Immune Checkpoint Inhibitor Treatment and Ophthalmologist Consultations in Patients with Malignant Melanoma or Lung Cancer—A Nationwide Cohort Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
2.1. The Danish Health Care System
2.2. Data Sources
2.3. Study Population
2.4. Cohorts
2.5. Treatment with Immune Checkpoint Inhibitors
2.6. Ophthalmologist Consultation and Ocular Inflammation
2.7. Comorbidities
2.8. Statistical Methods
3. Results
3.1. Initial ICI Treatment
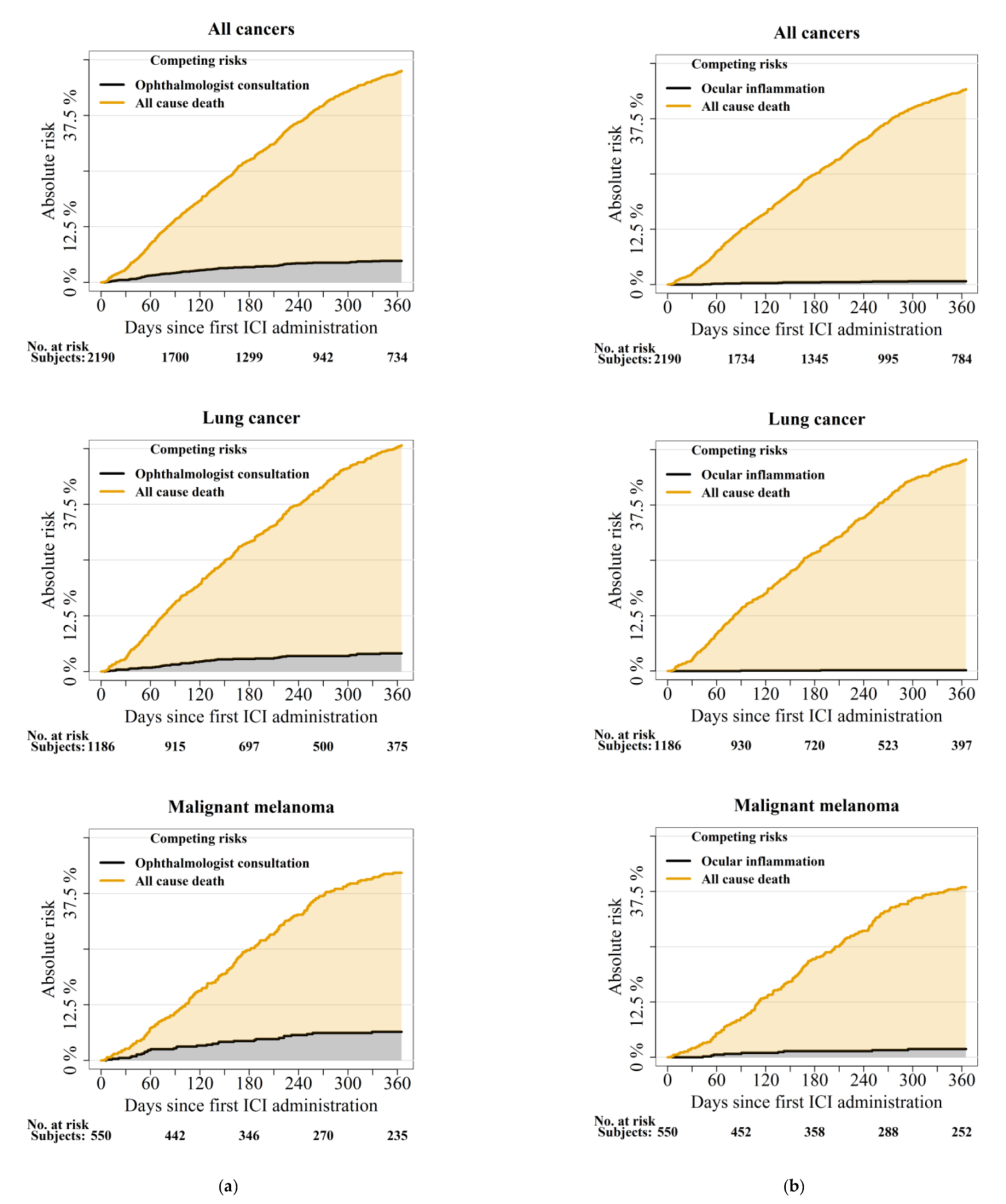
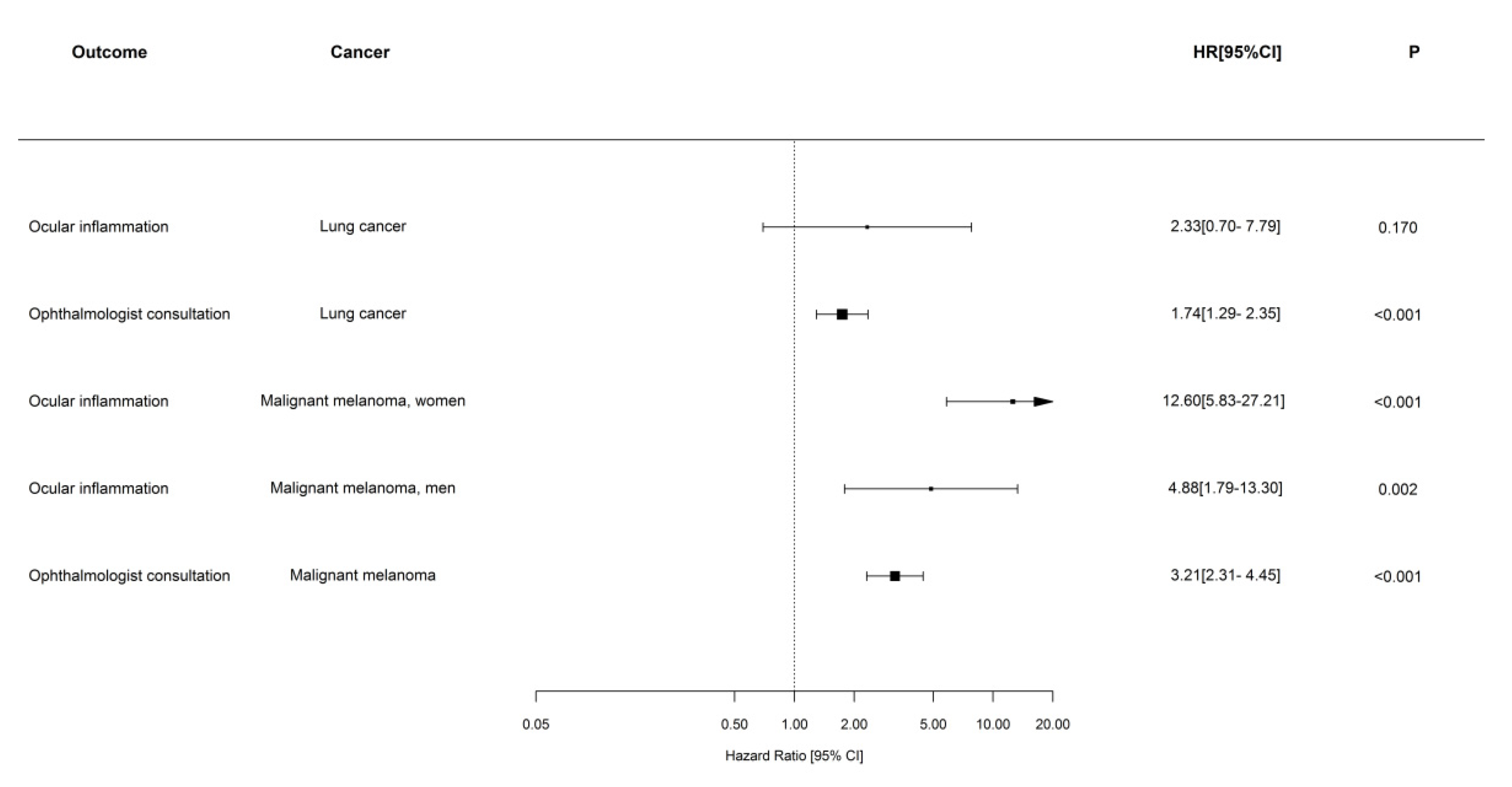
3.2. The Risk of Ophthalmologist Consultation and Ocular Inflammation
4. Discussion
4.1. One-Year Risks of First-Time Ophthalmologist Consultations and Incident Ocular Inflammation
4.2. Relative Rates
4.3. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Dummer, R.; Smylie, M.; Rutkowski, P.; et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N. Engl. J. Med. 2015, 373, 23–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus Chemotherapy for PD-L1–Positive Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.; Schachter, J.; Long, G.V.; Arance, A.; Grob, J.J.; Mortier, L.; Daud, A.; Carlino, M.S.; McNeil, C.; Lotem, M.; et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2015, 372, 2521–2532. [Google Scholar] [CrossRef]
- Haslam, A.; Prasad, V. Estimation of the Percentage of US Patients With Cancer Who Are Eligible for and Respond to Checkpoint Inhibitor Immunotherapy Drugs. JAMA Netw. Open 2019, 2, e192535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Youngnak, P.; Kozono, Y.; Kozono, H.; Iwai, H.; Otsuki, N.; Jin, H.; Omura, K.; Yagita, H.; Pardoll, D.M.; Chen, L.; et al. Differential Binding Properties of B7-H1 and B7-DC to Programmed Death-1. Biochem. Biophys. Res. Commun. 2003, 307, 672–677. [Google Scholar] [CrossRef]
- Huehn, M.; Gaebel, J.; Oeser, A.; Dietz, A.; Neumuth, T.; Wichmann, G.; Stoehr, M. Bayesian Networks to Support Decision-Making for Immune-Checkpoint Blockade in Recurrent/Metastatic (R/M) Head and Neck Squamous Cell Carcinoma (HNSCC). Cancers 2021, 13, 5890. [Google Scholar] [CrossRef] [PubMed]
- Pisibon, C.; Ouertani, A.; Bertolotto, C.; Ballotti, R.; Cheli, Y. Immune Checkpoints in Cancers: From Signaling to the Clinic. Cancers 2021, 13, 4573. [Google Scholar] [CrossRef] [PubMed]
- Michot, J.M.; Bigenwald, C.; Champiat, S.; Collins, M.; Carbonnel, F.; Postel-Vinay, S.; Berdelou, A.; Varga, A.; Bahleda, R.; Hollebecque, A.; et al. Immune-Related Adverse Events with Immune Checkpoint Blockade: A Comprehensive Review. Eur. J. Cancer 2016, 54, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Postow, M.A.; Sidlow, R.; Hellmann, M.D. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N. Engl. J. Med. 2018, 378, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Wang, C.; Ren, P.; Jiang, Y.; Tian, P.; Li, W. Treatment- and Immune-Related Adverse Events of Immune Checkpoint Inhibitors in Advanced Lung Cancer. Biosci. Rep. 2020, 40, BSR20192347. [Google Scholar] [CrossRef] [Green Version]
- Naidoo, J.; Page, D.B.; Li, B.T.; Connell, L.C.; Schindler, K.; Lacouture, M.E.; Postow, M.A.; Wolchok, J.D. Toxicities of the Anti-PD-1 and Anti-PD-L1 Immune Checkpoint Antibodies. Ann. Oncol. 2015, 26, 2375–2391. [Google Scholar] [CrossRef]
- Spiers, L.; Coupe, N.; Payne, M. Toxicities Associated with Checkpoint Inhibitors—An Overview. Rheumatology 2019, 58, vii7–vii16. [Google Scholar] [CrossRef]
- Brahmer, J.R.; Lacchetti, C.; Schneider, B.J.; Atkins, M.B.; Brassil, K.J.; Caterino, J.M.; Chau, I.; Ernstoff, M.S.; Gardner, J.M.; Ginex, P.; et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline. JCO 2018, 36, 1714–1768. [Google Scholar] [CrossRef]
- Parikh, R.A.; Chaon, B.C.; Berkenstock, M.K. Ocular Complications of Checkpoint Inhibitors and Immunotherapeutic Agents: A Case Series. Ocul. Immunol. Inflamm. 2020, 1–6. [Google Scholar] [CrossRef]
- Noble, C.W.; Gangaputra, S.S.; Thompson, I.A.; Yuan, A.; Apolo, A.B.; Lee, J.-M.; Papaliodis, G.N.; Kodati, S.; Bishop, R.; Magone, M.T.; et al. Ocular Adverse Events Following Use of Immune Checkpoint Inhibitors for Metastatic Malignancies. Ocul. Immunol. Inflamm. 2020, 28, 854–859. [Google Scholar] [CrossRef]
- Zhou, L.; Wei, X. Ocular Immune-Related Adverse Events Associated With Immune Checkpoint Inhibitors in Lung Cancer. Front. Immunol. 2021, 12, 701951. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Lee, J.S.; Lee, J.; Lee, S.C.; Kim, T.; Byeon, S.H.; Lee, C.S. Factors Associated with Ocular Adverse Event after Immune Checkpoint Inhibitor Treatment. Cancer Immunol. Immunother. 2020, 69, 2441–2452. [Google Scholar] [CrossRef] [PubMed]
- Pottegård, A.; Schmidt, S.A.J.; Wallach-Kildemoes, H.; Sørensen, H.T.; Hallas, J.; Schmidt, M. Data Resource Profile: The Danish National Prescription Registry. Int. J. Epidemiol. 2017, 46, 798–798f. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Schmidt, S.A.J.; Sandegaard, J.L.; Ehrenstein, V.; Pedersen, L.; Sørensen, H.T. The Danish National Patient Registry: A Review of Content, Data Quality, and Research Potential. Clin. Epidemiol. 2015, 7, 449–490. [Google Scholar] [CrossRef] [Green Version]
- Pedersen, C.B.; Gøtzsche, H.; Møller, J.Ø.; Mortensen, P.B. The Danish Civil Registration System. Dan. Med. Bull. 2006, 53, 441–449. [Google Scholar] [CrossRef]
- Helweg-Larsen, K. The Danish Register of Causes of Death. Scand. J. Public Health 2011, 39, 26–29. [Google Scholar] [CrossRef] [PubMed]
- Broe, M.; Mattson, T.; Bjødstrup, P.; Pottegård, A. Validity of Chemotherapy Procedure Codes in the Danish National Patient Registry. In Abstract Book; Danske Kræftforskningsdage: Odense, Denmark, 2019. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Abdel-Rahman, O.; Oweira, H.; Petrausch, U.; Helbling, D.; Schmidt, J.; Mannhart, M.; Mehrabi, A.; Schöb, O.; Giryes, A. Immune-Related Ocular Toxicities in Solid Tumor Patients Treated with Immune Checkpoint Inhibitors: A Systematic Review. Expert Rev. Anticancer. Ther. 2017, 17, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Wolchok, J.D.; Kluger, H.; Callahan, M.K.; Postow, M.A.; Rizvi, N.A.; Lesokhin, A.M.; Segal, N.H.; Ariyan, C.E.; Gordon, R.-A.; Reed, K.; et al. Nivolumab plus Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2013, 369, 122–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribas, A.; Hamid, O.; Daud, A.; Hodi, F.S.; Wolchok, J.D.; Kefford, R.; Joshua, A.M.; Patnaik, A.; Hwu, W.-J.; Weber, J.S.; et al. Association of Pembrolizumab With Tumor Response and Survival Among Patients With Advanced Melanoma. JAMA 2016, 315, 1600. [Google Scholar] [CrossRef]
- Bomze, D.; Meirson, T.; Hasan Ali, O.; Goldman, A.; Flatz, L.; Habot-Wilner, Z. Ocular Adverse Events Induced by Immune Checkpoint Inhibitors: A Comprehensive Pharmacovigilance Analysis. Ocul. Immunol. Inflamm. 2020, 1–7. [Google Scholar] [CrossRef]
- Bitton, K.; Michot, J.-M.; Barreau, E.; Lambotte, O.; Haigh, O.; Marabelle, A.; Voisin, A.-L.; Mateus, C.; Rémond, A.-L.; Couret, C.; et al. Prevalence and Clinical Patterns of Ocular Complications Associated With Anti-PD-1/PD-L1 Anticancer Immunotherapy. Am. J. Ophthalmol. 2019, 202, 109–117. [Google Scholar] [CrossRef]
- Fang, T.; Maberley, D.A.; Etminan, M. Ocular Adverse Events with Immune Checkpoint Inhibitors. J. Curr. Ophthalmol. 2019, 31, 319–322. [Google Scholar] [CrossRef]
- Dalvin, L.A.; Shields, C.L.; Orloff, M.; Sato, T.; Shields, J.A. Checkpoint Inhibitor Immune Therapy Systemic Indications and Ophthalmic Side Effects. Retina 2018, 38, 1063–1078. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.M.; Seleme, N.; Chen, J.J.; Zekeridou, A.; Sechi, E.; Walsh, R.D.; Beebe, J.D.; Sabbagh, O.; Mejico, L.J.; Gratton, S.; et al. Neuro-Ophthalmic Complications in Patients Treated With CTLA-4 and PD-1/PD-L1 Checkpoint Blockade. J. Neuro-Ophthalmol. 2021, 41, 519–530. [Google Scholar] [CrossRef]
- Reddy, M.; Chen, J.J.; Kalevar, A.; Terribilini, R.; Agarwal, A. Immune Retinopathy Associated with Nivolumab Administration for Metastatic Non-Small Cell Lung Cancer. Retin. Cases Brief Rep. 2020, 14, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Materin, M.A.; Sznol, M.; Kluger, H.M.; Weiss, S.; Chow, J.; Stoessel, K.; Kombo, N.; Del Priore, L.; Pointdujour-Lim, R. Ophthalmic Immune-Related Adverse Events of Immunotherapy: A Single-Site Case Series. Ophthalmology 2019, 126, 1058–1062. [Google Scholar] [CrossRef] [PubMed]
- Fortes, B.H.; Liou, H.; Dalvin, L.A. Ophthalmic Adverse Effects of Immune Checkpoint Inhibitors: The Mayo Clinic Experience. Br. J. Ophthalmol. 2020, 105, 1263–1271. [Google Scholar] [CrossRef] [PubMed]

| Patients with Cancer N (%) | 112,260 (100) |
|---|---|
| Cancer type, N (%) | |
| Skin cancer | 45,661 (40.7) |
| Lung cancer | 29,337 (26.1) |
| Malignant cutaneous melanoma | 16,023 (14.3) |
| Head and neck cancer | 6526 (5.8) |
| Urinary tract cancer | 7133 (6.4) |
| Kidney cancer | 6493 (5.8) |
| Hodgkin’s lymphoma | 1087 (1.0) |
| Age, median [p25–p75] | 69.4 (59.9–77.5) |
| Male sex, N (%) | 59,875 (53.3) |
| Medical history, N (%) | |
| Diabetes mellitus | 10,934 (9.7) |
| Morbus Bechterew | 233 (0.2) |
| Chronic tissue disease | 1647 (1.5) |
| Inflammatory arthritis | 3874 (3.5) |
| Hypertension | 37,055 (33.0) |
| Chronic kidney disease | 4095 (3.6) |
| Sarcoidosis | 250 (0.2) |
| Syphilis | 7 (0.0) |
| Multiple sclerosis | 314 (0.3) |
| Borrelia infection | 95 (0.1) |
| Human immunodeficiency virus infection | 5 (0.0) |
| Juvenile arthritis | 33 (0.0) |
| Patients Treated with Immune Checkpoint Inhibitor | |
|---|---|
| Immune checkpoint inhibitor, N (%) | |
| Ipilimumab | 239 (10.9) |
| Pembrolizumab | 806 (36.7) |
| Nivolumab | 942 (43.2) |
| Atezolizumab | 73 (3.3) |
| Durvalumab | 6 (0.3) |
| Ipilimumab + Nivolumab | 122 (43.2) |
| Cancer type, N (%) | |
| Lung cancer | 1186 (54.2) |
| Head and neck cancer | 59 (2.7) |
| Urinary tract cancer | 111(5.1) |
| Malignant cutaneous melanoma | 550 (25.1) |
| Kidney cancer | 220 (10.0) |
| Skin cancer | 54 (2.5) |
| Hodgkin’s lymphoma | 10 (0.5) |
| Age. Median [p25–p75] | 67 (59–73) |
| Male sex, N (%) | 1239 (56.4) |
| Medical history, N (%) | |
| Diabetes mellitus | 224 (10.4) |
| Morbus Bechterew | NA |
| Chronic tissue disease | 18 (0.8) |
| Inflammatory arthritis | 59 (2.7) |
| Hypertension | 612 (27.9) |
| Chronic kidney disease | 61 (2.8) |
| Sarcoidosis | 11 (0.5) |
| Syphilis | NA |
| Multiple sclerosis | 5 (0.2) |
| Borrelia infection | NA |
| Human immunodeficiency virus infection | NA |
| Juvenile arthritis | NA |
| Outcome | Subgroup (N/Included in Analysis) | N | Absolute Risk (95% CI) | N | Absolute Risk (95% CI) | N | Absolute Risk (95% CI) |
|---|---|---|---|---|---|---|---|
| 30d | 30 Days | 182d | 6 Months | 365d | 1 Year | ||
| Ocular inflammation | All cancers (2119/2190) | NA | NA | 10 | 0.5 (0.2–0.8) | 14 | 0.8 (0.4–1.2) |
| Ophthalmologist consultation | All cancers (1648/2190) | 12 | 0.7 (03–1.1) | 69 | 4.5 (3.4–5.5) | 92 | 6.3 (5–7.6) |
| Uveitis | All cancers (2179/2190) | NA | NA | 8 | 0.4 (0.1–0.7) | 9 | 0.5 (0.2–0.8) |
| All cause death | All cancers (2190/2190) | 74 | 97.5 (96.8–98.1) | 618 | 75.3 (73.4–77.2) | 1023 | 56.4 (54.0–58.7) |
| Ocular inflammation | Lung cancer (1144/1186) | NA | NA | NA | NA | NA | NA |
| Ophthalmologist consultation | Lung cancer (898/1186) | 5 | 0.6 (0.1–1.1) | 31 | 3.7 (2.4–5.0) | 42 | 5.4 (3.8–7.0) |
| All cause death | Lung cancer (1186/1186) | 41 | 97.4 (96.4–98.3) | 369 | 73.3 (70.6–75.9) | 614 | 52.3 (49.1–55.6) |
| Ocular inflammation | Malignant melanoma (531/550) | NA | NA | 7 | 1.5 (0.4–2.5) | 9 | 1.9 (0.7–3.2) |
| Ophthalmologist consultation | Malignant melanoma (428/550) | NA | NA | 22 | 5.6 (3.3–7.9) | 31 | 8.2 (5.4–11.0) |
| All cause death | Malignant melanoma (550/550) | 14 | 97.9 (96.7–99.1) | 124 | 78.7 (75.0–82.3) | 200 | 62.9 (58.5–67.3) |
| Outcome | N | Median | P25 | P75 | |
|---|---|---|---|---|---|
| Ocular inflammation | All cancers | 17 | 140 | 69 | 250 |
| Uveitis | All cancers | 10 | 102 | 54 | 142 |
| Ophthalmologist consultation | All cancers | 108 | 115 | 53 | 223 |
| All cause death | All cancers | 1399 | 212 | 101 | 391 |
| Ocular inflammation | Lung cancer | NA | NA | NA | NA |
| Ophthalmologist consultation | Lung cancer | 48 | 116 | 69 | 223 |
| All cause death | Lung cancer | 822 | 206 | 94 | 368 |
| Ocular inflammation | Malignant melanoma | 11 | 135 | 63 | 272 |
| Ophthalmologist consultation | Malignant melanoma | 38 | 139 | 54 | 245 |
| All cause death | Malignant melanoma | 294 | 223 | 117 | 265 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Souza, M.; Bagger, M.; Alberti, M.; Malmborg, M.; Schou, M.; Torp-Pedersen, C.; Gislason, G.; Svane, I.M.; Kiilgaard, J.F. Immune Checkpoint Inhibitor Treatment and Ophthalmologist Consultations in Patients with Malignant Melanoma or Lung Cancer—A Nationwide Cohort Study. Cancers 2022, 14, 49. https://doi.org/10.3390/cancers14010049
D’Souza M, Bagger M, Alberti M, Malmborg M, Schou M, Torp-Pedersen C, Gislason G, Svane IM, Kiilgaard JF. Immune Checkpoint Inhibitor Treatment and Ophthalmologist Consultations in Patients with Malignant Melanoma or Lung Cancer—A Nationwide Cohort Study. Cancers. 2022; 14(1):49. https://doi.org/10.3390/cancers14010049
Chicago/Turabian StyleD’Souza, Maria, Mette Bagger, Mark Alberti, Morten Malmborg, Morten Schou, Christian Torp-Pedersen, Gunnar Gislason, Inge Marie Svane, and Jens Folke Kiilgaard. 2022. "Immune Checkpoint Inhibitor Treatment and Ophthalmologist Consultations in Patients with Malignant Melanoma or Lung Cancer—A Nationwide Cohort Study" Cancers 14, no. 1: 49. https://doi.org/10.3390/cancers14010049
APA StyleD’Souza, M., Bagger, M., Alberti, M., Malmborg, M., Schou, M., Torp-Pedersen, C., Gislason, G., Svane, I. M., & Kiilgaard, J. F. (2022). Immune Checkpoint Inhibitor Treatment and Ophthalmologist Consultations in Patients with Malignant Melanoma or Lung Cancer—A Nationwide Cohort Study. Cancers, 14(1), 49. https://doi.org/10.3390/cancers14010049