The Relationship between Exercise Self-Efficacy, Intention, and Structural Barriers for Physical Activity after a Cancer Diagnosis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Design and Participants
2.2. Measures
2.3. Statistical Analyses
3. Results
3.1. Descriptive Statistics and Bivariate Correlation Analyses
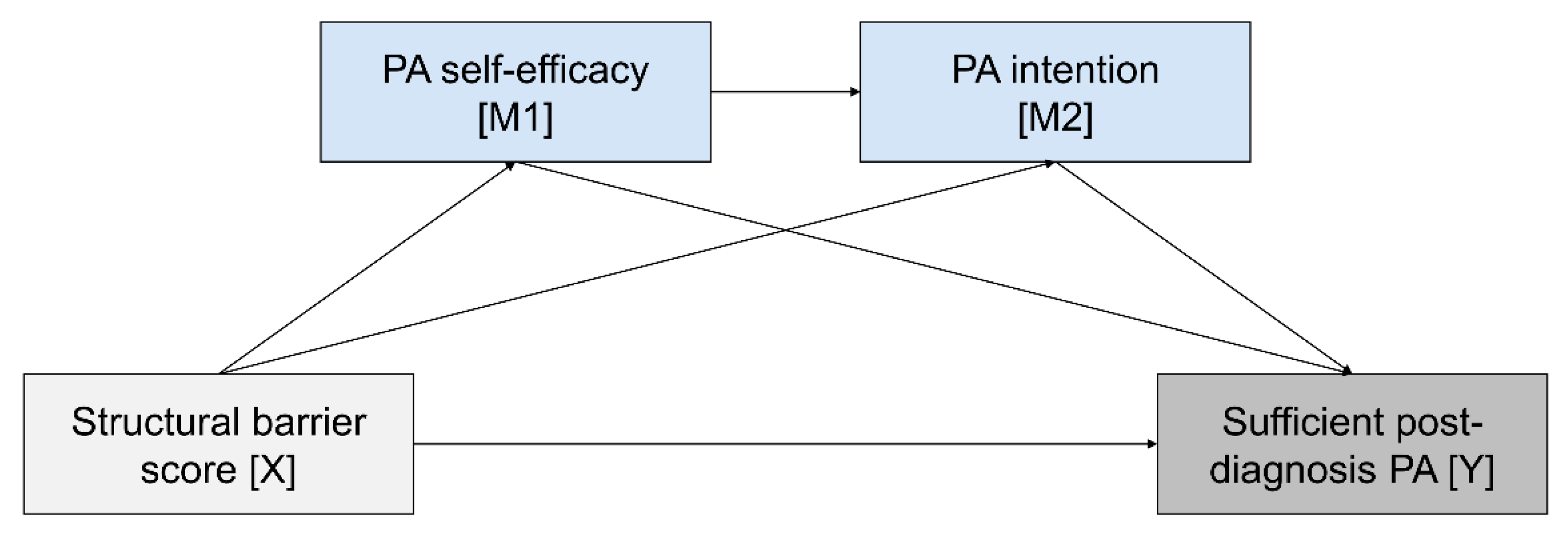
3.2. Mediation Model
3.3. Subgroup Analyses by Pre-Diagnosis PA
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sweegers, M.G.; Altenburg, T.M.; Brug, J.; May, A.M.; van Vulpen, J.K.; Aaronson, N.K.; Arbane, G.; Bohus, M.; Courneya, K.S.; Daley, A.J.; et al. Effects and moderators of exercise on muscle strength, muscle function and aerobic fitness in patients with cancer: A meta-analysis of individual patient data. Br. J. Sports Med. 2019, 53, 812. [Google Scholar] [CrossRef] [PubMed]
- Buffart, L.M.; Kalter, J.; Sweegers, M.G.; Courneya, K.S.; Newton, R.U.; Aaronson, N.K.; Jacobsen, P.B.; May, A.M.; Galvao, D.A.; Chinapaw, M.J.; et al. Effects and moderators of exercise on quality of life and physical function in patients with cancer: An individual patient data meta-analysis of 34 RCTs. Cancer Treat. Rev. 2017, 52, 91–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mustian, K.M.; Alfano, C.M.; Heckler, C.; Kleckner, A.S.; Kleckner, I.R.; Leach, C.R.; Mohr, D.; Palesh, O.G.; Peppone, L.J.; Piper, B.F.; et al. Comparison of Pharmaceutical, Psychological, and Exercise Treatments for Cancer-Related Fatigue: A Meta-analysis. JAMA Oncol. 2017, 3, 961–968. [Google Scholar] [CrossRef] [PubMed]
- Nakano, J.; Hashizume, K.; Fukushima, T.; Ueno, K.; Matsuura, E.; Ikio, Y.; Ishii, S.; Morishita, S.; Tanaka, K.; Kusuba, Y. Effects of Aerobic and Resistance Exercises on Physical Symptoms in Cancer Patients: A Meta-analysis. Integr. Cancer Ther. 2018, 17, 1048–1058. [Google Scholar] [CrossRef]
- McTiernan, A.; Friedenreich, C.M.; Katzmarzyk, P.T.; Powell, K.E.; Macko, R.; Buchner, D.; Pescatello, L.S.; Bloodgood, B.; Tennant, B.; Vaux-Bjerke, A.; et al. Physical Activity in Cancer Prevention and Survival: A Systematic Review. Med. Sci. Sports Exerc. 2019, 51, 1252–1261. [Google Scholar] [CrossRef]
- Campbell, K.L.; Winters-Stone, K.M.; Wiskemann, J.; May, A.M.; Schwartz, A.L.; Courneya, K.S.; Zucker, D.S.; Matthews, C.E.; Ligibel, J.A.; Gerber, L.H.; et al. Exercise Guidelines for Cancer Survivors: Consensus Statement from International Multidisciplinary Roundtable. Med. Sci. Sports Exerc. 2019, 51, 2375–2390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eng, L.; Pringle, D.; Su, J.; Shen, X.; Mahler, M.; Niu, C.; Charow, R.; Tiessen, K.; Lam, C.; Halytskyy, O.; et al. Patterns, perceptions, and perceived barriers to physical activity in adult cancer survivors. Support. Care Cancer 2018, 26, 3755–3763. [Google Scholar] [CrossRef]
- Thraen-Borowski, K.M.; Gennuso, K.P.; Cadmus-Bertram, L. Accelerometer-derived physical activity and sedentary time by cancer type in the United States. PLoS ONE 2017, 12, e0182554. [Google Scholar] [CrossRef]
- Steindorf, K.; Depenbusch, J.; Haussmann, A.; Tsiouris, A.; Schmidt, L.; Hermann, S.; Sieverding, M.; Wiskemann, J.; Ungar, N. Change patterns and determinants of physical activity differ between breast, prostate, and colorectal cancer patients. Support. Care Cancer 2020, 28, 3207–3218. [Google Scholar] [CrossRef]
- Fisher, A.; Wardle, J.; Beeken, R.J.; Croker, H.; Williams, K.; Grimmett, C. Perceived barriers and benefits to physical activity in colorectal cancer patients. Support. Care Cancer 2016, 24, 903–910. [Google Scholar] [CrossRef] [Green Version]
- Blaney, J.M.; Lowe-Strong, A.; Rankin-Watt, J.; Campbell, A.; Gracey, J.H. Cancer survivors’ exercise barriers, facilitators and preferences in the context of fatigue, quality of life and physical activity participation: A questionnaire-survey. Psychooncology 2013, 22, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Clifford, B.K.; Mizrahi, D.; Sandler, C.X.; Barry, B.K.; Simar, D.; Wakefield, C.E.; Goldstein, D. Barriers and facilitators of exercise experienced by cancer survivors: A mixed methods systematic review. Support. Care Cancer 2018, 26, 685–700. [Google Scholar] [CrossRef] [PubMed]
- Romero, S.A.D.; Brown, J.C.; Bauml, J.M.; Hay, J.L.; Li, Q.S.; Cohen, R.B.; Mao, J.J. Barriers to physical activity: A study of academic and community cancer survivors with pain. J. Cancer Surviv. 2018, 12, 744–752. [Google Scholar] [CrossRef]
- Ottenbacher, A.J.; Day, R.S.; Taylor, W.C.; Sharma, S.V.; Sloane, R.; Snyder, D.C.; Kraus, W.E.; Demark-Wahnefried, W. Exercise among breast and prostate cancer survivors-what are their barriers? J. Cancer Surviv. 2011, 5, 413–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lynch, B.M.; Owen, N.; Hawkes, A.L.; Aitken, J.F. Perceived barriers to physical activity for colorectal cancer survivors. Support. Care Cancer 2010, 18, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Depenbusch, J.; Wiskemann, J.; Haussmann, A.; Tsiouris, A.; Schmidt, L.; Ungar, N.; Sieverding, M.; Steindorf, K. Impact and determinants of structural barriers on physical activity in people with cancer. Int. J. Behav. Med. 2021. [Google Scholar] [CrossRef]
- Rogers, L.Q.; Courneya, K.S.; Verhulst, S.; Markwell, S.; Lanzotti, V.; Shah, P. Exercise barrier and task self-efficacy in breast cancer patients during treatment. Support. Care Cancer 2006, 14, 84–90. [Google Scholar] [CrossRef]
- Ajzen, I. Perceived Behavioral Control, Self-Efficacy, Locus of Control, and the Theory of Planned Behavior1. J. Appl. Soc. Psychol. 2002, 32, 665–683. [Google Scholar] [CrossRef]
- Bandura, A. Health promotion by social cognitive means. Health Educ. Behav. 2004, 31, 143–164. [Google Scholar] [CrossRef]
- Sheeran, P.; Klein, W.M.; Rothman, A.J. Health Behavior Change: Moving from Observation to Intervention. Annu. Rev. Psychol. 2017, 68, 573–600. [Google Scholar] [CrossRef]
- Hirschey, R.; Bryant, A.L.; Macek, C.; Battaglini, C.; Santacroce, S.; Courneya, K.S.; Walker, J.S.; Avishai, A.; Sheeran, P. Predicting physical activity among cancer survivors: Meta-analytic path modeling of longitudinal studies. Health Psychol. 2020, 39, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Chen, S.; Jiang, R.; Li, Y.; Chen, L.; Li, F.; Tai, J. The physical activity of colorectal cancer survivors during chemotherapy: Based on the theory of planned behavior. Support. Care Cancer 2020, 28, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Buffart, L.M.; de Bree, R.; Altena, M.; van der Werff, S.; Drossaert, C.H.C.; Speksnijder, C.M.; van den Brekel, M.W.; Jager-Wittenaar, H.; Aaronson, N.K.; Stuiver, M.M. Demographic, clinical, lifestyle-related, and social-cognitive correlates of physical activity in head and neck cancer survivors. Support. Care Cancer 2018, 26, 1447–1456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forbes, C.C.; Blanchard, C.M.; Mummery, W.K.; Courneya, K.S. A comparison of physical activity correlates across breast, prostate and colorectal cancer survivors in Nova Scotia, Canada. Support. Care Cancer 2014, 22, 891–903. [Google Scholar] [CrossRef]
- Husebø, A.M.; Dyrstad, S.M.; Søreide, J.A.; Bru, E. Predicting exercise adherence in cancer patients and survivors: A systematic review and meta-analysis of motivational and behavioural factors. J. Clin. Nurs. 2013, 22, 4–21. [Google Scholar] [CrossRef]
- Speed-Andrews, A.E.; Rhodes, R.E.; Blanchard, C.M.; Culos-Reed, S.N.; Friedenreich, C.M.; Belanger, L.J.; Courneya, K.S. Medical, demographic and social cognitive correlates of physical activity in a population-based sample of colorectal cancer survivors. Eur. J. Cancer Care 2012, 21, 187–196. [Google Scholar] [CrossRef]
- Ungar, N.; Wiskemann, J.; Sieverding, M. Physical Activity Enjoyment and Self-Efficacy As Predictors of Cancer Patients' Physical Activity Level. Front. Psychol. 2016, 7, 898. [Google Scholar] [CrossRef] [Green Version]
- Trinh, L.; Plotnikoff, R.C.; Rhodes, R.E.; North, S.; Courneya, K.S. Correlates of physical activity in a population-based sample of kidney cancer survivors: An application of the theory of planned behavior. Int. J. Behav. Nutr. Phys. Act. 2012, 9, 96. [Google Scholar] [CrossRef] [Green Version]
- Phillips, S.M.; McAuley, E. Social cognitive influences on physical activity participation in long-term breast cancer survivors. Psychooncology 2013, 22, 783–791. [Google Scholar] [CrossRef] [Green Version]
- Alharbi, M.; Gallagher, R.; Neubeck, L.; Bauman, A.; Prebill, G.; Kirkness, A.; Randall, S. Exercise barriers and the relationship to self-efficacy for exercise over 12 months of a lifestyle-change program for people with heart disease and/or diabetes. Eur. J. Cardiovasc. Nurs. 2017, 16, 309–317. [Google Scholar] [CrossRef]
- Olson, E.A.; Mullen, S.P.; Rogers, L.Q.; Courneya, K.S.; Verhulst, S.; McAuley, E. Meeting physical activity guidelines in rural breast cancer survivors. Am. J. Health Behav. 2014, 38, 890–899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rogers, L.Q.; McAuley, E.; Courneya, K.S.; Verhulst, S.J. Correlates of physical activity self-efficacy among breast cancer survivors. Am. J. Health Behav. 2008, 32, 594–603. [Google Scholar] [CrossRef] [PubMed]
- Boyle, T.; Vallance, J.K.; Ransom, E.K.; Lynch, B.M. How sedentary and physically active are breast cancer survivors, and which population subgroups have higher or lower levels of these behaviors? Support. Care Cancer 2016, 24, 2181–2190. [Google Scholar] [CrossRef] [PubMed]
- Hawkes, A.L.; Patrao, T.A.; Baade, P.; Lynch, B.M.; Courneya, K.S. Predictors of physical activity in colorectal cancer survivors after participation in a telephone-delivered multiple health behavior change intervention. J. Cancer Surviv. 2015, 9, 40–49. [Google Scholar] [CrossRef]
- Davies, N.J.; Batehup, L.; Thomas, R. The role of diet and physical activity in breast, colorectal, and prostate cancer survivorship: A review of the literature. Br. J. Cancer 2011, 105 (Suppl. 1), S52–S73. [Google Scholar] [CrossRef] [Green Version]
- Friedenreich, C.M.; Neilson, H.K.; Farris, M.S.; Courneya, K.S. Physical Activity and Cancer Outcomes: A Precision Medicine Approach. Clin. Cancer Res. 2016, 22, 4766–4775. [Google Scholar] [CrossRef] [Green Version]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Estabrooks, P.A.; Glasgow, R.E.; Dzewaltowski, D.A. Physical activity promotion through primary care. JAMA 2003, 289, 2913–2916. [Google Scholar] [CrossRef]
- Depenbusch, J.; Haussmann, A.; Tsiouris, A.; Schmidt, L.; Wiskemann, J.; Ungar, N.; Sieverding, M.; Steindorf, K. The association between physicians' exercise counseling and physical activity in patients with cancer: Which roles do patients’ satisfaction and previous physical activity levels play? Psychooncology 2020, 29, 1856–1863. [Google Scholar] [CrossRef]
- Godin, G. The Godin-Shephard Leisure-Time Physical Activity Questionnaire. Health Fit. J. Can. 2011, 4, 18–22. [Google Scholar] [CrossRef]
- Schmitz, K.H.; Courneya, K.S.; Matthews, C.; Demark-Wahnefried, W.; Galvão, D.A.; Pinto, B.M.; Irwin, M.L.; Wolin, K.Y.; Segal, R.J.; Lucia, A.; et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med. Sci. Sports Exerc. 2010, 42, 1409–1426. [Google Scholar] [CrossRef] [PubMed]
- Haussmann, A.; Ungar, N.; Gabrian, M.; Tsiouris, A.; Sieverding, M.; Wiskemann, J.; Steindorf, K. Are healthcare professionals being left in the lurch? The role of structural barriers and information resources to promote physical activity to cancer patients. Support. Care Cancer 2018, 26, 4087–4096. [Google Scholar] [CrossRef] [PubMed]
- Haussmann, A.; Gabrian, M.; Ungar, N.; Jooß, S.; Wiskemann, J.; Sieverding, M.; Steindorf, K. What hinders healthcare professionals in promoting physical activity towards cancer patients? The influencing role of healthcare professionals' concerns, perceived patient characteristics and perceived structural factors. Eur. J. Cancer Care 2018, 27, e12853. [Google Scholar] [CrossRef] [PubMed]
- Haughton McNeill, L.; Wyrwich, K.W.; Brownson, R.C.; Clark, E.M.; Kreuter, M.W. Individual, social environmental, and physical environmental influences on physical activity among black and white Adults: A Structural Equation Analysis. Ann. Behav. Med. 2006, 31, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Bandura, A.; Freeman, W.H.; Lightsey, R. Self-Efficacy: The Exercise of Control. J. Cogn. Psychother. J. Cogn. Psychother. 1999, 13, 158–166. [Google Scholar] [CrossRef]
- Speed-Andrews, A.E.; McGowan, E.L.; Rhodes, R.E.; Blanchard, C.M.; Culos-Reed, S.N.; Friedenreich, C.M.; Courneya, K.S. Identification and Evaluation of the Salient Physical Activity Beliefs of Colorectal Cancer Survivors. Cancer Nurs. 2014, 37, 14–22. [Google Scholar] [CrossRef]
- Lee, M.S.; Small, B.J.; Jacobsen, P.B. Rethinking barriers: A novel conceptualization of exercise barriers in cancer survivors. Psychol. Health Med. 2017, 22, 1248–1255. [Google Scholar] [CrossRef]
- Ajzen, I. The theory of planned behavior. Organ. Behav. Hum. Decis. Process. 1991, 50, 179–211. [Google Scholar] [CrossRef]
- Michie, S.; Richardson, M.; Johnston, M.; Abraham, C.; Francis, J.; Hardeman, W.; Eccles, M.P.; Cane, J.; Wood, C.E. The behavior change technique taxonomy (v1) of 93 hierarchically clustered techniques: Building an international consensus for the reporting of behavior change interventions. Ann. Behav. Med. 2013, 46, 81–95. [Google Scholar] [CrossRef]
- Warner, L.M.; Schüz, B.; Wolff, J.K.; Parschau, L.; Wurm, S.; Schwarzer, R. Sources of self-efficacy for physical activity. Health Psychol. 2014, 33, 1298–1308. [Google Scholar] [CrossRef]
- Williams, S.L.; French, D.P. What are the most effective intervention techniques for changing physical activity self-efficacy and physical activity behaviour-and are they the same? Health Educ. Res. 2011, 26, 308–322. [Google Scholar] [CrossRef] [PubMed]
- Meyer-Schwickerath, C.; Morawietz, C.; Baumann, F.T.; Huber, G.; Wiskemann, J. Efficacy of face-to-face behavior change counseling interventions on physical activity behavior in cancer survivors—A systematic review and meta-analysis. Disabil. Rehabil. 2021, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Finne, E.; Glausch, M.; Exner, A.-K.; Sauzet, O.; Stölzel, F.; Seidel, N. Behavior change techniques for increasing physical activity in cancer survivors: A systematic review and meta-analysis of randomized controlled trials. Cancer Manag. Res. 2018, 10, 5125–5143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lavallée, J.F.; Abdin, S.; Faulkner, J.; Husted, M. Barriers and facilitators to participating in physical activity for adults with breast cancer receiving adjuvant treatment: A qualitative metasynthesis. Psychooncology 2019, 28, 468–476. [Google Scholar] [CrossRef]
- Hardcastle, S.J.; Maxwell-Smith, C.; Hagger, M.S.; O’Connor, M.; Platell, C. Exploration of information and support needs in relation to health concerns, diet and physical activity in colorectal cancer survivors. Eur. J. Cancer Care 2018, 27, e12679. [Google Scholar] [CrossRef] [Green Version]
- Ottenbacher, A.; Sloane, R.; Snyder, D.C.; Kraus, W.; Sprod, L.; Demark-Wahnefried, W. Cancer-Specific Concerns and Physical Activity Among Recently Diagnosed Breast and Prostate Cancer Survivors. Integr. Cancer Ther. 2013, 12, 206–212. [Google Scholar] [CrossRef]
- Schmitz, K.H.; Campbell, A.M.; Stuiver, M.M.; Pinto, B.M.; Schwartz, A.L.; Morris, G.S.; Ligibel, J.A.; Cheville, A.; Galvão, D.A.; Alfano, C.M.; et al. Exercise is medicine in oncology: Engaging clinicians to help patients move through cancer. CA: A Cancer J. Clin. 2019, 69, 468–484. [Google Scholar] [CrossRef] [Green Version]
- Millet, N.; McDermott, H.J.; Moss, E.L.; Edwardson, C.L.; Munir, F. Increasing physical activity levels following treatment for cervical cancer: An intervention mapping approach. J. Cancer Surviv. 2021, 1–9. [Google Scholar] [CrossRef]
- Reale, S.; Turner, R.R.; Sutton, E.; Taylor, S.J.C.; Bourke, L.; Morrissey, D.; Brown, J.; Rosario, D.J.; Steed, L. Towards implementing exercise into the prostate cancer care pathway: Development of a theory and evidence-based intervention to train community-based exercise professionals to support change in patient exercise behaviour (The STAMINA trial). BMC Health Serv. Res. 2021, 21, 264. [Google Scholar] [CrossRef]
- Demark-Wahnefried, W.; Aziz, N.M.; Rowland, J.H.; Pinto, B.M. Riding the crest of the teachable moment: Promoting long-term health after the diagnosis of cancer. J. Clin. Oncol. 2005, 23, 5814–5830. [Google Scholar] [CrossRef] [Green Version]
- Haussmann, A.; Ungar, N.; Tsiouris, A.; Sieverding, M.; Wiskemann, J.; Steindorf, K. The Influence of Cancer Patient Characteristics on the Recommendation of Physical Activity by Healthcare Professionals. Int. J. Behav. Med. 2020, 27, 65–78. [Google Scholar] [CrossRef] [PubMed]
| Mean or abs. Number | SD or % | |
|---|---|---|
| Age (years) a,b | 58.2 | 12.4 |
| BMI (kg/m²) a | 26.3 | 4.8 |
| Sex | ||
| Female | 516 | 60.3% |
| Male | 314 | 39.7% |
| Educational level c | ||
| Lower | 445 | 52.0% |
| Higher | 411 | 48.0% |
| Cancer type | ||
| Breast cancer | 433 | 50.6% |
| Prostate cancer | 216 | 25.2% |
| Colorectal cancer | 207 | 24.2% |
| Time since diagnosis (months) a,d | 14.8 | 7.6 |
| Current treatment status | ||
| No treatment | 463 | 55.3% |
| Receiving treatment | 375 | 44.7% |
| Chemotherapy e | ||
| No | 469 | 55.3% |
| Yes | 379 | 44.7% |
| Radiotherapy e | ||
| No | 376 | 44.3% |
| Yes | 472 | 55.7% |
| Hormone therapy e | ||
| No | 543 | 64.6% |
| Yes | 297 | 35.4% |
| Co-morbidities | ||
| None | 405 | 47.3% |
| ≥1 | 451 | 52.7% |
| Pre-diagnosis MVPA | ||
| 0–149 min/week | 317 | 37.0% |
| ≥150 min/week | 539 | 63.0% |
| Post-diagnosis MVPA | ||
| 0–149 min/week | 389 | 45.4% |
| ≥150 min/week | 467 | 54.6% |
| 5A Score for PA counseling a,f | 1.0 | 0.9 |
| Structural barrier score a,g | 0.7 | 0.7 |
| PA intention a,h | 4.9 | 1.3 |
| PA self-efficacy a,i | 4.4 | 1.7 |
| Predictors | Criterion | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PA Self-Efficacy a | PA Intention a | Sufficient Post-Diagnosis PA b | ||||||||||
| Coefficient | SE | p-Value | 95% CI | Coefficient | SE | p-Value | 95% CI | Coefficient | SE | p-Value | 95% CI | |
| Structural barrier score c | −0.35 | 0.09 | <0.001 | (−0.53; −0.17) | −0.03 | 0.05 | 0.630 | (−0.13; 0.08) | −0.19 | 0.14 | 0.171 | (−0.47; 0.08) |
| PA self-efficacy | ---- | ---- | ---- | ---- | 0.55 | 0.03 | <0.001 | (0.50; 0.60) | 0.41 | 0.08 | <0.001 | (0.25; 0.57) |
| PA intention | ---- | ---- | ---- | ---- | ---- | ---- | ---- | ---- | 0.55 | 0.11 | <0.001 | (0.33; 0.78) |
| Age | 0.02 | 0.01 | 0.004 | (0.01; 0.03) | −0.00 | 0.00 | 0.486 | (−0.01; 0.00) | −0.01 | 0.01 | 0.180 | (−0.03; 0.01) |
| Sex d | 0.35 | 0.23 | 0.127 | (−010; 0.80) | −0.15 | 0.12 | 0.221 | (−0.39; 0.09) | 0.08 | 0.36 | 0.439 | (−0.42; 0.96) |
| Educational level | 0.42 | 0.11 | <0.001 | (0.20; 0.63) | 0.08 | 0.06 | 0.199 | (−0.04; 0.20) | 0.17 | 0.18 | 0.344 | (−0.18; 0.53) |
| BMI | −0.05 | 0.01 | <0.001 | (−0.07; −0.03) | −0.01 | 0.01 | 0.116 | (−0.03; 0.00) | −0.02 | 0.02 | 0.242 | (−0.06; 0.2) |
| Cancer type | ||||||||||||
| Prostate cancer e | −0.06 | 0.26 | 0.806 | (−0.57; 0.44) | 0.00 | 0.14 | 0.989 | (−0.28; 0.28) | −1.26 | 0.42 | 0.003 | (−2.08; −0.44) |
| Colorectal cancer f | −0.03 | 0.20 | 0.897 | (−0.41; 0.36) | −0.00 | 0.14 | 0.982 | (−0.20; 0.20) | −1.30 | 0.31 | <0.001 | (−1.91; −0.69) |
| Time since diagnosis | −0.01 | 0.01 | 0.322 | (−0.02; 0.01) | 0.00 | 0.00 | 0.893 | (−0.01; 0.01) | 0.04 | 0.01 | <0.001 | (0.02; 0.07) |
| Co-morbidities | −0.27 | 0.11 | 0.015 | (−0.50; −0.05) | 0.03 | 0.07 | 0.616 | (−0.10; 0.16) | −0.29 | 0.19 | 0.119 | (−0.66; 0.08) |
| 5A score g | 0.16 | 0.06 | 0.005 | (0.05; 0.27) | 0.06 | 0.03 | 0.034 | (0.00; 0.12) | 0.25 | 0.10 | 0.011 | (0.06; 0.44) |
| Pre-diagnosis PA h | 0.91 | 0.12 | <0.001 | (0.68; 1.14) | 0.18 | 0.07 | 0.013 | (0.04, 0.32) | 1.47 | 0.19 | <0.001 | (1.10, 1.84) |
| Considered section of the mediation model |  | 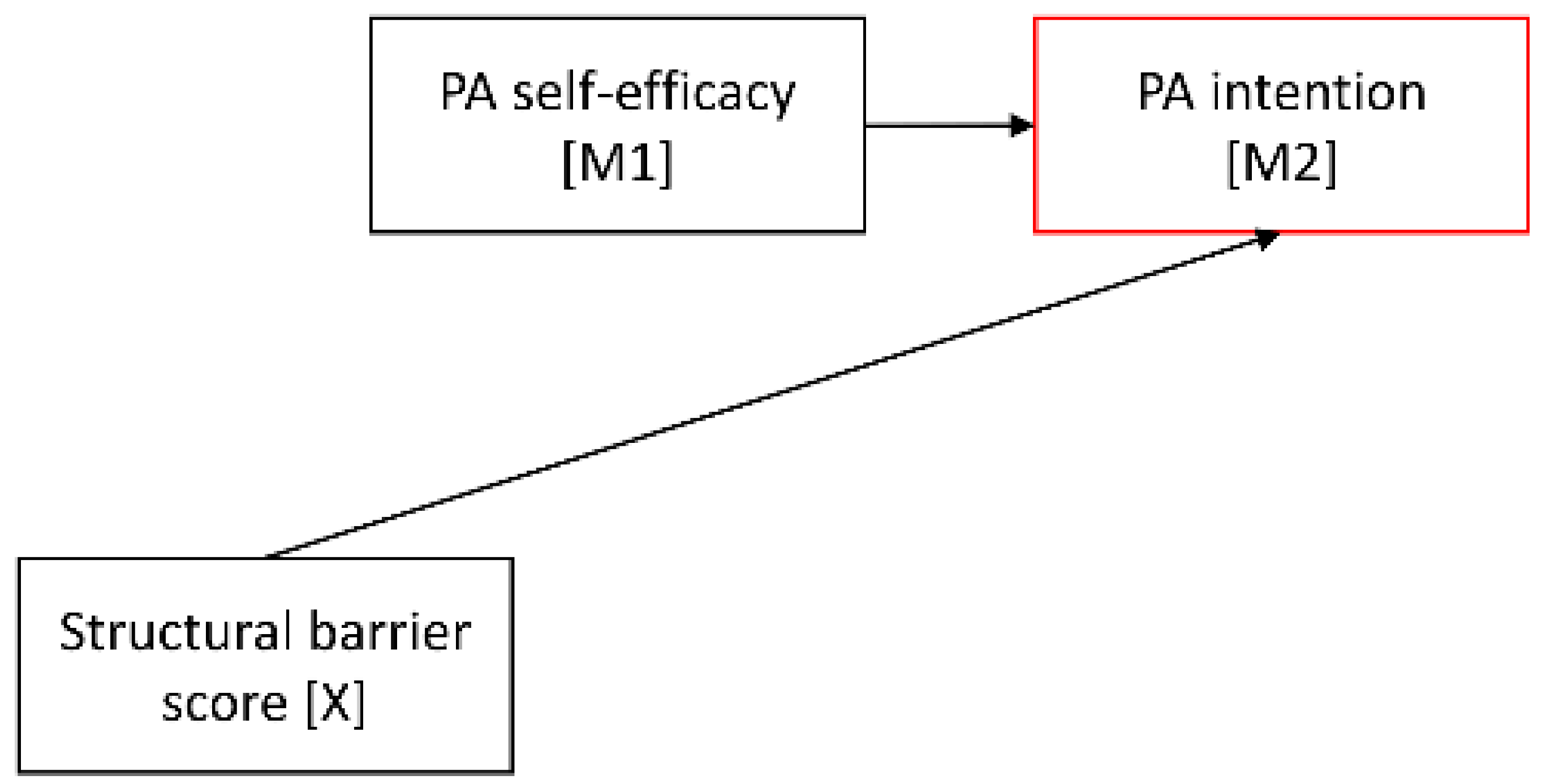 | 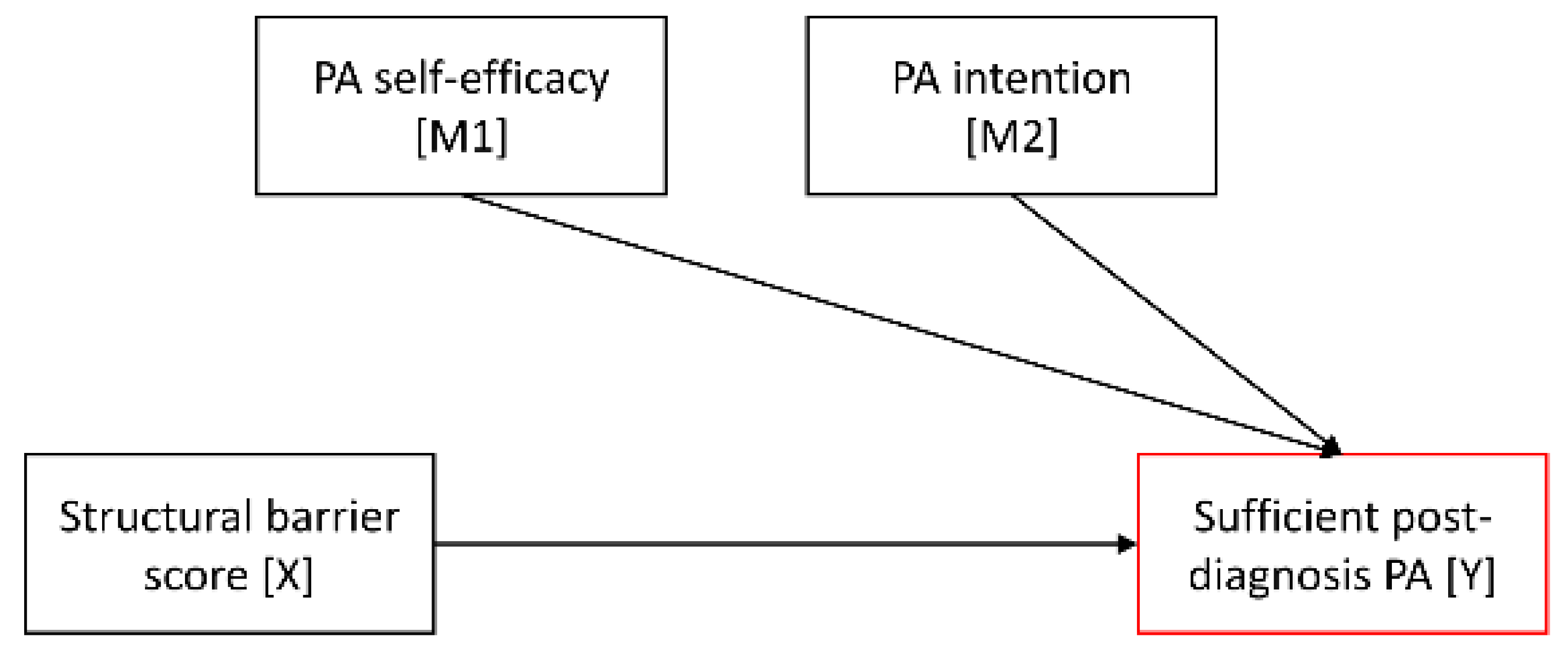 | |||||||||
| Analyzed Sample | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Statistical Model | Overall Sample | Previously Sufficiently Active a | Previously Insufficiently Active b | ||||||
| Direct Effect | Effect | SE | 95% CI | Effect | SE | 95% CI | Effect | SE | 95% CI |
| Structural barrier score c → Post-diagnosis PA d | −0.19 | 0.14 | (−0.47; 0.08) | −0.16 | 0.18 | (−0.52; 0.19) | −0.18 | 0.24 | (−0.64; 0.29) |
| Indirect effect(s) via | Effect | Boot SE | Boot 95% CI | Effect | Boot SE | Boot 95% CI | Effect | Boot SE | Boot 95% CI |
| a. PA self-efficacy | −0.14 | 0.05 | (−0.25; −0.06) | −0.23 | 0.08 | (−0.41; −0.10) | −0.05 | 0.05 | (−0.18; 0.02) |
| b. PA intention | 0.01 | 0.03 | (−0.08; 0.04) | 0.02 | 0.03 | (−0.04; 0.09) | −0.07 | 0.07 | (−0.24; 0.04) |
| c. PA self-efficacy and PA intention in serial | −0.11 | 0.04 | (−0.19; −0.05) | −0.11 | 0.05 | (−0.22; −0.03) | −0.07 | 0.06 | (−0.21; 0.03) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Depenbusch, J.; Haussmann, A.; Wiskemann, J.; Tsiouris, A.; Schmidt, L.; Sieverding, M.; Ungar, N.; Steindorf, K. The Relationship between Exercise Self-Efficacy, Intention, and Structural Barriers for Physical Activity after a Cancer Diagnosis. Cancers 2022, 14, 2480. https://doi.org/10.3390/cancers14102480
Depenbusch J, Haussmann A, Wiskemann J, Tsiouris A, Schmidt L, Sieverding M, Ungar N, Steindorf K. The Relationship between Exercise Self-Efficacy, Intention, and Structural Barriers for Physical Activity after a Cancer Diagnosis. Cancers. 2022; 14(10):2480. https://doi.org/10.3390/cancers14102480
Chicago/Turabian StyleDepenbusch, Johanna, Alexander Haussmann, Joachim Wiskemann, Angeliki Tsiouris, Laura Schmidt, Monika Sieverding, Nadine Ungar, and Karen Steindorf. 2022. "The Relationship between Exercise Self-Efficacy, Intention, and Structural Barriers for Physical Activity after a Cancer Diagnosis" Cancers 14, no. 10: 2480. https://doi.org/10.3390/cancers14102480
APA StyleDepenbusch, J., Haussmann, A., Wiskemann, J., Tsiouris, A., Schmidt, L., Sieverding, M., Ungar, N., & Steindorf, K. (2022). The Relationship between Exercise Self-Efficacy, Intention, and Structural Barriers for Physical Activity after a Cancer Diagnosis. Cancers, 14(10), 2480. https://doi.org/10.3390/cancers14102480







