Whole-Exome Sequencing of HPV Positive Tonsillar and Base of Tongue Squamous Cell Carcinomas Reveals a Global Mutational Pattern along with Relapse-Specific Somatic Variants
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients, Samples and Definition of HPV-Positive Status
2.2. Laser Microdissection and DNA Extraction
2.3. Library Preparation and Whole-Exome Sequencing
2.4. Alignment, Variant Calling and Filtering
2.5. Statistics and Data Analysis
3. Results
3.1. Dataset Summary
3.2. Per-Variant Analyses
| Patient Cohort | Patients with Recurrence (%) 1 | Patients without Recurrence (%) 1 | Total (%) | p-Values | |
|---|---|---|---|---|---|
| Number of patients | 17 | 18 | 35 | p-values | |
| Age | 63 | 63 | 63 | p = 0.66 | |
| Sex | Male | 14 (82%) | 13 (72%) | 27 (77%) | p = 0.7 |
| Female | 3 (18%) | 5 (28%) | 8 (23%) | ||
| Site | Tonsil Base of tongue | 14 (82%) 3 (18%) | 16 (89%) 2 (11%) | 30 (86%) 5 (14%) | p = 0.66 |
| T | T1 | 1 (6%) | 3 (17%) | 4 (11%) | T1 + T2 vs. |
| T2 | 7 (41%) | 9 (50%) | 16 (46%) | T3 + T4 | |
| T3 | 4 (24%) | 5 (27%) | 9 (26%) | p = 0.3 | |
| T4 | 5 (29%) | 1 (6%) | 6 (17%) | ||
| N | N0 | 1 (6%) | 3 (17%) | 4 (11%) | N0 + N1 vs. |
| N1 | 1 (6%) | 3 (17%) | 4 (11%) | N2 | |
| N2 | 15 (88%) | 12 (67%) | 27 (77%) | p = 0.23 | |
| M | M0 | 17 (100%) | 18 (100%) | 35 (100%) | p = 1 |
| M1 | 0 (0%) | 0 (0%) | 0 (0%) | ||
| TNM Stage | I | 0 (3%) | 0 (6%) | 0 (4%) | TNMI + II vs. |
| (AJCC 7th Edition) | II III | 0 (7%) 2 (25%) | 2 (6%) 3 (16%) | 2 (8%) 5 (24%) | TNMIII + IV p = 0.49 |
| IV | 15 (59%) | 13 (65%) | 28 (58%) | ||
| TNM Stage (AJCC 8th Edition) | I II III IV | 7 (41%) 5 (29%) 5 (29%) 0 (0%) | 12 (67%) 5 (28%) 1 (6%) 0 (0%) | 19 (54%) 10 (29%) 6 (17%) 0 (0%) | TNMI + II vs. TNMIII + IV p = 0.08 |
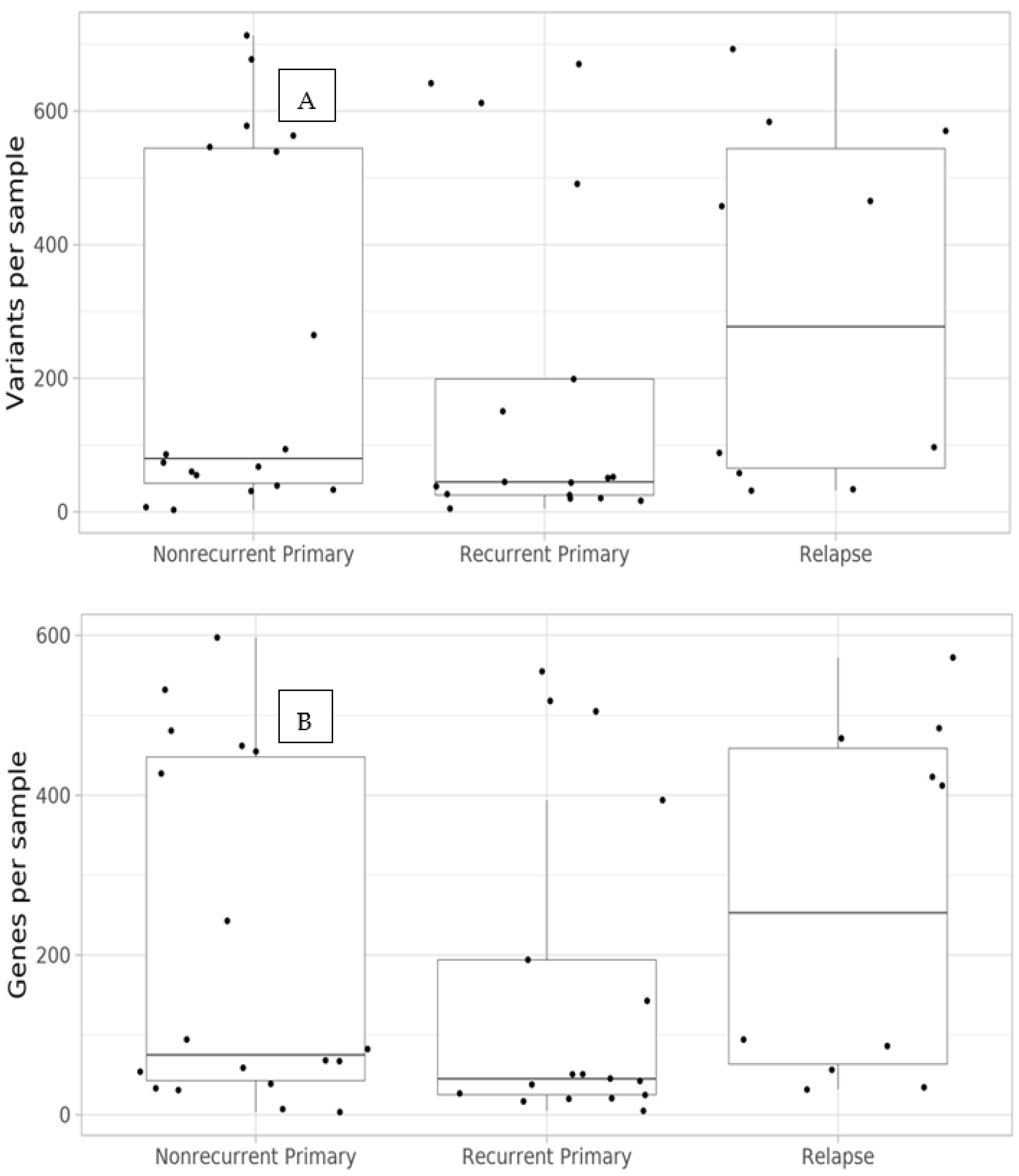
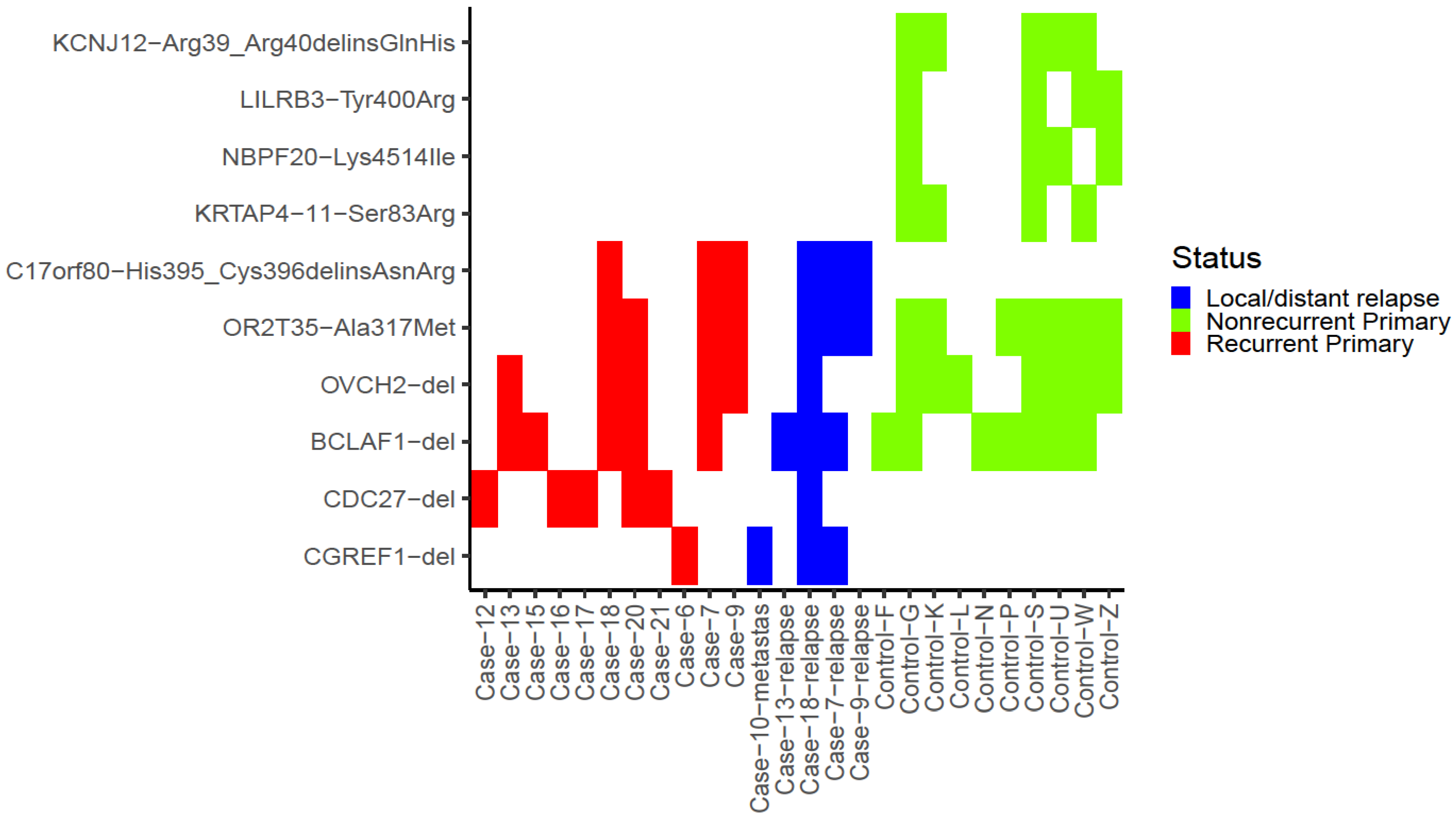
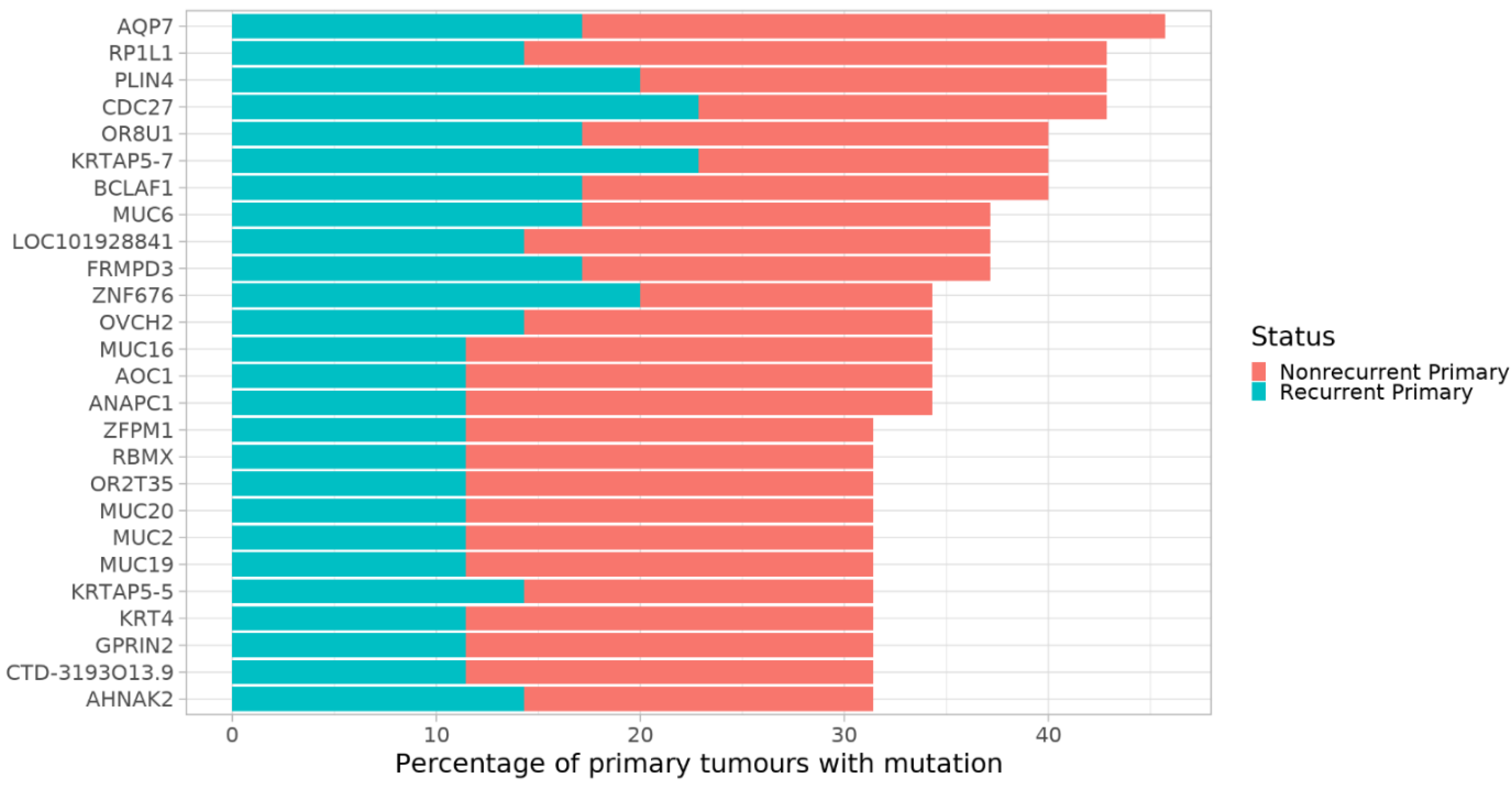
3.3. Per-Gene Analysis
3.4. Mutations in Hotspot Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dahlgren, L.; Dahlstrand, H.M.; Lindquist, D.; Hogmo, A.; Bjornestal, L.; Lindholm, J.; Lundberg, B.; Dalianis, T.; Munck-Wikland, E. Human papillomavirus is more common in base of tongue than in mobile tongue cancer and is a favorable prognostic factor in base of tongue cancer patients. Int. J. Cancer 2004, 112, 1015–1019. [Google Scholar] [CrossRef]
- Gillison, M.L.; Koch, W.M.; Capone, R.B.; Spafford, M.; Westra, W.H.; Wu, L.; Zahurak, M.L.; Daniel, R.W.; Viglione, M.; Symer, D.E.; et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J. Natl. Cancer Inst. 2000, 92, 709–720. [Google Scholar] [CrossRef] [PubMed]
- Mellin, H.; Friesland, S.; Lewensohn, R.; Dalianis, T.; Munck-Wikland, E. Human papillomavirus (HPV) DNA in tonsillar cancer: Clinical correlates, risk of relapse, and survival. Int. J. Cancer 2000, 89, 300–304. [Google Scholar] [CrossRef]
- Nygard, M.; Aagnes, B.; Bray, F.; Moller, B.; Mork, J. Population-based evidence of increased survival in human papillomavirus-related head and neck cancer. Eur. J. Cancer 2012, 48, 1341–1346. [Google Scholar] [CrossRef]
- Blomberg, M.; Nielsen, A.; Munk, C.; Kjaer, S.K. Trends in head and neck cancer incidence in Denmark, 1978–2007: Focus on human papillomavirus associated sites. Int. J. Cancer 2011, 129, 733–741. [Google Scholar] [CrossRef]
- Chaturvedi, A.K.; Engels, E.A.; Pfeiffer, R.M.; Hernandez, B.Y.; Xiao, W.; Kim, E.; Jiang, B.; Goodman, M.T.; Sibug-Saber, M.; Cozen, W.; et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J. Clin. Oncol. 2011, 29, 4294–4301. [Google Scholar] [CrossRef] [PubMed]
- Haeggblom, L.; Attoff, T.; Yu, J.; Holzhauser, S.; Vlastos, A.; Mirzae, L.; Ahrlund-Richter, A.; Munck-Wikland, E.; Marklund, L.; Hammarstedt-Nordenvall, L.; et al. Changes in incidence and prevalence of human papillomavirus in tonsillar and base of tongue cancer during 2000-2016 in the Stockholm region and Sweden. Head Neck 2019, 41, 1583–1590. [Google Scholar] [CrossRef] [PubMed]
- Hocking, J.S.; Stein, A.; Conway, E.L.; Regan, D.; Grulich, A.; Law, M.; Brotherton, J.M. Head and neck cancer in Australia between 1982 and 2005 show increasing incidence of potentially HPV-associated oropharyngeal cancers. Br. J. Cancer 2011, 104, 886–891. [Google Scholar] [CrossRef][Green Version]
- Marur, S.; D’Souza, G.; Westra, W.H.; Forastiere, A.A. HPV-associated head and neck cancer: A virus-related cancer epidemic. Lancet Oncol. 2010, 11, 781–789. [Google Scholar] [CrossRef]
- Nasman, A.; Attner, P.; Hammarstedt, L.; Du, J.; Eriksson, M.; Giraud, G.; Ahrlund-Richter, S.; Marklund, L.; Romanitan, M.; Lindquist, D.; et al. Incidence of human papillomavirus (HPV) positive tonsillar carcinoma in Stockholm, Sweden: An epidemic of viral-induced carcinoma? Int. J. Cancer 2009, 125, 362–366. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.M.; Cundall-Curry, D.; Bridger, M.W. Trends in the incidence rates of tonsil and base of tongue cancer in England, 1985–2006. Ann. R. Coll. Surg. Engl. 2010, 92, 655–659. [Google Scholar] [CrossRef]
- Tota, J.E.; Best, A.F.; Zumsteg, Z.S.; Gillison, M.L.; Rosenberg, P.S.; Chaturvedi, A.K. Evolution of the Oropharynx Cancer Epidemic in the United States: Moderation of Increasing Incidence in Younger Individuals and Shift in the Burden to Older Individuals. J. Clin. Oncol. 2019, 37, 1538–1546. [Google Scholar] [CrossRef]
- Oropharyngeal Cancer Treatment (Adult) (PDQ®)—Health Professional Version. Available online: https://www.cancer.gov/types/head-and-neck/hp/adult/oropharyngeal-treatment-pdq (accessed on 7 April 2021).
- Nasman, A.; Holzhauser, S.; Kostopoulou, O.N.; Zupancic, M.; Ahrlund-Richter, A.; Du, J.; Dalianis, T. Prognostic Markers and Driver Genes and Options for Targeted Therapy in Human-Papillomavirus-Positive Tonsillar and Base-of-Tongue Squamous Cell Carcinoma. Viruses 2021, 13, 910. [Google Scholar] [CrossRef]
- Beaty, B.T.; Moon, D.H.; Shen, C.J.; Amdur, R.J.; Weiss, J.; Grilley-Olson, J.; Patel, S.; Zanation, A.; Hackman, T.G.; Thorp, B.; et al. PIK3CA Mutation in HPV-Associated OPSCC Patients Receiving Deintensified Chemoradiation. J. Natl. Cancer Inst. 2020, 112, 855–858. [Google Scholar] [CrossRef]
- Bersani, C.; Mints, M.; Tertipis, N.; Haeggblom, L.; Nasman, A.; Romanitan, M.; Dalianis, T.; Ramqvist, T. MicroRNA-155, -185 and -193b as biomarkers in human papillomavirus positive and negative tonsillar and base of tongue squamous cell carcinoma. Oral Oncol. 2018, 82, 8–16. [Google Scholar] [CrossRef]
- Bersani, C.; Mints, M.; Tertipis, N.; Haeggblom, L.; Sivars, L.; Ahrlund-Richter, A.; Vlastos, A.; Smedberg, C.; Grun, N.; Munck-Wikland, E.; et al. A model using concomitant markers for predicting outcome in human papillomavirus positive oropharyngeal cancer. Oral Oncol. 2017, 68, 53–59. [Google Scholar] [CrossRef]
- Bersani, C.; Sivars, L.; Haeggblom, L.; DiLorenzo, S.; Mints, M.; Ahrlund-Richter, A.; Tertipis, N.; Munck-Wikland, E.; Nasman, A.; Ramqvist, T.; et al. Targeted sequencing of tonsillar and base of tongue cancer and human papillomavirus positive unknown primary of the head and neck reveals prognostic effects of mutated FGFR3. Oncotarget 2017, 8, 35339–35350. [Google Scholar] [CrossRef] [PubMed]
- Broutian, T.R.; Jiang, B.; Li, J.; Akagi, K.; Gui, S.; Zhou, Z.; Xiao, W.; Symer, D.E.; Gillison, M.L. Human papillomavirus insertions identify the PIM family of serine/threonine kinases as targetable driver genes in head and neck squamous cell carcinoma. Cancer Lett. 2020, 476, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Gronhoj, C.; Jensen, D.H.; Agander, T.; Kiss, K.; Hogdall, E.; Specht, L.; Bagger, F.O.; Nielsen, F.C.; von Buchwald, C. Deep sequencing of human papillomavirus positive loco-regionally advanced oropharyngeal squamous cell carcinomas reveals novel mutational signature. BMC Cancer 2018, 18, 640. [Google Scholar] [CrossRef]
- Gronhoj, C.; Jensen, D.H.; Dehlendorff, C.; Marklund, L.; Wagner, S.; Mehanna, H.; Munck-Wikland, E.; Ramqvist, T.; Nasman, A.; Wittekindt, C.; et al. Development and external validation of nomograms in oropharyngeal cancer patients with known HPV-DNA status: A European Multicentre Study (OroGrams). Br. J. Cancer 2018, 118, 1672–1681. [Google Scholar] [CrossRef] [PubMed]
- Harbison, R.A.; Kubik, M.; Konnick, E.Q.; Zhang, Q.; Lee, S.G.; Park, H.; Zhang, J.; Carlson, C.S.; Chen, C.; Schwartz, S.M.; et al. The mutational landscape of recurrent versus nonrecurrent human papillomavirus-related oropharyngeal cancer. JCI Insight 2018, 3, e99327. [Google Scholar] [CrossRef]
- Hess, A.K.; Muer, A.; Mairinger, F.D.; Weichert, W.; Stenzinger, A.; Hummel, M.; Budach, V.; Tinhofer, I. MiR-200b and miR-155 as predictive biomarkers for the efficacy of chemoradiation in locally advanced head and neck squamous cell carcinoma. Eur. J. Cancer 2017, 77, 3–12. [Google Scholar] [CrossRef]
- Hui, A.B.; Lin, A.; Xu, W.; Waldron, L.; Perez-Ordonez, B.; Weinreb, I.; Shi, W.; Bruce, J.; Huang, S.H.; O’Sullivan, B.; et al. Potentially prognostic miRNAs in HPV-associated oropharyngeal carcinoma. Clin. Cancer Res. 2013, 19, 2154–2162. [Google Scholar] [CrossRef]
- Lechner, M.; Frampton, G.M.; Fenton, T.; Feber, A.; Palmer, G.; Jay, A.; Pillay, N.; Forster, M.; Cronin, M.T.; Lipson, D.; et al. Targeted next-generation sequencing of head and neck squamous cell carcinoma identifies novel genetic alterations in HPV+ and HPV− tumors. Genome Med. 2013, 5, 49. [Google Scholar] [CrossRef]
- Lindquist, D.; Ahrlund-Richter, A.; Tarjan, M.; Tot, T.; Dalianis, T. Intense CD44 expression is a negative prognostic factor in tonsillar and base of tongue cancer. Anticancer Res 2012, 32, 153–161. [Google Scholar] [PubMed]
- Lindquist, D.; Nasman, A.; Tarjan, M.; Henriksson, R.; Tot, T.; Dalianis, T.; Hedman, H. Expression of LRIG1 is associated with good prognosis and human papillomavirus status in oropharyngeal cancer. Br. J. Cancer 2014, 110, 1793–1800. [Google Scholar] [CrossRef] [PubMed]
- Nasman, A.; Andersson, E.; Marklund, L.; Tertipis, N.; Hammarstedt-Nordenvall, L.; Attner, P.; Nyberg, T.; Masucci, G.V.; Munck-Wikland, E.; Ramqvist, T.; et al. HLA class I and II expression in oropharyngeal squamous cell carcinoma in relation to tumor HPV status and clinical outcome. PLoS ONE 2013, 8, e77025. [Google Scholar] [CrossRef] [PubMed]
- Nordfors, C.; Grun, N.; Tertipis, N.; Ahrlund-Richter, A.; Haeggblom, L.; Sivars, L.; Du, J.; Nyberg, T.; Marklund, L.; Munck-Wikland, E.; et al. CD8+ and CD4+ tumour infiltrating lymphocytes in relation to human papillomavirus status and clinical outcome in tonsillar and base of tongue squamous cell carcinoma. Eur. J. Cancer 2013, 49, 2522–2530. [Google Scholar] [CrossRef] [PubMed]
- Oguejiofor, K.; Galletta-Williams, H.; Dovedi, S.J.; Roberts, D.L.; Stern, P.L.; West, C.M. Distinct patterns of infiltrating CD8+ T cells in HPV+ and CD68 macrophages in HPV- oropharyngeal squamous cell carcinomas are associated with better clinical outcome but PD-L1 expression is not prognostic. Oncotarget 2017, 8, 14416–14427. [Google Scholar] [CrossRef] [PubMed]
- Oguejiofor, K.; Hall, J.; Slater, C.; Betts, G.; Hall, G.; Slevin, N.; Dovedi, S.; Stern, P.L.; West, C.M. Stromal infiltration of CD8 T cells is associated with improved clinical outcome in HPV-positive oropharyngeal squamous carcinoma. Br. J. Cancer 2015, 113, 886–893. [Google Scholar] [CrossRef] [PubMed]
- Reder, H.; Wagner, S.; Wuerdemann, N.; Langer, C.; Sandmann, S.; Braeuninger, A.; Dugas, M.; Gattenloehner, S.; Wittekindt, C.; Klussmann, J.P. Mutation patterns in recurrent and/or metastatic oropharyngeal squamous cell carcinomas in relation to human papillomavirus status. Cancer Med. 2021, 10, 1347–1356. [Google Scholar] [CrossRef] [PubMed]
- Rietbergen, M.M.; Martens-de Kemp, S.R.; Bloemena, E.; Witte, B.I.; Brink, A.; Baatenburg de Jong, R.J.; Leemans, C.R.; Braakhuis, B.J.; Brakenhoff, R.H. Cancer stem cell enrichment marker CD98: A prognostic factor for survival in patients with human papillomavirus-positive oropharyngeal cancer. Eur. J. Cancer 2014, 50, 765–773. [Google Scholar] [CrossRef]
- Sewell, A.; Brown, B.; Biktasova, A.; Mills, G.B.; Lu, Y.; Tyson, D.R.; Issaeva, N.; Yarbrough, W.G. Reverse-phase protein array profiling of oropharyngeal cancer and significance of PIK3CA mutations in HPV-associated head and neck cancer. Clin. Cancer Res. 2014, 20, 2300–2311. [Google Scholar] [CrossRef] [PubMed]
- Tertipis, N.; Hammar, U.; Nasman, A.; Vlastos, A.; Nordfors, C.; Grun, N.; Ahrlund-Richter, A.; Sivars, L.; Haeggblom, L.; Marklund, L.; et al. A model for predicting clinical outcome in patients with human papillomavirus-positive tonsillar and base of tongue cancer. Eur. J. Cancer 2015, 51, 1580–1587. [Google Scholar] [CrossRef] [PubMed]
- Tertipis, N.; Villabona, L.; Nordfors, C.; Nasman, A.; Ramqvist, T.; Vlastos, A.; Masucci, G.; Dalianis, T. HLA-A*02 in relation to outcome in human papillomavirus positive tonsillar and base of tongue cancer. Anticancer Res. 2014, 34, 2369–2375. [Google Scholar]
- Tinhofer, I.; Budach, V.; Saki, M.; Konschak, R.; Niehr, F.; Johrens, K.; Weichert, W.; Linge, A.; Lohaus, F.; Krause, M.; et al. Targeted next-generation sequencing of locally advanced squamous cell carcinomas of the head and neck reveals druggable targets for improving adjuvant chemoradiation. Eur. J. Cancer 2016, 57, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Zupancic, M.; Haeggblom, L.; Landin, D.; Marklund, L.; Dalianis, T.; Nasman, A. Psoriasin expression is associated with survival in patients with human papillomavirus-positive base of tongue squamous cell carcinoma. Oncol. Lett. 2021, 21, 277. [Google Scholar] [CrossRef]
- Bersani, C.; Haeggblom, L.; Ursu, R.G.; Giusca, S.E.; Marklund, L.; Ramqvist, T.; Nasman, A.; Dalianis, T. Overexpression of FGFR3 in HPV-positive Tonsillar and Base of Tongue Cancer Is Correlated to Outcome. Anticancer Res. 2018, 38, 4683–4690. [Google Scholar] [CrossRef]
- Leenhardt, F.; Alexandre, M.; Jacot, W. Alpelisib for the treatment of PIK3CA-mutated, hormone receptor-positive, HER2-negative metastatic breast cancer. Expert Opin. Pharmacother. 2021, 22, 667–675. [Google Scholar] [CrossRef]
- Tabernero, J.; Bahleda, R.; Dienstmann, R.; Infante, J.R.; Mita, A.; Italiano, A.; Calvo, E.; Moreno, V.; Adamo, B.; Gazzah, A.; et al. Phase I Dose-Escalation Study of JNJ-42756493, an Oral Pan-Fibroblast Growth Factor Receptor Inhibitor, in Patients With Advanced Solid Tumors. J. Clin. Oncol. 2015, 33, 3401–3408. [Google Scholar] [CrossRef]
- Smeets, S.J.; Hesselink, A.T.; Speel, E.J.; Haesevoets, A.; Snijders, P.J.; Pawlita, M.; Meijer, C.J.; Braakhuis, B.J.; Leemans, C.R.; Brakenhoff, R.H. A novel algorithm for reliable detection of human papillomavirus in paraffin embedded head and neck cancer specimen. Int. J. Cancer 2007, 121, 2465–2472. [Google Scholar] [CrossRef] [PubMed]
- Nasman, A.; Nordfors, C.; Holzhauser, S.; Vlastos, A.; Tertipis, N.; Hammar, U.; Hammarstedt-Nordenvall, L.; Marklund, L.; Munck-Wikland, E.; Ramqvist, T.; et al. Incidence of human papillomavirus positive tonsillar and base of tongue carcinoma: A stabilisation of an epidemic of viral induced carcinoma? Eur. J. Cancer 2015, 51, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Mints, M.; Landin, D.; Nasman, A.; Mirzaie, L.; Ursu, R.G.; Zupancic, M.; Marklund, L.; Dalianis, T.; Munck-Wikland, E.; Ramqvist, T. Tumour inflammation signature and expression of S100A12 and HLA class I improve survival in HPV-negative hypopharyngeal cancer. Sci. Rep. 2021, 11, 1782. [Google Scholar] [CrossRef]
- Foroughi Asl, H. Clinical-Genomics/BALSAMIC: Bioinformatic Analysis PipeLine for SomAtic Mutations in Cancer. v6.0. Available online: https://github.com/Clinical-Genomics/BALSAMIC (accessed on 21 December 2021).
- Babraham Bioinformatics—FastQC A Quality Control tool for High Throughput Sequence Data. Available online: https://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 22 June 2020).
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef]
- Li, H. A statistical framework for SNP calling, mutation discovery, association mapping and population genetical parameter estimation from sequencing data. Bioinformatics 2011, 27, 2987–2993. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R.; Genome Project Data Processing, S. The Sequence Alignment/Map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef]
- Picard Tools—By Broad Institute. Available online: https://broadinstitute.github.io/picard/ (accessed on 22 June 2020).
- Ewels, P.; Magnusson, M.; Lundin, S.; Kaller, M. MultiQC: Summarize analysis results for multiple tools and samples in a single report. Bioinformatics 2016, 32, 3047–3048. [Google Scholar] [CrossRef]
- Lai, Z.; Markovets, A.; Ahdesmaki, M.; Chapman, B.; Hofmann, O.; McEwen, R.; Johnson, J.; Dougherty, B.; Barrett, J.C.; Dry, J.R. VarDict: A novel and versatile variant caller for next-generation sequencing in cancer research. Nucleic Acids Res. 2016, 44, e108. [Google Scholar] [CrossRef]
- Chen, X.; Schulz-Trieglaff, O.; Shaw, R.; Barnes, B.; Schlesinger, F.; Kallberg, M.; Cox, A.J.; Kruglyak, S.; Saunders, C.T. Manta: Rapid detection of structural variants and indels for germline and cancer sequencing applications. Bioinformatics 2016, 32, 1220–1222. [Google Scholar] [CrossRef]
- McLaren, W.; Gil, L.; Hunt, S.E.; Riat, H.S.; Ritchie, G.R.; Thormann, A.; Flicek, P.; Cunningham, F. The Ensembl Variant Effect Predictor. Genome Biol. 2016, 17, 122. [Google Scholar] [CrossRef]
- Pedersen, B.S.; Layer, R.M.; Quinlan, A.R. Vcfanno: Fast, flexible annotation of genetic variants. Genome Biol. 2016, 17, 118. [Google Scholar] [CrossRef] [PubMed]
- Karczewski, K.J.; Francioli, L.C.; Tiao, G.; Cummings, B.B.; Alfoldi, J.; Wang, Q.; Collins, R.L.; Laricchia, K.M.; Ganna, A.; Birnbaum, D.P.; et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature 2020, 581, 434–443. [Google Scholar] [CrossRef]
- Yu, G.; Wang, L.G.; Han, Y.; He, Q.Y. clusterProfiler: An R package for comparing biological themes among gene clusters. OMICS 2012, 16, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Liberzon, A.; Subramanian, A.; Pinchback, R.; Thorvaldsdottir, H.; Tamayo, P.; Mesirov, J.P. Molecular signatures database (MSigDB) 3.0. Bioinformatics 2011, 27, 1739–1740. [Google Scholar] [CrossRef] [PubMed]
- Kazemi-Sefat, G.E.; Keramatipour, M.; Talebi, S.; Kavousi, K.; Sajed, R.; Kazemi-Sefat, N.A.; Mousavizadeh, K. The importance of CDC27 in cancer: Molecular pathology and clinical aspects. Cancer Cell Int. 2021, 21, 160. [Google Scholar] [CrossRef]
- Melloy, P.G. The anaphase-promoting complex: A key mitotic regulator associated with somatic mutations occurring in cancer. Genes Chromosomes Cancer 2020, 59, 189–202. [Google Scholar] [CrossRef]
- Lee, S.J.; Langhans, S.A. Anaphase-promoting complex/cyclosome protein Cdc27 is a target for curcumin-induced cell cycle arrest and apoptosis. BMC Cancer 2012, 12, 44. [Google Scholar] [CrossRef]
- Ren, Y.Q.; Fu, F.; Han, J. MiR-27a modulates radiosensitivity of triple-negative breast cancer (TNBC) cells by targeting CDC27. Med. Sci. Monit. 2015, 21, 1297–1303. [Google Scholar] [CrossRef]
- Berens, E.B.; Sharif, G.M.; Schmidt, M.O.; Yan, G.; Shuptrine, C.W.; Weiner, L.M.; Glasgow, E.; Riegel, A.T.; Wellstein, A. Keratin-associated protein 5-5 controls cytoskeletal function and cancer cell vascular invasion. Oncogene 2017, 36, 593–605. [Google Scholar] [CrossRef]
- Hemminki, K.; Chen, B.; Kumar, A.; Melander, O.; Manjer, J.; Hallmans, G.; Pettersson-Kymmer, U.; Ohlsson, C.; Folprecht, G.; Loffler, H.; et al. Germline genetics of cancer of unknown primary (CUP) and its specific subtypes. Oncotarget 2016, 7, 22140–22149. [Google Scholar] [CrossRef] [PubMed]
- Hollingsworth, M.A.; Swanson, B.J. Mucins in cancer: Protection and control of the cell surface. Nat. Rev. Cancer 2004, 4, 45–60. [Google Scholar] [CrossRef]
- Zhou, L.; Huang, L.; Xu, Q.; Lv, Y.; Wang, Z.; Zhan, P.; Han, H.; Shao, Y.; Lin, D.; Lv, T.; et al. Association of MUC19 Mutation with Clinical Benefits of Anti-PD-1 Inhibitors in Non-small Cell Lung Cancer. Front. Oncol. 2021, 11, 596542. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Wang, H.; Meng, F.; Han, Y.; Chen, Y.; Xiao, M.; Jiang, H.; Yu, Z.; Xu, B. Role of BCLAF-1 in PD-L1 stabilization in response to ionizing irradiation. Cancer Sci. 2021, 112, 4064–4074. [Google Scholar] [CrossRef]
- Jiang, T.; Liu, B.; Wu, D.; Zhang, F. BCLAF1 induces cisplatin resistance in lung cancer cells. Oncol. Lett. 2020, 20, 227. [Google Scholar] [CrossRef]
- Dai, C.; Charlestin, V.; Wang, M.; Walker, Z.T.; Miranda-Vergara, M.C.; Facchine, B.A.; Wu, J.; Kaliney, W.J.; Dovichi, N.J.; Li, J.; et al. Aquaporin-7 Regulates the Response to Cellular Stress in Breast Cancer. Cancer Res. 2020, 80, 4071–4086. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, L.; Lazar, V.; Michiels, S.; Ripoche, H.; Dessen, P.; Talbot, M.; Caillou, B.; Levillain, J.P.; Schlumberger, M.; Bidart, J.M. Follicular thyroid tumors with the PAX8-PPARgamma1 rearrangement display characteristic genetic alterations. Am. J. Pathol. 2005, 167, 223–231. [Google Scholar] [CrossRef]
- Wang, J.; Feng, L.; Zhu, Z.; Zheng, M.; Wang, D.; Chen, Z.; Sun, H. Aquaporins as diagnostic and therapeutic targets in cancer: How far we are? J. Transl. Med. 2015, 13, 96. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ährlund-Richter, A.; Holzhauser, S.; Dalianis, T.; Näsman, A.; Mints, M. Whole-Exome Sequencing of HPV Positive Tonsillar and Base of Tongue Squamous Cell Carcinomas Reveals a Global Mutational Pattern along with Relapse-Specific Somatic Variants. Cancers 2022, 14, 77. https://doi.org/10.3390/cancers14010077
Ährlund-Richter A, Holzhauser S, Dalianis T, Näsman A, Mints M. Whole-Exome Sequencing of HPV Positive Tonsillar and Base of Tongue Squamous Cell Carcinomas Reveals a Global Mutational Pattern along with Relapse-Specific Somatic Variants. Cancers. 2022; 14(1):77. https://doi.org/10.3390/cancers14010077
Chicago/Turabian StyleÄhrlund-Richter, Andreas, Stefan Holzhauser, Tina Dalianis, Anders Näsman, and Michael Mints. 2022. "Whole-Exome Sequencing of HPV Positive Tonsillar and Base of Tongue Squamous Cell Carcinomas Reveals a Global Mutational Pattern along with Relapse-Specific Somatic Variants" Cancers 14, no. 1: 77. https://doi.org/10.3390/cancers14010077
APA StyleÄhrlund-Richter, A., Holzhauser, S., Dalianis, T., Näsman, A., & Mints, M. (2022). Whole-Exome Sequencing of HPV Positive Tonsillar and Base of Tongue Squamous Cell Carcinomas Reveals a Global Mutational Pattern along with Relapse-Specific Somatic Variants. Cancers, 14(1), 77. https://doi.org/10.3390/cancers14010077








