A New Approach for a Safe and Reproducible Seeds Positioning for Diffusing Alpha-Emitters Radiation Therapy of Squamous Cell Skin Cancer: A Feasibility Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. DaRT Technique Overview
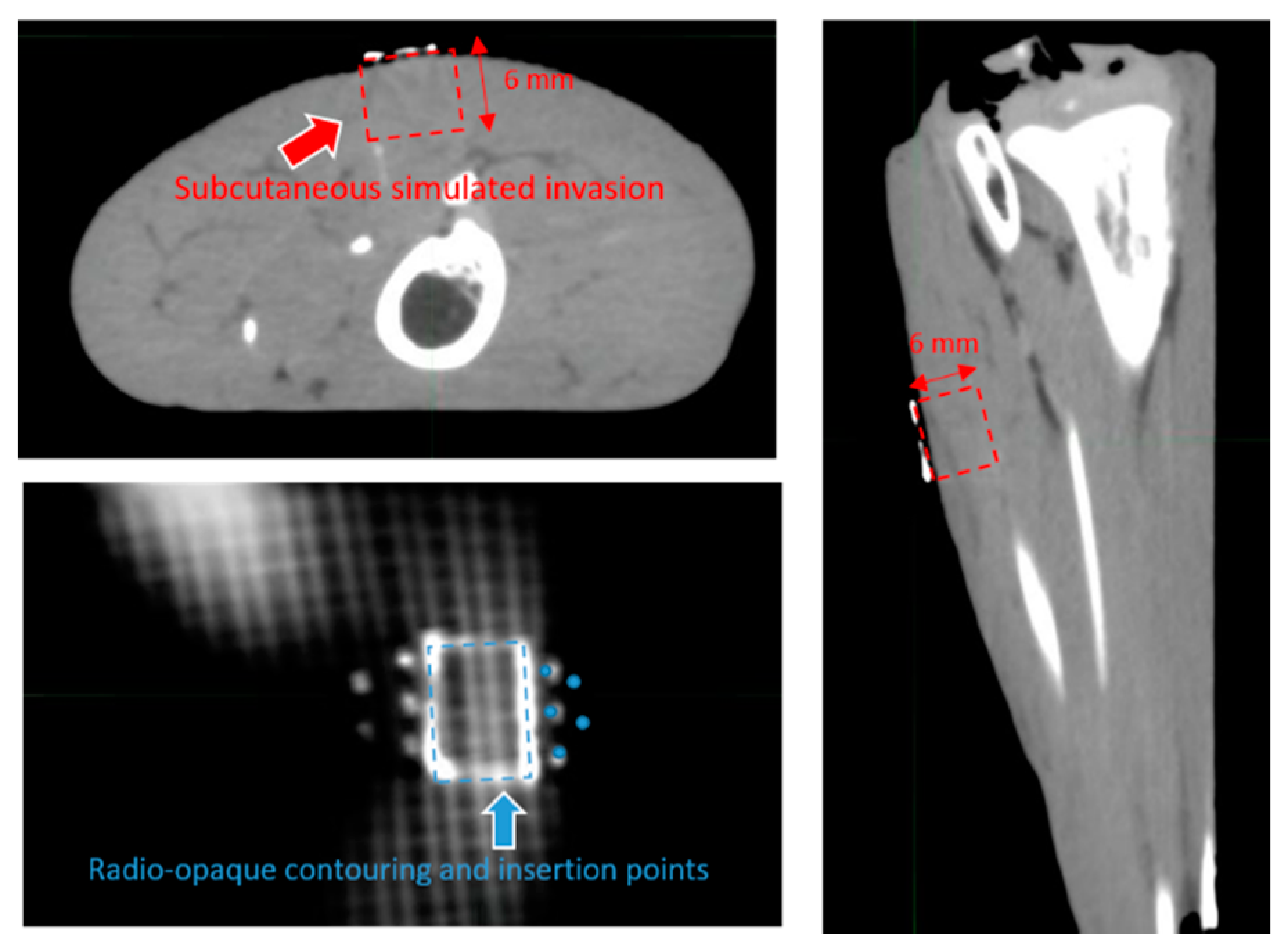
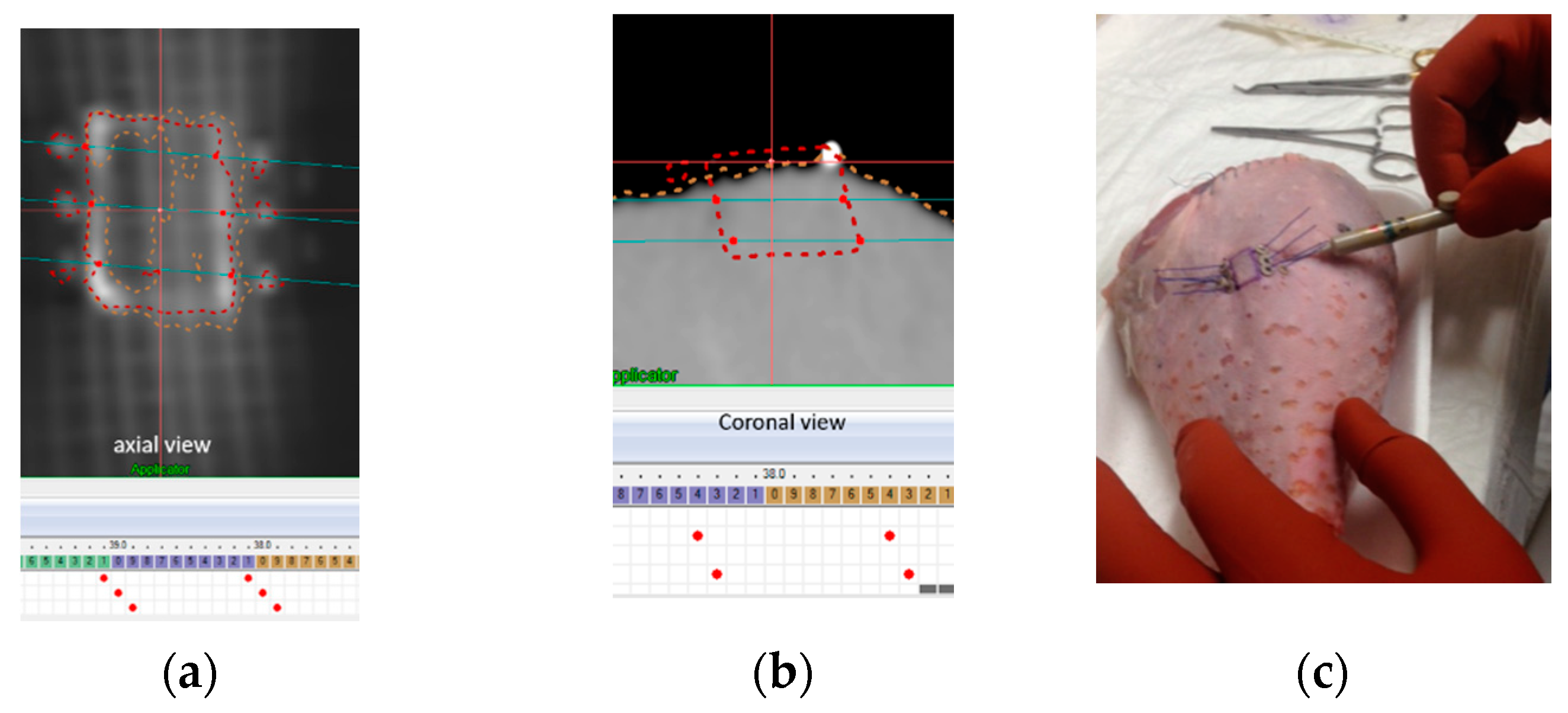
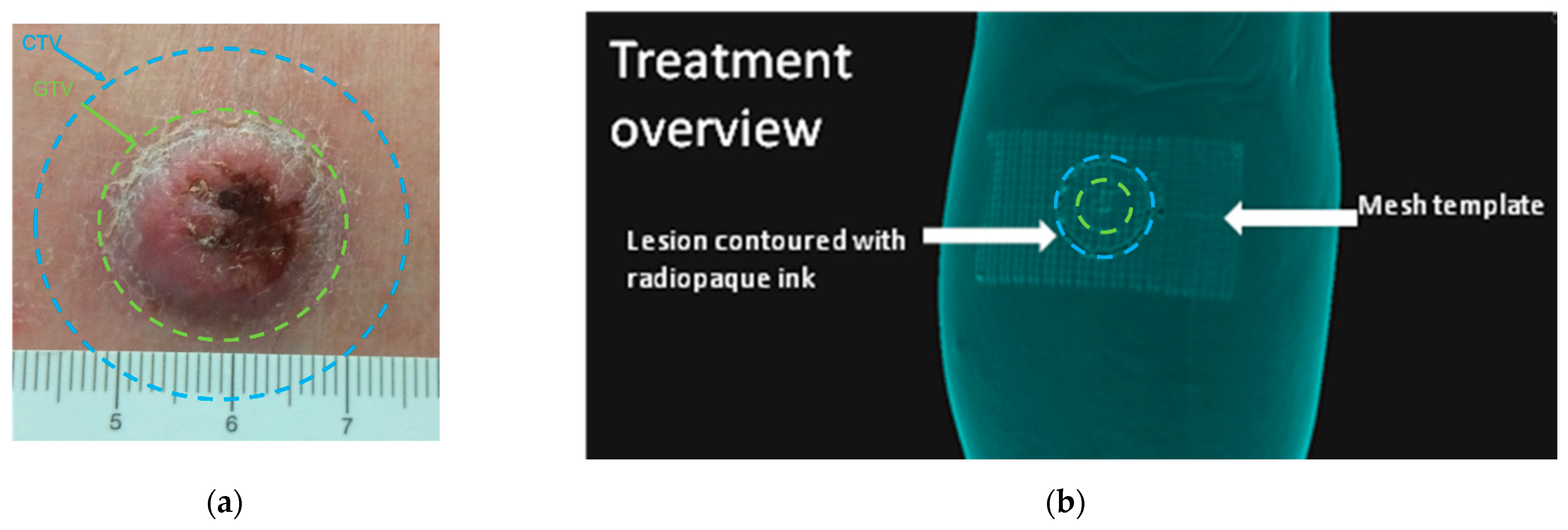
2.2. Template Based Planning Phase 1: Phantom Training
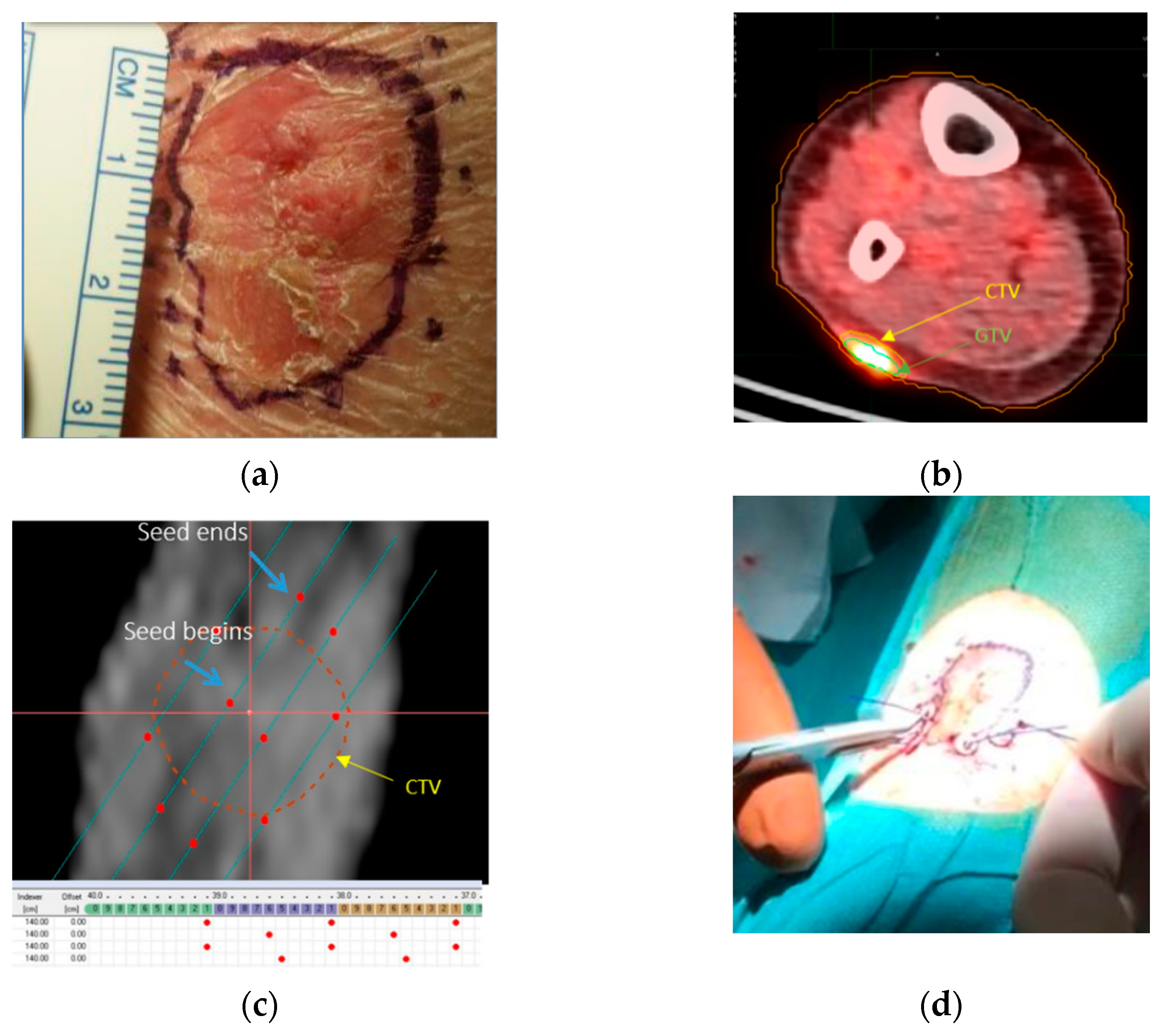
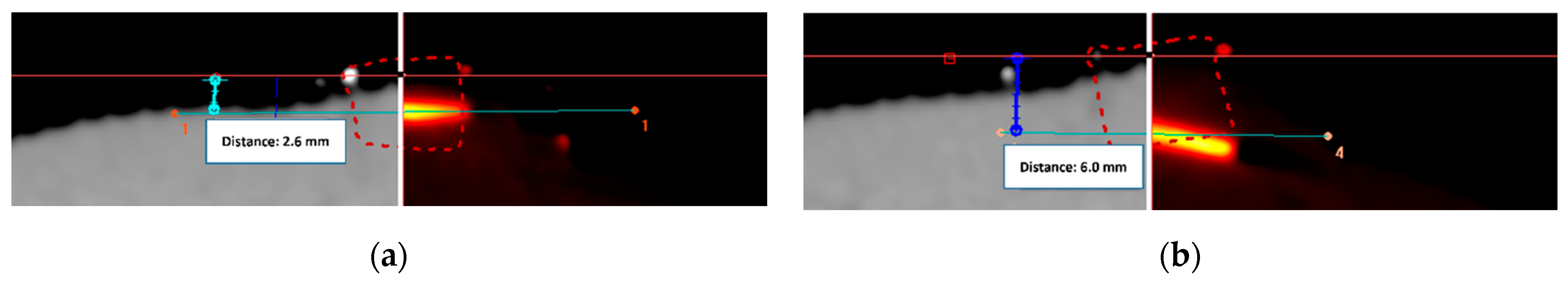
2.3. Template Based Planning Phase 2: Patient Application
3. Results
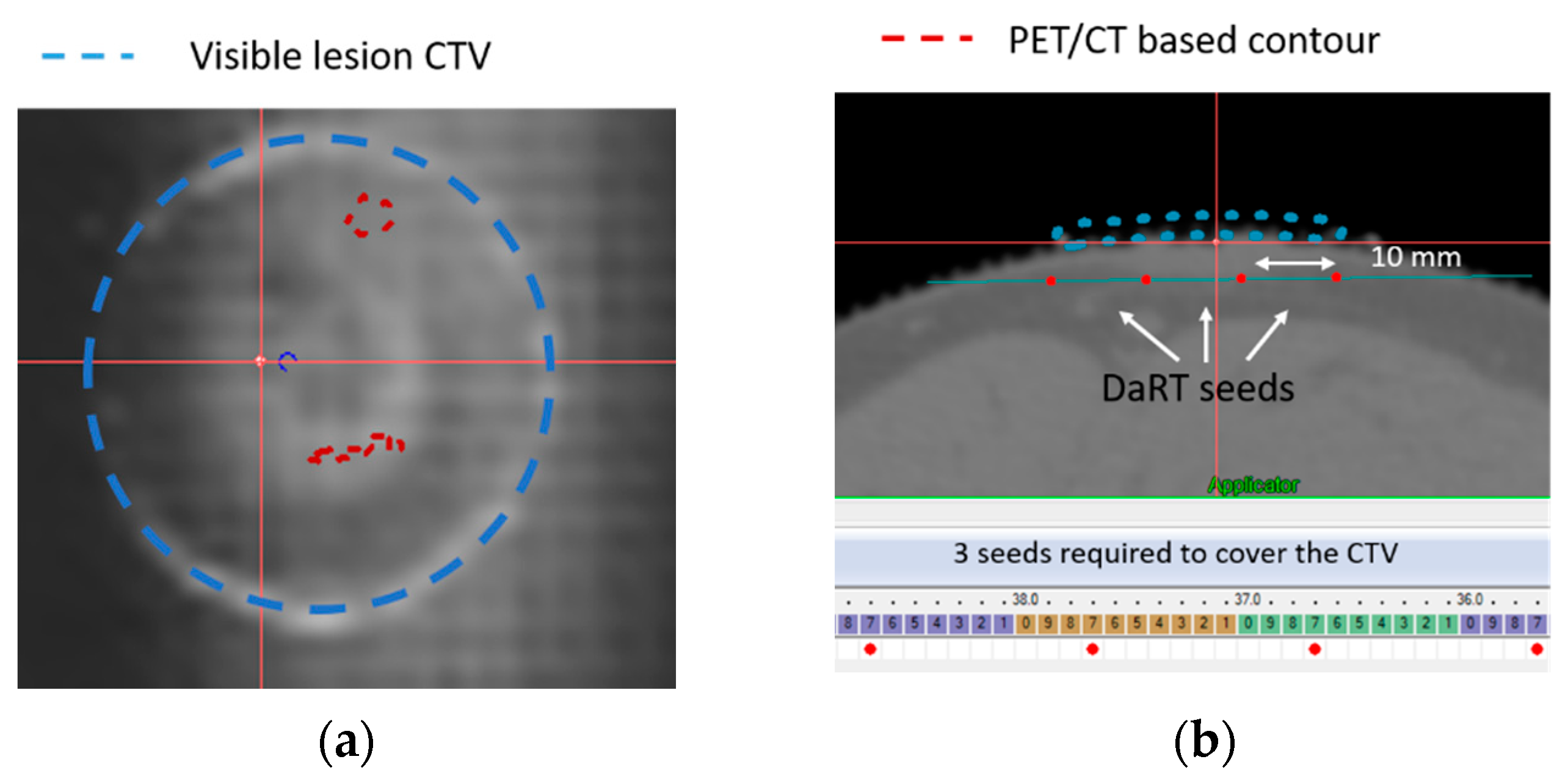
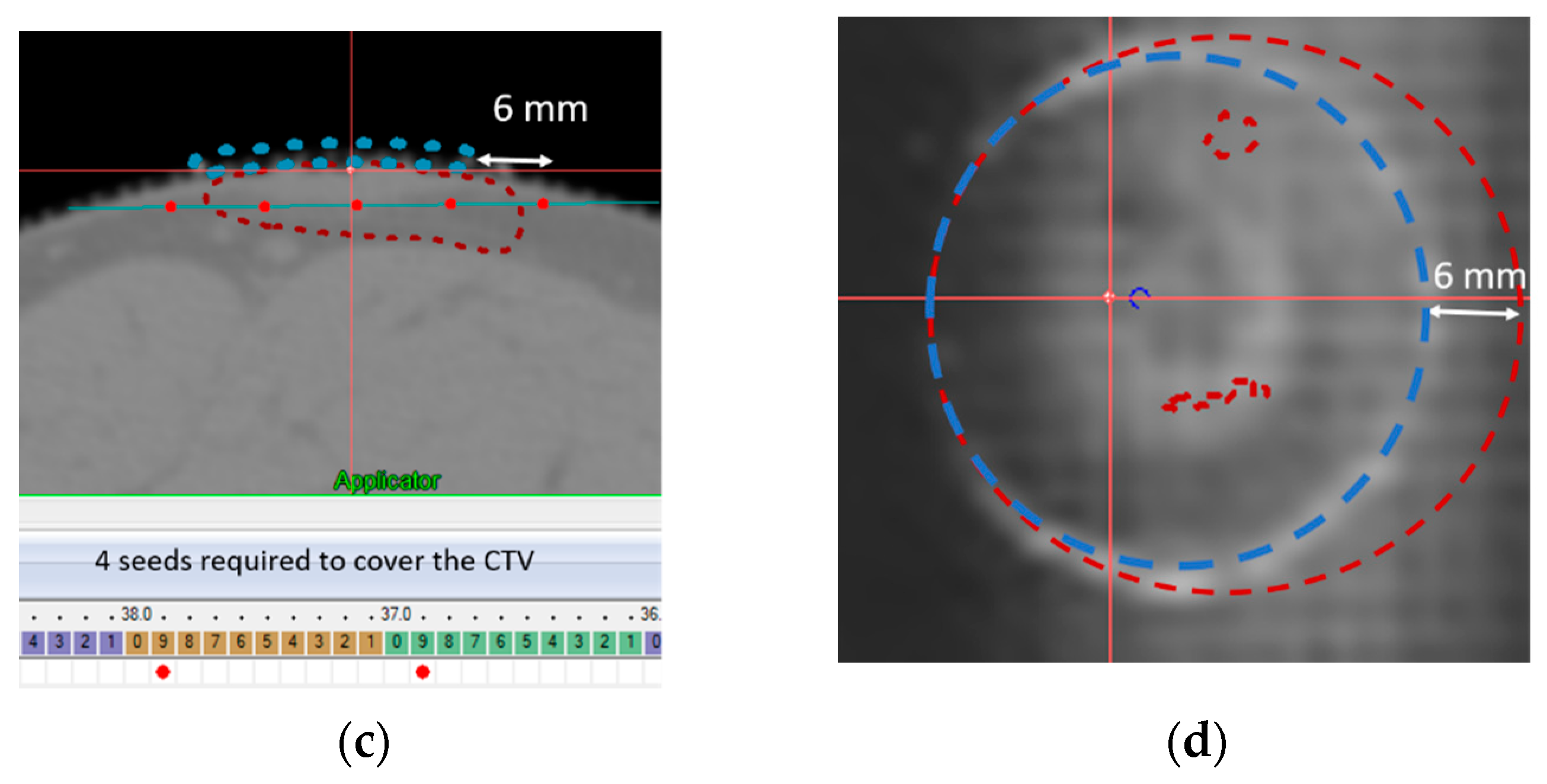
3.1. Template Based Planning Phase 1: Phantom Training
3.2. Template Based Planning Phase 2: Patient Application
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- NCCN. Squamous cell skin cancer Version 1. Medlin. Med. Encycl. 2021, 1–64. [Google Scholar]
- Schulte, K.-W.; Lippold, A.; Auras, C.; Bramkamp, G.; Breitkopf, C.; Elsmann, H.-J.; Habenicht, E.M.; Jasnoch, V.; Müller-Pannes, H.; Rupprecht, R.; et al. Soft X-ray therapy for cutaneous basal cell and squamous cell carcinomas. J. Am. Acad. Dermatol. 2005, 53, 993–1001. [Google Scholar] [CrossRef] [PubMed]
- Rowe, D.E.; Carroll, R.J.; Day, C.L.J. Prognostic factors for local recurrence, metastasis, and survival rates in squamous cell carcinoma of the skin, ear, and lip. Implications for treatment modality selection. J. Am. Acad. Dermatol. 1992, 26, 976–990. [Google Scholar] [CrossRef]
- Cognetta, A.B.; Howard, B.M.; Heaton, H.P.; Stoddard, E.R.; Hong, H.G.; Green, W.H. Superficial X-ray in the treatment of basal and squamous cell carcinomas: A viable option in select patients. J. Am. Acad. Dermatol. 2012, 67, 1235–1241. [Google Scholar] [CrossRef] [PubMed]
- Grossi Marconi, D.; da Costa Resende, B.; Rauber, E.; de Cassia Soares, P.; Fernandes, J.M.J.; Mehta, N.; Lopes Carvalho, A.; Kupelian, P.A.; Chen, A. Head and Neck Non-Melanoma Skin Cancer Treated By Superficial X-ray Therapy: An Analysis of 1021 Cases. PLoS ONE 2016, 11, e0156544. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Machin, B.; Borrego, L.; Gil-García, M.; Hernández, B.H. Office-based radiation therapy for cutaneous carcinoma: Evaluation of 710 treatments. Int. J. Dermatol. 2007, 46, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Lansbury, L.; Leonardi-Bee, J.; Perkins, W.; Goodacre, T.; Tweed, J.A.; Bath-Hextall, F.J. Interventions for non-metastatic squamous cell carcinoma of the skin. Cochrane Database Syst. Rev. 2010, 4, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Arazi, L.; Cooks, T.; Schmidt, M.; Keisari, Y.; Kelson, I. Treatment of solid tumors by interstitial release of recoiling short-lived alpha emitters. Phys. Med. Biol. 2007, 52, 5025–5042. [Google Scholar] [CrossRef] [PubMed]
- Arazi, L.; Efrati, M.; Kelson, I.; Keisari, Y. Local control of experimental malignant pancreatic tumors by treatment with a combination of chemotherapy and intratumoral 224Radium-loaded wires releasing alpha-emitting atoms. Transl. Res. 2012, 159, 32–41. [Google Scholar] [CrossRef]
- Confino, H.; Schmidt, M.; Hochman, I.; Umansky, V.; Kelson, I.; Keisari, Y. Inhibition of mouse breast adenocarcinoma growth by ablation with intratumoral alpha-irradiation combined with inhibitors of immunosuppression and CpG. Cancer Immunol. Immunother. 2016, 65, 1149. [Google Scholar] [CrossRef] [PubMed]
- Domankevich, V.; Cohen, A.; Efrati, M.; Schmidt, M.; Rammensee, H.-G.; Nair, S.S.; Tewari, A.; Kelson, I.; Keisari, Y. Combining alpha radiation-based brachytherapy with immunomodulators promotes complete tumor regression in mice via tumor-specific long-term immune response. Cancer Immunol. Immunother. 2019, 68, 1949–1958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellia, S.S.R.; Feliciani, G.; Del Duca, M.; Monti, M.; Turri, V.; Sarnelli, A.; Romeo, A.; Kelson, I.; Keisari, Y.; Popovtzer, A.; et al. Clinical evidence of abscopal effect in cutaneous squamous cell carcinoma treated with diffusing alpha emitters radiation therapy: A case report. J. Contemp. Brachyther. 2019, 11, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Keisari, Y.; Kelson, I. The Potentiation of Anti-Tumor Immunity by Tumor Abolition with Alpha Particles, Protons, or Carbon Ion Radiation and Its Enforcement by Combination with Immunoadjuvants or Inhibitors of Immune Suppressor Cells and Checkpoint Molecules. Cells 2021, 10, 228. [Google Scholar] [CrossRef] [PubMed]
- Cooks, T.; Arazi, L.; Efrati, M.; Schmidt, M.; Marshak, G. Interstitial Wires Releasing Diffusing Alpha Emitters Combined With Chemotherapy Improved Local Tumor Control and Survival in Squamous Cell Carcinoma-bearing Mice. Cancer 2009, 115, 1791–1801. [Google Scholar] [CrossRef] [PubMed]
- Arazi, L.; Cooks, T.; Schmidt, M.; Keisari, Y.; Kelson, I. The treatment of solid tumors by alpha emitters released from 224 Ra-loaded sources—Internal dosimetry analysis. Phys. Med. Biol. 2010, 55, 1203–1218. [Google Scholar] [CrossRef] [PubMed]
- Popovtzer, A.; Rosenfeld, E.; Mizrachi, A.; Bellia, S.R.; Ben-Hur, R.; Feliciani, G.; Sarnelli, A.; Arazi, L.; Deutsch, L.; Kelson, I.; et al. Initial Safety and Tumor Control Results From a “First-in-Human” Multicenter Prospective Trial Evaluating a Novel Alpha-Emitting Radionuclide for the Treatment of Locally Advanced Recurrent Squamous Cell Carcinomas of the Skin and Head and Neck. Int. J. Radiat. Oncol. Biol. Phys. 2020, 106, 571–578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guinot, J.L.; Rembielak, A.; Perez-Calatayud, J.; Rodríguez-Villalba, S.; Skowronek, J.; Tagliaferri, L.; Guix, B.; Gonzalez-Perez, V.; Valentini, V.; Kovacs, G. GEC-ESTRO ACROP recommendations in skin brachytherapy. Radiother. Oncol. 2018, 126, 377–385. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feliciani, G.; Bellia, S.R.; Del Duca, M.; Mazzotti, G.; Monti, M.; Stanganelli, I.; Keisari, Y.; Kelson, I.; Popovtzer, A.; Romeo, A.; et al. A New Approach for a Safe and Reproducible Seeds Positioning for Diffusing Alpha-Emitters Radiation Therapy of Squamous Cell Skin Cancer: A Feasibility Study. Cancers 2022, 14, 240. https://doi.org/10.3390/cancers14010240
Feliciani G, Bellia SR, Del Duca M, Mazzotti G, Monti M, Stanganelli I, Keisari Y, Kelson I, Popovtzer A, Romeo A, et al. A New Approach for a Safe and Reproducible Seeds Positioning for Diffusing Alpha-Emitters Radiation Therapy of Squamous Cell Skin Cancer: A Feasibility Study. Cancers. 2022; 14(1):240. https://doi.org/10.3390/cancers14010240
Chicago/Turabian StyleFeliciani, Giacomo, Salvatore Roberto Bellia, Massimo Del Duca, Giorgio Mazzotti, Manuela Monti, Ignazio Stanganelli, Yona Keisari, Itzhak Kelson, Aron Popovtzer, Antonino Romeo, and et al. 2022. "A New Approach for a Safe and Reproducible Seeds Positioning for Diffusing Alpha-Emitters Radiation Therapy of Squamous Cell Skin Cancer: A Feasibility Study" Cancers 14, no. 1: 240. https://doi.org/10.3390/cancers14010240
APA StyleFeliciani, G., Bellia, S. R., Del Duca, M., Mazzotti, G., Monti, M., Stanganelli, I., Keisari, Y., Kelson, I., Popovtzer, A., Romeo, A., & Sarnelli, A. (2022). A New Approach for a Safe and Reproducible Seeds Positioning for Diffusing Alpha-Emitters Radiation Therapy of Squamous Cell Skin Cancer: A Feasibility Study. Cancers, 14(1), 240. https://doi.org/10.3390/cancers14010240






