Intracellular Amplifiers of Reactive Oxygen Species Affecting Mitochondria as Radiosensitizers
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. General
2.2. Synthesis of Prodrugs and Their Characterization in Cell Free Settings
2.3. Cells, Cell Culture and Cellular Assays
3. Results and Discussion
3.1. Possible Reasons of Poor Anticancer Activity of Drug 1
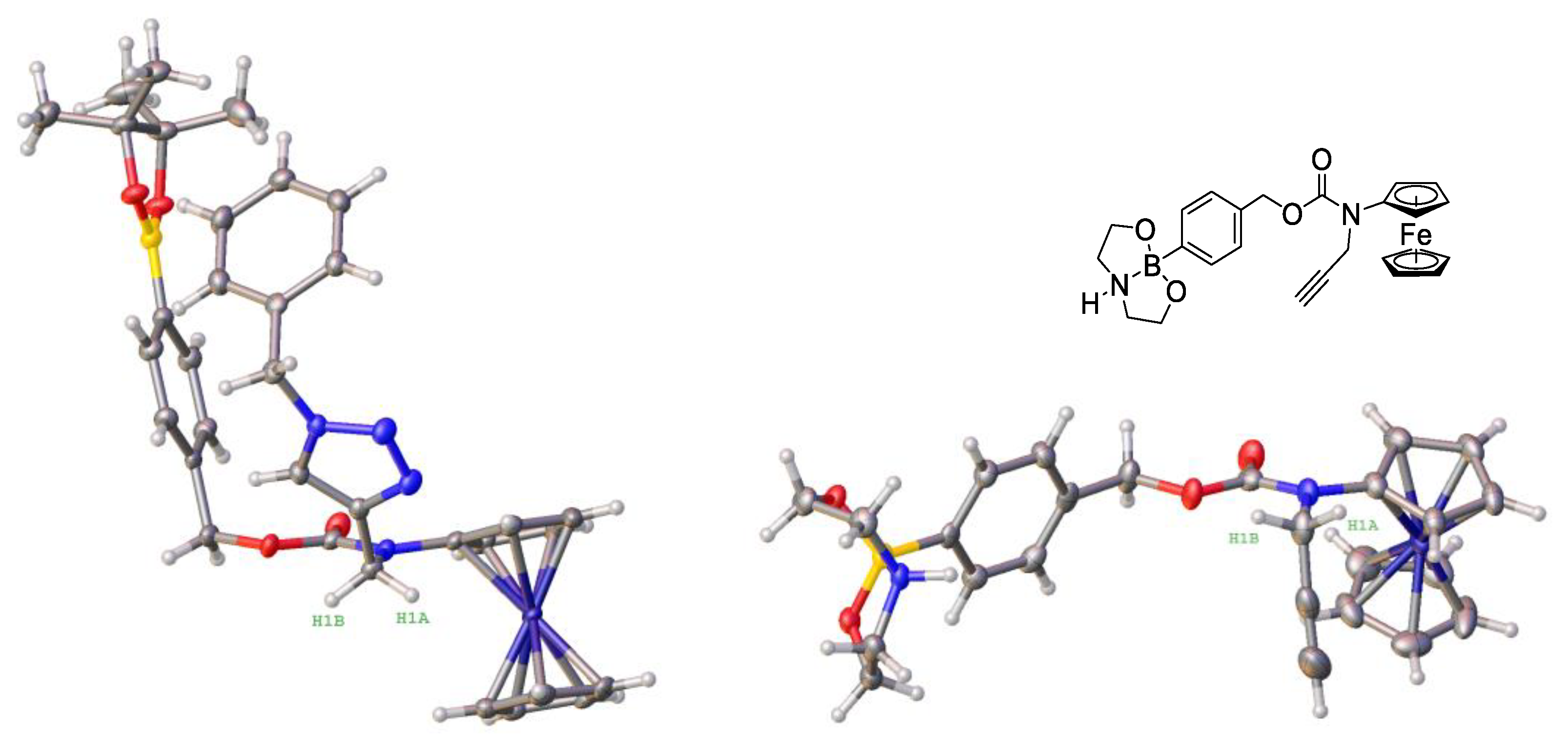
3.2. Design, Synthesis and Characterization of the Prodrugs
3.3. Stability and Solubility of Prodrugs 2a–2d in the Aqueous Neutral Solution and Their Lipophilicity
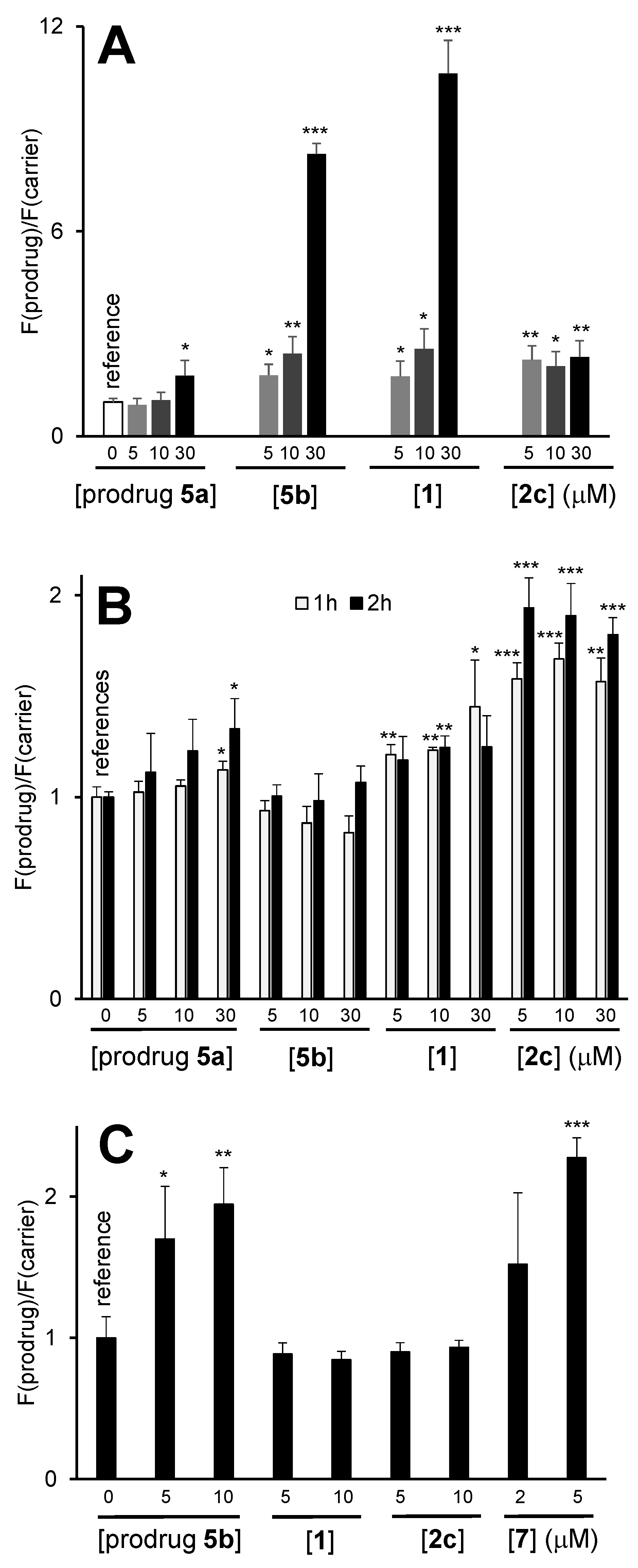
3.4. Generation of ROS in the Presence of the Prodrugs in Cell Free Settings
3.5. Anticancer Activity of the Prodrugs towards Different Cell Lines
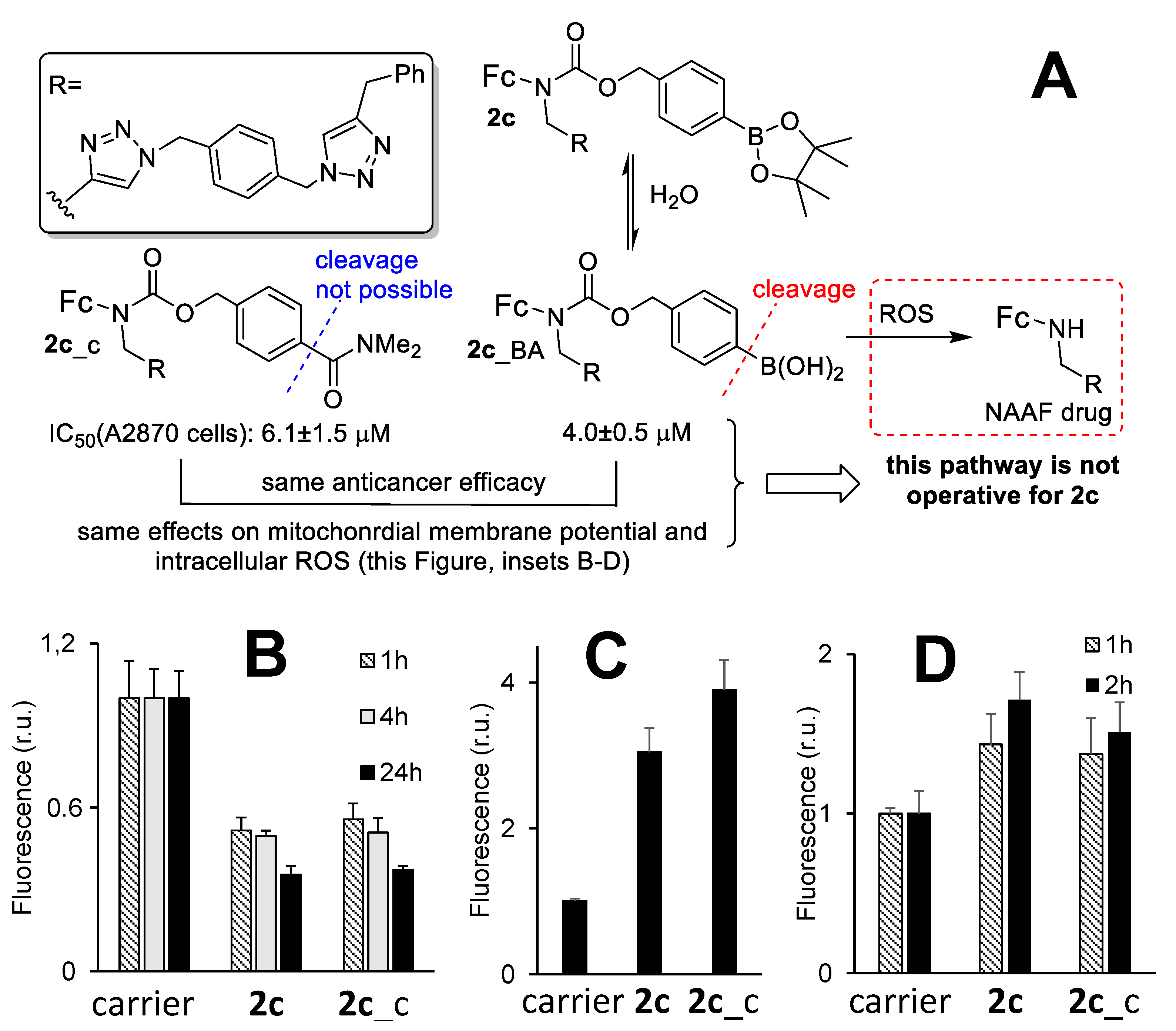
3.6. The Mechanism of the Anticancer Activity of Prodrug 2c
3.7. An Active Form of Prodrug 2c in Cells and the Role of Nitric Oxide in the Anticancer Activity of 2c
3.8. Evaluation of Cancer Cell Specificity of the Prodrug 2c
3.9. Synergy of the NAAF-Prodrugs with Radiotherapy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jagsi, R. Progress and controversies: Radiation therapy for invasive breast cancer. CA Cancer J. Clin. 2014, 64, 135–152. [Google Scholar] [CrossRef] [PubMed]
- Van-Hazel, G.A.; Heinemann, V.; Sharma, N.K.; Findlay, M.P.; Ricke, J.; Peeters, M.; Perez, D.; Robinson, B.A.; Strickland, A.H.; Ferguson, T.; et al. SIRFLOX: Randomized Phase III Trial Comparing First-Line mFOLFOX6 (Plus or Minus Bevacizumab) Versus mFOLFOX6 (Plus or Minus Bevacizumab) Plus Selective Internal Radiation Therapy in Patients with Metastatic Colorectal Cancer. J. Clin. Oncol. 2016, 34, 1723–1731. [Google Scholar] [CrossRef]
- Knipe, H.; Sheikh, Y. 3D Conformal Radiation Therapy. Reference Article, Radiopaedia.Org. Available online: https://radiopaedia.org/articles/3d-conformal-radiation-therapy?lang=us (accessed on 13 December 2021). [CrossRef]
- Rycaj, K.; Tang, D.G. Cancer stem cells and radioresistance. Int. J. Radiat. Biol. 2014, 90, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, T.S.; Blackstock, W.; McGinn, C. The mechanism of action of radiosensitization of conventional chemotherapeutic agents. Sem. Rad. Oncol. 2003, 13, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Wardman, P. Chemical radiosensitizers for use in radiotherapy. Clin. Oncol. 2007, 19, 397–417. [Google Scholar] [CrossRef] [PubMed]
- Gill, M.R.; Vallis, K.A. Transition metal compounds as cancer radiosensitizers. Chem. Soc. Rev. 2019, 48, 540–557. [Google Scholar] [CrossRef] [PubMed]
- Boussios, S.; Karihtala, P.; Moschetta, M.; Abson, C.; Karathanasi, A.; Zakynthinakis-Kyriakou, N.; Ryan, J.E.; Sheriff, M.; Rassy, E.; Pavlidis, N. Veliparib in ovarian cancer: A new synthetically lethal therapeutic approach. Investig. New Drugs 2020, 38, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Chiou, J.F.; Tai, C.J.; Wang, Y.H.; Liu, T.Z.; Jen, Y.M.; Shiau, C.Y. Sorafenib induces preferential apoptotic killing of a drug- and radio-resistant hep G2 cells through a mitochondria-dependent oxidative stress mechanism. Cancer Biol. Ther. 2009, 8, 1904–1913. [Google Scholar] [CrossRef] [PubMed]
- Hodgkiss, R.J.; Middleton, R.W. Enhancement of misonidazole radiosensitization by an inhibitor of glutathione biosynthesis, Radiother. Oncology 1983, 43, 179–183. [Google Scholar] [CrossRef]
- Ren, Q.; Tey, J.; Li, X.; Wu, Y.; Deng, H.; Han, L. Radiosensitization of cervical cancer xenografts by arsenic trioxide and the role of VEGF and Ku70. J. Radiat. Oncol. 2012, 1, 299–304. [Google Scholar] [CrossRef][Green Version]
- Dong, G.; Chen, Q.; Jiang, F.; Yu, D.; Mao, Q.; Xia, W.; Shi, R.; Wang, J.; Xu, L. Diisopropylamine dichloroacetate enhances radiosensitization in esophageal squamous cell carcinoma by increasing mitochondria-derived reactive oxygen species levels. Oncotarget 2016, 7, 68170–68178. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Chen, J.; Ge, C.; Liu, X.; He, J.; Ni, P.; Pan, Y. Synthesis of PEGylated ferrocene nanoconjugates as the radiosensitizer of cancer cells. Bioconj. Chem. 2016, 27, 1518–1524. [Google Scholar] [CrossRef]
- Skoupilova, H.; Rak, V.; Pinkas, J.; Karban, J.; Hrstka, R. The cytotoxic effect of newly synthesized ferrocenes against cervical carcinoma cells alone and in combination with radiotherapy. Appl. Sci. 2020, 10, 3728. [Google Scholar] [CrossRef]
- George, T.J.; Franke, A.J.; Chakravarthy, A.B.; Das, P.; Dasari, A.; El-Rayes, B.F.; Hong, T.S.; Kinsella, T.J.; Landry, J.C.; Lee, J.J.; et al. National Cancer Institute (NCI) state of the science: Targeted radiosensitizers in colorectal cancer. Cancer 2019, 125, 2732–2746. [Google Scholar] [CrossRef] [PubMed]
- Antunes, F.; Cadenas, R. Estimation of H2O2 gradients across biomembranes. FEBS Lett. 2000, 475, 121–126. [Google Scholar] [CrossRef]
- Szatrowski, T.P.; Nathan, C.F. Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Res. 1991, 51, 794–798. [Google Scholar]
- Halliwell, B. Oxidative stress and cancer: Have we moved forward? Biochem. J. 2007, 401, 1–11. [Google Scholar] [CrossRef]
- Marzenell, P.; Hagen, H.; Sellner, L.; Zenz, T.; Grinyte, R.; Pavlov, V.; Daum, S.; Mokhir, A. Aminoferrocene-based prodrugs and their effects on human normal and cancer cells as well as bacterial cells. J. Med. Chem. 2013, 56, 6935–6944. [Google Scholar] [CrossRef]
- Schikora, M.; Reznikov, A.; Chaykovskaya, L.; Sachinska, O.; Polyakova, L.; Mokhir, A. Activity of aminoferrocene-based prodrugs against prostate cancer. Bioorganic Med. Chem. Lett. 2015, 25, 3447–3450. [Google Scholar] [CrossRef]
- Daum, S.; Chekhun, V.; Todor, I.; Lukianova, N.; Shvets, Y.; Sellner, L.; Putzker, K.; Lewis, J.; Zenz, T.; Graaf, I.; et al. Improved synthesis of N-benzylaminoferrocene-based prodrugs and evaluation of their toxicity and antileukemic activity. J. Med. Chem. 2015, 58, 2015–2024. [Google Scholar] [CrossRef]
- Daum, S.; Reshetnikov, V.; Sisa, M.; Dumych, T.; Lootsik, M.D.; Bilyy, R.; Bila, E.; Janko, C.; Alexiou, C.; Herrmann, M.; et al. Lysosome-targeting amplifiers of reactive oxygen species as anticancer prodrugs. Angew. Chem. Int. Ed. 2017, 56, 15545–15549. [Google Scholar] [CrossRef]
- Reshetnikov, V.; Daum, S.; Mokhir, A. Cancer specific, intracellular, reductive activation of anticancer Pt(IV)-prodrugs. Chem. Eur. J. 2017, 23, 5678–5681. [Google Scholar] [CrossRef]
- Daum, S.; Babiy, S.; Konovalova, H.; Hofer, W.; Shtemenko, A.; Shtemenko, N.; Janko, C.; Alexiou, C.; Mokhir, A. Tuning the structure of aminoferrocene-based anticancer prodrugs to prevent their aggregation in aqueous solution. J. Inorg. Biochem. 2018, 178, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Reshetnikov, V.; Daum, S.; Janko, C.; Karawacka, W.; Tietze, R.; Alexiou, C.; Paryzhak, S.; Dumych, T.; Bilyy, R.; Tripal, P.; et al. ROS-responsive N-alkylaminoferrocenes for cancer cell specific targeting of mitochondria. Angew. Chem. Int. Ed. 2018, 57, 11943–11946. [Google Scholar] [CrossRef]
- Reshetnikov, V.; Arkhypov, A.; Julakanti, P.R.; Mokhir, A. A cancer specific oxaliplatin-releasing Pt(IV)-prodrug. Dalton Trans. 2018, 47, 6679–6682. [Google Scholar] [CrossRef]
- Daum, S.; Toms, J.; Reshetnikov, V.; Özkan, H.G.; Hampel, F.; Maschauer, S.; Hakimioun, A.; Beierlein, F.; Sellner, L.; Schmitt, M.; et al. Identification of boronic acid derivatives as an active form of N-alkylaminoferrocene-based anticancer prodrugs and their radiolabeling with 18F. Bioconjugate Chem. 2019, 30, 1077–1086. [Google Scholar] [CrossRef] [PubMed]
- Reshetnikov, V.; Özkan, H.G.; Daum, S.; Janko, C.; Alexiou, C.; Sauer, C.; Heinrich, M.R.; Mokhir, A. N-Alkylaminoferrocene-based prodrugs targeting mitochondria of cancer cells. Molecules 2020, 25, 2545. [Google Scholar] [CrossRef]
- Xu, H.-G.; Schikora, M.; Sisa, M.; Daum, S.; Klemt, I.; Janko, C.; Alexiou, C.; Bila, G.; Bilyy, R.; Gong, W.; et al. An endoplasmic reticulum specific pro-amplifier of reactive oxygen species in cancer cells. Angew. Chem. Int. Ed. 2021, 60, 11158–11162. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Wang, Y.; Peng, X. ROS-inducible DNA cross-linking agent as a new anticancer prodrug building block. Chem. Eur. J. 2012, 18, 3850–3854. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Fan, H.; Balakrishnan, K.; Lin, Z.; Cao, S.; Chen, W.; Fan, Y.; Guthrie, Q.A.; Sun, H.; Teske, K.A.; et al. Hydrogen peroxide activated quinone methide precursors with enhanced DNA cross-linking capability and cytotoxicity towards cancer cells. Eur. J. Med. Chem. 2017, 133, 197–207. [Google Scholar] [CrossRef]
- Jaouen, J.; Vessières, A.; Top, S. Ferrocifen type anticancer drugs. Chem. Soc. Rev. 2015, 44, 8802–8817. [Google Scholar] [CrossRef]
- Wang, Y.; Pigeon, P.; Top, S.; García, J.S.; Troufflard, C.; Ciofini, I.; McGlinchey, M.J.; Jaouen, J. Atypical lone pair-pi interaction with quinone methides in a series of imido-ferrociphenol anticancer drug candidates. Angew. Chem. Int. Ed. 2019, 58, 8421–8425. [Google Scholar] [CrossRef]
- Doering, M.; Ba, L.A.; Lilienthal, N.; Nicco, C.; Scherer, C.; Abbas, M.; Zada, A.A.P.; Coriat, R.; Burkholz, T.; Wessjohann, L.; et al. Synthesis and selective anticancer activity of organochalcogen based redox catalysts. J. Med. Chem. 2010, 53, 6954–6963. [Google Scholar] [CrossRef]
- Zeh, G.; Haines, P.; Miehlich, M.E.; Kienz, T.; Neidlinger, A.; Friedrich, R.P.; Özkan, H.G.; Alexiou, C.; Hampel, F.; Guldi, D.M.; et al. Anticancer effects of an electronically coupled oligoferrocene. Organometallics 2020, 38, 3112–3120. [Google Scholar] [CrossRef]
- McCann, E.; O’Sullivan, J.; Marcone, S. Targeting cancer-cell mitochondria and metabolism to improve radiotherapy response. Transl. Oncol. 2021, 14, 100905. [Google Scholar] [CrossRef]
- Rovini, A.; Heslop, K.; Hunt, E.G.; Morris, M.E.; Fang, D.; Gooz, M.; Gerencser, A.A.; Maldonado, E.N. Quantitative analysis of mitochondrial membrane potential heterogeneity in unsynchronized and synchronized cancer cells. FASEB J. 2020, 35, e21148. [Google Scholar] [CrossRef]
- Vander Heiden, M.G.; DeBerardinis, R.J. Understanding the intersections between metabolism and cancer biology. Cell 2017, 168, 657–669. [Google Scholar] [CrossRef]
- Peiris-Pagès, M.; Sotgia, F.; Lisanti, M.P. Doxycycline and therapeutic targeting of the DNA damage response in cancer cells: Old drug, new purpose. Oncoscience 2015, 2, 696–699. [Google Scholar] [CrossRef] [PubMed]
- Jean, S.R.; Ahmed, M.; Lei, E.K.; Wisnovsky, S.P.; Kelley, S.O. Peptide-mediated delivery of chemical probes and therapeutics to mitochondria. Acc. Chem. Res. 2016, 49, 1893–1902. [Google Scholar] [CrossRef] [PubMed]
- Tabish, T.A.; Hamblin, M.R. Mitochondria-targeted nanoparticles (mitoNANO): An emerging therapeutic shortcut for cancer. Biomat. Biosyst. 2021, 3, 100023. [Google Scholar] [CrossRef]
- Tabish, T.A.; Narayan, R.J. Mitochondria-targeted graphene for advanced cancer therapeutics. Acta Biomat. 2021, 129, 43–56. [Google Scholar] [CrossRef]
- Toms, J.; Reshetnikov, V.; Maschauer, S.; Mokhir, A.; Prante, O. Radiosynthesis of an 18F-fluoroglycosylated aminoferrocene for in-vivo imaging of reactive oxygen species by PET. J. Label. Compd. Radiopharm. 2018, 61, 1081–1088. [Google Scholar] [CrossRef] [PubMed]
- Eicheler, W.; Zips, D.; Dörfler, A.; Grénman, R.; Baumann, M. Splicing mutations in TP53 in human squamous cell carcinoma lines influence immunohistochemical detection. J. Histochem. Cytochem. 2002, 50, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Batsanov, S.S. Van der Waals radii of elements. Inorg. Mat. 2001, 37, 1031–1046. [Google Scholar] [CrossRef]
- Tengan, C.H.; Moraes, C.T. NO control of mitochondrial function in normal and transformed cells. Biochim. Biophys. Acta 2017, 1858, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Takabayashi, A.; Kawai, Y.; Iwata, S.; Kanai, M.; Denno, R.; Kawada, K.; Obama, K.; Taki, Y. Nitric oxide induces a decrease in the mitochondrial membrane potential of peripheral blood lymphocytes, especially in natural killer cells. Antioxid. Redox Signal. 2008, 2, 673–680. [Google Scholar] [CrossRef]
- Leemans, C.R.; Braakhuis, B.J.M.; Brakenhoff, R.H. The molecular biology of head and neck cancer. Nat. Rev. Cancer 2011, 11, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Digomann, D.; Kurth, I.; Tyutyunnykova, A.; Chen, O.; Löck, S.; Gorodetska, I.; Peitzsch, C.; Skvortsova, I.I.; Negro, G.; Aschenbrenner, B.; et al. The CD98 Heavy Chain Is a Marker and Regulator of Head and Neck Squamous Cell Carcinoma Radiosensitivity. Clin. Cancer Res. 2019, 25, 3152–3163. [Google Scholar] [CrossRef]


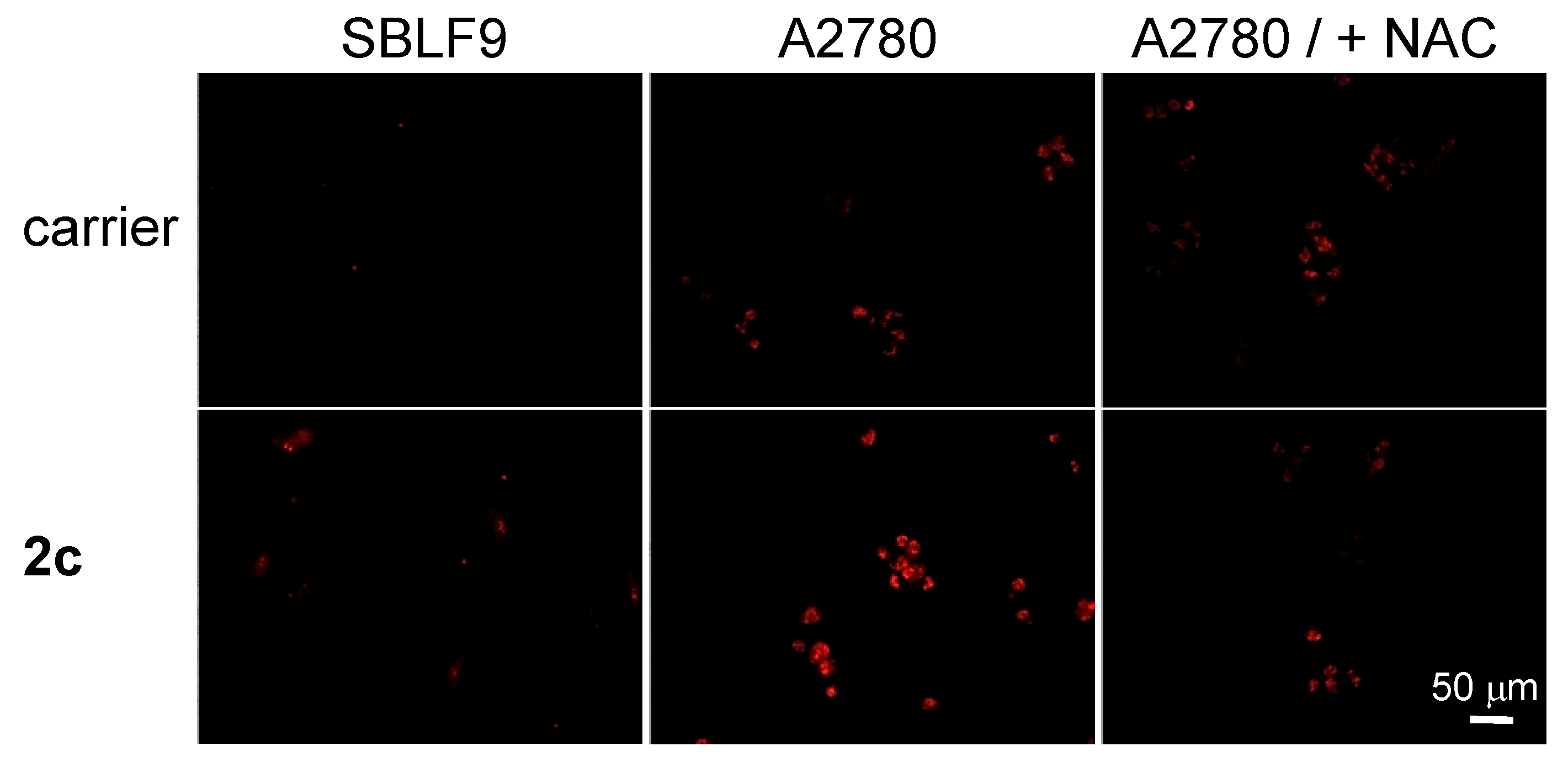

| Prodrug | logP (Prodrug/Hydrolyzed Prodrug) i | Solubility ii | ROS Release Efficacy iv |
|---|---|---|---|
| 1 | 4.87 ± 0.05/2.83 ± 0.07 | 67 ± 5 iii | 0.99 ± 0.16 |
| 2a | 5.27 ± 0.10/2.92 ± 0.14 | 100 (30) | 0.74 ± 0.19 |
| 2b | 5.37 ± 0.10/3.10 ± 0.12 | 100 (30) | 0.76 ± 0.19 |
| 2c | 5.37 ± 0.15/3.23 ± 0.11 | 30 (10) | 0.35 ± 0.17 |
| 2c_c | 3.90 ± 0.08 | 55 ± 5 | 0.050 ± 0.003 |
| 2d | 5.56 ± 0.14/3.42 ± 0.14 | 30 (10) | 0.37 ± 0.13 |
| Prodrug/Control | IC50 (μM)/Human Cancer Cell Lines i | |||
|---|---|---|---|---|
| A2780 | BL-2 | AsPC1 | Jurkat | |
| 1 | 30 ± 5 | 17 ± 6 | - | 30 ± 4 |
| 2c | 4.0 ± 0.5 | 5.7 ± 2.7 | 7.1 ± 1.0 | 5 ± 2 |
| 2c_c | 6.1 ± 1.5 | - | - | - |
| 5b | 7 ± 2 | 5 ± 3 | 6.7 ± 0.5 | 7.2 ± 0.1 |
| 6 | >100 | >100 | - | >100 |
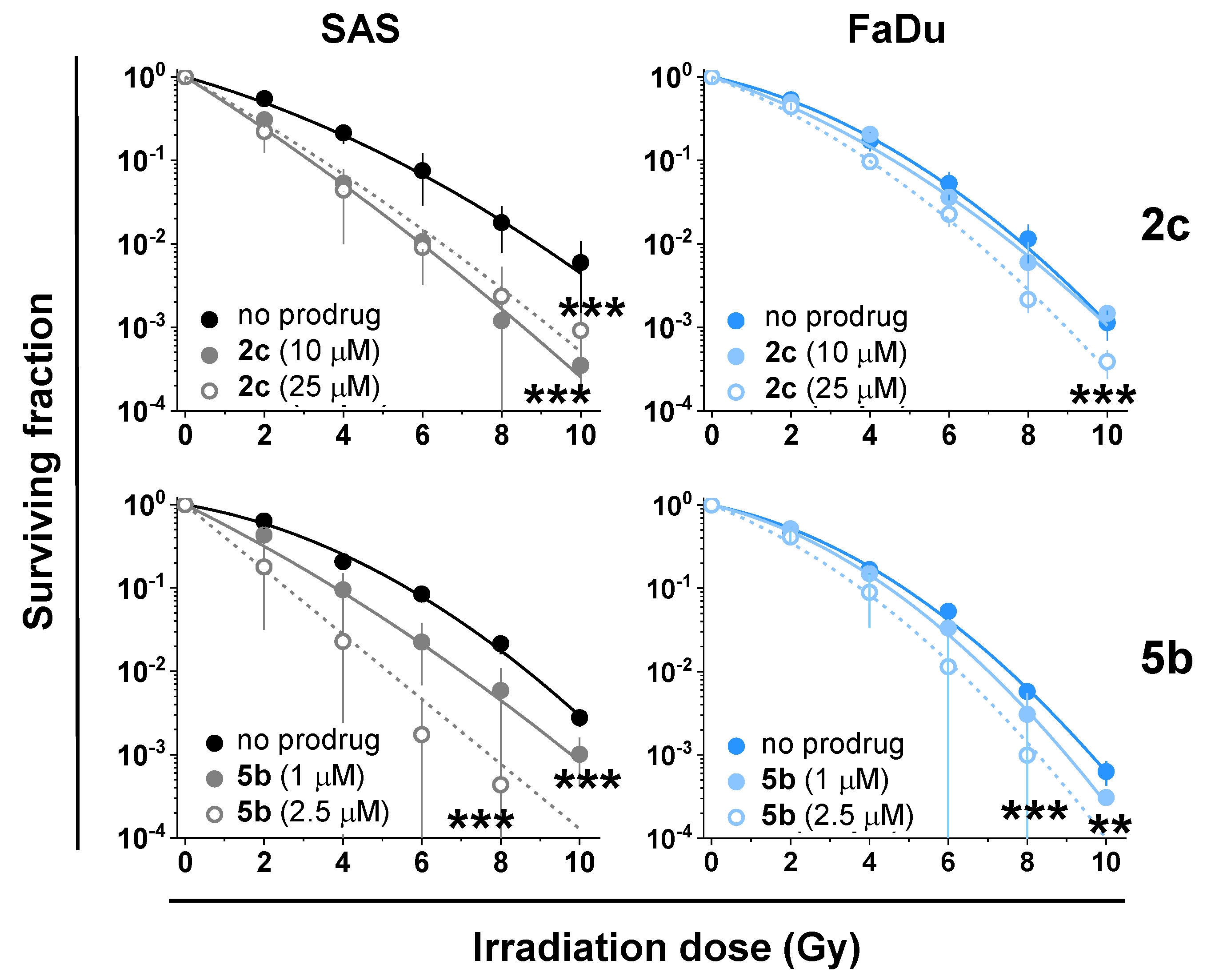
| Prodrug | Concentration (µM) | Radiosensitization i | p-Values ii | |
|---|---|---|---|---|
| SAS | FaDu | |||
| 2c | 10 | +/− | <0.001 | ns |
| 25 | + | <0.001 | <0.001 | |
| 5b | 1 | + | <0.001 | <0.01 |
| 2.5 | + | <0.001 | <0.001 | |
| 6 | 25 | - | ns | ns |
| 7 | 0.1 | +/− | ns | <0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, H.-G.; Reshetnikov, V.; Wondrak, M.; Eckhardt, L.; Kunz-Schughart, L.A.; Janko, C.; Tietze, R.; Alexiou, C.; Borchardt, H.; Aigner, A.; et al. Intracellular Amplifiers of Reactive Oxygen Species Affecting Mitochondria as Radiosensitizers. Cancers 2022, 14, 208. https://doi.org/10.3390/cancers14010208
Xu H-G, Reshetnikov V, Wondrak M, Eckhardt L, Kunz-Schughart LA, Janko C, Tietze R, Alexiou C, Borchardt H, Aigner A, et al. Intracellular Amplifiers of Reactive Oxygen Species Affecting Mitochondria as Radiosensitizers. Cancers. 2022; 14(1):208. https://doi.org/10.3390/cancers14010208
Chicago/Turabian StyleXu, Hong-Gui, Viktor Reshetnikov, Marit Wondrak, Lisa Eckhardt, Leoni A. Kunz-Schughart, Christina Janko, Rainer Tietze, Christoph Alexiou, Hannes Borchardt, Achim Aigner, and et al. 2022. "Intracellular Amplifiers of Reactive Oxygen Species Affecting Mitochondria as Radiosensitizers" Cancers 14, no. 1: 208. https://doi.org/10.3390/cancers14010208
APA StyleXu, H.-G., Reshetnikov, V., Wondrak, M., Eckhardt, L., Kunz-Schughart, L. A., Janko, C., Tietze, R., Alexiou, C., Borchardt, H., Aigner, A., Gong, W., Schmitt, M., Sellner, L., Daum, S., Özkan, H. G., & Mokhir, A. (2022). Intracellular Amplifiers of Reactive Oxygen Species Affecting Mitochondria as Radiosensitizers. Cancers, 14(1), 208. https://doi.org/10.3390/cancers14010208