Detection of Marker Associated with CTC in Colorectal Cancer in Mononuclear Cells of Patients with Benign Inflammatory Intestinal Diseases
Abstract
Simple Summary
Abstract
1. Introduction
2. Results
2.1. Patients and Demographics
2.2. Variable Positivity of CK20 and DEFA5 in Blood Samples of CRC and IID Patients as Well as Healthy Controls
2.3. Variable Expression Levels of CTC-Associated Markers in Blood Samples of CRC and IID Patients as well as Healthy Controls
2.4. Expression Levels of CTC-Associated Markers in Blood Samples of IID Patients Do Not Correlate with Severity of Inflammation
2.5. Suitability of a CTC-Associated Marker for Discrimination of Malignant versus Benign Instestinal Disease
3. Discussion
4. Conclusions
5. Material and Methods
5.1. Collection and Preparation of Patient/Donor Blood Samples
5.2. RNA Isolation and Semi-Quantitative RT-qPCR
5.3. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CD | Crohn’s disease |
| CK20 | Cytokeratin-20 |
| CRC | Colorectal cancer |
| CRP | C-Reactive protein |
| CTC | Circulating tumor cells |
| DEFA5 | Defensin alpha 5 |
| EMT | Epithelial–mesenchymal transition |
| EpCAM | Epithelial cell adhesion molecule |
| EU | expression units |
| IBD | Inflammatory bowel disease |
| IID | Inflammatory intestinal disease |
| LAD-1 | Ladinin-1 |
| MNC | Mononuclear cells |
| PLS3 | Plastin-3 |
| RT-qPCR | Real-time quantitative polymerase chain reaction |
| SD | Standard deviation |
| UC | Ulcerative colitis |
| UICC | Union Internationale Contre le Cancer |
References
- GBD 2017 Inflammatory Bowel Disease Collaborators. The Global, Regional, and National Burden of Inflammatory Bowel Disease in 195 Countries and Territories, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 17–30. [Google Scholar] [CrossRef]
- Lutgens, M.W.M.D.; Van Oijen, M.G.H.; Van der Heijden, G.J.M.G.; Vleggaar, F.P.; Siersema, P.D.; Oldenburg, B. Declining Risk of Colorectal Cancer in Inflammatory Bowel Disease: An Updated Meta-Analysis of Population-Based Cohort Studies. Inflamm. Bowel Dis. 2013, 19, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.S.; Han, M.; Park, S.; Kim, W.H.; Cheon, J.H. Cancer Risk in the Early Stages of Inflammatory Bowel Disease in Korean Patients: A Nationwide Population-Based Study. J. Crohn’s Colitis 2017, 11, 954–962. [Google Scholar] [CrossRef] [PubMed]
- Peyrin-Biroulet, L.; Lepage, C.; Jooste, V.; Guéant, J.-L.; Faivre, J.; Bouvier, A.-M. Colorectal Cancer in Inflammatory Bowel Diseases: A Population-Based Study (1976–2008). Inflamm. Bowel Dis. 2012, 18, 2247–2251. [Google Scholar] [CrossRef]
- Kappelman, M.D.; Farkas, D.K.; Long, M.D.; Erichsen, R.; Sandler, R.S.; Sørensen, H.T.; Baron, J.A. Risk of Cancer in Patients with Inflammatory Bowel Diseases: A Nationwide Population-Based Cohort Study with 30 Years of Follow-up Evaluation. Clin. Gastroenterol. Hepatol. 2014, 12, 265–273.e1. [Google Scholar] [CrossRef]
- Arnold, M.; Sierra, M.S.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Patterns and Trends in Colorectal Cancer Incidence and Mortality. Gut 2017, 66, 683–691. [Google Scholar] [CrossRef]
- Schreuders, E.H.; Ruco, A.; Rabeneck, L.; Schoen, R.E.; Sung, J.J.Y.; Young, G.P.; Kuipers, E.J. Colorectal Cancer Screening: A Global Overview of Existing Programmes. Gut 2015, 64, 1637–1649. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA A Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Hendricks, A.; Dall, K.; Brandt, B.; Geisen, R.; Röder, C.; Schafmayer, C.; Becker, T.; Hinz, S.; Sebens, S. Longitudinal Analysis of Circulating Tumor Cells in Colorectal Cancer Patients by a Cytological and Molecular Approach: Feasibility and Clinical Application. Front. Oncol. 2021, 11, 2038. [Google Scholar] [CrossRef]
- Pantel, K.; Alix-Panabières, C. Liquid Biopsy and Minimal Residual Disease—Latest Advances and Implications for Cure. Nat. Rev. Clin. Oncol. 2019, 16, 409–424. [Google Scholar] [CrossRef]
- Pantel, K.; Brakenhoff, R.H.; Brandt, B. Detection, Clinical Relevance and Specific Biological Properties of Disseminating Tumour Cells. Nat. Rev. Cancer 2008, 8, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Hinz, S.; Röder, C.; Tepel, J.; Hendricks, A.; Schafmayer, C.; Becker, T.; Kalthoff, H. Cytokeratin 20 Positive Circulating Tumor Cells Are a Marker for Response after Neoadjuvant Chemoradiation but Not for Prognosis in Patients with Rectal Cancer. BMC Cancer 2015, 15, 953. [Google Scholar] [CrossRef] [PubMed]
- Hinz, S.; Hendricks, A.; Wittig, A.; Schafmayer, C.; Tepel, J.; Kalthoff, H.; Becker, T.; Röder, C. Detection of Circulating Tumor Cells with CK20 RT-PCR Is an Independent Negative Prognostic Marker in Colon Cancer Patients—A Prospective Study. BMC Cancer 2017, 17, 53. [Google Scholar] [CrossRef]
- Hendricks, A.; Eggebrecht, G.L.; Bernsmeier, A.; Geisen, R.; Dall, K.; Trazold, A.; Becker, T.; Kalthoff, H.; Schafmayer, C.; Röder, C.; et al. Identifying Patients with an Unfavorable Prognosis in Early Stages of Colorectal Carcinoma. Oncotarget 2018, 9, 27423–27434. [Google Scholar] [CrossRef][Green Version]
- Yang, C.; Zou, K.; Zheng, L.; Xiong, B. Prognostic and Clinicopathological Significance of Circulating Tumor Cells Detected by RT-PCR in Non-Metastatic Colorectal Cancer: A Meta-Analysis and Systematic Review. BMC Cancer 2017, 17, 725. [Google Scholar] [CrossRef]
- Wang, S.; Zheng, G.; Cheng, B.; Chen, F.; Wang, Z.; Chen, Y.; Wang, Y.; Xiong, B. Circulating Tumor Cells (CTCs) Detected by RT-PCR and Its Prognostic Role in Gastric Cancer: A Meta-Analysis of Published Literature. PLoS ONE 2014, 9, e99259. [Google Scholar] [CrossRef] [PubMed]
- Hendricks, A.; Brandt, B.; Geisen, R.; Dall, K.; Röder, C.; Schafmayer, C.; Becker, T.; Hinz, S.; Sebens, S. Isolation and Enumeration of CTC in Colorectal Cancer Patients: Introduction of a Novel Cell Imaging Approach and Comparison to Cellular and Molecular Detection Techniques. Cancers 2020, 12, 2643. [Google Scholar] [CrossRef]
- Woestemeier, A.; Harms-Effenberger, K.; Karstens, K.-F.; Konczalla, L.; Ghadban, T.; Uzunoglu, F.G.; Izbicki, J.R.; Bockhorn, M.; Pantel, K.; Reeh, M. Clinical Relevance of Circulating Tumor Cells in Esophageal Cancer Detected by a Combined MACS Enrichment Method. Cancers 2020, 12, 718. [Google Scholar] [CrossRef]
- Cieślikowski, W.A.; Budna-Tukan, J.; Świerczewska, M.; Ida, A.; Hrab, M.; Jankowiak, A.; Mazel, M.; Nowicki, M.; Milecki, P.; Pantel, K.; et al. Circulating Tumor Cells as a Marker of Disseminated Disease in Patients with Newly Diagnosed High-Risk Prostate Cancer. Cancers 2020, 12, 160. [Google Scholar] [CrossRef]
- Abdalla, T.S.A.; Meiners, J.; Riethdorf, S.; König, A.; Melling, N.; Gorges, T.; Karstens, K.-F.; Izbicki, J.R.; Pantel, K.; Reeh, M. Prognostic Value of Preoperative Circulating Tumor Cells Counts in Patients with UICC Stage I-IV Colorectal Cancer. PLoS ONE 2021, 16, e0252897. [Google Scholar] [CrossRef]
- Mostert, B.; Sieuwerts, A.M.; Bolt-de Vries, J.; Kraan, J.; Lalmahomed, Z.; Van Galen, A.; Van der Spoel, P.; De Weerd, V.; Ramírez-Moreno, R.; Smid, M.; et al. MRNA Expression Profiles in Circulating Tumor Cells of Metastatic Colorectal Cancer Patients. Mol. Oncol. 2015, 9, 920–932. [Google Scholar] [CrossRef]
- Hernandez, B.Y.; Frierson, H.F.; Moskaluk, C.A.; Li, Y.J.; Clegg, L.; Cote, T.R.; McCusker, M.E.; Hankey, B.F.; Edwards, B.K.; Goodman, M.T. CK20 and CK7 Protein Expression in Colorectal Cancer: Demonstration of the Utility of a Population-Based Tissue Microarray. Hum. Pathol. 2005, 36, 275–281. [Google Scholar] [CrossRef]
- Schmalhofer, O.; Brabletz, S.; Brabletz, T. E-Cadherin, Beta-Catenin, and ZEB1 in Malignant Progression of Cancer. Cancer Metastasis Rev. 2009, 28, 151–166. [Google Scholar] [CrossRef]
- Genna, A.; Vanwynsberghe, A.M.; Villard, A.V.; Pottier, C.; Ancel, J.; Polette, M.; Gilles, C. EMT-Associated Heterogeneity in Circulating Tumor Cells: Sticky Friends on the Road to Metastasis. Cancers 2020, 12, 1632. [Google Scholar] [CrossRef]
- Yokobori, T.; Iinuma, H.; Shimamura, T.; Imoto, S.; Sugimachi, K.; Ishii, H.; Iwatsuki, M.; Ota, D.; Ohkuma, M.; Iwaya, T.; et al. Plastin3 Is a Novel Marker for Circulating Tumor Cells Undergoing the Epithelial-Mesenchymal Transition and Is Associated with Colorectal Cancer Prognosis. Cancer Res. 2013, 73, 2059–2069. [Google Scholar] [CrossRef]
- Kujawski, R.; Przybyłowska-Sygut, K.; Mik, M.; Lewandowski, M.; Trzciński, R.; Berut, M.; Dziki, Ł.; Majsterek, I.; Dziki, A. Expression of the PLS3 Gene in Circulating Cells in Patients with Colorectal Cancer. Pol. Przegl. Chir. 2015, 87, 59–64. [Google Scholar] [CrossRef]
- Kuriyama, K.; Yokobori, T.; Sohda, M.; Nakazawa, N.; Yajima, T.; Naruse, I.; Kuwano, H.; Shirabe, K.; Kaira, K.; Saeki, H. Plasma Plastin-3: A Tumor Marker in Patients with Non-Small-Cell Lung Cancer Treated with Nivolumab. Oncol. Lett. 2021, 21, 11. [Google Scholar] [CrossRef] [PubMed]
- Xiong, F.; Wu, G.-H.; Wang, B.; Chen, Y.-J. Plastin-3 Is a Diagnostic and Prognostic Marker for Pancreatic Adenocarcinoma and Distinguishes from Diffuse Large B-Cell Lymphoma. Cancer Cell Int. 2021, 21, 411. [Google Scholar] [CrossRef] [PubMed]
- Moon, B.; Yang, S.-J.; Park, S.M.; Lee, S.-H.; Song, K.S.; Jeong, E.-J.; Park, M.; Kim, J.-S.; Yeom, Y.I.; Kim, J.-A. LAD1 Expression Is Associated with the Metastatic Potential of Colorectal Cancer Cells. BMC Cancer 2020, 20, 1180. [Google Scholar] [CrossRef]
- Kim, J.M. Antimicrobial Proteins in Intestine and Inflammatory Bowel Diseases. Intest. Res. 2014, 12, 20–33. [Google Scholar] [CrossRef] [PubMed]
- Dobre, M.; Mănuc, T.E.; Milanesi, E.; Pleşea, I.E.; Ţieranu, E.N.; Popa, C.; Mănuc, M.; Preda, C.M.; Ţieranu, I.; Diculescu, M.M.; et al. Mucosal CCR1 Gene Expression as a Marker of Molecular Activity in Crohn’s Disease: Preliminary Data. Rom. J. Morphol. Embryol. 2017, 58, 1263–1268. [Google Scholar]
- Lawrance, I.C.; Fiocchi, C.; Chakravarti, S. Ulcerative Colitis and Crohn’s Disease: Distinctive Gene Expression Profiles and Novel Susceptibility Candidate Genes. Hum. Mol. Genet. 2001, 10, 445–456. [Google Scholar] [CrossRef]
- Noble, C.L.; Abbas, A.R.; Cornelius, J.; Lees, C.W.; Ho, G.-T.; Toy, K.; Modrusan, Z.; Pal, N.; Zhong, F.; Chalasani, S.; et al. Regional Variation in Gene Expression in the Healthy Colon Is Dysregulated in Ulcerative Colitis. Gut 2008, 57, 1398–1405. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, M.D.; Kulaylat, M.N. Gene Expression Profiles of Late Colonic Crohn’s Disease. J. Med. 2004, 35, 233–255. [Google Scholar] [PubMed]
- Nastase, A.; Pâslaru, L.; Niculescu, A.M.; Ionescu, M.; Dumitraşcu, T.; Herlea, V.; Dima, S.; Gheorghe, C.; Lazar, V.; Popescu, I. Prognostic and Predictive Potential Molecular Biomarkers in Colon Cancer. Chirurgia 2011, 106, 177–185. [Google Scholar] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fedewa, S.A.; Ahnen, D.J.; Meester, R.G.S.; Barzi, A.; Jemal, A. Colorectal Cancer Statistics, 2017. CA Cancer J. Clin. 2017, 67, 177–193. [Google Scholar] [CrossRef]
- Le Tourneau, C.; Delord, J.-P.; Gonçalves, A.; Gavoille, C.; Dubot, C.; Isambert, N.; Campone, M.; Trédan, O.; Massiani, M.-A.; Mauborgne, C.; et al. Molecularly Targeted Therapy Based on Tumour Molecular Profiling versus Conventional Therapy for Advanced Cancer (SHIVA): A Multicentre, Open-Label, Proof-of-Concept, Randomised, Controlled Phase 2 Trial. Lancet Oncol. 2015, 16, 1324–1334. [Google Scholar] [CrossRef]
- Sicklick, J.K.; Kato, S.; Okamura, R.; Schwaederle, M.; Hahn, M.E.; Williams, C.B.; De, P.; Krie, A.; Piccioni, D.E.; Miller, V.A.; et al. Molecular Profiling of Cancer Patients Enables Personalized Combination Therapy: The I-PREDICT Study. Nat. Med. 2019, 25, 744–750. [Google Scholar] [CrossRef]
- Alix-Panabieres, C.; Pantel, K. Circulating Tumor Cells: Liquid Biopsy of Cancer. Clin. Chem. 2013, 59, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Hardingham, J.E.; Hewett, P.J.; Sage, R.E.; Finch, J.L.; Nuttall, J.D.; Kotasek, D.; Dobrovic, A. Molecular Detection of Blood-Borne Epithelial Cells in Colorectal Cancer Patients and in Patients with Benign Bowel Disease. Int. J. Cancer 2000, 89, 8–13. [Google Scholar] [CrossRef]
- Pantel, K.; Denève, E.; Nocca, D.; Coffy, A.; Vendrell, J.-P.; Maudelonde, T.; Riethdorf, S.; Alix-Panabières, C. Circulating Epithelial Cells in Patients with Benign Colon Diseases. Clin. Chem. 2012, 58, 936–940. [Google Scholar] [CrossRef] [PubMed]
- Rhim, A.D.; Thege, F.I.; Santana, S.M.; Lannin, T.B.; Saha, T.N.; Tsai, S.; Maggs, L.R.; Kochman, M.L.; Ginsberg, G.G.; Lieb, J.G.; et al. Detection of Circulating Pancreas Epithelial Cells in Patients with Pancreatic Cystic Lesions. Gastroenterology 2014, 146, 647–651. [Google Scholar] [CrossRef] [PubMed]
- Rhim, A.D.; Mirek, E.T.; Aiello, N.M.; Maitra, A.; Bailey, J.M.; McAllister, F.; Reichert, M.; Beatty, G.L.; Rustgi, A.K.; Vonderheide, R.H.; et al. EMT and Dissemination Precede Pancreatic Tumor Formation. Cell 2012, 148, 349–361. [Google Scholar] [CrossRef] [PubMed]
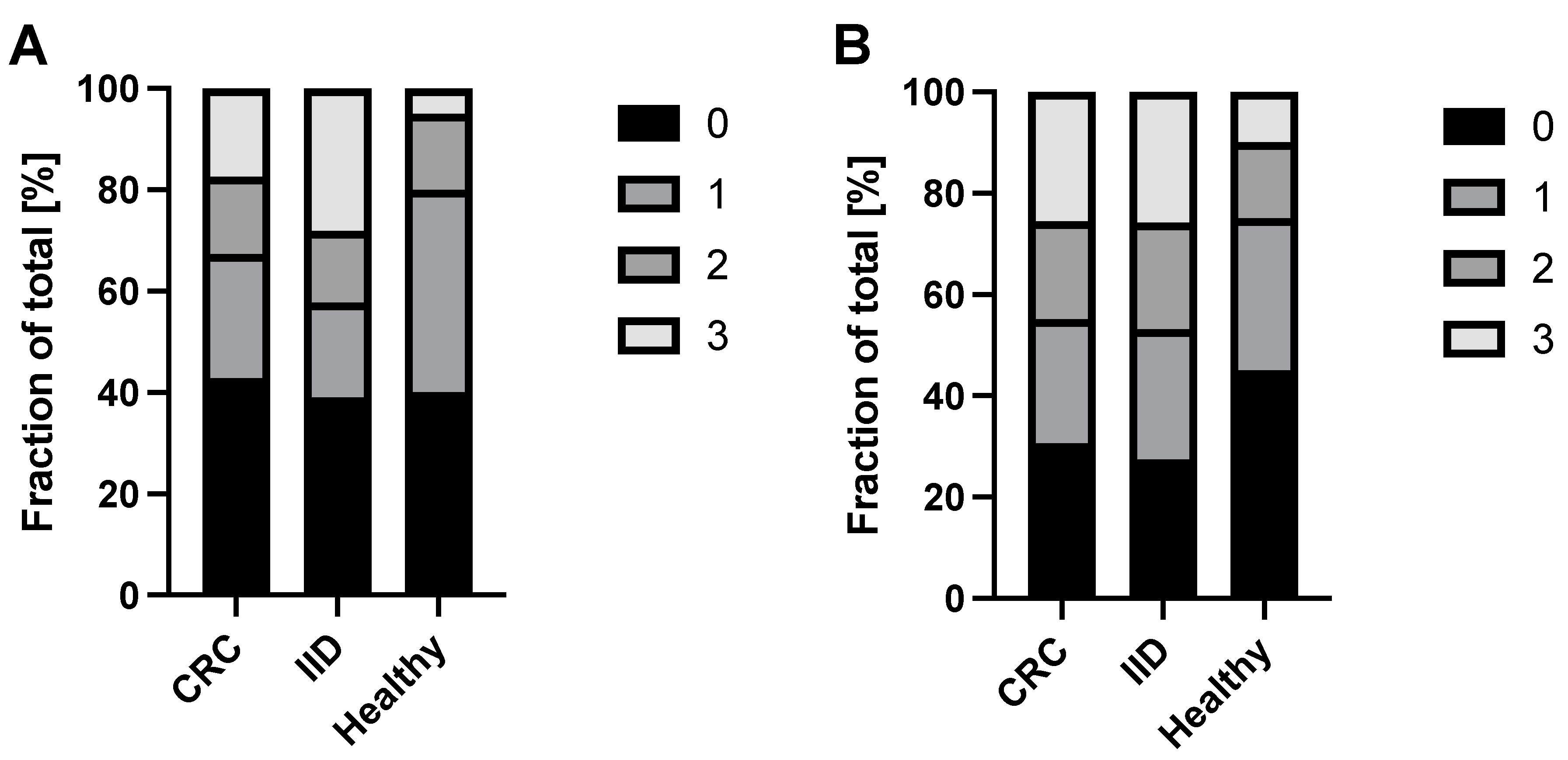

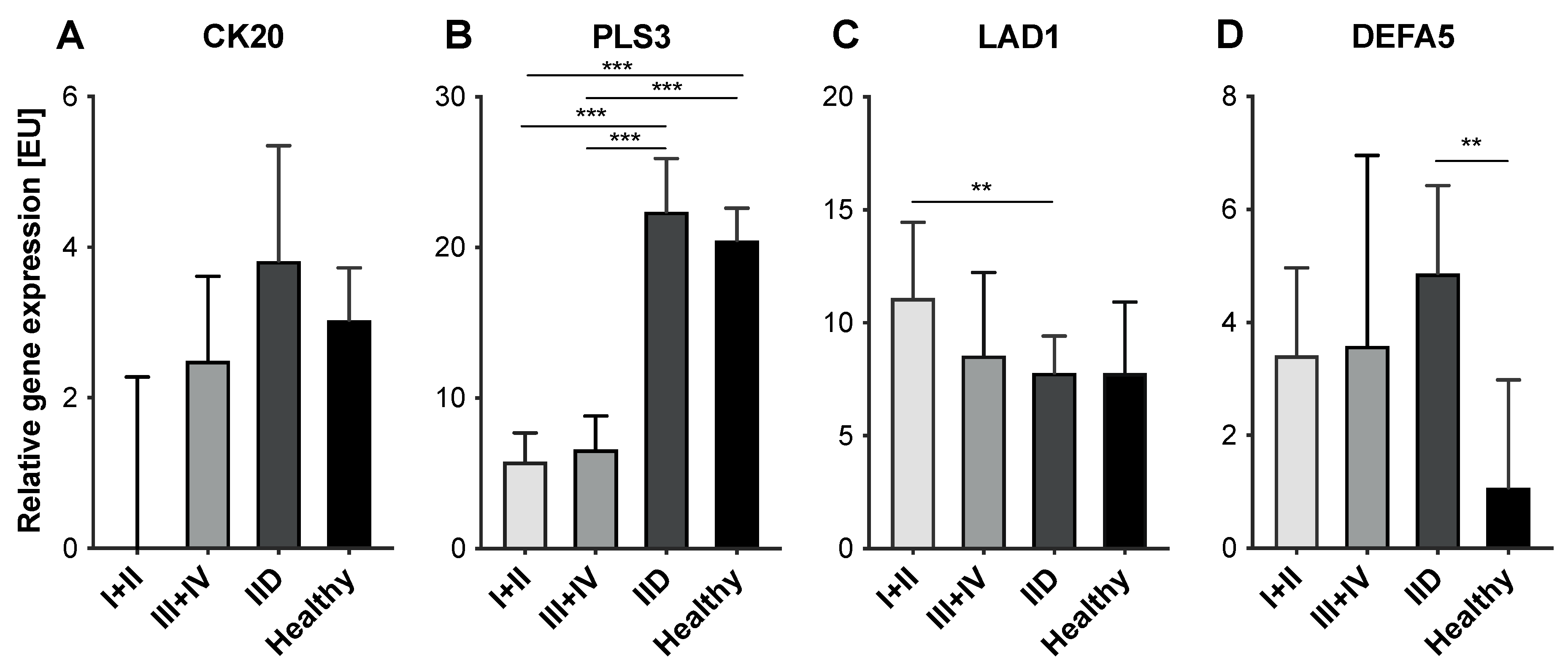
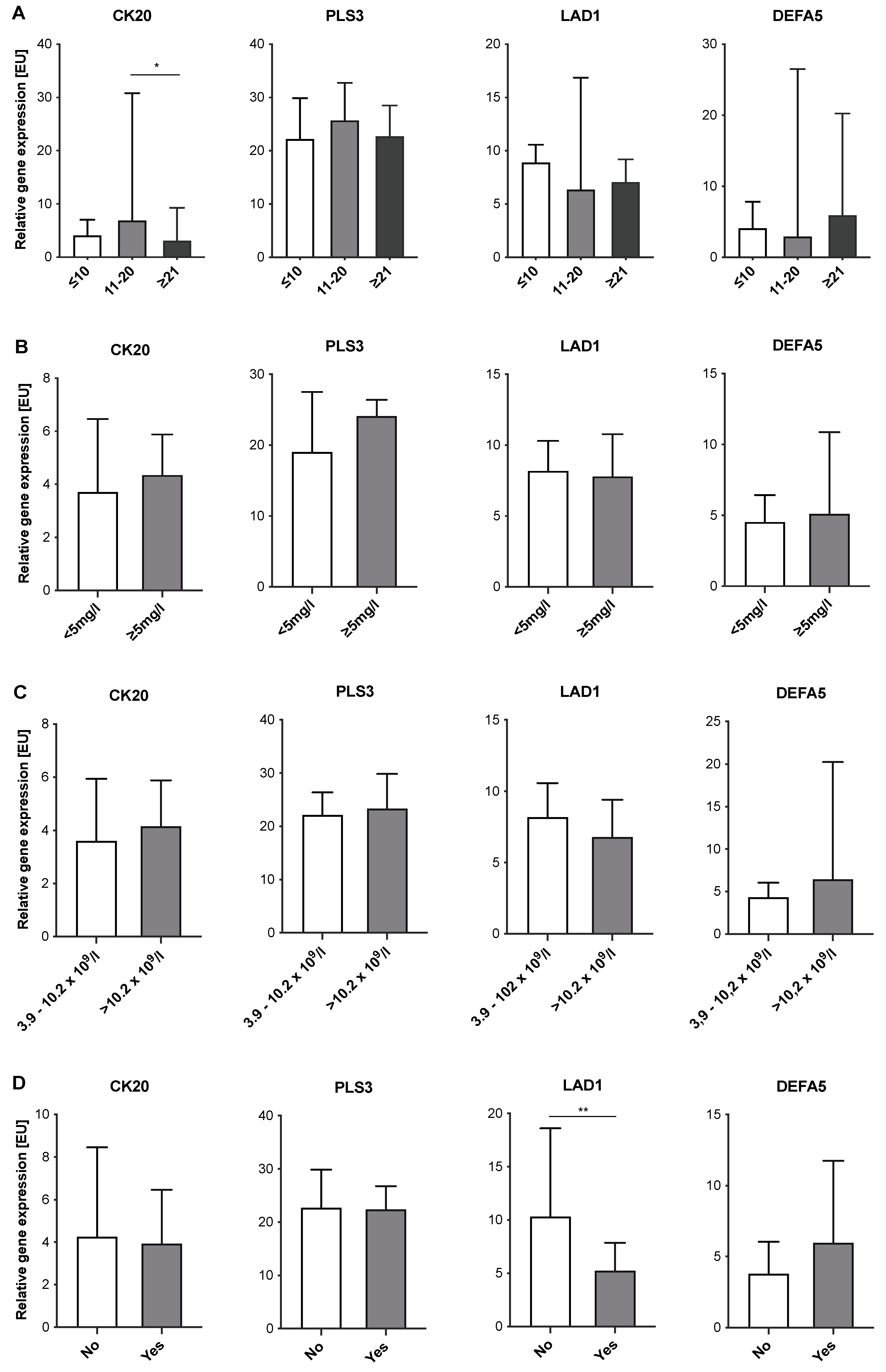
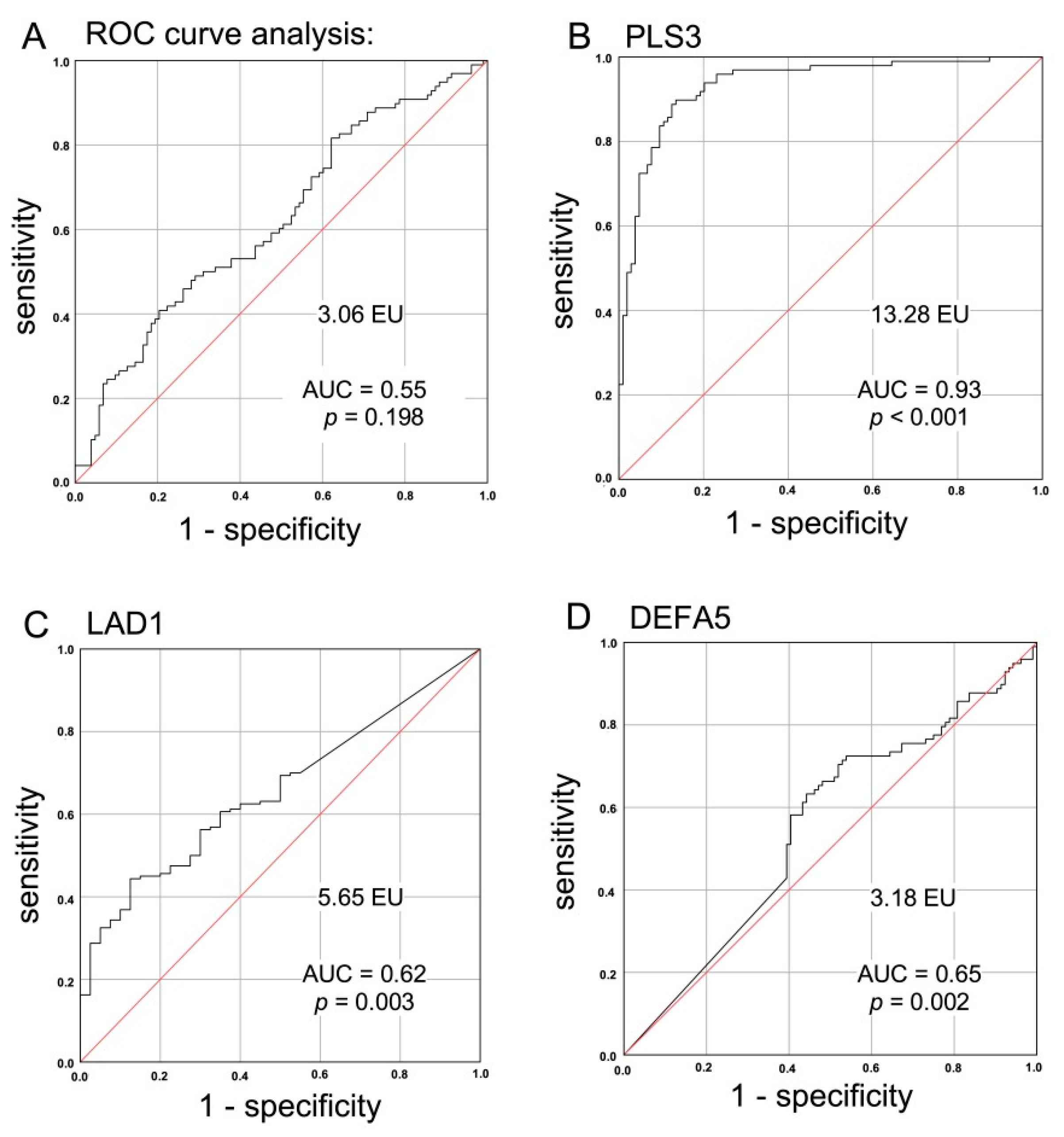
| Parameter | CRC Patients N (%) | IID Patients N (%) | Healthy Donors N (%) |
|---|---|---|---|
| 98 (100) | 64 (100) | 40 (100) | |
| Gender | |||
| Female | 41 (42) | 34 (53) | 25 (62,5) |
| Male | 57 (58) | 30 (47) | 15 (37,5) |
| Age in years | |||
| 20–29 | 0 (0) | 11 (17) | 10 (25) |
| 30–39 | 1 (1) | 9 (14) | 10 (25) |
| 40–49 | 5 (5) | 13 (20) | 8 (20) |
| 50–59 | 14 (14) | 15 (24) | 10 (25) |
| 60–69 | 24 (25) | 8 (13) | 2 (5) |
| 70–79 | 40 (41) | 6 (9) | 0 (0) |
| >80 | 14 (14) | 2 (3) | 0 (0) |
| mean | 69 | 48 | 40 |
| UICC stage | |||
| I | 7 (7) | - | - |
| II | 43 (44) | - | - |
| III | 6 (6) | - | - |
| IV | 42 (43) | - | - |
| Inflammatory intestinal disease | |||
| Crohn’s disease | - | 26 (41) | - |
| Ulcerative colitis | - | 22 (34) | - |
| Indeterminate colitis | - | 2 (3) | - |
| Sigmoid diverticulitis | - | 14 (22) | - |
| Parameter | CRC Patients N (%) | IID Patients N (%) | Healthy Donors N (%) | p |
|---|---|---|---|---|
| 98 (100) | 64 (100) | 40 (100) | ||
| CK20 | ||||
| + | 56 (57) | 39 (61) | 24 (60) | 0.880 |
| - | 42 (43) | 25 (39) | 16 (40) | |
| CK20 detection | ||||
| 0 | 42 (43) | 25 (39) | 16 (40) | 0.064 |
| 1 | 24 (25) | 12 (19) | 16(40) | |
| 2 | 15 (15) | 9 (14) | 6 (15) | |
| 3 | 17 (17) | 18 (28) | 2 (5) | |
| DEFA5 | ||||
| + | 68 (69) | 45 (73) | 22 (55) | 0.154 |
| - | 30 (31) | 17 (27) | 18 (45) | |
| DEFA5 detection | ||||
| 0 | 30 (31) | 17 (27) | 18 (45) | 0.340 |
| 1 | 24 (24) | 16 (26) | 12 (30) | |
| 2 | 19 (19) | 13 (21) | 6 (15) | |
| 3 | 25 (26) | 16 (26) | 4 (10) | |
| PLS3 | ||||
| + | 97 (99) | 64 (100) | 40 (100) | 1 |
| - | 1 (1) | 0 (0) | 0 (0) | |
| LAD1 | ||||
| + | 98 (100) | 63 (100) | 40 (100) | 1 |
| - | 0 (0) | 0 (0) | 0 (0) |
| Immunosuppressive Medication | Patient Count N (%) |
|---|---|
| 36 (100) | |
| Combination therapy | |
| Aminosalicylates + glucocorticoids | 9 (25) |
| Aminosalicylates + purine nucleoside analogues | 1 (3) |
| Aminosalicylates + monoclonal antibodies | 4 (11) |
| Glucocorticoids + purine nucleoside analogues | 3 (8) |
| Glucocorticoids + monoclonal antibodies | 10 (28) |
| Purine nucleoside analogues + monoclonal antibodies | 1 (3) |
| Aminosalicylates + glucocorticoids + monoclonal antibodies | 1 (3) |
| Aminosalicylates + glucocorticoids + purine nucleoside analogues | 1 (3) |
| Monotherapy | |
| Mesalazine | 3 (8) |
| Prednisolone | 1 (3) |
| Adalimumab | 1 (3) |
| Infliximab | 1 (3) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Born, J.; Hendricks, A.; Hauser, C.; Egberts, J.-H.; Becker, T.; Röder, C.; Sebens, S. Detection of Marker Associated with CTC in Colorectal Cancer in Mononuclear Cells of Patients with Benign Inflammatory Intestinal Diseases. Cancers 2022, 14, 47. https://doi.org/10.3390/cancers14010047
Born J, Hendricks A, Hauser C, Egberts J-H, Becker T, Röder C, Sebens S. Detection of Marker Associated with CTC in Colorectal Cancer in Mononuclear Cells of Patients with Benign Inflammatory Intestinal Diseases. Cancers. 2022; 14(1):47. https://doi.org/10.3390/cancers14010047
Chicago/Turabian StyleBorn, Johanna, Alexander Hendricks, Charlotte Hauser, Jan-Hendrik Egberts, Thomas Becker, Christian Röder, and Susanne Sebens. 2022. "Detection of Marker Associated with CTC in Colorectal Cancer in Mononuclear Cells of Patients with Benign Inflammatory Intestinal Diseases" Cancers 14, no. 1: 47. https://doi.org/10.3390/cancers14010047
APA StyleBorn, J., Hendricks, A., Hauser, C., Egberts, J.-H., Becker, T., Röder, C., & Sebens, S. (2022). Detection of Marker Associated with CTC in Colorectal Cancer in Mononuclear Cells of Patients with Benign Inflammatory Intestinal Diseases. Cancers, 14(1), 47. https://doi.org/10.3390/cancers14010047






