Adenoma to Colorectal Cancer Estimated Transition Rates Stratified by BMI Categories—A Cross-Sectional Analysis of Asymptomatic Individuals from Screening Colonoscopy Program
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Screening Procedures
2.3. Study Definitions
- (a).
- Early CRC is defined as CRC in clinical stage 1 and clinical stage 2 according to AJCC. Advanced CRC is defined as CRC in clinical stage 3 and clinical stage 4 according to AJCC.
- (b).
- Advanced adenoma is defined as adenomatous lesion that:—has histologically proven high-grade dysplasia OR/AND—is ≥10 mm large OR/AND—has villous or tubulovillous component [12].
- (c).
- Non-advanced adenoma is defined as any adenomatous lesion that was not advanced adenoma or colorectal cancer.
- (d).
- BMI was calculated using weight [kg]/(height [m])2 and stratified according to the WHO classification [13]. First, second and third class of obesity were pooled together to achieve appropriate power of analysis. Underweight category was excluded from analysis due to low number of patients.
2.4. Statistical Methods
- Adjusted prevalence of a colorectal lesion (non-advanced adenoma, advanced adenoma, CRC) is defined as number of individuals with a specific lesion divided by numbers of individuals screened multiplied by 100% and thereafter adjusted as specified above.
- Estimated transition rate (eTR) is defined as adjusted prevalence of more advanced lesion divided by adjusted prevalence of less advanced lesion (e.g., prevalence of CRC divided by prevalence of advanced adenomas etc.).PNAA—adjusted prevalence of non-advanced adenomasPAA—adjusted prevalence of advanced adenomasPECRC—adjusted prevalence of early CRCPACRC—adjusted prevalence of advanced CRCeTRNAAàAA = PAA/PNAA × 100%eTRAAàECRC = PECRC/PAA × 100%eTRECRCàACRC = PACRC/PECRS × 100% et cetera.
- Prevalence ratio was calculated as follows: adjusted prevalence of BMI subgroup (overweight or obese) was divided by adjusted prevalence of normal BMI subgroup. This calculation was performed for every type of lesion (non-advanced adenoma, advanced adenoma, early CRC, advanced CRC).
- Calculations were carried out with Stata Statistical Software, v. 13.1 (Stata Corporation, College Station, TX, USA).
3. Results
3.1. Adjusted Prevalence
3.2. Transition Rates
3.3. Prevalence Ratios
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Leslie, A.; Carey, F.A.; Pratt, N.R.; Steele, R.J. The colorectal adenoma–carcinoma sequence. BJS 2002, 89, 845–860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- AJCC. AJCC Colorectal Cancer Stages. Available online: https://www.cancer.org/cancer/colon-rectal-cancer/detection-diagnosis-staging/staged.html (accessed on 9 January 2019).
- Stryker, S.J.; Wolff, B.G.; Culp, C.E.; Libbe, S.D.; Ilstrup, D.M.; Maccarty, R.L. Natural history of untreated colonic polyps. Gastroenterology 1987, 93, 1009–1013. [Google Scholar] [CrossRef]
- Williams, A.R.; Balasooriya, B.A.; Day, D.W. Polyps and cancer of the large bowel: A necropsy study in Liverpool. Gut 1982, 23, 835–842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vatn, M.H.; Stalsberg, H. The prevalence of polyps of the large intestine in Oslo: An autopsy study. Cancer 1982, 49, 819–825. [Google Scholar] [CrossRef] [Green Version]
- Eide, T.J.; Stalsberg, H. Polyps of the large intestine in northern Norway. Cancer 1978, 42, 2839–2848. [Google Scholar] [CrossRef]
- Eide, T.J. Risk of colorectal cancer in adenoma-bearing individuals within a defined population. Int. J. Cancer 1986, 38, 173–176. [Google Scholar] [CrossRef] [PubMed]
- Kaminski, M.; Kraszewska, E.; Rupinski, M.; Laskowska, M.; Wieszczy, P.; Regula, J. Design of the Polish Colonoscopy Screen-ing Program: A randomized health services study. Endoscopy 2015, 47, 1144–1150. Available online: http://www.ncbi.nlm.nih.gov/pubmed/26517847 (accessed on 23 August 2018). [PubMed] [Green Version]
- Spychalski, P.; Kobiela, J.; Wieszczy, P.; Kaminski, M.; Regula, J. Clinical stages of colorectal cancer diagnosed in obese and overweight individuals in the Polish Colonoscopy Screening Program. United Eur. Gastroenterol. J. 2019, 7, 790–797. [Google Scholar] [CrossRef] [Green Version]
- Regula, J.; Rupinski, M.; Kraszewska, E.; Polkowski, M.; Pachlewski, J.; Orlowska, J.; Nowacki, M.P.; Butruk, E. Colonoscopy in Colorectal-Cancer Screening for Detection of Advanced Neoplasia. N. Engl. J. Med. 2006, 355, 1863–1872. [Google Scholar] [CrossRef] [PubMed]
- Kaminski, M.; Polkowski, M.; Kraszewska, E.; Rupinski, M.; Butruk, E.; Regula, J. A score to estimate the likelihood of detecting advanced colorectal neoplasia at colonoscopy. Gut 2014, 63, 1112–1119. [Google Scholar] [CrossRef] [PubMed]
- Atkin, W.; Valori, R.; Kuipers, E.; Hoff, G.; Senore, C.; Segnan, N.R.; Jover, W.; Schmiegel, R.; Pox, R.L.C.; International Agency for Research on Cancer. European guidelines for quality assurance in colorectal cancer screening and diagnosis. First Edition—Colonoscopic surveillance following adenoma removal. Endoscopy 2012, 44, SE151-63. Available online: http://www.ncbi.nlm.nih.gov/pubmed/23012119 (accessed on 28 September 2019). [CrossRef] [PubMed] [Green Version]
- WHO. Body Mass Index—BMI. World Health Organization. 2018. Available online: https://www.euro.who.int/en/health-topics/disease-prevention/nutrition/a-healthy-lifestyle/body-mass-index-bmi (accessed on 21 August 2021).
- Brenner, H.; Hoffmeister, M.; Stegmaier, C.; Brenner, G.; Altenhofen, L.; Haug, U. Risk of progression of advanced adenomas to colorectal cancer by age and sex: Estimates based on 840 149 screening colonoscopies. Gut 2007, 56, 1585–1589. [Google Scholar] [CrossRef] [Green Version]
- Im, J.P.; Kim, D.; Chung, S.J.; Jin, E.H.; Han, Y.M.; Park, M.J.; Song, J.H.; Yang, S.Y.; Kim, Y.S.; Yim, J.Y.; et al. Visceral obesity as a risk factor for colorectal adenoma occur-rence in surveillance colonoscopy. Gastrointest. Endosc. 2018, 88, 119–127.e4. Available online: http://www.ncbi.nlm.nih.gov/pubmed/29510147 (accessed on 19 March 2019). [CrossRef]
- Sinn, D.H.; Min, Y.W.; Son, H.J.; Rhee, P.-L.; Paik, S.W.; Hong, S.N.; Gwak, G.-Y. Metabolically-healthy obesity is associated with higher prevalence of colorectal adenoma. PLoS ONE 2017, 12, e0179480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, T.J.; Kim, J.E.; Choi, Y.-H.; Hong, S.N.; Kim, Y.-H.; Chang, D.K.; Rhee, P.-L.; Kim, M.-J.; Jung, S.-H.; Son, H.J. Obesity-related parameters and colorectal adenoma development. J. Gastroenterol. 2017, 52, 1221–1229. [Google Scholar] [CrossRef]
- Shapero, T.F.; Chen, G.I.; Devlin, T.; Gibbs, A.; Murray, I.C.; Tran, S.; Weigensberg, C. Obesity Increases Prevalence of Colonic Adenomas at Screening Colonoscopy: A Canadian Community-Based Study. Can. J. Gastroenterol. Hepatol. 2017, 2017, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Ladabaum, U.; Song, K. Projected National Impact of Colorectal Cancer Screening on Clinical and Economic Outcomes and Health Services Demand. Gastroenterology 2005, 129, 1151–1162. [Google Scholar] [CrossRef] [PubMed]
- Ijspeert, J.E.G.; Bevan, R.; Senore, C.; Kaminski, M.; Kuipers, E.J.; Mróz, A.; Bessa, X.; Cassoni, P.; Hassan, C.; Repici, A.; et al. Detection rate of serrated polyps and serrated polyposis syndrome in colorectal cancer screening cohorts: A European overview. Gut 2017, 66, 1225–1232. [Google Scholar] [CrossRef]
- Renehan, A.G. The ‘obesity paradox’ and survival after colorectal cancer: True or false? Cancer Causes Control 2014, 25, 1419–1422. [Google Scholar] [CrossRef]
- Caan, B.J.; Meyerhardt, J.A.; Kroenke, C.H.; Alexeeff, S.; Xiao, J.; Weltzien, E.; Weltzien, E.; Feliciano, E.C.; Castillo, A.L.; Quesenberry, C.P.; et al. Explaining the Obesity Paradox: The Associa-tion between Body Composition and Colorectal Cancer Survival (C-SCANS Study). Cancer Epidemiol. Biomark. Prev. 2017, 26, 1008–1015. Available online: http://www.ncbi.nlm.nih.gov/pubmed/28506965 (accessed on 10 March 2019). [CrossRef] [Green Version]
- Renehan, A.G.; Sperrin, M. The Obesity Paradox and Mortality After Colorectal Cancer. JAMA Oncol. 2016, 2, 1127–1129. [Google Scholar] [CrossRef] [PubMed]
- Sperrin, M.; Candlish, J.; Badrick, E.; Renehan, A.; Buchan, I. Collider Bias Is Only a Partial Explanation for the Obesity Para-dox. Epidemiology 2016, 27, 525–530. Available online: http://www.ncbi.nlm.nih.gov/pubmed/27075676 (accessed on 10 March 2019). [CrossRef] [PubMed] [Green Version]
- Kroenke, C.H.; Neugebauer, R.; Meyerhardt, J.; Prado, C.M.; Weltzien, E.; Kwan, M.L.; Xiao, J.; Caan, B.J. Analysis of Body Mass Index and Mortality in Patients with Colorectal Cancer Using Causal Diagrams. JAMA Oncol. 2016, 2, 1137–1145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woo Park, S.; Lak Lee, H.; Young Doo, E.; Nyeong Lee, K.; Won Jun, D.; Young Lee, O.; Han, D.S.; Yoon, B.C.; Choi, H.S.; Lee, K.H. Visceral Obesity Predicts Fewer Lymph Node Metastases and Better Overall Survival in Colon Cancer. J. Gastrointest. Surg. 2015, 19, 1513–1521. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4503880/pdf/11605_2015_Article_2834.pdf (accessed on 21 June 2018). [CrossRef] [PubMed] [Green Version]
- Murono, K.; Kitayama, J.; Tsuno, N.H.; Nozawa, H.; Kawai, K.; Sunami, E.; Akahane, M.; Watanabe, T. Hepatic steatosis is associated with lower inci-dence of liver metastasis from colorectal cancer. Int. J. Colorectal. Dis. 2013, 28, 1065–1072. Available online: http://www.ncbi.nlm.nih.gov/pubmed/23392476 (accessed on 1 July 2018). [CrossRef]
- Yamaji, T.; Iwasaki, M.; Sasazuki, S.; Tsugane, S. Gender difference in the association of insulin and the insulin-like growth factor axis with colorectal neoplasia. Int. J. Obes. 2011, 36, 440–447. [Google Scholar] [CrossRef] [Green Version]
- Spix, C.; Berthold, F.; Hero, B.; Michaelis, J.; Schilling, F.H. Correction factors for self-selection when evaluating screening programmes. J. Med Screen. 2016, 23, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Maukonen, M.; Männistö, S.; Tolonen, H. A comparison of measured versus self-reported anthropometrics for assessing obesity in adults: A literature review. Scand. J. Public Health 2018, 46, 565–579. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| BMI Category | Normal n = 46,294 | Overweight n = 67,528 | Obese n = 33,563 | ALL n = 147,385 |
|---|---|---|---|---|
| Age mean (SD) | 55.35 (5.52) | 56.39 (5.20) | 56.84 (5.01) | 56.17 (5.20) |
| Male gender n (%) | 12,702 (27.44%) | 30,286 (44.85%) | 13,990 (41.68%) | 56,978 (38.66%) |
| Family history of CRC n (%) | 9667 (20.88%) | 12,112 (17.94%) | 5562 (16.57%) | 27,341 (18.55%) |
| Diabetes n (%) | 757 (1.64%) | 2613 (3.87%) | 3424 (10.20%) | 6794 (4.61%) |
| Never smokers n (%) * | 26,817 (57.93%) | 37,650 (55.75%) | 17,969 (53.54%) | 82,436 (55.93%) |
| PPI use n (%) | 6031 (13.03%) | 8769 (12.99%) | 4532 (13.5%) | 19,332 (13.12%) |
| Aspirin use n (%) | 4183 (9.04%) | 9044 (13.39%) | 6461 (19.25%) | 19,688 (13.36%) |
| HRT use n (%) | 7790 (23.19%) | 8094 (21.73%) | 3323 (16.98%) | 19,207 (21.25%) |
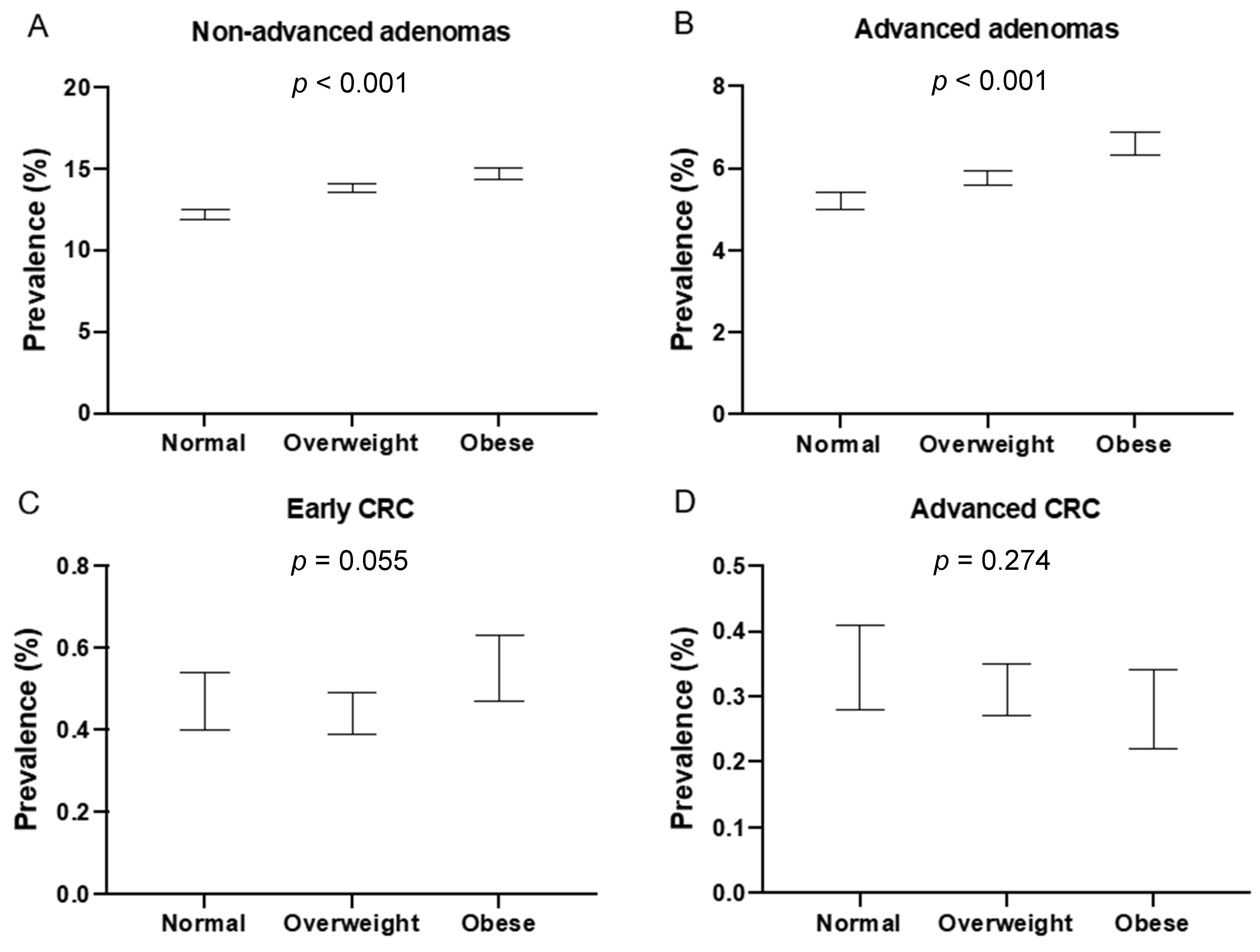
| n Diagnosed | Crude Prevalence | Adjusted Prevalence | 95% CI | p-Value * | |
|---|---|---|---|---|---|
| nAA | 19,974 | <0.001 | |||
| Normal | 5304 | 11.46% | 12.19% | 11.86–12.52 | |
| Overweight | 9600 | 14.22% | 13.81% | 13.55–14.07 | |
| Obese | 5070 | 15.11% | 14.70% | 14.31–15.08 | |
| AA | 8594 | <0.001 | |||
| Normal | 2295 | 4.96% | 5.20% | 4.98–5.42 | |
| Overweight | 4029 | 5.97% | 5.77% | 5.60–5.95 | |
| Obese | 2270 | 6.76% | 6.61% | 6.33–6.88 | |
| eCRC ** | 709 | 0.055 | |||
| Normal | 199 | 0.43% | 0.47% | 0.40–0.54 | |
| Overweight | 314 | 0.47% | 0.44% | 0.39–0.49 | |
| Obese | 196 | 0.59% | 0.55% | 0.47–0.63 | |
| aCRC ** | 445 | 0.274 | |||
| Normal | 134 | 0.29% | 0.35% | 0.28–0.41 | |
| Overweight | 213 | 0.32% | 0.31% | 0.27–0.35 | |
| Obese | 98 | 0.29% | 0.28% | 0.22–0.34 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spychalski, P.; Kobiela, J.; Wieszczy, P.; Bugajski, M.; Reguła, J.; Kaminski, M.F. Adenoma to Colorectal Cancer Estimated Transition Rates Stratified by BMI Categories—A Cross-Sectional Analysis of Asymptomatic Individuals from Screening Colonoscopy Program. Cancers 2022, 14, 62. https://doi.org/10.3390/cancers14010062
Spychalski P, Kobiela J, Wieszczy P, Bugajski M, Reguła J, Kaminski MF. Adenoma to Colorectal Cancer Estimated Transition Rates Stratified by BMI Categories—A Cross-Sectional Analysis of Asymptomatic Individuals from Screening Colonoscopy Program. Cancers. 2022; 14(1):62. https://doi.org/10.3390/cancers14010062
Chicago/Turabian StyleSpychalski, Piotr, Jarek Kobiela, Paulina Wieszczy, Marek Bugajski, Jaroslaw Reguła, and Michał F. Kaminski. 2022. "Adenoma to Colorectal Cancer Estimated Transition Rates Stratified by BMI Categories—A Cross-Sectional Analysis of Asymptomatic Individuals from Screening Colonoscopy Program" Cancers 14, no. 1: 62. https://doi.org/10.3390/cancers14010062
APA StyleSpychalski, P., Kobiela, J., Wieszczy, P., Bugajski, M., Reguła, J., & Kaminski, M. F. (2022). Adenoma to Colorectal Cancer Estimated Transition Rates Stratified by BMI Categories—A Cross-Sectional Analysis of Asymptomatic Individuals from Screening Colonoscopy Program. Cancers, 14(1), 62. https://doi.org/10.3390/cancers14010062






