Proton Pump Inhibitors Enhance the Antitumor Effect of Chemotherapy for Esophageal Squamous Cell Carcinoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Western Blotting
2.3. Cell Viability Assay
2.4. Measurement of Intracellular pH
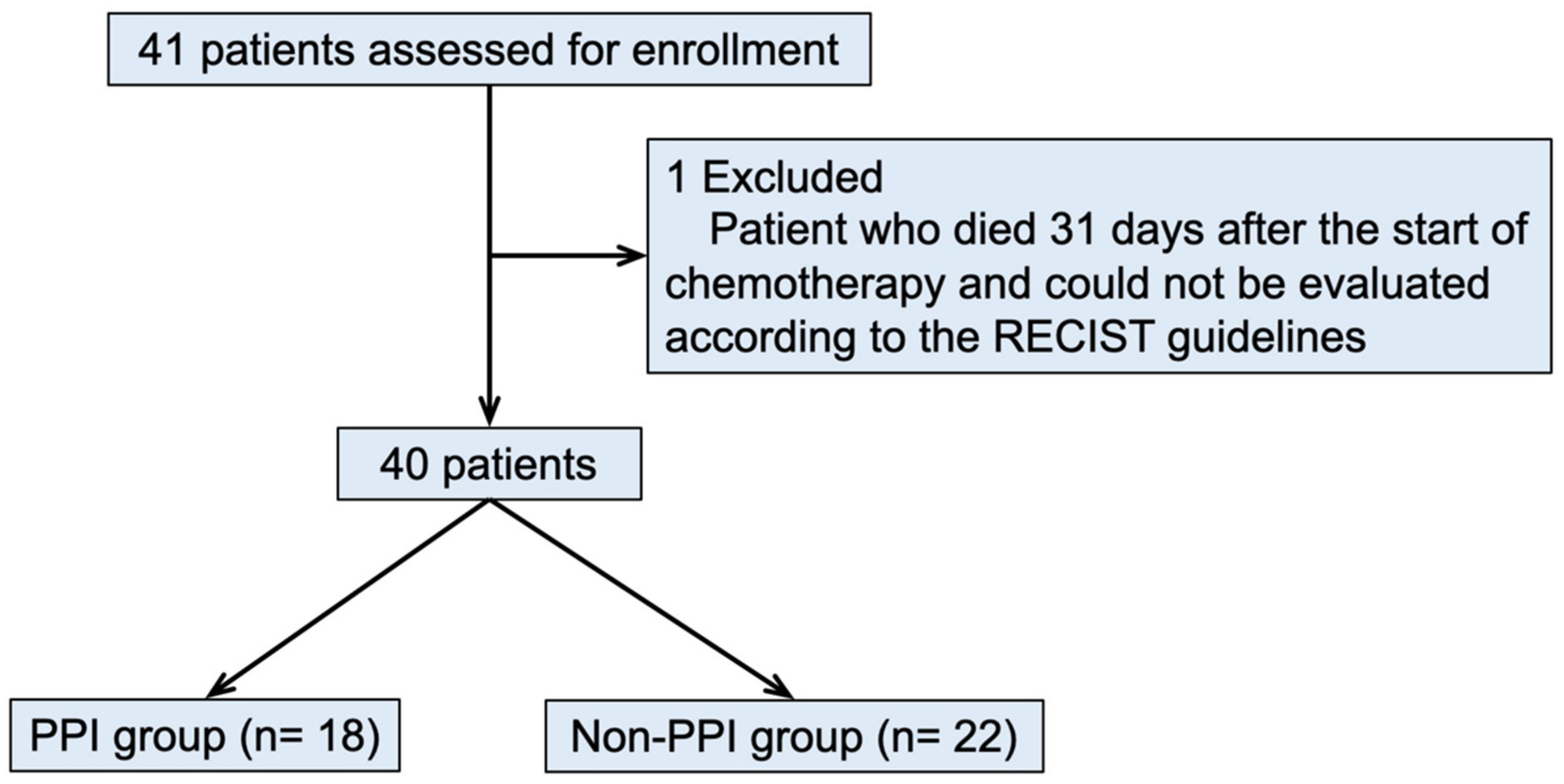
2.5. Clinical Research
2.6. Statistical Analysis
3. Results
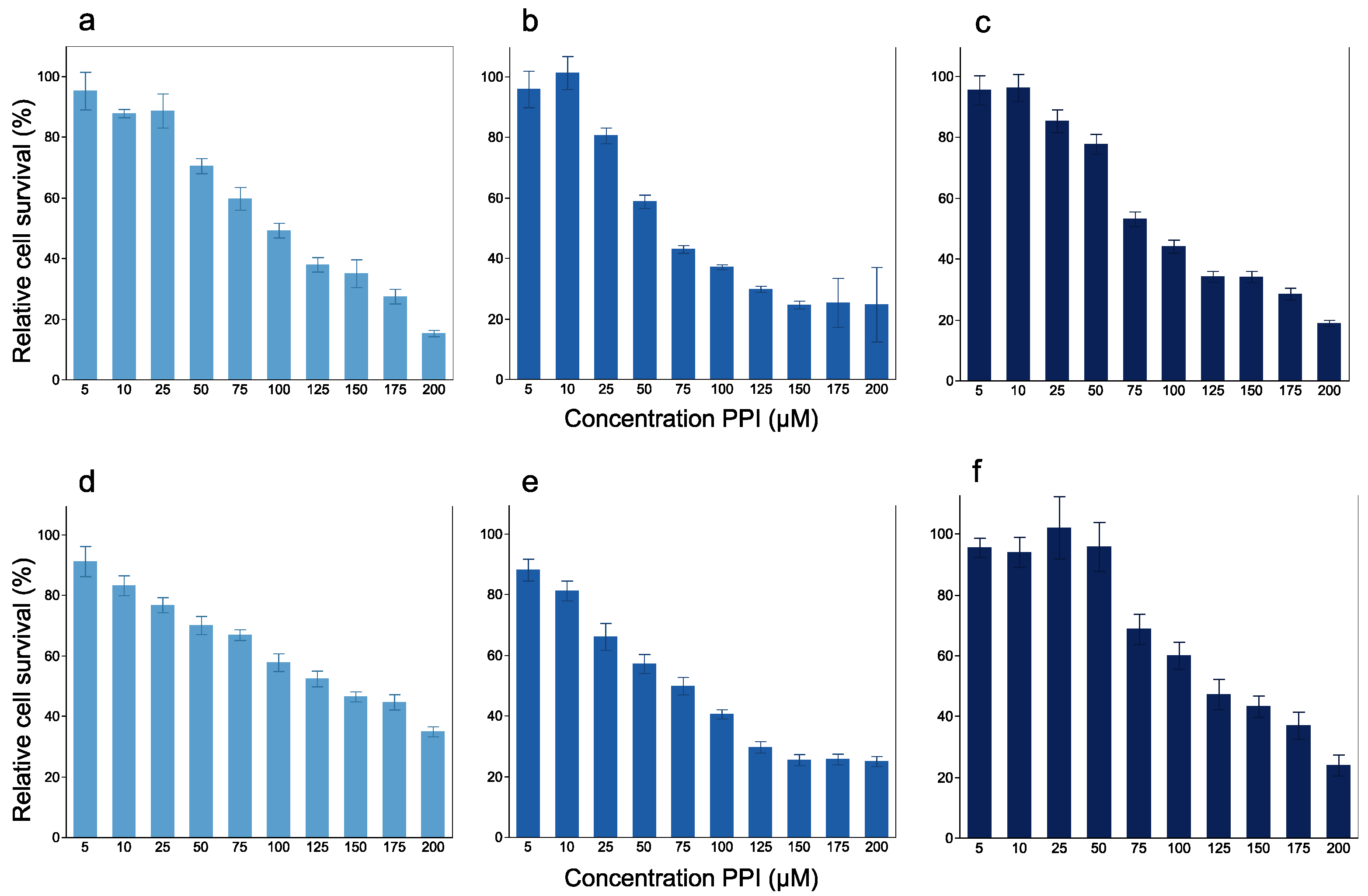
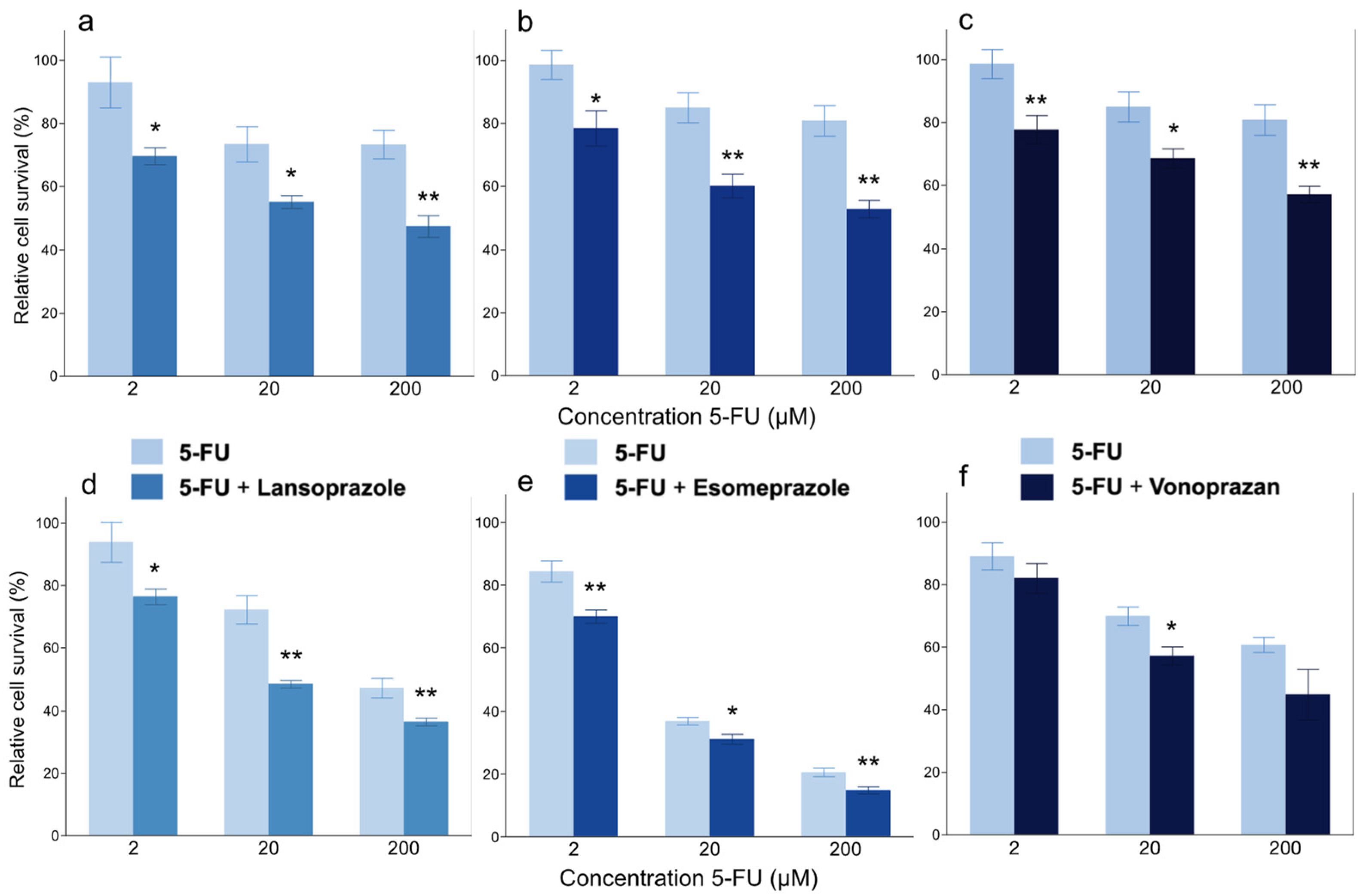
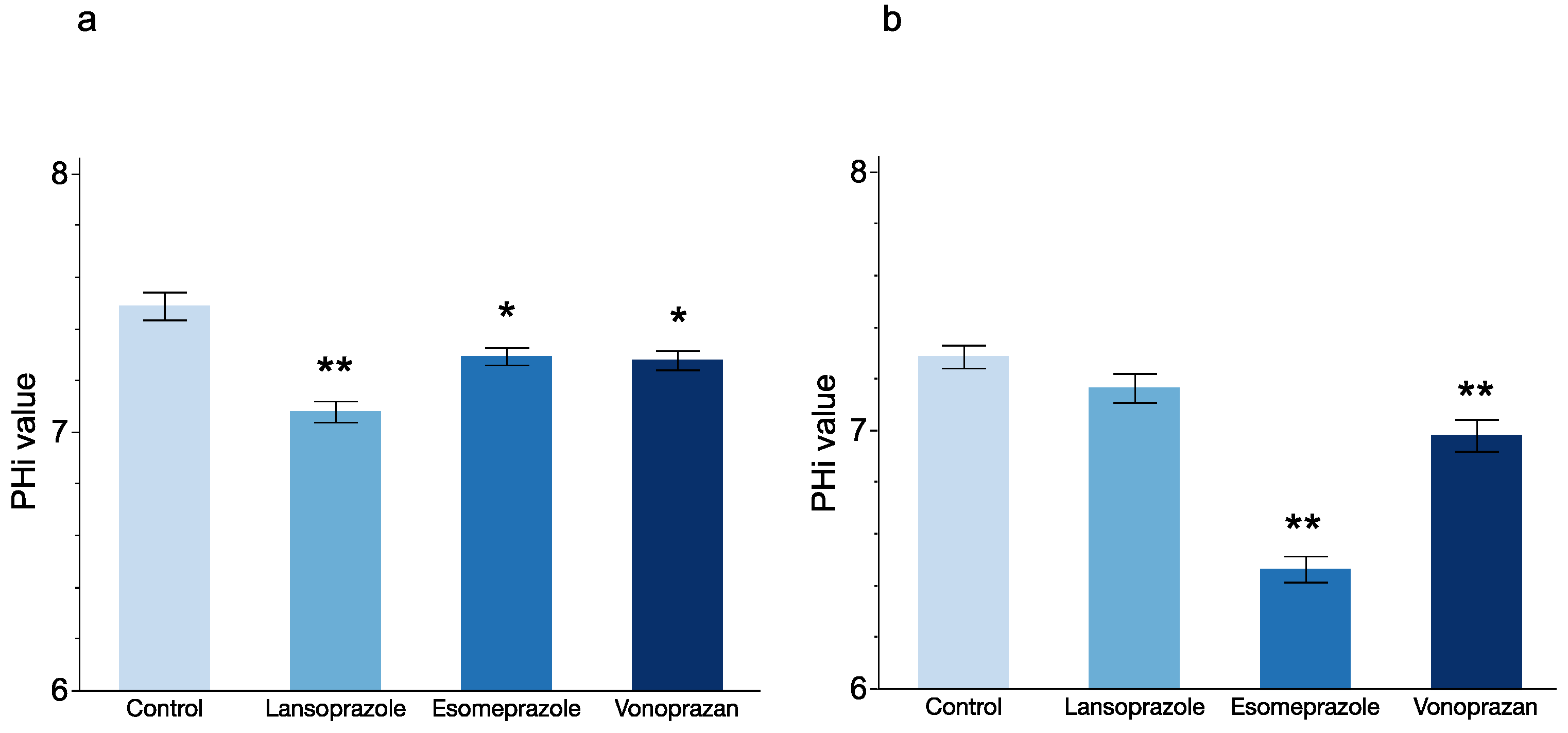
3.1. In Vitro Experiment
3.2. Clinical Research
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arnold, M.; Ferlay, J.; van Berge Henegouwen, M.I.; Soerjomataram, I. Global Burden of Oesophageal and Gastric Cancer by Histology and Subsite in 2018. Gut 2020, 69, 1564–1571. Available online: https://gut.bmj.com/content/gutjnl/69/9/1564.full.pdf (accessed on 30 April 2022). [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, 68, 394–424. Available online: https://acsjournals.onlinelibrary.wiley.com/doi/pdfdirect/10.3322/caac.21492?download=true (accessed on 10 November 2021). [CrossRef] [PubMed]
- Miyata, H.; Yamasaki, M.; Kurokawa, Y.; Takiguchi, S.; Nakajima, K.; Fujiwara, Y.; Konishi, K.; Mori, M.; Doki, Y. Survival Factors in Patients with Recurrence after Curative Resection of Esophageal Squamous Cell Carcinomas. Ann. Surg. Oncol. 2011, 18, 3353–3361. Available online: https://link.springer.com/article/10.1245/s10434-011-1747-7 (accessed on 1 May 2022). [CrossRef] [PubMed]
- Yoshida, T.; Miyoshi, T.; Seike, J.; Yamai, H.; Takechi, H.; Yuasa, Y.; Tangoku, A. Gene Expression Changes in a Chemoresistant Model with Human Esophageal Cancer Xenografts Using cDNA Microarray. Anticancer Res. 2009, 29, 1163–1168. Available online: https://ar.iiarjournals.org/content/anticanres/29/4/1163.full.pdf (accessed on 24 May 2021). [PubMed]
- Gatenby, R.A.; Gillies, R.J. Why Do Cancers Have High Aerobic Glycolysis? Nat. Rev. Cancer 2004, 4, 891–899. Available online: https://www.nature.com/articles/nrc1478 (accessed on 21 May 2021). [CrossRef] [PubMed]
- Spugnini, E.P.; Baldi, A.; Buglioni, S.; Carocci, F.; de Bazzichini, G.M.; Betti, G.; Pantaleo, I.; Menicagli, F.; Citro, G.; Fais, S. Lansoprazole as a Rescue Agent in Chemoresistant Tumors: A Phase I/II Study in Companion Animals with Spontaneously Occurring Tumors. J. Transl. Med. 2011, 9, 221. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3264547/pdf/1479-5876-9-221.pdf (accessed on 20 May 2021). [CrossRef] [PubMed]
- Cipriano, D.J.; Wang, Y.; Bond, S.; Hinton, A.; Jefferies, K.C.; Qi, J.; Forgac, M. Structure and Regulation of the Vacuolar ATPases. Biochim. Biophys. Acta 2008, 1777, 599–604. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2467516/pdf/nihms57639.pdf (accessed on 12 November 2021). [CrossRef]
- Trédan, O.; Galmarini, C.M.; Patel, K.; Tannock, I.F. Drug resistance and the solid tumor microenvironment. J. Natl. Cancer Inst. 2007, 99, 1441–1454. [Google Scholar] [CrossRef]
- Huang, L.; Lu, Q.; Han, Y.; Li, Z.; Zhang, Z.; Li, X. ABCG2/V-ATPase was Associated with the Drug Resistance and Tumor Metastasis of Esophageal Squamous Cancer Cells. Diagn. Pathol. 2012, 7, 180. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3542252/pdf/1746-1596-7-180.pdf (accessed on 23 May 2021). [CrossRef]
- Raghunand, N.; He, X.; van Sluis, R.; Mahoney, B.; Baggett, B.; Taylor, C.W.; Paine-Murrieta, G.; Roe, D.; Bhujwalla, Z.M.; Gillies, R.J. Enhancement of Chemotherapy by Manipulation of Tumour pH. Br. J. Cancer 1999, 80, 1005–1011. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2363059/pdf/80-6690455a.pdf (accessed on 23 May 2021). [CrossRef]
- Eaton, A.F.; Merkulova, M.; Brown, D. The H(+)-ATPase (V-ATPase): From proton pump to signaling complex in health and disease. Am. J. Physiol. Cell Physiol. 2021, 320, C392–C414. [Google Scholar] [CrossRef] [PubMed]
- Kolosenko, I.; Avnet, S.; Baldini, N.; Viklund, J.; De Milito, A. Therapeutic implications of tumor interstitial acidification. Semin. Cancer Biol. 2017, 43, 119–133. [Google Scholar] [CrossRef]
- You, H.; Jin, J.; Shu, H.; Yu, B.; De Milito, A.; Lozupone, F.; Deng, Y.; Tang, N.; Yao, G.; Fais, S.; et al. Small interfering RNA targeting the subunit ATP6L of proton pump V-ATPase overcomes chemoresistance of breast cancer cells. Cancer Lett. 2009, 280, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Wallmark, B.; Larsson, H.; Humble, L. The relationship between gastric acid secretion and gastric H+, K+-ATPase activity. J. Biol. Chem. 1985, 260, 13681–13684. [Google Scholar] [CrossRef]
- Puscas, I.; Coltau, M.; Baican, M.; Domuta, G. Omeprazole Has a Dual Mechanism of Action: It Inhibits Both H(+)K(+)ATPase and Gastric Mucosa Carbonic Anhydrase Enzyme in Humans (In Vitro and In Vivo Experiments). J. Pharmacol. Exp. Ther. 1999, 290, 530–534. Available online: https://jpet.aspetjournals.org/content/290/2/530.long (accessed on 23 May 2021).
- Horn, J. The Proton-Pump Inhibitors: Similarities and Differences. Clin. Ther. 2000, 22, 266–280. Available online: https://www.clinicaltherapeutics.com/article/S0149-2918(00)80032-6/pdf (accessed on 23 May 2021). [CrossRef]
- Mizunashi, K.; Furukawa, Y.; Katano, K.; Abe, K. Effect of Omeprazole, an Inhibitor of H+,K(+)-ATPase, on Bone Resorption in Humans. Calcif. Tissue Int. 1993, 53, 21–25. Available online: https://link.springer.com/article/10.1007/BF01352010 (accessed on 23 May 2021). [CrossRef]
- Graber, M.L.; Devine, P. Omeprazole and SCH 28080 Inhibit Acid Secretion by the Turtle Urinary Bladder. Ren. Physiol. Biochem. 1993, 16, 257–267. Available online: https://www.karger.com/Article/Abstract/173771 (accessed on 23 May 2021). [CrossRef]
- Sabolić, I.; Brown, D.; Verbavatz, J.M.; Kleinman, J. H(+)-ATPases of Renal Cortical and Medullary Endosomes are Differentially Sensitive to Sch-28080 and Omeprazole. Am. J. Physiol. 1994, 266, F868–F877. Available online: https://journals.physiology.org/doi/abs/10.1152/ajprenal.1994.266.6.F868 (accessed on 23 May 2021). [CrossRef]
- Lu, Z.N.; Shi, Z.Y.; Dang, Y.F.; Cheng, Y.N.; Guan, Y.H.; Hao, Z.J.; Tian, B.; He, H.W.; Guo, X.L. Pantoprazole pretreatment elevates sensitivity to vincristine in drug-resistant oral epidermoid carcinoma in vitro and in vivo. Biomed. Pharmacother. 2019, 120, 109478. [Google Scholar] [CrossRef]
- Avnet, S.; Lemma, S.; Cortini, M.; Pellegrini, P.; Perut, F.; Zini, N.; Kusuzaki, K.; Chano, T.; Grisendi, G.; Dominici, M.; et al. Altered pH Gradient at the Plasma Membrane of Osteosarcoma Cells is a Key Mechanism of Drug Resistance. Oncotarget 2016, 7, 63408–63423. Available online: https://www.oncotarget.com/article/11503/pdf/ (accessed on 1 May 2022). [CrossRef] [PubMed]
- Lee, Y.Y.; Jeon, H.K.; Hong, J.E.; Cho, Y.J.; Ryu, J.Y.; Choi, J.J.; Lee, S.H.; Yoon, G.; Kim, W.Y.; Do, I.G.; et al. Proton pump Inhibitors Enhance the Effects of Cytotoxic Agents in Chemoresistant Epithelial Ovarian Carcinoma. Oncotarget 2015, 6, 35040–35050. Available online: https://www.oncotarget.com/article/5319/pdf/ (accessed on 29 December 2020). [CrossRef] [PubMed]
- Chou, T.C. Drug combination studies and their synergy quantification using the chou-talalay method. Cancer Res. 2020, 70, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Rice, T.W.; Patil, D.T.; Blackstone, E.H. 8th Edition AJCC/UICC Staging of Cancers of the Esophagus and Esophagogastric Junction: Application to Clinical Practice. Ann. Cardiothorac. Surg. 2017, 6, 119–130. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5387145/pdf/acs-06-02-119.pdf (accessed on 30 April 2022). [CrossRef] [PubMed]
- De Milito, A.; Fais, S. Tumor Acidity, Chemoresistance and proton Pump Inhibitors. Future Oncol. 2005, 1, 779–786. Available online: https://www.futuremedicine.com/doi/10.2217/14796694.1.6.779?url_ver=Z39.88-2003&rfr_id=ori%3Arid%3Acrossref.org&rfr_dat=cr_pub%3Dpubmed (accessed on 7 November 2021). [CrossRef]
- Hashim, A.I.; Zhang, X.; Wojtkowiak, J.W.; Martinez, G.V.; Gillies, R.J. Imaging pH and Metastasis. NMR Biomed. 2011, 24, 582–591. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3740268/pdf/nihms-385819.pdf (accessed on 12 November 2021). [CrossRef]
- Webb, B.A.; Chimenti, M.; Jacobson, M.P.; Barber, D.L. Dysregulated pH: A Perfect Storm for Cancer Progression. Nat. Rev. Cancer 2011, 11, 671–677. Available online: https://www.nature.com/articles/nrc3110 (accessed on 11 November 2021). [CrossRef]
- Hindenburg, A.A.; Gervasoni, J.E., Jr.; Krishna, S.; Stewart, V.J.; Rosado, M.; Lutzky, J.; Bhalla, K.; Baker, M.A.; Taub, R.N. Intracellular Distribution and Pharmacokinetics of Daunorubicin in Anthracycline-Sensitive and -Resistant HL-60 cells. Cancer Res. 1989, 49, 4607–4614. Available online: https://cancerres.aacrjournals.org/content/canres/49/16/4607.full.pdf (accessed on 2 November 2021).
- Vukovic, V.; Tannock, I.F. Influence of Low pH on Cytotoxicity of Paclitaxel, Mitoxantrone and Topotecan. Br. J. Cancer 1997, 75, 1167–1172. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2222779/pdf/brjcancer00185-0081.pdf (accessed on 2 November 2021). [CrossRef]
- Bour-Dill, C.; Gramain, M.P.; Merlin, J.L.; Marchal, S.; Guillemin, F. Determination of Intracellular Organelles Implicated in Daunorubicin Cytoplasmic Sequestration in Multidrug-Resistant MCF-7 Cells Using Fluorescence Microscopy Image Analysis. Cytometry 2000, 39, 16–25. Available online: https://onlinelibrary.wiley.com/doi/pdfdirect/10.1002/%28SICI%291097-0320%2820000101%2939%3A1%3C16%3A%3AAID-CYTO4%3E3.0.CO%3B2-I?download=true (accessed on 30 April 2022). [CrossRef]
- Liao, C.; Hu, B.; Arno, M.J.; Panaretou, B. Genomic Screening In Vivo Reveals the Role Played by Vacuolar H+ ATPase and Cytosolic Acidification in Sensitivity to DNA-Damaging Agents such as Cisplatin. Mol. Pharmacol. 2007, 71, 416–425. Available online: https://molpharm.aspetjournals.org/content/71/2/416.long (accessed on 2 November 2021). [CrossRef] [PubMed]
- Ishisaki, A.; Hashimoto, S.; Amagasa, T.; Nishihara, T. Caspase-3 Activation during the Process of Apoptosis Induced by a Vacuolar Type H(+)-ATPase inhibitor. Biol. Cell 1999, 91, 507–513. Available online: https://www.sciencedirect.com/science/article/abs/pii/S0248490000882076?via%3Dihub (accessed on 7 November 2021). [CrossRef]
- Wang, B.Y.; Zhang, J.; Wang, J.L.; Sun, S.; Wang, Z.H.; Wang, L.P.; Zhang, Q.L.; Lv, F.F.; Cao, E.Y.; Shao, Z.M.; et al. Intermittent High Dose Proton Pump Inhibitor Enhances the Antitumor Effects of Chemotherapy in Metastatic Breast Cancer. J. Exp. Clin. Cancer Res. 2015, 34, 85. Available online: https://jeccr.biomedcentral.com/track/pdf/10.1186/s13046-015-0194-x.pdf (accessed on 6 November 2021). [CrossRef] [PubMed]
- Wang, X.; Liu, C.; Wang, J.; Fan, Y.; Wang, Z.; Wang, Y. Proton Pump Inhibitors Increase the Chemosensitivity of Patients with Advanced Colorectal Cancer. Oncotarget 2017, 8, 58801–58808. Available online: https://www.oncotarget.com/article/18522/pdf/ (accessed on 28 December 2020). [CrossRef][Green Version]
- Papagerakis, S.; Bellile, E.; Peterson, L.A.; Pliakas, M.; Balaskas, K.; Selman, S.; Hanauer, D.; Taylor, J.M.; Duffy, S.; Wolf, G. Proton Pump Inhibitors and Histamine 2 Blockers Are Associated with Improved Overall Survival in Patients with Head and Neck Squamous Carcinoma. Cancer Prev. Res. 2014, 7, 1258–1269. Available online: https://cancerpreventionresearch.aacrjournals.org/content/canprevres/7/12/1258.full.pdf (accessed on 4 January 2021). [CrossRef]
- Miyazaki, H.; Igarashi, A.; Takeuchi, T.; Teng, L.; Uda, A.; Deguchi, H.; Higuchi, K.; Tango, T. Vonoprazan Versus Proton-Pump Inhibitors for Healing Gastroesophageal Reflux Disease: A Systematic Review. J. Gastroenterol. Hepatol. 2019, 34, 1316–1328. Available online: https://onlinelibrary.wiley.com/doi/10.1111/jgh.14664 (accessed on 10 November 2021). [CrossRef]
- Murakami, K.; Sakurai, Y.; Shiino, M.; Funao, N.; Nishimura, A.; Asaka, M. Vonoprazan, a Novel Potassium-Competitive acid Blocker, as a Component of First-Line and Second-Line Triple Therapy for Helicobacter Pylori Eradication: A Phase III, Randomised, Double-Blind Study. Gut 2016, 65, 1439–1446. Available online: https://gut.bmj.com/content/gutjnl/65/9/1439.full.pdf (accessed on 10 November 2021). [CrossRef]


| Characteristics | PPI (n = 18) | Non-PPI (n = 22) | p Value | ||
|---|---|---|---|---|---|
| Age, mean (SD), years | 67.9 (7.83) | 65.6 (7.90) | 0.364 | ||
| Sex, n | |||||
| Male | 16 | 17 | 0.328 | ||
| Female | 2 | 5 | |||
| PS, n | |||||
| 0 | 6 | 11 | 0.264 | ||
| 1 | 12 | 10 | |||
| 2 | 0 | 1 | |||
| BMI, mean (SD), kg/m2 | 21.2 (3.90) | 19.4 (3.12) | 0.112 | ||
| Location, n | |||||
| U | 2 | 4 | 0.490 | ||
| M | 7 | 11 | |||
| L | 9 | 7 | |||
| Disease status, n | |||||
| Metastatic | 17 | 18 | 0.212 | ||
| Recurrent | 1 | 4 | |||
| Prognostic factors | |||||
| CCI, n | |||||
| 0 | 9 | 14 | 0.596 | ||
| 1–2 | 7 | 7 | |||
| ≥3 | 2 | 1 | |||
| GPS, n | |||||
| 0 | 9 | 11 | 0.548 | ||
| 1 | 7 | 6 | |||
| 2 | 2 | 5 | |||
| PNI, mean (SD) | 44.8 (4.23) | 43.6 (7.53) | 0.529 | ||
| NLR, mean (SD) | 3.6 (2.32) | 5.2 (7.84) | 0.409 | ||
| Regimen (1st course), n | |||||
| FP | 11 | 16 | 0.500 | ||
| DCF | 5 | 4 | |||
| Nedaplatin FU | 1 | 2 | |||
| FOLFOX | 1 | 0 | |||
| Radiation therapy, n | |||||
| Yes | 13 | 11 | 0.150 | ||
| No | 5 | 11 | |||
| PPI subtypes, daily dose, n | |||||
| Lansoprazole | - | - | |||
| 15 mg | 2 | ||||
| 30 mg | 6 | ||||
| Rabeprazole | |||||
| 10 mg | 3 | ||||
| Esomeprazole | |||||
| 20 mg | 4 | ||||
| Vonoprazan | |||||
| 10 mg | 1 | ||||
| 20 mg | 2 | ||||
| Response to Treatment | PPI (n = 18) | Non-PPI (n = 22) | Treatment Difference (95% CI) | p Value |
|---|---|---|---|---|
| CR, n (%) | 0 | 1 (4.5) | 0.269 | |
| PR, n (%) | 12 (66.7) | 8 (36.4) | ||
| SD, n (%) | 5 (27.8) | 8 (36.4) | ||
| PD, n (%) | 1 (5.6) | 5 (22.7) | ||
| Response rate, n (%) | 12 (66.7) | 9 (40.9) | 0.35 (0.09–1.27) | 0.102 |
| Disease control rate, n (%) | 17 (94.4) | 17 (77.3) | 0.2 (0.02–1.90) | 0.113 |
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p Value | HR | 95% CI | p Value | |
| Age | ||||||
| <75 years | 1 | |||||
| ≥75 years | 0.70 | 0.20–1.84 | 0.496 | |||
| Sex | ||||||
| Female | 1 | |||||
| Male | 2.05 | 0.78–7.07 | 0.161 | |||
| PS | ||||||
| =0 | 1 | 1 | ||||
| ≥1 | 1.20 | 0.56–2.59 | 0.642 | 1.32 | 0.53–3.30 | 0.549 |
| Regimen (1st course) | ||||||
| DCF | 1 | |||||
| Other | 0.91 | 0.42–2.15 | 0.825 | |||
| CCI | ||||||
| 0 | 1 | |||||
| ≥1 | 1.38 | 0.65–2.95 | 0.408 | |||
| GPS | ||||||
| =0–1 | 1 | 1 | ||||
| =2 | 2.32 | 0.76–5.90 | 0.130 | 2.59 | 0.68–8.88 | 0.154 |
| PNI | ||||||
| ≥45 | 1 | |||||
| <45 | 1.18 | 0.55–2.59 | 0.670 | |||
| NLR | ||||||
| <5 | 1 | 1 | ||||
| ≥5 | 1.93 | 0.74–4.50 | 0.169 | 1.88 | 0.64–5.08 | 0.236 |
| PPI | ||||||
| No | 1 | 1 | ||||
| Yes | 0.41 | 0.17–0.92 | 0.029 | 0.35 | 0.13–0.80 | 0.012 |
| PPI (n = 18) | Non-PPI (n = 22) | Odds Ratio (95% CI) | p Value | |
|---|---|---|---|---|
| Myelosuppression, n | 7 | 9 | 0.92 (0.26–3.28) | 0.897 |
| Gastrointestinal toxicity, n | 1 | 1 | 1.24 (0.07–21.2) | 0.884 |
| Heart failure, n | 1 | 0 | - | 0.202 |
| Renal toxicity, n | 1 | 2 | 0.59 (0.05–7.07) | 0.669 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsumura, S.; Ishikawa, T.; Yoshida, J.; Morita, R.; Sakakida, T.; Endo, Y.; Doi, T.; Hirose, R.; Inoue, K.; Dohi, O.; et al. Proton Pump Inhibitors Enhance the Antitumor Effect of Chemotherapy for Esophageal Squamous Cell Carcinoma. Cancers 2022, 14, 2395. https://doi.org/10.3390/cancers14102395
Matsumura S, Ishikawa T, Yoshida J, Morita R, Sakakida T, Endo Y, Doi T, Hirose R, Inoue K, Dohi O, et al. Proton Pump Inhibitors Enhance the Antitumor Effect of Chemotherapy for Esophageal Squamous Cell Carcinoma. Cancers. 2022; 14(10):2395. https://doi.org/10.3390/cancers14102395
Chicago/Turabian StyleMatsumura, Shinya, Takeshi Ishikawa, Juichiro Yoshida, Ryuichi Morita, Tomoki Sakakida, Yuki Endo, Toshifumi Doi, Ryohei Hirose, Ken Inoue, Osamu Dohi, and et al. 2022. "Proton Pump Inhibitors Enhance the Antitumor Effect of Chemotherapy for Esophageal Squamous Cell Carcinoma" Cancers 14, no. 10: 2395. https://doi.org/10.3390/cancers14102395
APA StyleMatsumura, S., Ishikawa, T., Yoshida, J., Morita, R., Sakakida, T., Endo, Y., Doi, T., Hirose, R., Inoue, K., Dohi, O., Yoshida, N., Uchiyama, K., Takagi, T., Konishi, H., Yasui, K., Naito, Y., & Itoh, Y. (2022). Proton Pump Inhibitors Enhance the Antitumor Effect of Chemotherapy for Esophageal Squamous Cell Carcinoma. Cancers, 14(10), 2395. https://doi.org/10.3390/cancers14102395









