Synaptophysin, CD117, and GATA3 as a Diagnostic Immunohistochemical Panel for Small Cell Neuroendocrine Carcinoma of the Urinary Tract
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Samples
2.2. Tissue Microarray Construction
2.3. IHC
2.4. Establishment of the Decision Tree Model
2.5. Transmission Electron Microscopy (TEM) Analysis
3. Results
3.1. Patients’ Characteristics
3.2. Expression of NE, Luminal, and Basal Markers in the Discovery Cohort
3.3. Decision Tree-Based Diagnostic NE IHC Model
3.4. Application of the Diagnostic NE IHC Model on an External Cohort
3.5. Ultrastructural Validation of NE Differentiation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chau, C.; Rimmer, Y.; Choudhury, A.; Leaning, D.; Law, A.; Enting, D.; Lim, J.H.; Hafeez, S.; Khoo, V.; Huddart, R.; et al. Treatment outcomes for small cell carcinoma of the bladder: Results from a UK patient retrospective cohort study. Int. J. Radiat. Oncol. Biol. Phys. 2021, 110, 1143–1150. [Google Scholar] [CrossRef] [PubMed]
- Kouba, E.; Cheng, L. Neuroendocrine tumors of the urinary bladder according to the 2016 World Health Organization Classification: Molecular and clinical characteristics. Endocr. Pathol. 2016, 27, 188–199. [Google Scholar] [CrossRef]
- Royce, T.J.; Lin, C.C.; Gray, P.J.; Shipley, W.U.; Jemal, A.; Efstathiou, J.A. Clinical characteristics and outcomes of nonurothelial cell carcinoma of the bladder: Results from the National Cancer Data Base. Urol. Oncol. 2018, 36, e71–e78. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Flynn, E.A. Small cell carcinoma of the urinary bladder: A rare, aggressive neuroendocrine malignancy. Arch. Pathol. Lab. Med. 2012, 136, 1451–1459. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Thompson, R.H.; Boorjian, S.A.; Thapa, P.; Hernandez, L.P.; Jimenez, R.E.; Costello, B.A.; Frank, I.; Cheville, J.C. High grade neuroendocrine carcinoma of the urinary bladder treated by radical cystectomy: A series of small cell, mixed neuroendocrine and large cell neuroendocrine carcinoma. Pathology 2015, 47, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Lynch, S.P.; Shen, Y.; Kamat, A.; Grossman, H.B.; Shah, J.B.; Millikan, R.E.; Dinney, C.P.; Siefker-Radtke, A. Neoadjuvant chemotherapy in small cell urothelial cancer improves pathologic downstaging and long-term outcomes: Results from a retrospective study at the MD Anderson Cancer Center. Eur. Urol. 2013, 64, 307–313. [Google Scholar] [CrossRef] [Green Version]
- Al-Ahmadie, H.; Netto, G.J. Updates on the genomics of bladder cancer and novel molecular taxonomy. Adv. Anat. Pathol. 2020, 27, 36–43. [Google Scholar] [CrossRef]
- WHO Classification of Tumours Editorial Board. Thoracic Tumours (WHO Classification of Tumours Series), 5th ed.; International Agency for Research on Cancer (IARC): Lyon, France, 2021; Volume 5.
- Rooper, L.M.; Sharma, R.; Li, Q.K.; Illei, P.B.; Westra, W.H. INSM1 demonstrates superior performance to the individual and combined use of synaptophysin, chromogranin and CD56 for diagnosing neuroendocrine tumors of the thoracic cavity. Am. J. Surg. Pathol. 2017, 41, 1561–1569. [Google Scholar] [CrossRef]
- Travis, W.D. Update on small cell carcinoma and its differentiation from squamous cell carcinoma and other non-small cell carcinomas. Mod. Pathol. 2012, 25 (Suppl. 1), S18–S30. [Google Scholar] [CrossRef] [Green Version]
- Batista da Costa, J.; Gibb, E.A.; Bivalacqua, T.J.; Liu, Y.; Oo, H.Z.; Miyamoto, D.T.; Alshalalfa, M.; Davicioni, E.; Wright, J.; Dall’Era, M.A.; et al. Molecular characterization of neuroendocrine-like bladder cancer. Clin. Cancer Res. 2019, 25, 3908–3920. [Google Scholar] [CrossRef] [Green Version]
- Erler, B.S.; Presby, M.M.; Finch, M.; Hodges, A.; Horowitz, K.; Topilow, A.A.; Matulewicz, T. CD117, Ki-67, and p53 predict survival in neuroendocrine carcinomas, but not within the subgroup of small cell lung carcinoma. Tumour Biol. 2011, 32, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Papouchado, B.; Erickson, L.A.; Rohlinger, A.L.; Hobday, T.J.; Erlichman, C.; Ames, M.M.; Lloyd, R.V. Epidermal growth factor receptor and activated epidermal growth factor receptor expression in gastrointestinal carcinoids and pancreatic endocrine carcinomas. Mod. Pathol. 2005, 18, 1329–1335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qian, Z.R.; Li, T.; Ter-Minassian, M.; Yang, J.; Chan, J.A.; Brais, L.K.; Masugi, Y.; Thiaglingam, A.; Brooks, N.; Nishihara, R.; et al. Association between somatostatin receptor expression and clinical outcomes in neuroendocrine tumors. Pancreas 2016, 45, 1386–1393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, W.; Porten, S.; Kim, S.; Willis, D.; Plimack, E.R.; Hoffman-Censits, J.; Roth, B.; Cheng, T.; Tran, M.; Lee, I.L.; et al. Identification of distinct basal and luminal subtypes of muscle-invasive bladder cancer with different sensitivities to frontline chemotherapy. Cancer Cell 2014, 25, 152–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robertson, A.G.; Kim, J.; Al-Ahmadie, H.; Bellmunt, J.; Guo, G.; Cherniack, A.D.; Hinoue, T.; Laird, P.W.; Hoadley, K.A.; Akbani, R.; et al. Comprehensive molecular characterization of muscle-invasive bladder cancer. Cell 2017, 171, 540–556.e525. [Google Scholar] [CrossRef] [Green Version]
- De Felice, F.; Crocetti, D.; Parisi, M.; Maiuri, V.; Moscarelli, E.; Caiazzo, R.; Bulzonetti, N.; Musio, D.; Tombolini, V. Decision tree algorithm in locally advanced rectal cancer: An example of over-interpretation and misuse of a machine learning approach. J. Cancer Res. Clin. Oncol. 2020, 146, 761–765. [Google Scholar] [CrossRef]
- Kim, M.; Ro, J.Y.; Amin, M.B.; de Peralta-Venturina, M.; Kwon, G.Y.; Park, Y.W.; Cho, Y.M. Urothelial eddies in papillary urothelial neoplasms: A distinct morphologic pattern with low risk for progression. Int. J. Clin. Exp. Pathol. 2013, 6, 1458–1466. [Google Scholar]
- Ohwada, M.; Wada, T.; Saga, Y.; Tsunoda, S.; Jobo, T.; Kuramoto, H.; Konno, R.; Suzuki, M. C-kit overexpression in neuroendocrine small cell carcinoma of the uterine cervix. Eur. J. Gynaecol. Oncol. 2006, 27, 53–55. [Google Scholar]
- Terada, T. Small cell neuroendocrine carcinoma of the esophagus: Report of 6 cases with immunohistochemical and molecular genetic analysis of KIT and PDGFRA. Int. J. Clin. Exp. Pathol. 2013, 6, 485–491. [Google Scholar]
- Potti, A.; Moazzam, N.; Ramar, K.; Hanekom, D.S.; Kargas, S.; Koch, M. CD117 (c-KIT) overexpression in patients with extensive-stage small-cell lung carcinoma. Ann. Oncol. 2003, 14, 894–897. [Google Scholar] [CrossRef]
- Pan, C.X.; Yang, X.J.; Lopez-Beltran, A.; MacLennan, G.T.; Eble, J.N.; Koch, M.O.; Jones, T.D.; Lin, H.; Nigro, K.; Papavero, V.; et al. c-kit Expression in small cell carcinoma of the urinary bladder: Prognostic and therapeutic implications. Mod. Pathol. 2005, 18, 320–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heinrich, M.C. Is KIT an important therapeutic target in small cell lung cancer? Clin. Cancer Res. 2003, 9, 5825–5828. [Google Scholar] [PubMed]
- Foster, B.M.; Zaidi, D.; Young, T.R.; Mobley, M.E.; Kerr, B.A. CD117/c-kit in cancer stem cell-mediated progression and therapeutic resistance. Biomedicines 2018, 6, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, G.; Xiao, L.; Zhang, M.; Kamat, A.M.; Siefker-Radtke, A.; Dinney, C.P.; Czerniak, B.; Guo, C.C. Small cell carcinoma of the urinary bladder: A clinicopathological and immunohistochemical analysis of 81 cases. Hum. Pathol. 2018, 79, 57–65. [Google Scholar] [CrossRef]
- Chen, J.F.; Yang, C.; Sun, Y.; Cao, D. Expression of novel neuroendocrine marker insulinoma-associated protein 1 (INSM1) in genitourinary high-grade neuroendocrine carcinomas: An immunohistochemical study with specificity analysis and comparison to chromogranin, synaptophysin, and CD56. Pathol. Res. Pract. 2020, 216, 152993. [Google Scholar] [CrossRef]
- Kim, I.E., Jr.; Amin, A.; Wang, L.J.; Cheng, L.; Perrino, C.M. Insulinoma-associated protein 1 (INSM1) expression in small cell neuroendocrine carcinoma of the urinary tract. Appl. Immunohistochem. Mol. Morphol. 2020, 28, 687–693. [Google Scholar] [CrossRef]
- Fortarezza, F.; Della Barbera, M.; Pezzuto, F.; Lunardi, F.; Faccioli, E.; Pasello, G.; Rea, F.; Rizzo, S.; Calabrese, F. Diagnostic challenges in epithelioid pleural mesothelioma: Case series with support from electron microscopy. Diagnostics 2021, 11, 841. [Google Scholar] [CrossRef]
- Sjödahl, G.; Eriksson, P.; Liedberg, F.; Höglund, M. Molecular classification of urothelial carcinoma: Global mRNA classification versus tumour-cell phenotype classification. J. Pathol. 2017, 242, 113–125. [Google Scholar] [CrossRef]
- Kamoun, A.; de Reyniès, A.; Allory, Y.; Sjödahl, G.; Robertson, A.G.; Seiler, R.; Hoadley, K.A.; Groeneveld, C.S.; Al-Ahmadie, H.; Choi, W.; et al. A consensus molecular classification of muscle-invasive bladder cancer. Eur. Urol. 2020, 77, 420–433. [Google Scholar] [CrossRef]
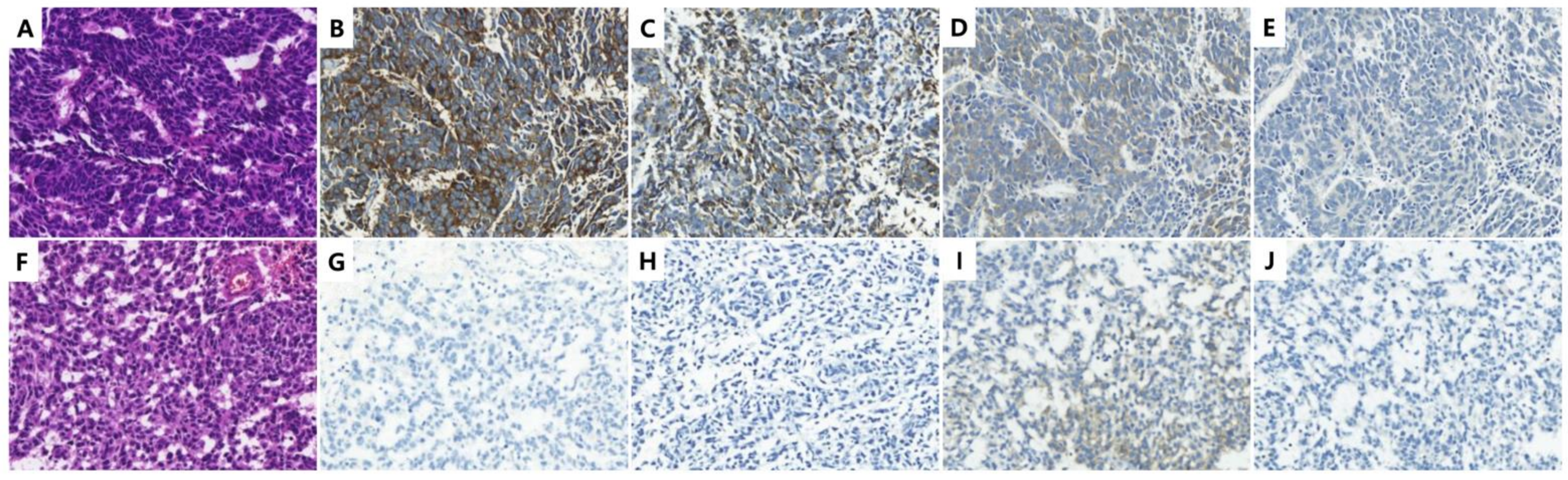
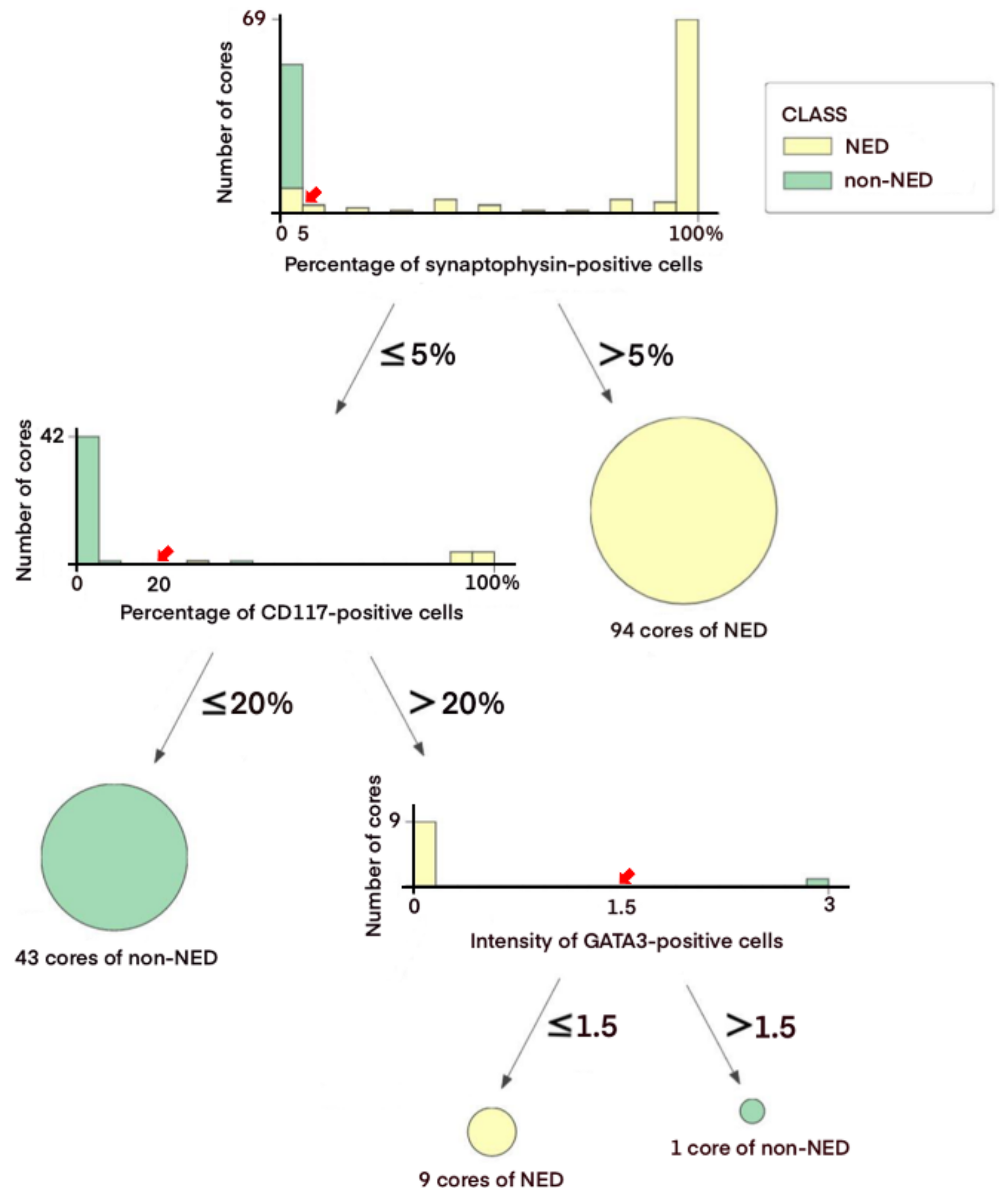
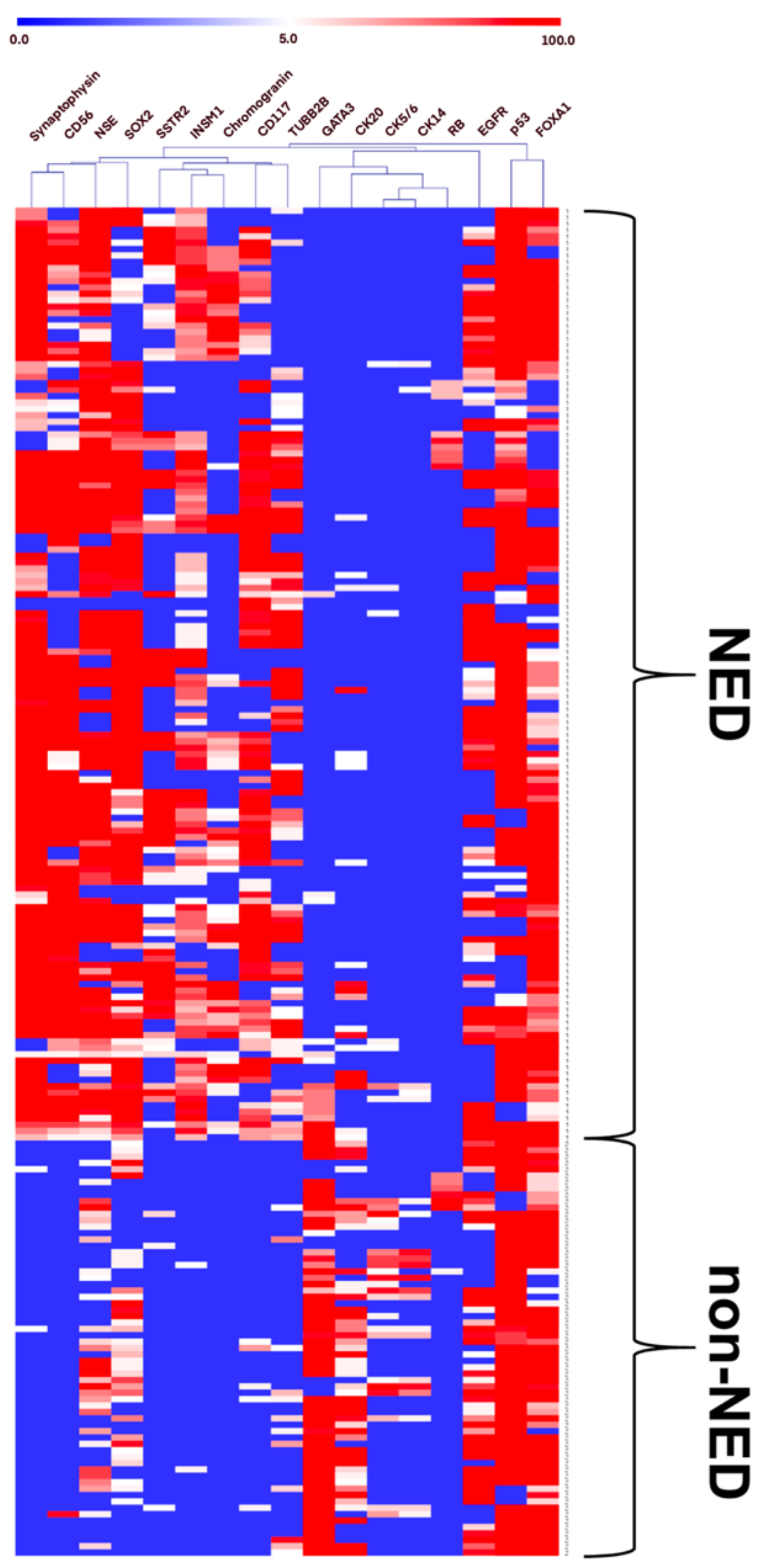
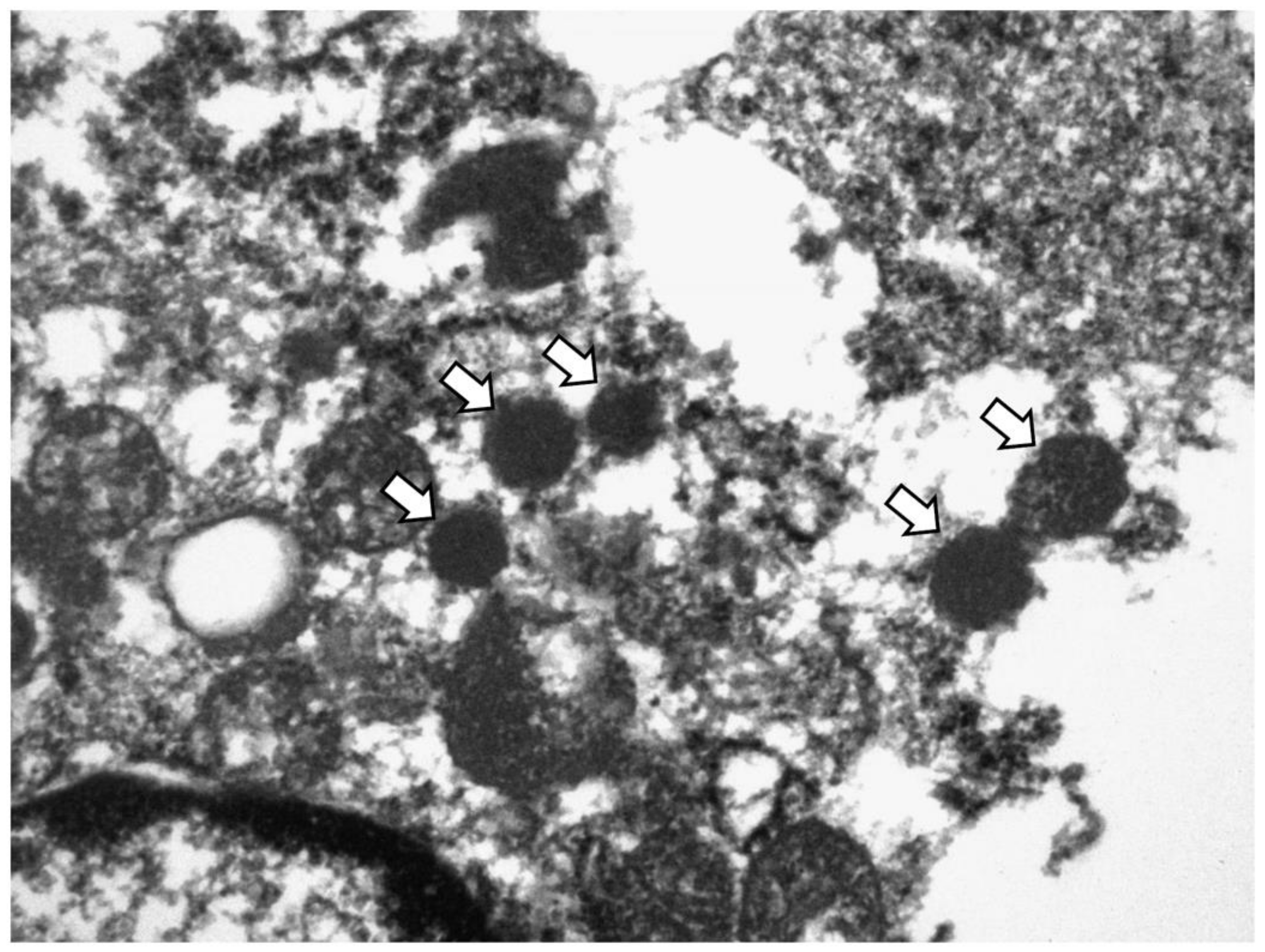
| Features | Value | |
|---|---|---|
| Patients (n = 34) | ||
| Age at initial diagnosis (years) | 66.1 (31–86) | |
| Sex | Male | 29 (85.3) |
| Female | 5 (14.7) | |
| All cases (n = 47) | ||
| Tumor size (cm) | 4.36 (1.0–11.4) | |
| Location | Urinary bladder | 45 (95.7) |
| Ureter | 2 (4.3) | |
| Procedure | Cystoscopic biopsy | 1 (2.1) |
| Transurethral resection | 34 (72.3) | |
| Partial cystectomy | 2 (4.3) | |
| Radical cystectomy/ureterectomy | 10 (21.3) | |
| Histology | Pure NEC | 29 (61.7) |
| Mixed NEC and non-NEC | 15 (31.9) | |
| Non-NEC | 3 (6.4) | |
| Invasion depth | Non-invasive | 0 (0.0) |
| Subepithelial connective tissue | 9 (19.1) | |
| Muscularis propria | 28 (59.6) | |
| Perivesical tissue | 9 (19.1) | |
| Other organs * | 1 (2.1) | |
| Lymphovascular invasion | Present | 25 (53.2) |
| Absent | 22 (46.8) | |
| Cystectomy cases (n = 10) | ||
| Tumor stage | pT1 | 0 (0.0) |
| pT2 | 1 (10.0) | |
| pT3 | 8 (80.0) | |
| pT4 | 1 (10.0) | |
| N stage | NX | 1 (10.0) |
| N0 | 4 (40.0) | |
| N1-3 | 5 (50.0) |
| Neuroendocrine Cores (n = 146) | Non-Neuroendocrine Cores (n = 65) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intensity | Extent | Intensity | Extent | ||||||||||||||||||
| 0 and 1 | 2 and 3 | ≤5% | >5–≤50% | >50% | 0 and 1 | 2 and 3 | ≤5% | >5–≤50% | >50% | ||||||||||||
| SYP | 29 | (19.9) | 117 | (80.1) | 12 | (8.2) | 18 | (12.3) | 116 | (79.5) | 65 | (100) | 0 | (0.0) | 65 | (100) | 0 | (0.0) | 0 | (0.0) | |
| CGA | 84 | (57.5) | 62 | (42.5) | 89 | (61.0) | 27 | (18.5) | 30 | (20.5) | 65 | (100) | 0 | (0.0) | 65 | (100) | 0 | (0.0) | 0 | (0.0) | |
| CD56 | 47 | (32.2) | 99 | (67.8) | 32 | (21.9) | 25 | (17.1) | 89 | (61.0) | 63 | (96.9) | 2 | (3.1) | 64 | (98.5) | 0 | (0.0) | 1 | (1.5) | |
| CD117 | 74 | (50.7) | 72 | (49.3) | 38 | (26.0) | 23 | (15.8) | 85 | (58.2) | 61 | (93.8) | 4 | (6.2) | 62 | (95.4) | 3 | (4.6) | 0 | (0.0) | |
| INSM1 | 43 | (29.5) | 103 | (70.5) | 33 | (22.6) | 49 | (33.6) | 64 | (43.8) | 65 | (100) | 0 | (0.0) | 64 | (98.5) | 1 | (1.5) | 0 | (0.0) | |
| NSE | 35 | (24.0) | 111 | (76.0) | 20 | (13.7) | 15 | (10.3) | 111 | (76.0) | 45 | (69.2) | 20 | (30.8) | 37 | (56.9) | 19 | (29.2) | 9 | (13.8) | |
| SOX2 | 25 | (17.1) | 121 | (82.9) | 30 | (20.5) | 16 | (11.0) | 100 | (68.5) | 27 | (41.5) | 38 | (58.5) | 36 | (55.4) | 21 | (32.3) | 8 | (12.3) | |
| TUBB2B | 78 | (53.4) | 68 | (46.6) | 68 | (46.6) | 27 | (18.5) | 51 | (34.9) | 54 | (83.1) | 11 | (16.9) | 56 | (86.2) | 8 | (12.3) | 1 | (1.5) | |
| SSTR2 | 78 | (53.4) | 68 | (46.6) | 81 | (55.5) | 23 | (15.8) | 42 | (28.8) | 62 | (95.4) | 3 | (4.6) | 63 | (96.9) | 2 | (3.1) | 0 | (0.0) | |
| p53 | 17 | (11.6) | 129 | (88.4) | 26 | (17.8) | 9 | (6.2) | 111 | (76.0) | 15 | (23.1) | 50 | (76.9) | 9 | (13.8) | 0 | (0.0) | 56 | (86.2) | |
| Rb | 131 | (89.7) | 15 | (10.3) | 130 | (89.0) | 8 | (5.5) | 8 | (5.5) | 65 | (100) | 0 | (0.0) | 65 | (100) | 0 | (0.0) | 0 | (0.0) | |
| EGFR | 95 | (65.1) | 51 | (34.9) | 81 | (55.5) | 19 | (13.0) | 46 | (31.5) | 10 | (15.4) | 55 | (84.6) | 6 | (9.2) | 11 | (16.9) | 48 | (73.8) | |
| CK5/6 | 138 | (94.5) | 8 | (5.5) | 142 | (97.3) | 4 | (2.7) | 0 | (0.0) | 41 | (63.1) | 24 | (36.9) | 46 | (70.8) | 11 | (16.9) | 8 | (12.3) | |
| CK14 | 137 | (93.8) | 9 | (6.2) | 143 | (97.9) | 3 | (2.1) | 0 | (0.0) | 43 | (66.2) | 22 | (33.8) | 50 | (76.9) | 9 | (13.8) | 6 | (9.2) | |
| CK20 | 119 | (81.5) | 27 | (18.5) | 135 | (92.5) | 4 | (2.7) | 7 | (4.8) | 17 | (26.2) | 48 | (73.8) | 21 | (32.3) | 22 | (33.8) | 22 | (33.8) | |
| FOXA1 | 39 | (26.7) | 107 | (73.3) | 18 | (12.3) | 23 | (15.8) | 105 | (71.9) | 23 | (35.4) | 42 | (64.6) | 14 | (21.5) | 15 | (23.1) | 36 | (55.4) | |
| GATA3 | 131 | (89.7) | 15 | (10.3) | 134 | (91.8) | 8 | (5.5) | 4 | (2.7) | 8 | (12.3) | 57 | (87.7) | 9 | (13.8) | 4 | (6.2) | 52 | (80.0) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, G.H.; Cho, Y.M.; Kim, S.-W.; Park, J.-M.; Yoon, S.Y.; Jeong, G.; Shin, D.-M.; Ju, H.; Jeong, S.U. Synaptophysin, CD117, and GATA3 as a Diagnostic Immunohistochemical Panel for Small Cell Neuroendocrine Carcinoma of the Urinary Tract. Cancers 2022, 14, 2495. https://doi.org/10.3390/cancers14102495
Kim GH, Cho YM, Kim S-W, Park J-M, Yoon SY, Jeong G, Shin D-M, Ju H, Jeong SU. Synaptophysin, CD117, and GATA3 as a Diagnostic Immunohistochemical Panel for Small Cell Neuroendocrine Carcinoma of the Urinary Tract. Cancers. 2022; 14(10):2495. https://doi.org/10.3390/cancers14102495
Chicago/Turabian StyleKim, Gi Hwan, Yong Mee Cho, So-Woon Kim, Ja-Min Park, Sun Young Yoon, Gowun Jeong, Dong-Myung Shin, Hyein Ju, and Se Un Jeong. 2022. "Synaptophysin, CD117, and GATA3 as a Diagnostic Immunohistochemical Panel for Small Cell Neuroendocrine Carcinoma of the Urinary Tract" Cancers 14, no. 10: 2495. https://doi.org/10.3390/cancers14102495
APA StyleKim, G. H., Cho, Y. M., Kim, S.-W., Park, J.-M., Yoon, S. Y., Jeong, G., Shin, D.-M., Ju, H., & Jeong, S. U. (2022). Synaptophysin, CD117, and GATA3 as a Diagnostic Immunohistochemical Panel for Small Cell Neuroendocrine Carcinoma of the Urinary Tract. Cancers, 14(10), 2495. https://doi.org/10.3390/cancers14102495







