Simple Summary
TP53-mutated acute myeloid leukemia (AML) represents one of the most informative examples of adverse risk AML. As the currently available therapies have not translated to meaningful advances in the survival of these patients, a clinical trial should be the recommendation for all newly diagnosed patients. CD47/SIRPα axis and TIM-3 inhibition appear to be some of the more promising strategies, but other agents with novel mechanisms of action are in development. We review the pathobiology of TP53-mutated AML, the possible heterogeneity among patients with this disease and how some of the novel and emerging therapies may fit into the treatment landscape in the hopefully not-so-distant future.
Abstract
The currently available therapeutic options for patients with TP53-mutated acute myeloid leukemia (AML) are insufficient, as they translate to a median overall of only 6–9 months, and less than 10% of patients undergoing the most aggressive treatments, such as intensive induction therapy and allogeneic hematopoietic stem cell transplantation, will be cured. The lack of clear differences in outcomes with different treatments precludes the designation of a standard of care. Recently, there has been growing attention on this critical area of need by way of better understanding the biology of TP53 alterations and the disparities in outcomes among patients in this molecular subgroup, reflected in the development and testing of agents with novel mechanisms of action. Promising preclinical and efficacy data exist for therapies that are directed at the p53 protein rendered dysfunctional via mutation or that inhibit the CD47/SIRPα axis or other immune checkpoints such as TIM-3. In this review, we discuss recently attractive and emerging therapeutic agents, their preclinical rationale and the available clinical data as a monotherapy or in combination with the currently accepted backbones in frontline and relapsed/refractory settings for patients with TP53-mutated AML.
1. Introduction
TP53 is a critical tumor suppressor gene located on chromosome 17p13.1 that encodes the p53 protein, which, in response to cellular stress, including deoxyribonucleic acid (DNA) damage, increases in level and ultimately induces the transcription of the genes responsible for DNA damage repair and cell cycle arrest/apoptosis, among others [1]. As a result, deficiency in the functional p53 protein predicted by mutations in or deletions of this “guardian of the genome” allows cells that would otherwise be destined for programmed cell death (apoptosis) to escape it and foster progression of the malignant disease.
Among patients with a new diagnosis of acute myeloid leukemia (AML), at least 10% will have disease-harboring mutations in TP53 (TP53m-AML) but up to 30% in certain subpopulations such as those with secondary AML, therapy-related AML (t-AML) or acute erythroid leukemia (and nearly all patients with the pure erythroid leukemia subtype) [2,3,4,5,6]. Stemming from the grave deficits in cellular regulation imparted by mutations in TP53, TP53m-AML is associated with a limited response to traditional AML-directed therapy and poor overall survival (OS). With the currently available therapies, the median OS of a patient with newly diagnosed TP53m-AML is approximately 6–9 months, and only approximately 10% of patients will be alive three years after allogeneic hematopoietic stem cell transplantation (alloHCT) [7,8,9,10,11]. As such, the presence of TP53 mutations has been included in the adverse risk category in the 2017 European LeukemiaNet recommendations [12]. The poor outcomes observed with this subgroup of AML has prompted the development and study of novel agents and combinations to address this critical area of need.
2. Mechanisms and the Landscape of TP53 Alteration
Alterations of TP53 may occur via several mechanisms, including TP53 mutations and chromosomal aberrations imparting aberrant protein function and loss of TP53, respectively. All classes of TP53 mutations have been reported in patients with TP53m-AML, but nearly all occur in the DNA-binding domain (encoded by exons 5–8), and the vast majority will be missense mutations, which occur in 80–90% of cases [13,14,15]. Approximately 5–10%, 2–5%, 2–5% and 1–2% of mutations are found to be splice site, frameshift, nonsense and indel variants, respectively [13,14,15]. TP53 mutations in AML classically involve arginine residues and occur at hot spots (codon positions 175, 220, 245, 248 and 273), specifically R175H, Y220C, R248Q and R273C. P72R mutations outside of the DNA-binding domain are also recurringly identified [2,14,15]. These lesions induce conformational changes in the TP53 protein or induce degradation of the DNA-binding domain that mostly result in a dominant-negative effect, in which the remaining wild-type allele is impaired by the product of the mutated allele, allowing for a selection advantage of the affected clones exposed to cellular stress [16]. Although mutations in TP53 are largely loss-of-function variants, some predict a partially functional protein [17,18], and others, such as those involving R282, are gain-of-function variants [19].
TP53m-AML is more likely than the TP53 wild-type AML to harbor complex karyotype (>3 chromosomal abnormalities), which is namely detected in up to 90% of cases of t-AML [20,21,22]. An increased rate of monosomy 17/abnormal 17p, monosomy 7 and monosomy 5, each found in about 70% of cases, is observed [21,23]. However, for unclear reasons, the rate of classical AML driver mutations (found in approximately 30% of TP53 wild-type cases) is low, with only 2–7% as cases of TP53m-AML-harboring mutations in NPM1 or FLT3 [10,21,23,24,25].
The loss of band 17p13.1 on which TP53 is located, either by del(17p) or monosomy 17, leads to an allelic and functional loss of the TP53 allele. Indeed, AML with del(17p)/monosomy 17 is associated with a median OS similar to AML harboring a TP53 mutation, and these two lesions should be considered the same for the purposes of risk assessment [26]. In addition, the TP53 protein can be rendered dysfunctional via the overexpression of its chief negative regular, murine double minute 2 (MDM2) [27,28].
The clinical impact of the TP53 alteration in AML/MDS depends on whether the allelic disruption is monoallelic or biallelic, which determines the amount of functional TP53 protein present. Elegant analyses of patients with TP53-mutated MDS demonstrated that approximately 40% of the population harbors disease with a copy-neutral loss of heterozygosity, which, based on the predicted absence of the functional TP53 protein, was significantly associated with inferior survival; conversely, patients with a monoallelic loss of TP53 fared similar to patients with TP53 wild-type disease [29]. However, less stringent data support this effect on survival among patients with TP53m-AML, which is affected by biallelic TP53 loss in 55–75% of cases [21,29,30,31]. Lastly, the inability to firmly establish copy number alterations or assess the loss of heterozygosity with the current “standard” techniques leaves the prediction of TP53 biallelic loss to surrogates such as the detection of dual TP53 mutations, concurrent chromosome 17/17p abnormality or high mutant VAF (i.e., >50%), which have limitations when applied to TP53m-AML.
3. Current and Insufficient Standards-of-Care
Intensive, multi-cytotoxic agent regimens, typically comprised of cytarabine and an anthracycline, have been the mainstay of AML-directed therapy for decades. Unplanned subgroup analyses and post hoc evaluations have demonstrated that the rate of complete remission (CR)/CR with incomplete count recovery (CRi) for patients with TP53m-AML after cytarabine–anthracycline therapy, either classical “7 + 3” or the liposomal formulation CPX-351, is 20–40% [8,21,23,32,33]; this is in contrast to the 80% rate of CR that is observed for patients with the TP53 wild-type disease (Table 1) [21,23,24,32].

Table 1.
Summary of the experiences with the currently available frontline therapies for TP53-mutated AML.
In addition to the inherently poor rates of remission, there is a clear disconnect between the rates of remission and long-term survival for patients with TP53m-AML, as an improvement in the former does not consistently translate into an improvement in the latter. However, remission is the first hurdle that must be jumped to ultimately improve long-term outcomes. Definitive consolidation for patients in remission represents the second hurdle. AlloHCT as a consolidative strategy for patients with TP53m-AML has been debated, given that only 5–10% of patients will benefit from long-term post-alloHCT OS [13,23,37,44]; however, multivariable analyses have found that alloHCT in the first remission still appears to impart benefits [8,24]. The enthusiasm for proceeding with alloHCT during the first remission likely depends on several factors, including the depth of response to therapy, as estimated by the achievement of molecular remission/mutational clearance.
No intensive therapy is clearly superior to the other in getting patients with TP53m-AML into first remission. A randomized, multicenter, phase 3 trial found that CPX-351 demonstrated the superior rates of CR, followed by alloHCT, as well as better median event-free survival (EFS) (2.53 vs. 1.31 months; p = 0.021) and median OS (9.6 vs. 5.9 months, p = 0.005) when compared with “7 + 3” in older patients with newly diagnosed AML with myelodysplasia-related changes and t-AML [34]. However, post hoc analyses of this trial found that any superiority of CPX-351 appears to be abrogated when specifically evaluating patients with TP53m-AML with CR/CRi rates of about 30–40% [9,33]. These figures have been affirmed in multivariable analyses of other retrospective and real-world studies of CPX-351 for TP53m-AML [35,46].
The unsatisfactory remission rates observed after intensive therapy must also be viewed in the context of the toxicities with which it is associated. Although no prospective trials restricted to intensively treated patients with TP53m-AML are available, unselected patients treated with intensive therapy accept a 10–20% risk of treatment-related mortality by 60 days [34,47]. Advanced age and frailty also increase this risk, with up to 30% and 60% of patients aged >60 years and an Eastern Cooperative Oncology Group (ECOG) performance status >3, respectively, experiencing early mortality [48,49,50,51,52,53,54]. Age is an imperfect surrogate for intensive therapy appropriateness but is often associated with a decreased end-organ reserve and decreased performance status [49,55,56,57,58,59,60]. It is for these reasons that many older patients will be deemed inappropriate to receive intensive therapy and be treated with less intensive, less toxic, disease-modifying therapy. This risk:benefit calculation is further clarified in the context of the dismal outcomes associated with a diagnosis of TP53m-AML, and many providers may wish to offer less-intensive therapy to the patient who may actually be appropriate for intensive therapy.
Azacitidine and decitabine are the standard hypomethylating agents (HMAs) that have been available since the early 2000s and the standard option for the patients inappropriate for intensive therapy [61]. Randomized trials and large, population-based analyses of unselected patients have found azacitidine and decitabine monotherapy to be equally effective in treating AML [62,63]. No differences are apparent when employing indirect comparisons between studies evaluating patients with TP53m-AML who appear to have approximately 30–40% rates of CR/CRi with either [10,38,39,40,41,42]. Attempts have been made to replicate the initial promising data for decitabine on a schedule extended 10 days for TP53m-AML [43], but no improvement in the remission rates has been realized when compared with classical 5-day decitabine in unselected patients [38,39,40,41,42]. The only randomized, prospective study comparing the two regimens found that 10-day decitabine induced a numerically higher rate of CR/CRi (47% vs. 29%, p = 0.40) and better OS (8.5 vs. 5.5 months, p = 0.55), but these were not statistically significant (Table 1) [64].
The introduction of venetoclax-inclusive combinations represents a paradigm shift in the treatment of patients with AML who are not appropriate for intensive therapy.
The randomized phase 3 VIALE-A trial demonstrated that azacitidine + venetoclax was associated with a superior OS when compared with azacitidine monotherapy in mostly older patients with a median age of 76 years (14.7 vs. 9.6 months, p < 0.001) [36]. However, patients with TP53-mutated AML treated during the initial noncomparative study of azacitidine/decitabine + venetoclax had a 47% rate of CR/CRi [7]; an unplanned and underpowered post hoc subgroup analysis of VIALE-A showed no clear benefit in the OS when evaluating 52 patients with TP53m-AML (hazard ratio (HR) = 0.76, 95% CI: 0.40–1.45) [36]. Subsequent retrospective studies have also shown no clear benefit associated with the addition of venetoclax to HMA monotherapy for patient with TP53m-AML [65]. The addition of venetoclax to 10-day decitabine has also been studied in TP53m-AML and demonstrated a 57% rate of CR/CRi, but the median OS was only 5 months and indistinguishable from that expected with HMA monotherapy [25]. Despite these analogous outcomes in mostly older patients, improved rates of remission may improve the chance of a patient able to proceed to potentially curative alloHCT.
In sum, the optimal frontline regimen for a patient with newly diagnosed TP53m-AML is unclear. Intensive regimens and HMA + venetoclax regimens appear to induce the same rates of remission and long-term outcomes. The largest retrospective study of 174 patients with TP53m-AML found no difference in the OS between patients treated with azacitidine + venetoclax and intensive therapies [8]. Another retrospective study of 95 patients with TP53m-AML found that CPX-351 induction was associated with a better relapse-free survival (RFS) (HR = 0.37, p = 0.04) and OS (OR = 0.41, p = 0.003), but CPX-351-treated patients were more likely to proceed to alloHCT, invoking a selection bias and the likelihood that patients destined to do better because of less comorbidity and frailty received CPX-351 [66]. It remains unclear whether intensive therapy is the standard for TP53m-AML when compared with less-intensive regimens and, among less-intensive regimens, whether venetoclax provides a benefit. Unfortunately, these patients appear to have a near uniform poor prognosis irrespective of the induction strategy chosen. The persistent knowledge gap and poor outcomes make enrollment in clinical trials the unequivocal recommendation for patients with TP53m-AML who have the option.
4. Mutant p53 Protein “Refolding” or “Reactivating” Therapies
Several scientific and clinical advancements have provided confidence that TP53m-AML may soon be more appropriately treated (Figure 1). Efforts to identify a small molecule capable of restoring a wild-type conformation of the mutant p53 protein identified “p53 reactivation and induction of massive apoptosis” or PRIMA-1, which was noted to do so. Specifically, the structural analog PRIMA-1met or eprenetapropt (APR-246) has demonstrated impressive clinical results consistent with the first molecule to restore functional p53. Eprenetapropt has demonstrated dose-dependent apoptotic effects in the AML cell lines, as well as primary leukemic cells from AML patients [67]. Leukemic cells from patients with TP53m-AML, although more chemoresistant, were found to be equally sensitive to the cytotoxic effect of eprenetapropt, which was also found to upregulate the levels of p53 [67].
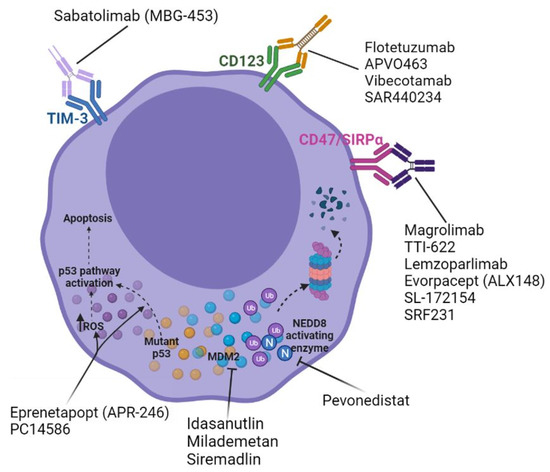
Figure 1.
Emerging frontline agents for the treatment of TP53-mutated acute myeloid leukemia.
Transcriptome analyses using homozygous TP53-mutated myeloid malignancy cell lines and primary AML/myelodysplastic syndrome (MDS) patient samples have demonstrated that even low doses of eprenetapropt can reactivate the p53 pathway and apoptotic programs when administered in combination with a HMA [68]. Cytotoxicity and apoptosis assays have confirmed that eprenetapropt alone is unable to induce apoptosis and, although HMA monotherapy can induce low levels, a combination HMA + eprenetapropt therapy significantly increases apoptosis; to a lesser extent, eprenetapropt is also able to increase the G0/G1 arrest observed with HMA [68]. A subsequent phase 2 study evaluated eprenetapropt (dosed at the recommended phase 2 dose of 100 mg/kg lean body mass (equivalent to 4500 mg/day fixed dosing) daily for four days each 28-day cycle, as identified in the phase 1b portion of the study) with azacitidine 75 mg/m2 on a 7-day schedule in patients with higher-risk MDS or oligoblastic (20–30% bone marrow blasts) AML with mutated TP53 [15]. In the intention-to-treat analysis of the AML population, the median OS was 10.8 months, with improved outcomes observed in the responding patients [15]. In evaluating the entire study population, the responding patients were noted to have significant reductions in the TP53 variant allele frequency (VAF), with 38% of all patients experiencing TP53 molecular remission by next-generation sequencing [15]. However, the results of the primary data cut from the phase 3 trial comparing azacitidine + eprenetapropt vs. azacitidine monotherapy in patients with TP53-mutated higher-risk MDS, a biologically similar disease, demonstrated no difference in CR, the primary endpoint, between groups (33.3% vs. 22.2%, respectively; p = 0.13). The OS was also similar between the arms [69]. An evaluation of the secondary endpoints with maturing data is ongoing. A recent analysis of the phase 2 trial of azacitidine + eprenetapropt with more than two years of median follow-up demonstrated that patients achieving TP53 mutation clearance prior to alloHCT had the most favorable survival, with a median OS not yet reached [70]. An ongoing phase 2 trial of up to 12 cycles of azacitidine + eprenetapropt post-alloHCT maintenance for patients with TP53-mutated AML/MDS demonstrated a 1-year RFS and OS of 58% and 79%, respectively, in the interim analysis of 33 patients (only 12% of whom had pre-alloHCT mutational clearance) [71]. The triplet combination of eprenetapropt with azacitidine + venetoclax is currently being studied in a single-arm trial (NCT04214860). Interim data for this trial demonstrated that, among 30 evaluable patients, the rate of CR and CR/CRi was 37% and 53%, respectively, with a 4-month median duration of response [72]. These data do not appear very different than that associated with azacitidine + venetoclax doublets [7,25,65] and are derived from a single-arm study with a limited number of patients and follow-up. A randomized study is needed before any strong conclusions can be drawn.
PC14586 is another oral small molecule “reactivator” of the mutant p53 protein specifically characterized by a Y220C mutation, which constitutes <5% of all mutations in TP53m-AML [2]. This agent is currently being studied in a phase 1/2 trial of patients with advanced solid tumors with TP53 Y220C (NCT04585750) but may soon extend to TP53m-AML. Despite these efforts, more recent data has suggested that mutant TP53 may not be a biomarker of the response to these types of agents. Preclinical data demonstrated that the mutated p53 protein binds to NRF2, an antioxidant transcription factor that ultimately decreases the expression of the cysteine/glutamate antiporter SLC7A11 [73]. As an important negative regulator of ferroptosis, a nonapoptotic cell death mechanism, decreased SLC7A11 may be an appropriate biomarker of the response, which is also associated with a decrease in glutathione and, thus, an increase in reactive oxygen species (ROS) with resultant cellular stress and death [73,74]. Indeed, preclinical experiments on the AML cell lines have shown that eprenetapropt leads to glutathione depletion and the induction of ferroptosis irrespective of the TP53 status [75]. In sum, these agents may not be mutant p53 “reactivators” or “refolders”, and more study is needed to determine more accurate predictive biomarkers.
5. Leveraging the Immune System
The use of alloHCT, specifically the hypothesized “graft versus leukemia effect” exerted by adoptively transferred donor T cells, to consolidate AML in remission is classically regarded as the analog to immunotherapy for solid tumors [76]. The heterogeneous nature of AML and lack of highly specific targets as a whole have made clear success hard to come by with regards to the various forms of immunotherapy. However, recent endeavors have supported optimism when evaluating patients with TP53m-AML, specifically.
5.1. CD47/SIRPα Axis Inhibition
The transmembrane protein CD47 (“’don’t eat me’ signal”) interacts with macrophage-expressed signal-regulatory protein alpha (SIRPα) to dampen macrophage-mediated phagocytosis and cellular destruction [77,78]. CD47 is commonly expressed on normal human cells but is found to be overexpressed on the surfaces of malignant cells from a number of tumor types, including hematologic malignancies, as well as aged erythrocytes [79,80,81,82]. Consequently, the tumor cell overexpression of CD47 prompts its evasion from innate immunosurveillance. Leukemic HSCs isolated from AML patient marrow and peripheral blood samples have been found to uniformly be enriched for CD47 expression when compared with normal controls; furthermore, increased AML HSC CD47 expression is independently correlated with inferior survival [82,83,84]. Murine studies have demonstrated that treatment with monoclonal antibodies directed against CD47 inhibit the in vivo engraftment of AML HSCs and also restore the physiologic level of AML HSC phagocytosis by macrophages [82,83]. In vitro studies have also shown that treatment with HMA or venetoclax increases the cell surface expression of CD47 but, also, the prophagocytic marker calreticulin (“‘eat me’ signal”), supporting synergy with anti-CD47 agents [85]. Many agents are being developed that consist of the CD47-binding domain of human SIRPα and the Fc region of human IgG1 and/or IgG4, thus blocking the CD47 interaction with macrophage SIRPα and overriding the inhibition of phagocytosis. The exact mechanism by which TP53m-AML appears to be more susceptible to CD47/SIRPα axis inhibition is unclear but may be related to the differential expression of the aforementioned prophagocytic in this particular disease subgroup [86].
Magrolimab (Hu5F9-G4), a humanized anti-CD47-IgG4 monoclonal antibody, has demonstrated an early, promising efficacy when administered as a priming/intra-patient dose escalation regimen (1–30 mg/kg weekly) in combination with a standard dose azacitidine for patients with AML [87]. Interim analyses from the phase 1b trial of this combination in 52 patients demonstrated a favorable safety profile and, ultimately, a 56% rate of CR/CRi; when specifically evaluating TP53m-AML, for which the study population was enriched, 48% of patients achieved CR and 19% achieved CRi for a composite CR/CRi rate of 67% [87]. The median duration of the response was 9.9 months and median OS was 12.9 months [87]. It should be emphasized that these data stem from a single-arm study with a relatively small number of patients and limited follow-up. A phase 1/2 trial of frontline triplet therapy with magrolimab + azacitidine + venetoclax for unselected patients with AML is underway (NCT04435691). The interim data from this trial demonstrated a CR/CRi rate of 100% in seven evaluable patients with TP53m-AML and a 57% rate of measurable residual disease (MRD) negativity by a multiparameter flow cytometric analysis (MFC); five patients (71%) successfully proceeded to alloHCT, and all patients were alive at six months after a median 3.9 months follow-up (Table 2) [88].

Table 2.
Summary of known clinical data for emerging/novel therapies for TP53-mutated AML.
Other agents, differing in their Fc isotype and/or their molecular weight, have shown a promise of efficacy in TP53m-AML. SRF231, a fully humanized anti-CD47 antibody, has demonstrated an ability to increase phagocytosis in AML cell lines, including the p53-null HL60 cell line, as well as in primary bone marrow samples from patients with AML [96]. SRF231 appears to have no effect on hemagglutination or erythrocyte phagocytosis and is currently being studied in a phase 1 basket trial, including patients with hematologic malignancies (NCT03512340) [96]. Evorpacept (ALX148) has demonstrated its ability to increase tumor cell phagocytosis in TP53m-AML cell lines and in several mouse xenograft models, which also demonstrated better survival with combination treatments, namely evorpacept + HMA or evorpacept + HMA + venetoclax [85]. For these reasons, evorpacept is currently being studied in a trial in combination with azacitidine + venetoclax for patients with newly diagnosed AML (NCT04755244) (Table 3). Data from the phase 1 portion of the combination of evorpacept + azacitidine for patients with higher-risk MDS demonstrated marrow CR in one out of five patients with TP53-mutated disease; the phase 2 portion is ongoing [97].

Table 3.
Ongoing frontline clinical trials including TP53-mutated AML patients without clinical data.
Anti-CD47 agents with emerging data might soon apply to patients with TP53m-AML. TTI-621 and TTI-622 are human recombinant soluble fusion proteins that only minimally bind to normal erythrocytes with reduced transient hemolysis, which is observed with other anti-CD47 agents and has less interference with crossmatching [98,99,100]. The TTI-621 treatment of primary samples from patients with AML amongst patients with MDS and other lymphohematopoietic malignancies led to an increase in macrophage-related phagocytosis in 97% of samples. Murine AML xenograft models have demonstrated similar antitumor activity [98]. The interim data from the first-in-human phase 1 study (NCT02663518) of TTI-621 administered IV weekly in a basket hematologic malignancy trial that included 20 patients with AML determined the maximum tolerated dose (MTD) to be 0.2 mg/kg after an episode of transient grade 4 thrombocytopenia, with 20% of patients experiencing grade >3 thrombocytopenia [101]. None of the 20 patients with AML achieved remission, although one patient in CRi with MRD positivity by next-generation sequencing at the time of enrollment achieved MRD negativity [101]. The absence of a strong clinical efficacy demonstrated in this trial must be tempered by the possibility that the established MTD was inaccurate based on transient thrombocytopenia, as well as the growing knowledge regarding the synergy with combination therapy, such as that with HMA, venetoclax and/or other leukemia-directed therapy, which also provide the mandatory “eat me” signals for efficacy. Notably, TTI-622 has been well-tolerated with dosing up to 18 mg/kg, with both improved pharmacokinetics and objective responses in the ongoing trial with lymphoma [102]. Given that combination therapy is required to optimize the efficacy, TTI-622 is the preferred SIRPα fusion protein to proceed in phase 1b/2 testing for patients with AML, including TP53m-AML. An ongoing trial will evaluate TTI-622 in combination with azacitidine for patients with TP53m-AML and in combination with azacitidine + venetoclax in patients with TP53 wild-type AML, both starting at a TTI-622 dose of 8 mg/kg (NCT03530683) [102]. Lemzoparlimab is another anti-CD47 agent currently being dose-escalated in an ongoing trial of patients with relapsed/refractory AML and MDS (NCT04202003). As of the last data cutoff, five patients with AML were enrolled at up to 10 mg/kg without a MTD and, specifically, no dose-dependent hematologic toxicities, although one patient developed grade 3 thrombocytopenia [103]. A CD47 receptor occupancy of 85% was achieved at 10 mg/kg, supporting the likelihood of tumor cell phagocytosis and antileukemia activity. Indeed, one patient treated at 1 mg/kg achieved a morphologic leukemia-free state (MLFS) after two cycles of therapy [103]. Lemzoparlimab is also being studied in an ongoing phase 1 study in combination with azacitidine + venetoclax in patients with newly diagnosed AML/MDS (NCT04912063). Other products being studied in AML include AK117 (NCT04980885), DSP107 (NCT04937166), SL-172154 (NCT05275439) and IBI188 (NCT04485052) (Table 3).
5.2. Immune Checkpoint Inhibition and Other Immunotherapy
In addition to its previously described functions, TP53 influences the induction of interferon-α and -β [104,105,106]; other proinflammatory cytokines (e.g., IL-1, IL-6, IL-12 and TNF) production [107,108] and immune checkpoint regulation; tumor cell TP53-dependent PD-1 and PD-L1 upregulation are observed as a response to DNA damage and other genotoxic stress [109,110,111]. This immune checkpoint modulation is hypothesized to be the consequence of several TP53-regulated microRNAs (miRs), such as miR-145, the miR-17-92 cluster and miR-34 [112,113,114,115]. TP53 transcriptionally targets miR-34, which has been shown to directly bind the 3′ untranslated region of CD274 or the gene encoding PD-L1 [115]. Mutations in TP53 might also prompt genetic instability, which prompts the generation of other neoantigens that influences the immune homeostasis within the tumor microenvironment. Indeed, a recent transcriptomic analysis of 10,817 tumor aliquots of over 30 tumor types from The Cancer Genome Atlas identified mutations in TP53 as a predictor of an increased tumor-infiltrating leukocyte fraction, particularly interferon-γ dominant subtypes and a Th2 cell bias to the adaptive immune infiltrate [116]. When compared with TP53 wild-type AML/MDS bone marrow mononuclear cells, those with TP53 mutations exhibited increased PD-L1 expression, as well as the downregulation of miR-34 [117]. However, no convincing clinical data exist to suggest that PD-1 or PD-L1 inhibition is effective in patients with TP53m-AML.
Recent attention has turned to alternative immune checkpoint molecules. T-cell immunoglobulin and mucin domain-containing 3 (TIM-3) is a T-cell-negative regulator that is expressed on both CD4+ helper T cells and interferon-γ-secreting CD8+ cytotoxic T cells [118,119]. TIM-3 is constitutively expressed on innate immune cells, such as monocytes/macrophages, natural killer cells and dendritic cells; this key negative immune regulator is also enriched on FoxP3+ regulatory T cells [120,121]. Similar to the PD-1/PD-L1 axis, leukemic blasts in AML have been found to overexpress TIM-3, which ultimately inhibits CD8+ T-cell recognition and, thus, the destruction of these malignant cells [118,119,122,123]. The interim data from an ongoing phase 1 trial of the TIM-3 inhibitor sabatolimab (MBG453) in combination with azacitidine demonstrated CR/CRi in 2 out of 5 patients with TP53m-AML and an ORR of 71% (10 out of 14) in patients with TP53-mutated higher-risk MDS [91]. The median duration of response in the latter population was 21.5 months (95% confidence interval: 6.7—not evaluable) but with a small sample size of 11 patients [91]. These data provide confidence that this combination might be effective for TP53m-AML. A single-arm triplet trial of sabatolimab + azacitidine + venetoclax is underway (NCT04150029). Other early phase clinical trials of sabatolimab (MBG453) in combination with HMA therapy and/or BCL-2 inhibitors for patients with AML/MDS are also currently underway (NCT04266301, NCT03946670 and NCT03940352) (Table 2 and Table 3).
Exploratory analyses from a trial of flotetuzumab, an investigational CD123 × CD3 bispecific dual-affinity retargeting antibody therapy, in AML demonstrated that TP53-mutated primary bone marrow samples exhibit greater CD8+ T-cell infiltration and inflammatory cytokine levels [92]. TP53-altered AML marrow samples are enriched for interferon-γ and IL-17/tumor necrosis factor signaling programs by gene expression profiling [92]. Intermediate-to-high baseline immune infiltration, a higher tumor inflammation signature and higher interferon-γ levels were predictive of the response to flotetuzumab; in contrast, although PD-L1 and markers of CD8+ T-cell exhaustion and senescence-like CD244, EOMES, LAG3 and PTGER4 were overexpressed in the TP53-mutated population, they did not predict the response to flotetuzumab [92]. These data suggest that the T-cell engager-derived inflammatory tumor microenvironment in AML is not dampened by the presence of TP53 alterations. Additionally, the observation that protracted interferon-γ signaling induces a polygenic program that is associated with the resistance to conventional therapies in solid tumors invokes a similar concern for TP53m-AML [124]. Other anti-CD123 therapies are in development, such as APVO463, XmAb14045/vibecotamab and SAR440234, among others, but none are currently any further along than the phase 1 stage of development.
6. MDM2–p53 Interaction Destabilization
As an E3 ligase, MDM2 promotes the ubiquitination and proteosome-mediated degradation of p53, which remains at relatively low levels under normal cellular conditions [27]. Cellular stress such as the generation of ROS or DNA damage leads to the phosphorylation of MDM2 and p53, disrupting their interaction and preventing p53 degradation [1,27]. High levels of MDM2 are observed in approximately 30% of AML, with MDM2 expression found to correlate with wild-type TP53 [28]. Although mutations in TP53 are largely loss of function, some will predict functional proteins [17,18]. The study of the oral MDM2 inhibitor idasanutlin was met with discouraging results, including in the few patients included with TP53m-AML [93,125], and might limit the further study of this agent in AML. However, MDM2 inhibition also indirectly promotes the degradation of MCL1, the BH3 family antiapoptotic protein that increases in response to and promotes resistance to venetoclax-containing therapy and a target of some currently studied inhibitory agents (e.g., AMG176, AMG397, S64315 and AZD5991) [126]. These data provide the rationale for a MDM2 inhibitor combination with venetoclax. The MDM2 inhibitor milademetan + low-dose cytarabine with or without venetoclax is being studied in patients with relapsed/refractory AML (NCT03634228), and siremadlin (HDM201) will be studied in a two-arm study in combination with azacitidine + ventoclax and allowed to enroll with TP53m-AML (NCT05155709). Other MDM2 inhibitors such as RG7112 (RO5045337), APG-115, BI-907828 and CGM097 are in development and may soon have data for their efficacy in TP53m-AML.
Similar to disruption of the MDM2-p53 interaction, other attempts to leverage the ubiquitin–proteosome system to modulate p53 protein activity have been explored as options to effectively treat TP53m-AML. The ubiquitin-like protein neural cell developmentally downregulated 8 (NEDD8) is critical to the activity of the Cullin-RING E3 ligases to which it binds and NEDDylates, eventually promoting the proteosome-medicated degradation of proteins such as Nrf-2 and p27. The NEDD8 pathway also appears to influence the activity of the p53 protein via the NEDDylation of MDM2. MDM2, along with NUB1, promotes the nuclear exportation of monoubiquitinated p53 and, thus, its inactivation. The NEDD8-activating enzyme processes NEDD8, rendering it able to bind to its target substrates, and it is for this reason that NEDD8-activating enzyme inhibition has been studied for TP53m-AML. The first-in-class NEDD8-activating enzyme inhibitor pevonedistat was studied in combination with azacitidine in patients with AML, and among the eight evaluable patients with TP53m-AML, six achieved CR/CRi/PR (75%), with the majority remaining on the treatment at 10 cycles [95]. Ex vivo experiments using AML cell lines, primary patient samples and patient-derived xenograft models, demonstrated that the combination of pevonedistat + azacitidine enhanced venetoclax-mediated apoptosis via the neutralization of MCL-1, a known mechanism of resistance to azacitidine + venetoclax therapy [126,127]. However, the interim analysis of an ongoing phase 1/2 study of the triplet combination of pevonedistat + azacitidine + venetoclax (NCT04266795) demonstrated CR/CRi in six out of eight (75%) patients with TP53m-AML but only a median OS of 9 months, limiting the enthusiasm for this triplet combination beyond what is expected with HMA + venetoclax doublet therapy (Table 2) [128]. In sum, the initial attempts to exploit the preclinical data in support of targeting the MDM2–p53 interaction or the ubiquitin–proteosome system to modulate p53 protein activity have been discouraging, but subsequent iterations guided by improved biomarkers and agents may still hold promise for TP53m-AML.
7. Conclusions
The standard of care for the treatment of TP53m-AML is unknown, but the current options available to providers charged with the care of patients afflicted with this disease are inadequate. A meaningful effort is being put forth to develop and study agents, either as monotherapies or in combination with the currently available regimens, with novel or optimized mechanisms of action to address this critical gap. Clinical data for agents exploiting MDM2–p53 homeostasis, such as MDM2 and NEDD8-activating enzyme inhibitors, have recently been met with disappointment. However, among the most promising are agents that inhibit the CD47/SIRPα axis or other immune checkpoints, such as TIM-3. Agents hypothesized to “reactivate” mutant and dysfunctional p53 proteins are also in development. A better understanding of the mechanisms of TP53 alterations and the nuances that influence the amount of functional TP53 protein, such as higher fidelity and universal methods or copy number determination, are required to identify the variance within the molecular subgroup of TP53m-AML. The full profiling of TP53 alterations should occur in large databases and in larger clinical trials to fully understand their differences in behavior and where research efforts can be more efficiently directed. With a more sophisticated understanding of TP53 alteration biology and its determinants of the patient outcomes, future randomized trials employing novel agents could soon establish a true standard of care for TP53m-AML, a disease that, to date, has served as the chief example of poor-risk AML.
Author Contributions
Conceptualization, R.M.S. and A.M.Z.; writing—original draft preparation, R.M.S. and writing—review and editing, R.M.S., J.P.B., M.F.S., S.H. and A.M.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded in part by the Edward P. Evans Foundation (to S.H.), The Frederick A. DeLuca Foundation (to S.H. and A.Z.), the Vera and Joseph Dresner Foundation (to S.H.), by the NIH/NIDDK R01DK102792, the NIH/NCI R01CA222518, R01CA253981, and R01CA266604 (to S.H.).
Conflicts of Interest
R.M.S. participated in Advisory Boards for Bristol Myers Squibb and Gilead Sciences, Inc. J.P.B. has no conflicts to disclose. M.F.S. consulted for Curis Oncology, Haymarket Media and Boston Consulting and participated in Advisory Boards for Novartis. S.H. consulted for Forma Therapeutics. A.Z. has consulted for AbbVie, Acceleron, Agios, Amgen, Aprea, Astellas, AstraZeneca, BeyondSpring, Boehringer Ingelheim, Cardiff Oncology, BMS, Daiichi Sankyo, Epizyme, Genentech, Gilead, Kura, Incyte, Ionis, Loxo Oncology, Janssen and Novartis; served on clinical trial committees for AbbVie, BioCryst, BMS, Geron, Gilead, Kura, Loxo Oncology and Novartis and received research funding from AbbVie, Acceleron, ADC Therapeutics, Amgen, Aprea, Astex, Boehringer Ingelheim, Cardiff Oncology, BMS, Incyte, Jasper, Jazz, Novartis and Pfizer. None of these relationships were related to this work.
References
- Bykov, V.J.N.; Eriksson, S.E.; Bianchi, J.; Wiman, K.G. Targeting mutant p53 for efficient cancer therapy. Nat. Rev. Cancer 2018, 18, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Papaemmanuil, E.; Gerstung, M.; Bullinger, L.; Gaidzik, V.I.; Paschka, P.; Roberts, N.D.; Potter, N.E.; Heuser, M.; Thol, F.; Bolli, N.; et al. Genomic classification and prognosis in acute myeloid leukemia. N. Engl. J. Med. 2016, 374, 2209–2221. [Google Scholar] [CrossRef] [PubMed]
- Kandoth, C.; McLellan, M.D.; Vandin, F.; Ye, K.; Niu, B.; Lu, C.; Xie, M.; Zhang, Q.; McMichael, J.F.; Wyczalkowski, M.A.; et al. Mutational landscape and significance across 12 major cancer types. Nature 2013, 502, 333–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cancer Genome Atlas Research, N.; Ley, T.J.; Miller, C.; Ding, L.; Raphael, B.J.; Mungall, A.J.; Robertson, A.; Hoadley, K.; Triche, T.J., Jr.; Laird, P.W.; et al. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N. Engl. J. Med. 2013, 368, 2059–2074. [Google Scholar] [CrossRef] [Green Version]
- Montalban-Bravo, G.; Benton, C.B.; Wang, S.A.; Ravandi, F.; Kadia, T.; Cortes, J.; Daver, N.; Takahashi, K.; DiNardo, C.; Jabbour, E.; et al. More than 1 tp53 abnormality is a dominant characteristic of pure erythroid leukemia. Blood 2017, 129, 2584–2587. [Google Scholar] [CrossRef] [Green Version]
- Rose, D.; Haferlach, T.; Schnittger, S.; Perglerova, K.; Kern, W.; Haferlach, C. Subtype-specific patterns of molecular mutations in acute myeloid leukemia. Leukemia 2017, 31, 11–17. [Google Scholar] [CrossRef] [Green Version]
- DiNardo, C.D.; Pratz, K.; Pullarkat, V.; Jonas, B.A.; Arellano, M.; Becker, P.S.; Frankfurt, O.; Konopleva, M.; Wei, A.H.; Kantarjian, H.M.; et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood 2019, 133, 7–17. [Google Scholar] [CrossRef] [Green Version]
- Badar, T.; Atallah, E.; Shallis, R.M.; Goldberg, A.D.; Patel, A.; Abaza, Y.; Bewersdorf, J.P.; Saliba, A.N.; Correia, G.S.C.; Murthy, G.; et al. Outcomes of tp53-mutated aml with evolving frontline therapies: Impact of allogeneic stem cell transplantation on survival. Am. J. Hematol. 2022. [Google Scholar] [CrossRef]
- Goldberg, A.D.; Talati, C.; Desai, P.; Famulare, C.; Devlin, S.M.; Farnoud, N.; Sallman, D.A.; Lancet, J.E.; Roboz, G.J.; Sweet, K.L.; et al. Tp53 mutations predict poorer responses to cpx-351 in acute myeloid leukemia. Blood 2018, 132, 1433. [Google Scholar] [CrossRef]
- Kadia, T.M.; Jain, P.; Ravandi, F.; Garcia-Manero, G.; Andreef, M.; Takahashi, K.; Borthakur, G.; Jabbour, E.; Konopleva, M.; Daver, N.G.; et al. Tp53 mutations in newly diagnosed acute myeloid leukemia: Clinicomolecular characteristics, response to therapy, and outcomes. Cancer 2016, 122, 3484–3491. [Google Scholar] [CrossRef] [Green Version]
- Middeke, J.M.; Herold, S.; Rucker-Braun, E.; Berdel, W.E.; Stelljes, M.; Kaufmann, M.; Schafer-Eckart, K.; Baldus, C.D.; Stuhlmann, R.; Ho, A.D.; et al. Tp53 mutation in patients with high-risk acute myeloid leukaemia treated with allogeneic haematopoietic stem cell transplantation. Br. J. Haematol. 2016, 172, 914–922. [Google Scholar] [CrossRef] [PubMed]
- Dohner, H.; Estey, E.; Grimwade, D.; Amadori, S.; Appelbaum, F.R.; Buchner, T.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Larson, R.A.; et al. Diagnosis and management of aml in adults: 2017 eln recommendations from an international expert panel. Blood 2017, 129, 424–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bewersdorf, J.P.; Shallis, R.M.; Gowda, L.; Wei, W.; Hager, K.; Isufi, I.; Kim, T.K.; Pillai, M.M.; Seropian, S.; Podoltsev, N.A.; et al. Clinical outcomes and characteristics of patients with tp53-mutated acute myeloid leukemia or myelodysplastic syndromes: A single center experience. Leuk. Lymphoma 2020, 61, 2180–2190. [Google Scholar] [CrossRef] [PubMed]
- Cluzeau, T.; Sebert, M.; Rahme, R.; Cuzzubbo, S.; Lehmann-Che, J.; Madelaine, I.; Peterlin, P.; Beve, B.; Attalah, H.; Chermat, F.; et al. Eprenetapopt plus azacitidine in tp53-mutated myelodysplastic syndromes and acute myeloid leukemia: A phase ii study by the groupe francophone des myelodysplasies (gfm). J. Clin. Oncol. 2021, 39, 1575–1583. [Google Scholar] [CrossRef]
- Sallman, D.A.; DeZern, A.E.; Garcia-Manero, G.; Steensma, D.P.; Roboz, G.J.; Sekeres, M.A.; Cluzeau, T.; Sweet, K.L.; McLemore, A.; McGraw, K.L.; et al. Eprenetapopt (apr-246) and azacitidine in tp53-mutant myelodysplastic syndromes. J. Clin. Oncol. 2021, 39, 1584–1594. [Google Scholar] [CrossRef]
- Boettcher, S.; Miller, P.G.; Sharma, R.; McConkey, M.; Leventhal, M.; Krivtsov, A.V.; Giacomelli, A.O.; Wong, W.; Kim, J.; Chao, S.; et al. A dominant-negative effect drives selection of tp53 missense mutations in myeloid malignancies. Science 2019, 365, 599–604. [Google Scholar] [CrossRef]
- Kato, S.; Han, S.Y.; Liu, W.; Otsuka, K.; Shibata, H.; Kanamaru, R.; Ishioka, C. Understanding the function-structure and function-mutation relationships of p53 tumor suppressor protein by high-resolution missense mutation analysis. Proc. Natl. Acad. Sci. USA 2003, 100, 8424–8429. [Google Scholar] [CrossRef] [Green Version]
- Kotler, E.; Shani, O.; Goldfeld, G.; Lotan-Pompan, M.; Tarcic, O.; Gershoni, A.; Hopf, T.A.; Marks, D.S.; Oren, M.; Segal, E. A systematic p53 mutation library links differential functional impact to cancer mutation pattern and evolutionary conservation. Mol. Cell 2018, 71, 178–190. [Google Scholar] [CrossRef] [Green Version]
- Bode, A.M.; Dong, Z. Post-translational modification of p53 in tumorigenesis. Nat. Rev. Cancer 2004, 4, 793–805. [Google Scholar] [CrossRef]
- Kayser, S.; Dohner, K.; Krauter, J.; Kohne, C.H.; Horst, H.A.; Held, G.; von Lilienfeld-Toal, M.; Wilhelm, S.; Kundgen, A.; Gotze, K.; et al. The impact of therapy-related acute myeloid leukemia (aml) on outcome in 2853 adult patients with newly diagnosed aml. Blood 2011, 117, 2137–2145. [Google Scholar] [CrossRef] [Green Version]
- Rucker, F.G.; Schlenk, R.F.; Bullinger, L.; Kayser, S.; Teleanu, V.; Kett, H.; Habdank, M.; Kugler, C.M.; Holzmann, K.; Gaidzik, V.I.; et al. Tp53 alterations in acute myeloid leukemia with complex karyotype correlate with specific copy number alterations, monosomal karyotype, and dismal outcome. Blood 2012, 119, 2114–2121. [Google Scholar] [CrossRef] [PubMed]
- Schoch, C.; Kern, W.; Kohlmann, A.; Hiddemann, W.; Schnittger, S.; Haferlach, T. Acute myeloid leukemia with a complex aberrant karyotype is a distinct biological entity characterized by genomic imbalances and a specific gene expression profile. Genes Chromosom. Cancer 2005, 43, 227–238. [Google Scholar] [CrossRef]
- Bowen, D.; Groves, M.J.; Burnett, A.K.; Patel, Y.; Allen, C.; Green, C.; Gale, R.E.; Hills, R.; Linch, D.C. Tp53 gene mutation is frequent in patients with acute myeloid leukemia and complex karyotype, and is associated with very poor prognosis. Leukemia 2009, 23, 203–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prochazka, K.T.; Pregartner, G.; Rucker, F.G.; Heitzer, E.; Pabst, G.; Wolfler, A.; Zebisch, A.; Berghold, A.; Dohner, K.; Sill, H. Clinical implications of subclonal tp53 mutations in acute myeloid leukemia. Haematologica 2019, 104, 516–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.; Maiti, A.; Loghavi, S.; Pourebrahim, R.; Kadia, T.M.; Rausch, C.R.; Furudate, K.; Daver, N.G.; Alvarado, Y.; Ohanian, M.; et al. Outcomes of tp53-mutant acute myeloid leukemia with decitabine and venetoclax. Cancer 2021, 127, 3772–3781. [Google Scholar] [CrossRef]
- Seifert, H.; Mohr, B.; Thiede, C.; Oelschlagel, U.; Schakel, U.; Illmer, T.; Soucek, S.; Ehninger, G.; Schaich, M.; Study Alliance, L. The prognostic impact of 17p (p53) deletion in 2272 adults with acute myeloid leukemia. Leukemia 2009, 23, 656–663. [Google Scholar] [CrossRef] [Green Version]
- Moll, U.M.; Petrenko, O. The mdm2-p53 interaction. Mol. Cancer Res. 2003, 1, 1001–1008. [Google Scholar]
- Quintas-Cardama, A.; Hu, C.; Qutub, A.; Qiu, Y.H.; Zhang, X.; Post, S.M.; Zhang, N.; Coombes, K.; Kornblau, S.M. P53 pathway dysfunction is highly prevalent in acute myeloid leukemia independent of tp53 mutational status. Leukemia 2017, 31, 1296–1305. [Google Scholar] [CrossRef]
- Bernard, E.; Nannya, Y.; Hasserjian, R.P.; Devlin, S.M.; Tuechler, H.; Medina-Martinez, J.S.; Yoshizato, T.; Shiozawa, Y.; Saiki, R.; Malcovati, L.; et al. Implications of tp53 allelic state for genome stability, clinical presentation and outcomes in myelodysplastic syndromes. Nat. Med. 2020, 26, 1549–1556. [Google Scholar] [CrossRef]
- Grob, T.; Al Hinai, A.S.; Sanders, M.A.; Kavelaars, F.; Rijken, M.; Gradowska, P.; Biemond, B.J.; Breems, D.A.; Maertens, J.; van Marwijk Kooy, M.; et al. Molecular characterization of mutant tp53 acute myeloid leukemia and high-risk myelodysplastic syndrome. Blood 2022, 139, 2347–2354. [Google Scholar] [CrossRef]
- Weinberg, O.K.; Siddon, A.J.; Madanat, Y.; Gagan, J.; Arber, D.A.; Dal Cin, P.; Narayanan, D.; Ouseph, M.M.; Kurzer, J.H.; Hasserjian, R.P. Tp53 mutation defines a unique subgroup within complex karyotype de novo and therapy-related mds/aml. Blood Adv. 2022, 6, 2847–2853. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.A.; Chou, W.C.; Kuo, Y.Y.; Liu, C.Y.; Lin, L.I.; Tseng, M.H.; Chiang, Y.C.; Liu, M.C.; Liu, C.W.; Tang, J.L.; et al. Tp53 mutations in de novo acute myeloid leukemia patients: Longitudinal follow-ups show the mutation is stable during disease evolution. Blood Cancer J. 2015, 5, e331. [Google Scholar] [CrossRef] [PubMed]
- Lindsley, R.C.; Gibson, C.J.; Murdock, H.M.; Stone, R.M.; Cortes, J.E.; Uy, G.L.; Lin, T.L.; Ritchie, E.K.; Prebet, T.; Ryan, R.J.; et al. Genetic characteristics and outcomes by mutation status in a phase 3 study of cpx-351 versus 7+3 in older adults with newly diagnosed, high-risk/secondary acute myeloid leukemia (aml). Blood 2019, 134, 15. [Google Scholar] [CrossRef]
- Lancet, J.E.; Uy, G.L.; Cortes, J.E.; Newell, L.F.; Lin, T.L.; Ritchie, E.K.; Stuart, R.K.; Strickland, S.A.; Hogge, D.; Solomon, S.R.; et al. Cpx-351 (cytarabine and daunorubicin) liposome for injection versus conventional cytarabine plus daunorubicin in older patients with newly diagnosed secondary acute myeloid leukemia. J. Clin. Oncol. 2018, 36, 2684–2692. [Google Scholar] [CrossRef] [PubMed]
- Madarang, E.; Lykon, J.; Nguyen, N.; Watts, J.M.; Bradley, T.J.; Chandhok, N.S. Real world outcomes of liposomal daunorubicin and cytarabine versus 7+3 in patients with secondary acute myeloid leukemia. Blood 2020, 136, 5–6. [Google Scholar] [CrossRef]
- DiNardo, C.D.; Jonas, B.A.; Pullarkat, V.; Thirman, M.J.; Garcia, J.S.; Wei, A.H.; Konopleva, M.; Dohner, H.; Letai, A.; Fenaux, P.; et al. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N. Engl. J. Med. 2020, 383, 617–629. [Google Scholar] [CrossRef] [PubMed]
- Dohner, H.; Dolnik, A.; Tang, L.; Seymour, J.F.; Minden, M.D.; Stone, R.M.; Del Castillo, T.B.; Al-Ali, H.K.; Santini, V.; Vyas, P.; et al. Cytogenetics and gene mutations influence survival in older patients with acute myeloid leukemia treated with azacitidine or conventional care. Leukemia 2018, 32, 2546–2557. [Google Scholar] [CrossRef]
- Short, N.J.; Kantarjian, H.M.; Loghavi, S.; Huang, X.; Qiao, W.; Borthakur, G.; Kadia, T.M.; Daver, N.; Ohanian, M.; Dinardo, C.D.; et al. Treatment with a 5-day versus a 10-day schedule of decitabine in older patients with newly diagnosed acute myeloid leukaemia: A randomised phase 2 trial. Lancet Haematol. 2019, 6, e29–e37. [Google Scholar] [CrossRef]
- Kantarjian, H.M.; Thomas, X.G.; Dmoszynska, A.; Wierzbowska, A.; Mazur, G.; Mayer, J.; Gau, J.P.; Chou, W.C.; Buckstein, R.; Cermak, J.; et al. Multicenter, randomized, open-label, phase iii trial of decitabine versus patient choice, with physician advice, of either supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia. J. Clin. Oncol. 2012, 30, 2670–2677. [Google Scholar] [CrossRef] [Green Version]
- Boddu, P.; Kantarjian, H.; Ravandi, F.; Garcia-Manero, G.; Borthakur, G.; Andreeff, M.; Jabbour, E.J.; Benton, C.B.; DiNardo, C.D.; Konopleva, M.; et al. Outcomes with lower intensity therapy in tp53-mutated acute myeloid leukemia. Leuk. Lymphoma 2018, 59, 2238–2241. [Google Scholar] [CrossRef]
- Cashen, A.F.; Schiller, G.J.; O’Donnell, M.R.; DiPersio, J.F. Multicenter, phase ii study of decitabine for the first-line treatment of older patients with acute myeloid leukemia. J. Clin. Oncol. 2010, 28, 556–561. [Google Scholar] [CrossRef] [PubMed]
- Blum, W.; Garzon, R.; Klisovic, R.B.; Schwind, S.; Walker, A.; Geyer, S.; Liu, S.; Havelange, V.; Becker, H.; Schaaf, L.; et al. Clinical response and mir-29b predictive significance in older aml patients treated with a 10-day schedule of decitabine. Proc. Natl. Acad. Sci. USA 2010, 107, 7473–7478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Welch, J.S.; Petti, A.A.; Miller, C.A.; Fronick, C.C.; O’Laughlin, M.; Fulton, R.S.; Wilson, R.K.; Baty, J.D.; Duncavage, E.J.; Tandon, B.; et al. Tp53 and decitabine in acute myeloid leukemia and myelodysplastic syndromes. N. Engl. J. Med. 2016, 375, 2023–2036. [Google Scholar] [CrossRef] [PubMed]
- Aldoss, I.; Zhang, J.; Pillai, R.; Shouse, G.; Sanchez, J.F.; Mei, M.; Nakamura, R.; Stein, A.S.; Forman, S.J.; Marcucci, G.; et al. Venetoclax and hypomethylating agents in tp53-mutated acute myeloid leukaemia. Br. J. Haematol. 2019, 187, e45–e48. [Google Scholar] [CrossRef] [Green Version]
- DiNardo, C.D.; Maiti, A.; Rausch, C.R.; Pemmaraju, N.; Naqvi, K.; Daver, N.G.; Kadia, T.M.; Borthakur, G.; Ohanian, M.; Alvarado, Y.; et al. 10-day decitabine with venetoclax for newly diagnosed intensive chemotherapy ineligible, and relapsed or refractory acute myeloid leukaemia: A single-centre, phase 2 trial. Lancet Haematol. 2020, 7, e724–e736. [Google Scholar] [CrossRef]
- Chiche, E.; Rahme, R.; Bertoli, S.; Dumas, P.Y.; Micol, J.B.; Hicheri, Y.; Pasquier, F.; Peterlin, P.; Chevallier, P.; Thomas, X.; et al. Real-life experience with cpx-351 and impact on the outcome of high-risk aml patients: A multicentric french cohort. Blood Adv. 2021, 5, 176–184. [Google Scholar] [CrossRef]
- Zeidan, A.M.; Podoltsev, N.A.; Wang, X.; Zhang, C.; Bewersdorf, J.P.; Shallis, R.M.; Huntington, S.F.; Neparidze, N.; Giri, S.; Gore, S.D.; et al. Patterns of care and clinical outcomes with cytarabine-anthracycline induction chemotherapy for aml patients in the united states. Blood Adv. 2020, 4, 1615–1623. [Google Scholar] [CrossRef] [Green Version]
- Kantarjian, H.; Ravandi, F.; O’Brien, S.; Cortes, J.; Faderl, S.; Garcia-Manero, G.; Jabbour, E.; Wierda, W.; Kadia, T.; Pierce, S.; et al. Intensive chemotherapy does not benefit most older patients (age 70 years or older) with acute myeloid leukemia. Blood 2010, 116, 4422–4429. [Google Scholar] [CrossRef]
- Kantarjian, H.; O’Brien, S.; Cortes, J.; Giles, F.; Faderl, S.; Jabbour, E.; Garcia-Manero, G.; Wierda, W.; Pierce, S.; Shan, J.; et al. Results of intensive chemotherapy in 998 patients age 65 years or older with acute myeloid leukemia or high-risk myelodysplastic syndrome: Predictive prognostic models for outcome. Cancer 2006, 106, 1090–1098. [Google Scholar] [CrossRef]
- Klepin, H.D.; Geiger, A.M.; Tooze, J.A.; Kritchevsky, S.B.; Williamson, J.D.; Pardee, T.S.; Ellis, L.R.; Powell, B.L. Geriatric assessment predicts survival for older adults receiving induction chemotherapy for acute myelogenous leukemia. Blood 2013, 121, 4287–4294. [Google Scholar] [CrossRef]
- Lowenberg, B.; Ossenkoppele, G.J.; van Putten, W.; Schouten, H.C.; Graux, C.; Ferrant, A.; Sonneveld, P.; Maertens, J.; Jongen-Lavrencic, M.; von Lilienfeld-Toal, M.; et al. High-dose daunorubicin in older patients with acute myeloid leukemia. N. Engl. J. Med. 2009, 361, 1235–1248. [Google Scholar] [CrossRef] [PubMed]
- Burnett, A.K.; Milligan, D.; Goldstone, A.; Prentice, A.; McMullin, M.F.; Dennis, M.; Sellwood, E.; Pallis, M.; Russell, N.; Hills, R.K.; et al. The impact of dose escalation and resistance modulation in older patients with acute myeloid leukaemia and high risk myelodysplastic syndrome: The results of the lrf aml14 trial. Br. J. Haematol. 2009, 145, 318–332. [Google Scholar] [CrossRef] [PubMed]
- Goldstone, A.H.; Burnett, A.K.; Wheatley, K.; Smith, A.G.; Hutchinson, R.M.; Clark, R.E. Attempts to improve treatment outcomes in acute myeloid leukemia (aml) in older patients: The results of the united kingdom medical research council aml11 trial. Blood 2001, 98, 1302–1311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buchner, T.; Berdel, W.E.; Haferlach, C.; Haferlach, T.; Schnittger, S.; Muller-Tidow, C.; Braess, J.; Spiekermann, K.; Kienast, J.; Staib, P.; et al. Age-related risk profile and chemotherapy dose response in acute myeloid leukemia: A study by the german acute myeloid leukemia cooperative group. J. Clin. Oncol. 2009, 27, 61–69. [Google Scholar] [CrossRef] [Green Version]
- Shallis, R.M.; Boddu, P.C.; Bewersdorf, J.P.; Zeidan, A.M. The golden age for patients in their golden years: The progressive upheaval of age and the treatment of newly-diagnosed acute myeloid leukemia. Blood Rev. 2020, 40, 100639. [Google Scholar] [CrossRef]
- Shallis, R.M.; Wang, R.; Davidoff, A.; Ma, X.; Zeidan, A.M. Epidemiology of acute myeloid leukemia: Recent progress and enduring challenges. Blood Rev. 2019, 36, 70–87. [Google Scholar] [CrossRef]
- Juliusson, G. Older patients with acute myeloid leukemia benefit from intensive chemotherapy: An update from the swedish acute leukemia registry. Clin. Lymphoma Myeloma Leuk. 2011, 11, S54–S59. [Google Scholar] [CrossRef]
- Juliusson, G.; Antunovic, P.; Derolf, A.; Lehmann, S.; Mollgard, L.; Stockelberg, D.; Tidefelt, U.; Wahlin, A.; Hoglund, M. Age and acute myeloid leukemia: Real world data on decision to treat and outcomes from the swedish acute leukemia registry. Blood 2009, 113, 4179–4187. [Google Scholar] [CrossRef] [Green Version]
- Walter, R.B.; Othus, M.; Borthakur, G.; Ravandi, F.; Cortes, J.E.; Pierce, S.A.; Appelbaum, F.R.; Kantarjian, H.A.; Estey, E.H. Prediction of early death after induction therapy for newly diagnosed acute myeloid leukemia with pretreatment risk scores: A novel paradigm for treatment assignment. J. Clin. Oncol. 2011, 29, 4417–4423. [Google Scholar] [CrossRef]
- Appelbaum, F.R.; Gundacker, H.; Head, D.R.; Slovak, M.L.; Willman, C.L.; Godwin, J.E.; Anderson, J.E.; Petersdorf, S.H. Age and acute myeloid leukemia. Blood 2006, 107, 3481–3485. [Google Scholar] [CrossRef]
- Oran, B.; Weisdorf, D.J. Survival for older patients with acute myeloid leukemia: A population-based study. Haematologica 2012, 97, 1916–1924. [Google Scholar] [CrossRef] [PubMed]
- Zeidan, A.M.; Wang, R.; Wang, X.; Shallis, R.M.; Podoltsev, N.A.; Bewersdorf, J.P.; Huntington, S.F.; Neparidze, N.; Giri, S.; Gore, S.D.; et al. Clinical outcomes of older patients with aml receiving hypomethylating agents: A large population-based study in the united states. Blood Adv. 2020, 4, 2192–2201. [Google Scholar] [CrossRef] [PubMed]
- Zeidan, A.M.; Fenaux, P.; Gobbi, M.; Mayer, J.; Roboz, G.J.; Krauter, J.; Robak, T.; Kantarjian, H.M.; Novak, J.; Jedrzejczaket, W.W. Comparative results of azacitidine and decitabine from a large prospective phase 3 study in treatment naive acute myeloid leukemia (tn-aml) not eligible for intensive therapy. In Proceedings of the European Hematology Association 2020 Meeting, Hamburg, Germany, 12 June 2020; Abstract s142. pp. 11–14. [Google Scholar]
- Short, N.J.; Kantarjian, H.M.; Loghavi, S.; Huang, X.; Qiao, W.; Borthakur, G.; Kadia, T.M.; Daver, N.G.; Ohanian, M.N.; DiNardo, C.D.; et al. Five-day versus ten-day schedules of decitabine in older patients with newly diagnosed acute myeloid leukemia: Results of a randomized phase ii study. Blood 2018, 132, 84. [Google Scholar] [CrossRef]
- Pollyea, D.A.; Pratz, K.W.; Wei, A.H.; Pullarkat, V.A.; Jonas, B.A.; Recher, C.; Babu, S.; Schuh, A.C.; Dail, M.; Sun, Y.; et al. Outcomes in patients with poor-risk cytogenetics with or without tp53 mutations treated with venetoclax combined with hypomethylating agents. In Proceedings of the American Society of Hematology 2021 Meeting, Atlanta, GA, USA, 11 December 2021; Abstract 224. p. 224. [Google Scholar]
- Grenet, J.; Jain, A.G.; Burkart, M.; Waksal, J.; Famulare, C.; Numan, Y.; Stahl, M.; Mckinnell, Z.; Ball, B.; Ma, X.; et al. Comparing outcomes between liposomal daunorubicin/cytarabine (cpx-351) and hma+venetoclax as frontline therapy in acute myeloid leukemia. In Proceedings of the American Society of Hematology 2021 Meeting, Atlanta, GA, USA, 10–14 December 2021; Abstract 32. p. 32. [Google Scholar]
- Ali, D.; Jonsson-Videsater, K.; Deneberg, S.; Bengtzen, S.; Nahi, H.; Paul, C.; Lehmann, S. Apr-246 exhibits anti-leukemic activity and synergism with conventional chemotherapeutic drugs in acute myeloid leukemia cells. Eur. J. Haematol. 2011, 86, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Maslah, N.; Salomao, N.; Drevon, L.; Verger, E.; Partouche, N.; Ly, P.; Aubin, P.; Naoui, N.; Schlageter, M.H.; Bally, C.; et al. Synergistic effects of prima-1(met) (apr-246) and 5-azacitidine in tp53-mutated myelodysplastic syndromes and acute myeloid leukemia. Haematologica 2020, 105, 1539–1551. [Google Scholar] [CrossRef] [Green Version]
- Aprea Therapeutics Announces Results of Primary Endpoint from Phase 3 Trial of Eprenetapopt in Tp53 Mutant myelodysPlastic Syndromes (Mds). Aprea Therapeutics. Available online: https://ir.Aprea.Com/news-releases/news-release-details/aprea-therapeutics-announces-results-primary-endpoint-phase-3 (accessed on 28 December 2020).
- Sallman, D.A.; Komrokji, R.S.; Dezern, A.E.; Sebert, M.; Garcia-Manero, G.; Rahmé, R.; Steensma, D.P.; Rahmé, R.; Lehmann, J.; Roboz, G.J.; et al. Long term follow-up and combined phase 2 results of eprenetapopt (apr-246) and azacitidine (aza) in patients with tp53 mutant myelodysplastic syndromes (mds) and oligoblastic acute myeloid leukemia (aml). In Proceedings of the American Society of Hematology 2021 Meeting, Atlanta, GA, USA, 11 December 2021; Abstract 246. p. 246. [Google Scholar]
- Mishra, A.; Tamari, R.; DeZern, A.E.; Byrne, M.T.; Gooptu, M.; Chen, Y.-B.; Deeg, H.J.; Gallacher, P.; Wennborg, A.; Kaylor Hickman, D.; et al. Phase ii trial of eprenetapopt (apr-246) in combination with azacitidine (aza) as maintenance therapy for tp53 mutated aml or mds following allogeneic stem cell transplantation (sct). Blood 2021, 138, 409. [Google Scholar] [CrossRef]
- Garcia-Manero, G.; Goldberg, A.D.; Winer, E.S.; Altman, J.K.; Fathi, A.T.; Odenike, O.; Roboz, G.J.; Gallacher, P.; Wennborg, A.; Kaylor Hickman, D.; et al. Phase i and expansion study of eprenetapopt (apr-246) in combination with venetoclax (ven) and azacitidine (aza) in tp53-mutant acute myeloid leukemia (aml). Blood 2021, 138, 3409. [Google Scholar] [CrossRef]
- Liu, D.S.; Duong, C.P.; Haupt, S.; Montgomery, K.G.; House, C.M.; Azar, W.J.; Pearson, H.B.; Fisher, O.M.; Read, M.; Guerra, G.R.; et al. Inhibiting the system xc(-)/glutathione axis selectively targets cancers with mutant-p53 accumulation. Nat. Commun. 2017, 8, 14844. [Google Scholar] [CrossRef] [Green Version]
- Fujihara, K.M.; Corrales Benitez, M.; Cabalag, C.S.; Zhang, B.Z.; Ko, H.S.; Liu, D.S.; Simpson, K.J.; Haupt, Y.; Lipton, L.; Haupt, S.; et al. Slc7a11 is a superior determinant of apr-246 (eprenetapopt) response than tp53 mutation status. Mol. Cancer Ther. 2021, 20, 1858–1867. [Google Scholar] [CrossRef]
- Birsen, R.; Larrue, C.; Decroocq, J.; Johnson, N.; Guiraud, N.; Gotanegre, M.; Cantero-Aguilar, L.; Grignano, E.; Huynh, T.; Fontenay, M.; et al. Apr-246 induces early cell death by ferroptosis in acute myeloid leukemia. Haematologica 2022, 107, 403–416. [Google Scholar] [CrossRef]
- Sweeney, C.; Vyas, P. The graft-versus-leukemia effect in aml. Front. Oncol. 2019, 9, 1217. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Wang, J.; Willingham, S.B.; Martin, R.; Wernig, G.; Weissman, I.L. Anti-cd47 antibodies promote phagocytosis and inhibit the growth of human myeloma cells. Leukemia 2012, 26, 2538–2545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Theocharides, A.P.; Jin, L.; Cheng, P.Y.; Prasolava, T.K.; Malko, A.V.; Ho, J.M.; Poeppl, A.G.; van Rooijen, N.; Minden, M.D.; Danska, J.S.; et al. Disruption of sirpalpha signaling in macrophages eliminates human acute myeloid leukemia stem cells in xenografts. J. Exp. Med. 2012, 209, 1883–1899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chao, M.P.; Weissman, I.L.; Majeti, R. The cd47-sirpalpha pathway in cancer immune evasion and potential therapeutic implications. Curr. Opin. Immunol. 2012, 24, 225–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willingham, S.B.; Volkmer, J.P.; Gentles, A.J.; Sahoo, D.; Dalerba, P.; Mitra, S.S.; Wang, J.; Contreras-Trujillo, H.; Martin, R.; Cohen, J.D.; et al. The cd47-signal regulatory protein alpha (sirpa) interaction is a therapeutic target for human solid tumors. Proc. Natl. Acad. Sci. USA 2012, 109, 6662–6667. [Google Scholar] [CrossRef] [Green Version]
- Edris, B.; Weiskopf, K.; Volkmer, A.K.; Volkmer, J.P.; Willingham, S.B.; Contreras-Trujillo, H.; Liu, J.; Majeti, R.; West, R.B.; Fletcher, J.A.; et al. Antibody therapy targeting the cd47 protein is effective in a model of aggressive metastatic leiomyosarcoma. Proc. Natl. Acad. Sci. USA 2012, 109, 6656–6661. [Google Scholar] [CrossRef] [Green Version]
- Jaiswal, S.; Jamieson, C.H.; Pang, W.W.; Park, C.Y.; Chao, M.P.; Majeti, R.; Traver, D.; van Rooijen, N.; Weissman, I.L. Cd47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell 2009, 138, 271–285. [Google Scholar] [CrossRef] [Green Version]
- Majeti, R.; Chao, M.P.; Alizadeh, A.A.; Pang, W.W.; Jaiswal, S.; Gibbs, K.D., Jr.; van Rooijen, N.; Weissman, I.L. Cd47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell 2009, 138, 286–299. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Sun, C.; Li, M.; Xia, B.; Wang, Y.; Zhang, L.; Zhang, Y.; Wang, J.; Sun, F.; Lu, S.; et al. Novel fully human anti-cd47 antibodies stimulate phagocytosis and promote elimination of aml cells. J. Cell. Physiol. 2021, 236, 4470–4481. [Google Scholar] [CrossRef]
- Chen, A.; Harrabi, O.; Fong, A.P.; Ruffner, K.L.; Forgie, A.J.; Sim, J.; Randolph, S.S.; Wan, H.; Pons, J.; Kuo, T.C. Alx148 enhances the depth and durability of response to multiple aml therapies. Blood 2020, 136, 15–16. [Google Scholar] [CrossRef]
- Wang, C.; Sallman, D.A. Targeting the cluster of differentiation 47/signal-regulatory protein alpha axis in myeloid malignancies. Curr. Opin. Hematol. 2022, 29, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Sallman, D.A.; Asch, A.S.; Al Malki, M.M.; Lee, D.J.; Donnellan, W.B.; Marcucci, G.; Kambhampati, S.; Daver, N.G.; Garcia-Manero, G.; Komrokji, R.S.; et al. The first-in-class anti-cd47 antibody magrolimab (5f9) in combination with azacitidine is effective in mds and aml patients: Ongoing phase 1b results. Blood 2019, 134, 569. [Google Scholar] [CrossRef]
- Daver, N.; Konopleva, M.; Maiti, A.; Kadia, T.M.; Dinardo, C.D.; Loghavi, S.; Pemmaraju, N.; Jabbour, E.J.; Montalban-Bravo, G.; Tang, G.; et al. Phase i/ii study of azacitidine (aza) with venetoclax (ven) and magrolimab (magro) in patients (pts) with newly diagnosed older/unfit or high-risk acute myeloid leukemia (aml) and relapsed/refractory (r/r) aml. In Proceedings of the American Society of Hematology 2021 Meeting, Atlanta, GA, USA, 12 December 2021; Abstract 371. p. 371. [Google Scholar]
- Chen, X.; Othus, M.; Wood, B.L.; Walter, R.B.; Becker, P.S.; Percival, M.E.; Abkowitz, J.L.; Appelbaum, F.R.; Estey, E.H. Comparison of myeloid blast counts and variant allele frequencies of gene mutations in myelodysplastic syndrome with excess blasts and secondary acute myeloid leukemia. Leuk. Lymphoma 2021, 62, 1226–1233. [Google Scholar] [CrossRef] [PubMed]
- Groschel, S.; Schlenk, R.F.; Engelmann, J.; Rockova, V.; Teleanu, V.; Kuhn, M.W.; Eiwen, K.; Erpelinck, C.; Havermans, M.; Lubbert, M.; et al. Deregulated expression of evi1 defines a poor prognostic subset of mll-rearranged acute myeloid leukemias: A study of the german-austrian acute myeloid leukemia study group and the dutch-belgian-swiss hovon/sakk cooperative group. J. Clin. Oncol. 2013, 31, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Brunner, A.M.; Esteve, J.; Porkka, K.; Knapper, S.; Traer, E.; Scholl, S.; Garcia-Manero, G.; Vey, N.; Wermke, M.; Janssen, J.; et al. Efficacy and safety of sabatolimab (mbg453) in combination with hypomethylating agents (hmas) in patients (pts) with very high/high-risk myelodysplastic syndrome (vhr/hr-mds) and acute myeloid leukemia (aml): Final analysis from a phase ib study. Blood 2021, 138, 244. [Google Scholar] [CrossRef]
- Vadakekolathu, J.; Lai, C.; Reeder, S.; Church, S.E.; Hood, T.; Lourdusamy, A.; Rettig, M.P.; Aldoss, I.; Advani, A.S.; Godwin, J.; et al. Tp53 abnormalities correlate with immune infiltration and associate with response to flotetuzumab immunotherapy in aml. Blood Adv. 2020, 4, 5011–5024. [Google Scholar] [CrossRef]
- Yee, K.; Papayannidis, C.; Vey, N.; Dickinson, M.J.; Kelly, K.R.; Assouline, S.; Kasner, M.; Seiter, K.; Drummond, M.W.; Yoon, S.S.; et al. Murine double minute 2 inhibition alone or with cytarabine in acute myeloid leukemia: Results from an idasanutlin phase 1/1b study small star, filled. Leuk. Res. 2021, 100, 106489. [Google Scholar] [CrossRef]
- Erba, H.P.; Becker, P.S.; Shami, P.J.; Grunwald, M.R.; Flesher, D.L.; Zhu, M.; Rasmussen, E.; Henary, H.A.; Anderson, A.A.; Wang, E.S. Phase 1b study of the mdm2 inhibitor amg 232 with or without trametinib in relapsed/refractory acute myeloid leukemia. Blood Adv. 2019, 3, 1939–1949. [Google Scholar] [CrossRef]
- Swords, R.T.; Coutre, S.; Maris, M.B.; Zeidner, J.F.; Foran, J.M.; Cruz, J.; Erba, H.P.; Berdeja, J.G.; Tam, W.; Vardhanabhuti, S.; et al. Pevonedistat, a first-in-class nedd8-activating enzyme inhibitor, combined with azacitidine in patients with aml. Blood 2018, 131, 1415–1424. [Google Scholar] [CrossRef] [Green Version]
- Peluso, M.O.; Adam, A.; Armet, C.M.; Zhang, L.; O’Connor, R.W.; Lee, B.H.; Lake, A.C.; Normant, E.; Chappel, S.C.; Hill, J.A.; et al. The fully human anti-cd47 antibody srf231 exerts dual-mechanism antitumor activity via engagement of the activating receptor cd32a. J. Immunother. Cancer 2020, 8, e000413. [Google Scholar] [CrossRef]
- Garcia-Manero, G.; Erba, H.P.; Sanikommu, S.R.; Altman, J.K.; Sayar, H.; Scott, B.L.; Fond, A.P. Evorpacept (alx148), a cd47-blocking myeloid checkpoint inhibitor, in combination with azacitidine: A phase 1/2 study in patients with myelodysplastic syndrome (aspen-02). In Proceedings of the American Society of Hematology Meeting, Atlanta, GA, USA, 12 December 2021; Abstract 2601. p. 2601. [Google Scholar]
- Petrova, P.S.; Viller, N.N.; Wong, M.; Pang, X.; Lin, G.H.; Dodge, K.; Chai, V.; Chen, H.; Lee, V.; House, V.; et al. Tti-621 (sirpalphafc): A cd47-blocking innate immune checkpoint inhibitor with broad antitumor activity and minimal erythrocyte binding. Clin. Cancer Res. 2017, 23, 1068–1079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCullough, K.; Sharma, P.; Ali, T.; Khan, I.; Smith, W.C.; MacLeod, A.; Black, C. Measuring the population burden of chronic kidney disease: A systematic literature review of the estimated prevalence of impaired kidney function. Nephrol. Dial. Transplant. 2012, 27, 1812–1821. [Google Scholar] [CrossRef] [PubMed]
- Fenaux, P.; Mufti, G.J.; Hellstrom-Lindberg, E.; Santini, V.; Finelli, C.; Giagounidis, A.; Schoch, R.; Gattermann, N.; Sanz, G.; List, A.; et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: A randomised, open-label, phase iii study. Lancet Oncol. 2009, 10, 223–232. [Google Scholar] [CrossRef] [Green Version]
- Ansell, S.M.; Maris, M.B.; Lesokhin, A.M.; Chen, R.W.; Flinn, I.W.; Sawas, A.; Minden, M.D.; Villa, D.; Percival, M.M.; Advani, A.S.; et al. Phase i study of the cd47 blocker tti-621 in patients with relapsed or refractory hematologic malignancies. Clin. Cancer Res. 2021, 27, 2190–2199. [Google Scholar] [CrossRef] [PubMed]
- Trillium Therapeutics, Inc. Trillium Therapeutics Provides Data Update, Announces Phase 1b/2 Program Priorities Across Hematologic Malignancies and Solid Tumors, and Reports Governance Changes. Available online: https://s22.Q4cdn.Com/183592819/files/doc_news/2021/04/2021-04-28-rd-day-press-release-final (accessed on 10 June 2021).
- Qi, J.; Li, J.; Jiang, B.; Jiang, B.; Liu, H.; Cao, X.; Zhang, M.; Meng, Y.; MA, X.; Jia, Y.; et al. A phase i/iia study of lemzoparlimab, a monoclonal antibody targeting cd47, in patients with relapsed and/or refractory acute myeloid leukemia (aml) and myelodysplastic syndrome (mds): Initial phase i results. Blood 2020, 136, 30–31. [Google Scholar] [CrossRef]
- Munoz-Fontela, C.; Macip, S.; Martinez-Sobrido, L.; Brown, L.; Ashour, J.; Garcia-Sastre, A.; Lee, S.W.; Aaronson, S.A. Transcriptional role of p53 in interferon-mediated antiviral immunity. J. Exp. Med. 2008, 205, 1929–1938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munoz-Fontela, C.; Mandinova, A.; Aaronson, S.A.; Lee, S.W. Emerging roles of p53 and other tumour-suppressor genes in immune regulation. Nat. Rev. Immunol. 2016, 16, 741–750. [Google Scholar] [CrossRef] [Green Version]
- Shatz, M.; Menendez, D.; Resnick, M.A. The human tlr innate immune gene family is differentially influenced by DNA stress and p53 status in cancer cells. Cancer Res. 2012, 72, 3948–3957. [Google Scholar] [CrossRef] [Green Version]
- Gudkov, A.V.; Gurova, K.V.; Komarova, E.A. Inflammation and p53: A tale of two stresses. Genes Cancer 2011, 2, 503–516. [Google Scholar] [CrossRef]
- Komarova, E.A.; Krivokrysenko, V.; Wang, K.; Neznanov, N.; Chernov, M.V.; Komarov, P.G.; Brennan, M.L.; Golovkina, T.V.; Rokhlin, O.W.; Kuprash, D.V.; et al. P53 is a suppressor of inflammatory response in mice. FASEB J. 2005, 19, 1030–1032. [Google Scholar] [CrossRef]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, W.; Wolchok, J.D.; Chen, L. Pd-l1 (b7-h1) and pd-1 pathway blockade for cancer therapy: Mechanisms, response biomarkers, and combinations. Sci. Transl. Med. 2016, 8, 328rv4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baumeister, S.H.; Freeman, G.J.; Dranoff, G.; Sharpe, A.H. Coinhibitory pathways in immunotherapy for cancer. Annu. Rev. Immunol. 2016, 34, 539–573. [Google Scholar] [CrossRef] [PubMed]
- Nittner, D.; Lambertz, I.; Clermont, F.; Mestdagh, P.; Kohler, C.; Nielsen, S.J.; Jochemsen, A.; Speleman, F.; Vandesompele, J.; Dyer, M.A.; et al. Synthetic lethality between rb, p53 and dicer or mir-17-92 in retinal progenitors suppresses retinoblastoma formation. Nat. Cell Biol. 2012, 14, 958–965. [Google Scholar] [CrossRef]
- Sachdeva, M.; Zhu, S.; Wu, F.; Wu, H.; Walia, V.; Kumar, S.; Elble, R.; Watabe, K.; Mo, Y.Y. P53 represses c-myc through induction of the tumor suppressor mir-145. Proc. Natl. Acad. Sci. USA 2009, 106, 3207–3212. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, H.I.; Yamagata, K.; Sugimoto, K.; Iwamoto, T.; Kato, S.; Miyazono, K. Modulation of microrna processing by p53. Nature 2009, 460, 529–533. [Google Scholar] [CrossRef]
- Cortez, M.A.; Ivan, C.; Valdecanas, D.; Wang, X.; Peltier, H.J.; Ye, Y.; Araujo, L.; Carbone, D.P.; Shilo, K.; Giri, D.K.; et al. Pdl1 regulation by p53 via mir-34. J. Natl. Cancer Inst. 2016, 108, djv303. [Google Scholar] [CrossRef] [Green Version]
- Thorsson, V.; Gibbs, D.L.; Brown, S.D.; Wolf, D.; Bortone, D.S.; Ou Yang, T.H.; Porta-Pardo, E.; Gao, G.F.; Plaisier, C.L.; Eddy, J.A.; et al. The immune landscape of cancer. Immunity 2018, 48, 812–830.e814. [Google Scholar] [CrossRef] [Green Version]
- Sallman, D.A.; McLemore, A.F.; Aldrich, A.L.; Komrokji, R.S.; McGraw, K.L.; Dhawan, A.; Geyer, S.; Hou, H.A.; Eksioglu, E.A.; Sullivan, A.; et al. Tp53 mutations in myelodysplastic syndromes and secondary aml confer an immunosuppressive phenotype. Blood 2020, 136, 2812–2823. [Google Scholar] [CrossRef]
- Sakuishi, K.; Jayaraman, P.; Behar, S.M.; Anderson, A.C.; Kuchroo, V.K. Emerging tim-3 functions in antimicrobial and tumor immunity. Trends Immunol. 2011, 32, 345–349. [Google Scholar] [CrossRef] [Green Version]
- Sakuishi, K.; Apetoh, L.; Sullivan, J.M.; Blazar, B.R.; Kuchroo, V.K.; Anderson, A.C. Targeting tim-3 and pd-1 pathways to reverse t cell exhaustion and restore anti-tumor immunity. J. Exp. Med. 2010, 207, 2187–2194. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.C.; Anderson, D.E.; Bregoli, L.; Hastings, W.D.; Kassam, N.; Lei, C.; Chandwaskar, R.; Karman, J.; Su, E.W.; Hirashima, M.; et al. Promotion of tissue inflammation by the immune receptor tim-3 expressed on innate immune cells. Science 2007, 318, 1141–1143. [Google Scholar] [CrossRef] [PubMed]
- Ndhlovu, L.C.; Lopez-Verges, S.; Barbour, J.D.; Jones, R.B.; Jha, A.R.; Long, B.R.; Schoeffler, E.C.; Fujita, T.; Nixon, D.F.; Lanier, L.L. Tim-3 marks human natural killer cell maturation and suppresses cell-mediated cytotoxicity. Blood 2012, 119, 3734–3743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asayama, T.; Tamura, H.; Ishibashi, M.; Kuribayashi-Hamada, Y.; Onodera-Kondo, A.; Okuyama, N.; Yamada, A.; Shimizu, M.; Moriya, K.; Takahashi, H.; et al. Functional expression of tim-3 on blasts and clinical impact of its ligand galectin-9 in myelodysplastic syndromes. Oncotarget 2017, 8, 88904–88917. [Google Scholar] [CrossRef] [Green Version]
- Kikushige, Y.; Miyamoto, T.; Yuda, J.; Jabbarzadeh-Tabrizi, S.; Shima, T.; Takayanagi, S.; Niiro, H.; Yurino, A.; Miyawaki, K.; Takenaka, K.; et al. A tim-3/gal-9 autocrine stimulatory loop drives self-renewal of human myeloid leukemia stem cells and leukemic progression. Cell Stem Cell 2015, 17, 341–352. [Google Scholar] [CrossRef] [Green Version]
- Benci, J.L.; Xu, B.; Qiu, Y.; Wu, T.J.; Dada, H.; Twyman-Saint Victor, C.; Cucolo, L.; Lee, D.S.M.; Pauken, K.E.; Huang, A.C.; et al. Tumor interferon signaling regulates a multigenic resistance program to immune checkpoint blockade. Cell 2016, 167, 1540–1554.e12. [Google Scholar] [CrossRef] [Green Version]
- Konopleva, M.Y.; Rollig, C.; Cavenagh, J.; Deeren, D.; Girshova, L.; Krauter, J.; Martinelli, G.; Montesinos, P. A randomized double-blind phase 3 trial of cytarabine with mdm2 inhibitor idasanutlin or placebo in relapsed/refractory acute myeloid leukemia (r/r aml): Primary analysis results of the mirros study. In Proceedings of the 25th Congress of the European Hematology Association 2020, Frankfurt, Germany, 12 June 2020. Abstract ep532. [Google Scholar]
- DiNardo, C.D.; Tiong, I.S.; Quaglieri, A.; MacRaild, S.; Loghavi, S.; Brown, F.C.; Thijssen, R.; Pomilio, G.; Ivey, A.; Salmon, J.M.; et al. Molecular patterns of response and treatment failure after frontline venetoclax combinations in older patients with aml. Blood 2020, 135, 791–803. [Google Scholar] [CrossRef]
- Cojocari, D.; Smith, B.N.; Purkal, J.J.; Arrate, M.P.; Huska, J.D.; Xiao, Y.; Gorska, A.; Hogdal, L.J.; Ramsey, H.E.; Boghaert, E.R.; et al. Pevonedistat and azacitidine upregulate noxa (pmaip1) to increase sensitivity to venetoclax in preclinical models of acute myeloid leukemia. Haematologica 2021, 107, 825–835. [Google Scholar] [CrossRef]
- Short, N.J.; Montalban-Bravo, G.; Alvarado, Y.; Konopleva, M.; Jabbour, E.J.; Garcia-Manero, G.; Yilmaz, M.; Jain, N.; Borthakur, G.; DiNardo, C.D.; et al. Azacitidine, venetoclax and pevonedistat as frontline therapy for patients with secondary acute myeloid leukemia who are unfit for intensive chemotherapy: Results from a phase i/ii study. Blood 2021, 138, 2349. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).