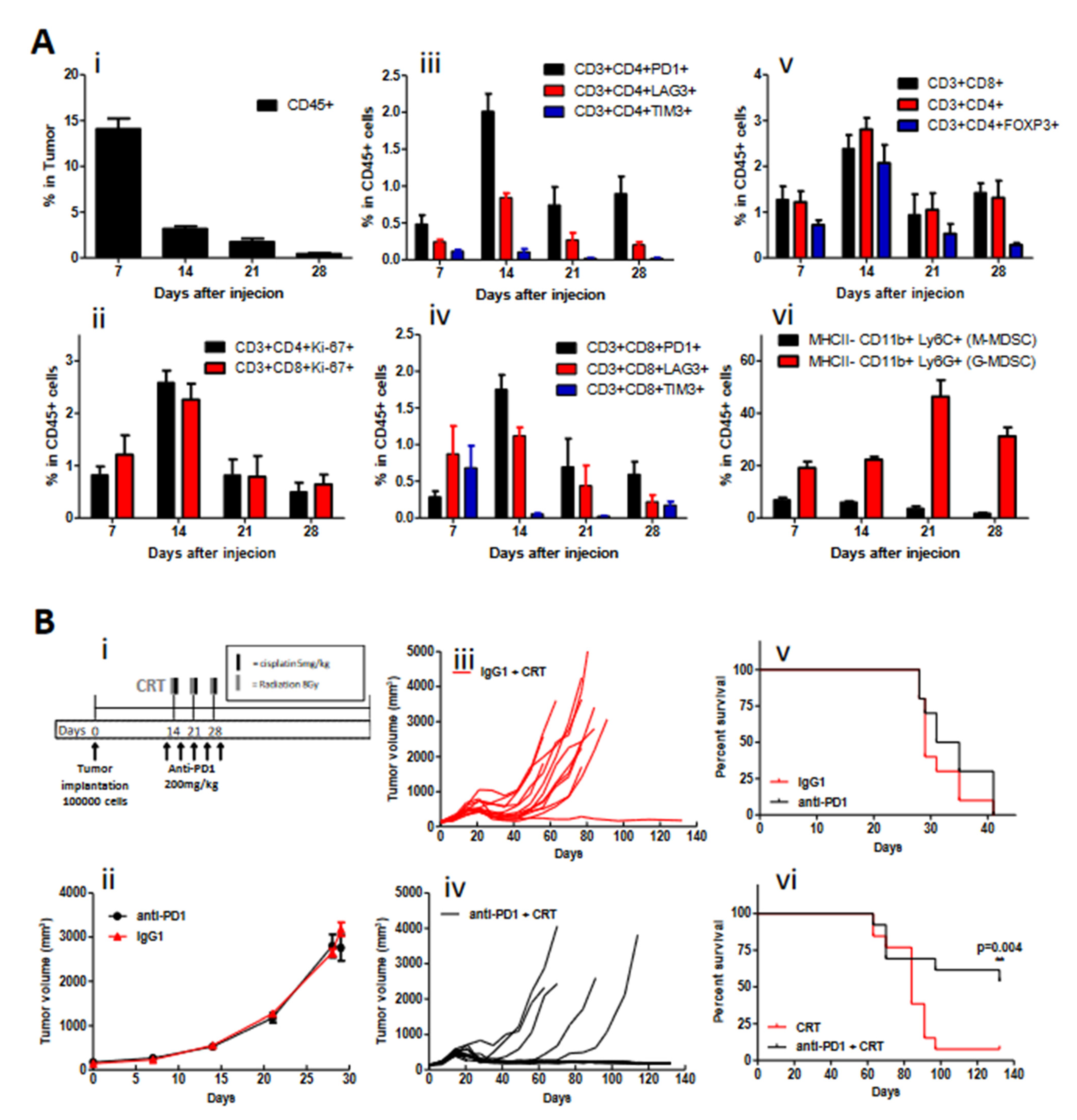
3.1. PD-1 Blockade Synergizes with Chemoradiotherapy in an HPV+ HNSCC Syngeneic Model
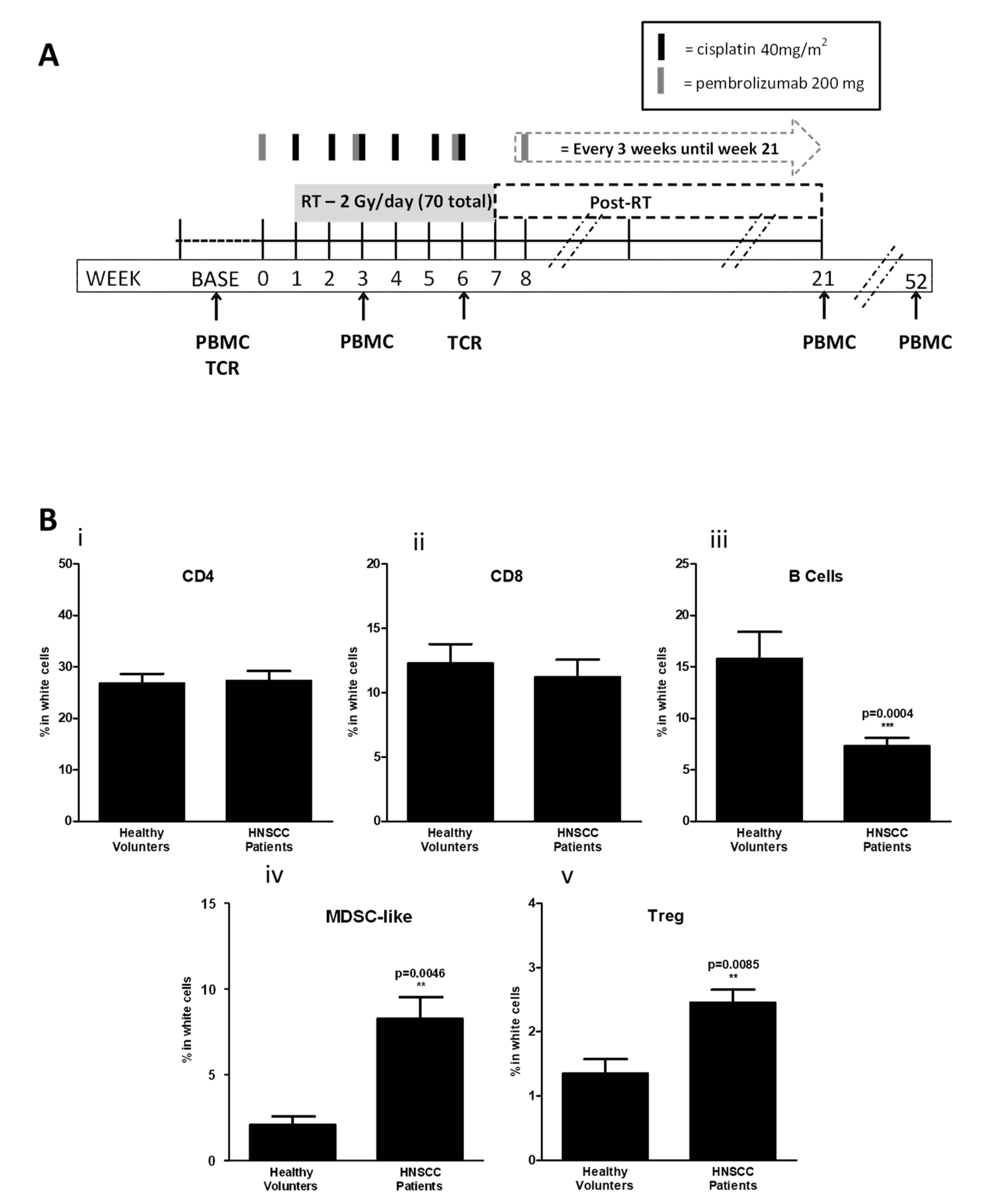
3.2. Circulating T-Cell Populations Decline with an Apparent Rise in Immunosuppressive Monocytes during Chemoradiotherapy despite PD-1 Blockade in HNSCC Patients
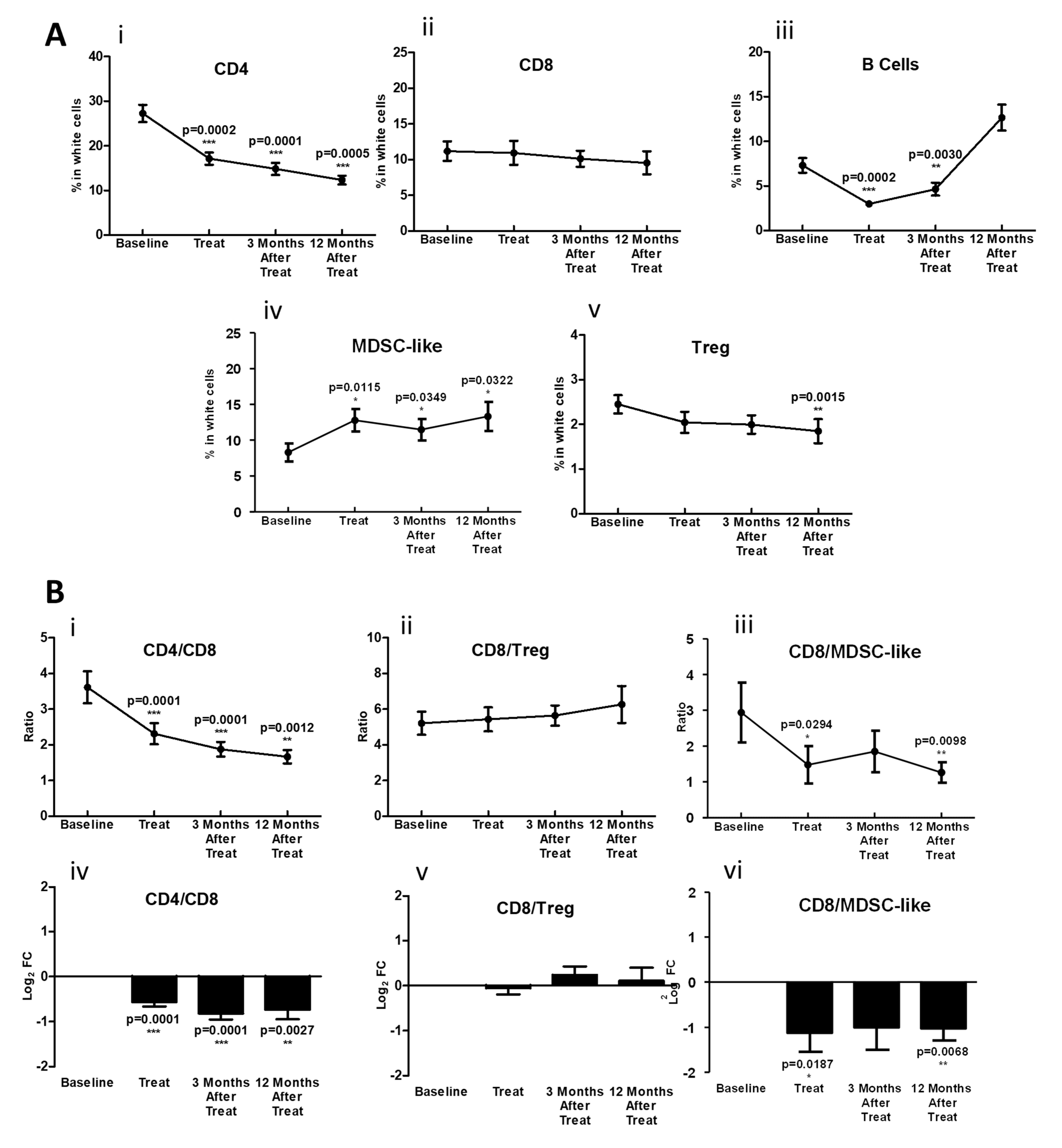
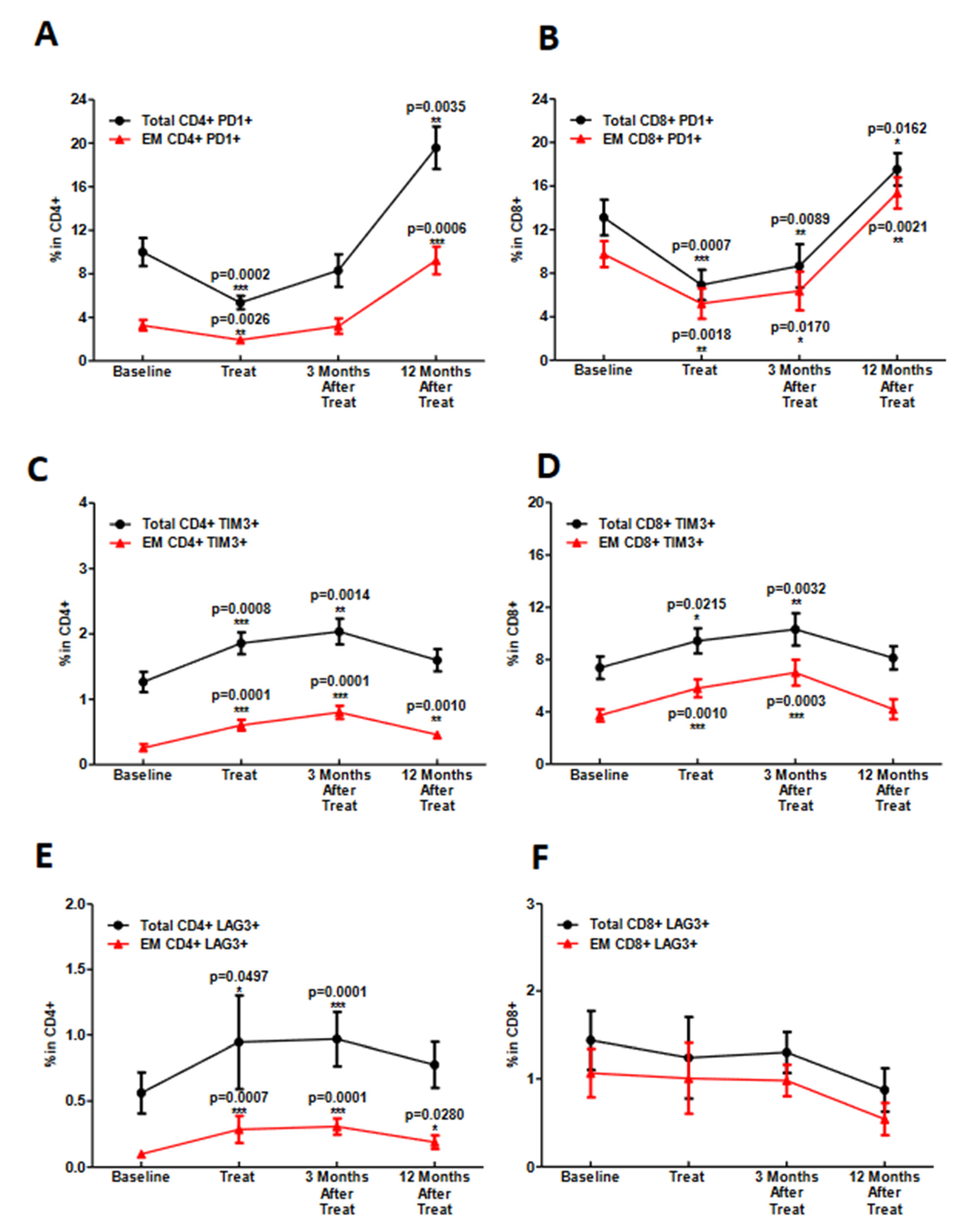
3.3. During Chemoradiotherapy, PD-1 Blockade Reduces PD-1 Expressing T-Cell Populations, However, with a Concordant Rise in Other Exhaustive Checkpoint Expression
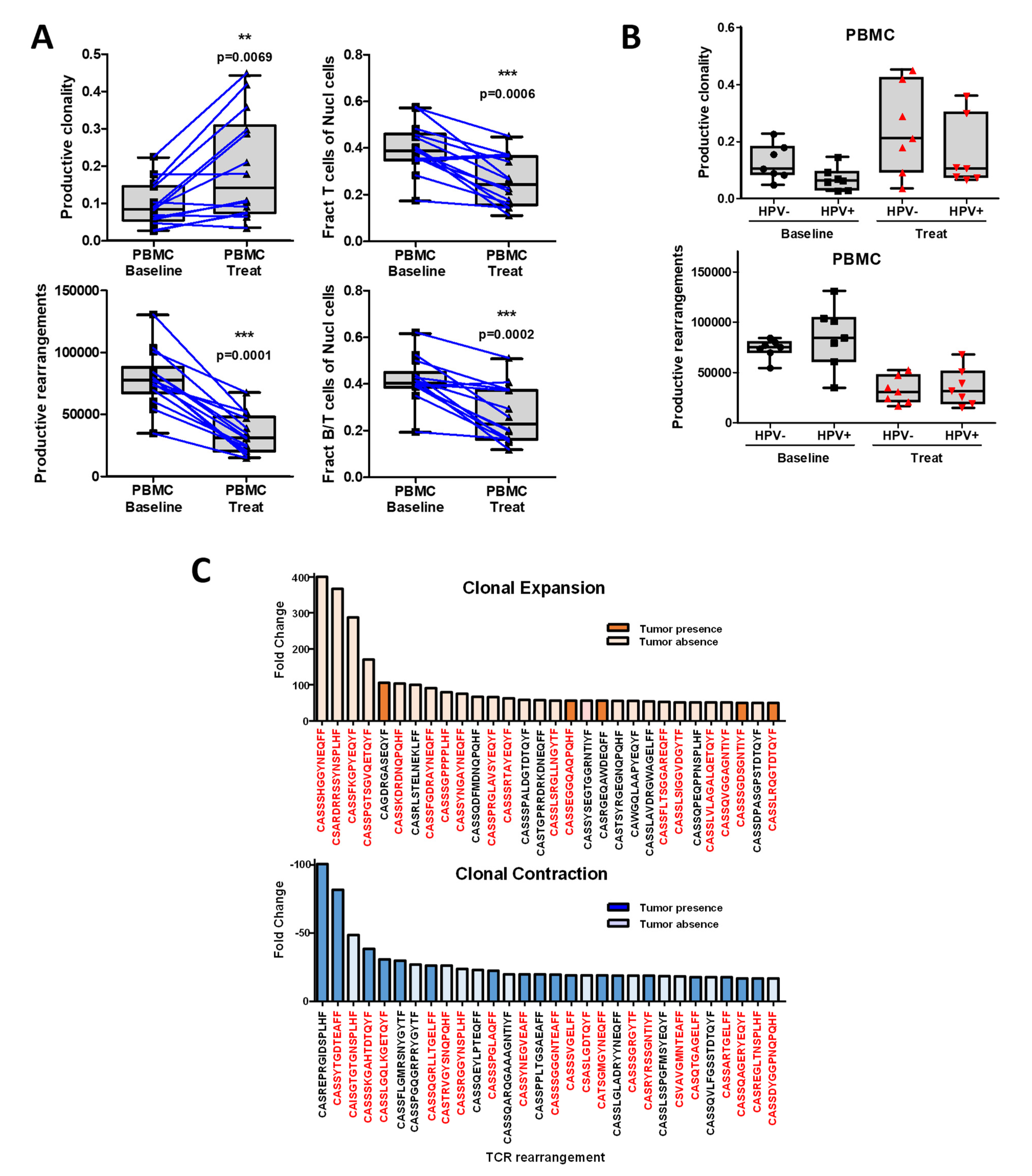
3.4. Chemoradiotherapy in Combination with PD-1 Blockade Increases Clonal Selection in PBMC T-Cell Repertoire
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Sankaranarayanan, R.; Masuyer, E.; Swaminathan, R.; Ferlay, J.; Whelan, S. Head and Neck Cancer: A Global Perspective on Epidemiology and Prognosis. Anticancer Res. 1998, 18, 4779–4786. [Google Scholar] [PubMed]
- Vokes, E.E.; Agrawal, N.; Seiwert, T.Y. HPV-Associated Head and Neck Cancer. J. Natl. Cancer Inst. 2015, 107, djv344. [Google Scholar] [CrossRef] [PubMed]
- Ang, K.K.; Harris, J.; Wheeler, R.; Weber, R.; Rosenthal, D.I.; Nguyen-Tân, P.F.; Westra, W.H.; Chung, C.H.; Jordan, R.C.; Lu, C.; et al. Human Papillomavirus and Survival of Patients with Oropharyngeal Cancer. N. Engl. J. Med. 2010, 363, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Pignon, J.-P.; le Maître, A.; Maillard, E.; Bourhis, J.; on behalf of the MACH-NC Collaborative Group. Meta-Analysis of Chemotherapy in Head and Neck Cancer (MACH-NC): An Update on 93 Randomised Trials and 17,346 Patients. Radiother. Oncol. 2009, 92, 4–14. [Google Scholar] [CrossRef]
- Spanos, W.C.; Nowicki, P.; Lee, D.W.; Hoover, A.; Hostager, B.; Gupta, A.; Anderson, M.E.; Lee, J.H. Immune Response During Therapy With Cisplatin or Radiation for Human Papillomavirus–Related Head and Neck Cancer. Arch. Otolaryngol. Head Neck Surg. 2009, 135, 1137. [Google Scholar] [CrossRef]
- Vermeer, D.W.; Spanos, W.C.; Vermeer, P.D.; Bruns, A.M.; Lee, K.M.; Lee, J.H. Radiation-Induced Loss of Cell Surface CD47 Enhances Immune-Mediated Clearance of Human Papillomavirus-Positive Cancer. Int. J. Cancer 2013, 133, 120–129. [Google Scholar] [CrossRef]
- Lucido, C.; Vermeer, P.; Wieking, B.; Vermeer, D.; Lee, J. CD137 Enhancement of HPV Positive Head and Neck Squamous Cell Carcinoma Tumor Clearance. Vaccines 2014, 2, 841–853. [Google Scholar] [CrossRef]
- Lipson, E.J.; Forde, P.M.; Hammers, H.-J.; Emens, L.A.; Taube, J.M.; Topalian, S.L. Antagonists of PD-1 and PD-L1 in Cancer Treatment. Semin. Oncol. 2015, 42, 587–600. [Google Scholar] [CrossRef]
- Francisco, L.M.; Sage, P.T.; Sharpe, A.H. The PD-1 Pathway in Tolerance and Autoimmunity: PD-1 Pathway, Tregs, and Autoimmune Diseases. Immunol. Rev. 2010, 236, 219–242. [Google Scholar] [CrossRef]
- Kim, J.W.; Eder, J.P. Prospects for Targeting PD-1 and PD-L1 in Various Tumor Types. Oncology 2014, 28 (Suppl. S3), 15–28. [Google Scholar] [PubMed]
- Lyford-Pike, S.; Peng, S.; Young, G.D.; Taube, J.M.; Westra, W.H.; Akpeng, B.; Bruno, T.C.; Richmon, J.D.; Wang, H.; Bishop, J.A.; et al. Evidence for a Role of the PD-1:PD-L1 Pathway in Immune Resistance of HPV-Associated Head and Neck Squamous Cell Carcinoma. Cancer Res. 2013, 73, 1733–1741. [Google Scholar] [CrossRef] [PubMed]
- Dovedi, S.; Adlard, A.; Lipowska-Bhalla, G.; McKenna, C.; Jones, S.; Cheadle, E.; Stratford, I.; Poon, E.; Morrow, M.; Stewart, R.; et al. The Anti-Tumor Immune Response Generated by Radiation Therapy May Be Limited by Tumor Cell Adaptive Resistance and Can Be Circumvented by PD-L1 Blockade. J. Immunother. Cancer 2014, 2, O9. [Google Scholar] [CrossRef]
- Deng, L.; Liang, H.; Burnette, B.; Beckett, M.; Darga, T.; Weichselbaum, R.R.; Fu, Y.-X. Irradiation and Anti–PD-L1 Treatment Synergistically Promote Antitumor Immunity in Mice. J. Clin. Investig. 2014, 124, 687–695. [Google Scholar] [CrossRef]
- Qin, X.; Liu, C.; Zhou, Y.; Wang, G. Cisplatin Induces Programmed Death-1-Ligand 1(PD-L1) over-Expression in Hepatoma H22 Cells via Erk /MAPK Signaling Pathway. Cell. Mol. Biol. 2010, 56, 1366–1372. [Google Scholar]
- Tran, L.; Allen, C.T.; Xiao, R.; Moore, E.; Davis, R.; Park, S.-J.; Spielbauer, K.; Van Waes, C.; Schmitt, N.C. Cisplatin Alters Antitumor Immunity and Synergizes with PD-1/PD-L1 Inhibition in Head and Neck Squamous Cell Carcinoma. Cancer Immunol. Res. 2017, 5, 1141–1151. [Google Scholar] [CrossRef]
- Parikh, F.; Duluc, D.; Imai, N.; Clark, A.; Misiukiewicz, K.; Bonomi, M.; Gupta, V.; Patsias, A.; Parides, M.; Demicco, E.G.; et al. Chemoradiotherapy-Induced Upregulation of PD-1 Antagonizes Immunity to HPV-Related Oropharyngeal Cancer. Cancer Res. 2014, 74, 7205–7216. [Google Scholar] [CrossRef]
- Gao, X.; Zhu, Y.; Li, G.; Huang, H.; Zhang, G.; Wang, F.; Sun, J.; Yang, Q.; Zhang, X.; Lu, B. TIM-3 Expression Characterizes Regulatory T Cells in Tumor Tissues and Is Associated with Lung Cancer Progression. PLoS ONE 2012, 7, e30676. [Google Scholar] [CrossRef]
- Fourcade, J.; Sun, Z.; Benallaoua, M.; Guillaume, P.; Luescher, I.F.; Sander, C.; Kirkwood, J.M.; Kuchroo, V.; Zarour, H.M. Upregulation of Tim-3 and PD-1 Expression Is Associated with Tumor Antigen–Specific CD8+ T Cell Dysfunction in Melanoma Patients. J. Exp. Med. 2010, 207, 2175–2186. [Google Scholar] [CrossRef]
- Jie, H.-B.; Gildener-Leapman, N.; Li, J.; Srivastava, R.M.; Gibson, S.P.; Whiteside, T.L.; Ferris, R.L. Intratumoral Regulatory T Cells Upregulate Immunosuppressive Molecules in Head and Neck Cancer Patients. Br. J. Cancer 2013, 109, 2629–2635. [Google Scholar] [CrossRef]
- Andrews, L.P.; Marciscano, A.E.; Drake, C.G.; Vignali, D.A.A. LAG3 (CD223) as a Cancer Immunotherapy Target. Immunol. Rev. 2017, 276, 80–96. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.-W.; Mao, L.; Yu, G.-T.; Bu, L.-L.; Ma, S.-R.; Liu, B.; Gutkind, J.S.; Kulkarni, A.B.; Zhang, W.-F.; Sun, Z.-J. LAG-3 Confers Poor Prognosis and Its Blockade Reshapes Antitumor Response in Head and Neck Squamous Cell Carcinoma. Oncoimmunology 2016, 5, e1239005. [Google Scholar] [CrossRef] [PubMed]
- Tesaro, Inc. A Phase 1 Dose Escalation and Cohort Expansion Study of TSR-033, an Anti-LAG-3 Monoclonal Antibody, Alone and in Combination with an Anti-PD-1 in Patients With Advanced Solid Tumors; Clinical trial registration NCT03250832; clinicaltrials.gov, 2022. Available online: http://clinicaltrials.gov/ct2/show/NCT03250832 (accessed on 13 May 2022).
- Nguyen, L.T.; Ohashi, P.S. Clinical Blockade of PD1 and LAG3--Potential Mechanisms of Action. Nat. Rev. Immunol. 2015, 15, 45–56. [Google Scholar] [CrossRef]
- Vaddepally, R.K.; Kharel, P.; Pandey, R.; Garje, R.; Chandra, A.B. Review of Indications of FDA-Approved Immune Checkpoint Inhibitors per NCCN Guidelines with the Level of Evidence. Cancers 2020, 12, 738. [Google Scholar] [CrossRef] [PubMed]
- Hoover, A.C.; Strand, G.L.; Nowicki, P.N.; Anderson, M.E.; Vermeer, P.D.; Klingelhutz, A.J.; Bossler, A.D.; Pottala, J.V.; Hendriks, W.; Lee, J.H. Impaired PTPN13 Phosphatase Activity in Spontaneous or HPV-Induced Squamous Cell Carcinomas Potentiates Oncogene Signaling through the MAP Kinase Pathway. Oncogene 2009, 28, 3960–3970. [Google Scholar] [CrossRef] [PubMed]
- Hoover, A.C.; Spanos, W.C.; Harris, G.F.; Anderson, M.E.; Klingelhutz, A.J.; Lee, J.H. The Role of Human Papillomavirus 16 E6 in Anchorage-Independent and Invasive Growth of Mouse Tonsil Epithelium. Arch. Otolaryngol. Head Neck Surg. 2007, 133, 495. [Google Scholar] [CrossRef] [PubMed]
- Spanos, W.C.; Geiger, J.; Anderson, M.E.; Harris, G.F.; Bossler, A.D.; Smith, R.B.; Klingelhutz, A.J.; Lee, J.H. Deletion of the PDZ Motif of HPV16 E6 Preventing Immortalization and Anchorage-Independent Growth in Human Tonsil Epithelial Cells. Head Neck 2008, 30, 139–147. [Google Scholar] [CrossRef]
- Uphoff, C.C.; Drexler, H.G. Comparative PCR Analysis for Detection of Mycoplasma Infections in Continuous Cell Lines. In Vitro Cell. Dev. Biol. Anim. 2002, 38, 79–85. [Google Scholar] [CrossRef]
- Powell, S.F.; Gold, K.A.; Gitau, M.M.; Sumey, C.J.; Lohr, M.M.; McGraw, S.C.; Nowak, R.K.; Jensen, A.W.; Blanchard, M.J.; Fischer, C.D.; et al. Safety and Efficacy of Pembrolizumab With Chemoradiotherapy in Locally Advanced Head and Neck Squamous Cell Carcinoma: A Phase IB Study. J. Clin. Oncol. 2020, 38, 2427–2437. [Google Scholar] [CrossRef]
- Donahue, R.N.; Lepone, L.M.; Grenga, I.; Jochems, C.; Fantini, M.; Madan, R.A.; Heery, C.R.; Gulley, J.L.; Schlom, J. Analyses of the Peripheral Immunome Following Multiple Administrations of Avelumab, a Human IgG1 Anti-PD-L1 Monoclonal Antibody. J. Immunother. Cancer 2017, 5, 20. [Google Scholar] [CrossRef]
- Bonaventura, P.; Shekarian, T.; Alcazer, V.; Valladeau-Guilemond, J.; Valsesia-Wittmann, S.; Amigorena, S.; Caux, C.; Depil, S. Cold Tumors: A Therapeutic Challenge for Immunotherapy. Front. Immunol. 2019, 10, 168. [Google Scholar] [CrossRef] [PubMed]
- Krupar, R.; Hautmann, M.G.; Pathak, R.R.; Varier, I.; McLaren, C.; Gaag, D.; Hellerbrand, C.; Evert, M.; Laban, S.; Idel, C.; et al. Immunometabolic Determinants of Chemoradiotherapy Response and Survival in Head and Neck Squamous Cell Carcinoma. Am. J. Pathol. 2018, 188, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Pilones, K.A.; Vanpouille-Box, C.; Demaria, S. Combination of Radiotherapy and Immune Checkpoint Inhibitors. Semin. Radiat. Oncol. 2015, 25, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Edge, S.B.; Compton, C.C. The American Joint Committee on Cancer: The 7th Edition of the AJCC Cancer Staging Manual and the Future of TNM. Ann. Surg. Oncol. 2010, 17, 1471–1474. [Google Scholar] [CrossRef]
- Wargo, J.A.; Reuben, A.; Cooper, Z.A.; Oh, K.S.; Sullivan, R.J. Immune Effects of Chemotherapy, Radiation, and Targeted Therapy and Opportunities for Combination With Immunotherapy. Semin. Oncol. 2015, 42, 601–616. [Google Scholar] [CrossRef]
- Seiwert, T.Y.; Burtness, B.; Mehra, R.; Weiss, J.; Berger, R.; Eder, J.P.; Heath, K.; McClanahan, T.; Lunceford, J.; Gause, C.; et al. Safety and Clinical Activity of Pembrolizumab for Treatment of Recurrent or Metastatic Squamous Cell Carcinoma of the Head and Neck (KEYNOTE-012): An Open-Label, Multicentre, Phase 1b Trial. Lancet Oncol. 2016, 17, 956–965. [Google Scholar] [CrossRef]
- Cohen, E.E.W. Pembrolizumab versus Methotrexate, Docetaxel, or Cetuximab for Recurrent or Metastatic Head-and-Neck Squamous Cell Carcinoma (KEYNOTE-040): A Randomised, Open-Label, Phase 3 Study. Lancet 2019, 393, 156–167. [Google Scholar] [CrossRef]
- Ferris, R.L.; Blumenschein, G.; Fayette, J.; Guigay, J.; Colevas, A.D.; Licitra, L.; Harrington, K.; Kasper, S.; Vokes, E.E.; Even, C.; et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 2016, 375, 1856–1867. [Google Scholar] [CrossRef]
- Teng, F.; Meng, X.; Kong, L.; Mu, D.; Zhu, H.; Liu, S.; Zhang, J.; Yu, J. Tumor-Infiltrating Lymphocytes, Forkhead Box P3, Programmed Death Ligand-1, and Cytotoxic T Lymphocyte–Associated Antigen-4 Expressions before and after Neoadjuvant Chemoradiation in Rectal Cancer. Transl. Res. 2015, 166, 721–732.e1. [Google Scholar] [CrossRef]
- Lugade, A.A.; Moran, J.P.; Gerber, S.A.; Rose, R.C.; Frelinger, J.G.; Lord, E.M. Local Radiation Therapy of B16 Melanoma Tumors Increases the Generation of Tumor Antigen-Specific Effector Cells That Traffic to the Tumor. J. Immunol. 2005, 174, 7516–7523. [Google Scholar] [CrossRef]
- Miyauchi, S.; Kim, S.S.; Pang, J.; Gold, K.A.; Gutkind, J.S.; Califano, J.A.; Mell, L.K.; Cohen, E.E.W.; Sharabi, A.B. Immune Modulation of Head and Neck Squamous Cell Carcinoma and the Tumor Microenvironment by Conventional Therapeutics. Clin. Cancer Res. 2019, 25, 4211–4223. [Google Scholar] [CrossRef]
- Sendo, S.; Saegusa, J.; Morinobu, A. Myeloid-Derived Suppressor Cells in Non-Neoplastic Inflamed Organs. Inflamm. Regen. 2018, 38, 19. [Google Scholar] [CrossRef] [PubMed]
- Weber, R.; Fleming, V.; Hu, X.; Nagibin, V.; Groth, C.; Altevogt, P.; Utikal, J.; Umansky, V. Myeloid-Derived Suppressor Cells Hinder the Anti-Cancer Activity of Immune Checkpoint Inhibitors. Front. Immunol. 2018, 9, 1310. [Google Scholar] [CrossRef] [PubMed]
- Shayan, G.; Srivastava, R.; Li, J.; Schmitt, N.; Kane, L.P.; Ferris, R.L. Adaptive Resistance to Anti-PD1 Therapy by Tim-3 Upregulation Is Mediated by the PI3K-Akt Pathway in Head and Neck Cancer. Oncoimmunology 2017, 6, e1261779. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.-R.; Turnis, M.E.; Goldberg, M.V.; Bankoti, J.; Selby, M.; Nirschl, C.J.; Bettini, M.L.; Gravano, D.M.; Vogel, P.; Liu, C.L.; et al. Immune Inhibitory Molecules LAG-3 and PD-1 Synergistically Regulate T-Cell Function to Promote Tumoral Immune Escape. Cancer Res. 2012, 72, 917–927. [Google Scholar] [CrossRef] [PubMed]
- Tawbi, H.A.; Schadendorf, D.; Lipson, E.J.; Ascierto, P.A.; Matamala, L.; Castillo Gutiérrez, E.; Rutkowski, P.; Gogas, H.J.; Lao, C.D.; De Menezes, J.J.; et al. Relatlimab and Nivolumab versus Nivolumab in Untreated Advanced Melanoma. N. Engl. J. Med. 2022, 386, 24–34. [Google Scholar] [CrossRef]
- Oweida, A.; Hararah, M.K.; Phan, A.; Binder, D.; Bhatia, S.; Lennon, S.; Bukkapatnam, S.; Van Court, B.; Uyanga, N.; Darragh, L.; et al. Resistance to Radiotherapy and PD-L1 Blockade Is Mediated by TIM-3 Upregulation and Regulatory T-Cell Infiltration. Clin. Cancer Res. 2018, 24, 5368–5380. [Google Scholar] [CrossRef]
- Lee, N.Y.; Ferris, R.L.; Beck, J.T.; Harrington, K.; Haddad, R.I.; Bourhis, J.; Tahara, M.; Geraldes, M.; Nuyten, D.S.A.; Goldberg, Z.; et al. JAVELIN Head and Neck 100: A Phase 3 Trial of Avelumab in Combination with Chemoradiotherapy (CRT) vs CRT for 1st-Line Treatment of Locally Advanced Squamous Cell Carcinoma of the Head and Neck (LA SCCHN). J. Clin. Oncol. 2017, 35, TPS6093. [Google Scholar] [CrossRef]
- Machiels, J.P.H.; Licitra, L.; Rischin, D.; Waldron, J.; Burtness, B.; Gregoire, V.; Shekar, T.; Brown, H.M.; Cheng, J.D.; Siu, L.L. KEYNOTE-412: Pembrolizumab (Pembro) in Combination with Chemoradiation versus Chemoradiation Alone in Locally Advanced Head and Neck Squamous Cell Carcinoma (LA-HNSCC). J. Clin. Oncol. 2017, 35, TPS6090. [Google Scholar] [CrossRef]
- Cohen, E.E.; Ferris, R.L.; Psyrri, A.; Haddad, R.; Tahara, M.; Bourhis, J.; Harrington, K.J.; Chang, P.M.-H.; Lin, J.-C.; Razaq, M.; et al. 910O Primary Results of the Phase III JAVELIN Head & Neck 100 Trial: Avelumab plus Chemoradiotherapy (CRT) Followed by Avelumab Maintenance vs CRT in Patients with Locally Advanced Squamous Cell Carcinoma of the Head and Neck (LA SCCHN). Ann. Oncol. 2020, 31, S658. [Google Scholar] [CrossRef]
| Characteristic | Total Patients |
|---|---|
| (N = 35) | |
| Median Age, Years | 61.6 |
| Range | (38–81) |
| Sex | |
| Male | 30 (85.7%) |
| Female | 5 (14.3%) |
| Race | |
| White, Non-Hispanic | 35 (100%) |
| Primary Site | |
| Oropharynx | 22 (62.8%) |
| Larynx | 9 (25.7%) |
| Hypopharynx | 4 (11.5%) |
| TNM Stage (AJCC 7th ed.) [35] | |
| III | 8 (22.8%) |
| IVA | 27 (77.2%) |
| T0–1 * | 8 (22.8%) |
| T2 | 11 (31.4%) |
| T3 | 11 (31.4%) |
| T4 | 5 (14.4%) |
| N0 | 3 (8.6%) |
| N1 | 4 (11.4%) |
| N2 | 28 (80.0%) |
| p16 (HPV) Status | |
| Positive | 21 (60.0%) |
| Negative | 14 (40.0%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Callejas-Valera, J.L.; Vermeer, D.W.; Lucido, C.T.; Williamson, C.; Killian, M.; Vermeer, P.D.; Spanos, W.C.; Powell, S.F. Characterization of the Immune Response to PD-1 Blockade during Chemoradiotherapy for Head and Neck Squamous Cell Carcinoma. Cancers 2022, 14, 2499. https://doi.org/10.3390/cancers14102499
Callejas-Valera JL, Vermeer DW, Lucido CT, Williamson C, Killian M, Vermeer PD, Spanos WC, Powell SF. Characterization of the Immune Response to PD-1 Blockade during Chemoradiotherapy for Head and Neck Squamous Cell Carcinoma. Cancers. 2022; 14(10):2499. https://doi.org/10.3390/cancers14102499
Chicago/Turabian StyleCallejas-Valera, Juan L., Daniel W. Vermeer, Christopher T. Lucido, Caitlin Williamson, Marisela Killian, Paola D. Vermeer, William C. Spanos, and Steven F. Powell. 2022. "Characterization of the Immune Response to PD-1 Blockade during Chemoradiotherapy for Head and Neck Squamous Cell Carcinoma" Cancers 14, no. 10: 2499. https://doi.org/10.3390/cancers14102499
APA StyleCallejas-Valera, J. L., Vermeer, D. W., Lucido, C. T., Williamson, C., Killian, M., Vermeer, P. D., Spanos, W. C., & Powell, S. F. (2022). Characterization of the Immune Response to PD-1 Blockade during Chemoradiotherapy for Head and Neck Squamous Cell Carcinoma. Cancers, 14(10), 2499. https://doi.org/10.3390/cancers14102499