Correlation Analysis of Protein Expression of 10 HDAC/Sirtuin Isoenzymes with Sensitivities of 23 Anticancer Drugs in 17 Cancer Cell Lines and Potentiation of Drug Activity by Co-Treatment with HDAC Inhibitors
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Chemicals
2.2. Cell Culture
2.3. Determination of Antiproliferative Activity (GI50 Values)
2.4. Determination of Expression Profiles
2.5. Data from the National Cancer Institute 60 Cell Line Program
2.6. Statistics
3. Results
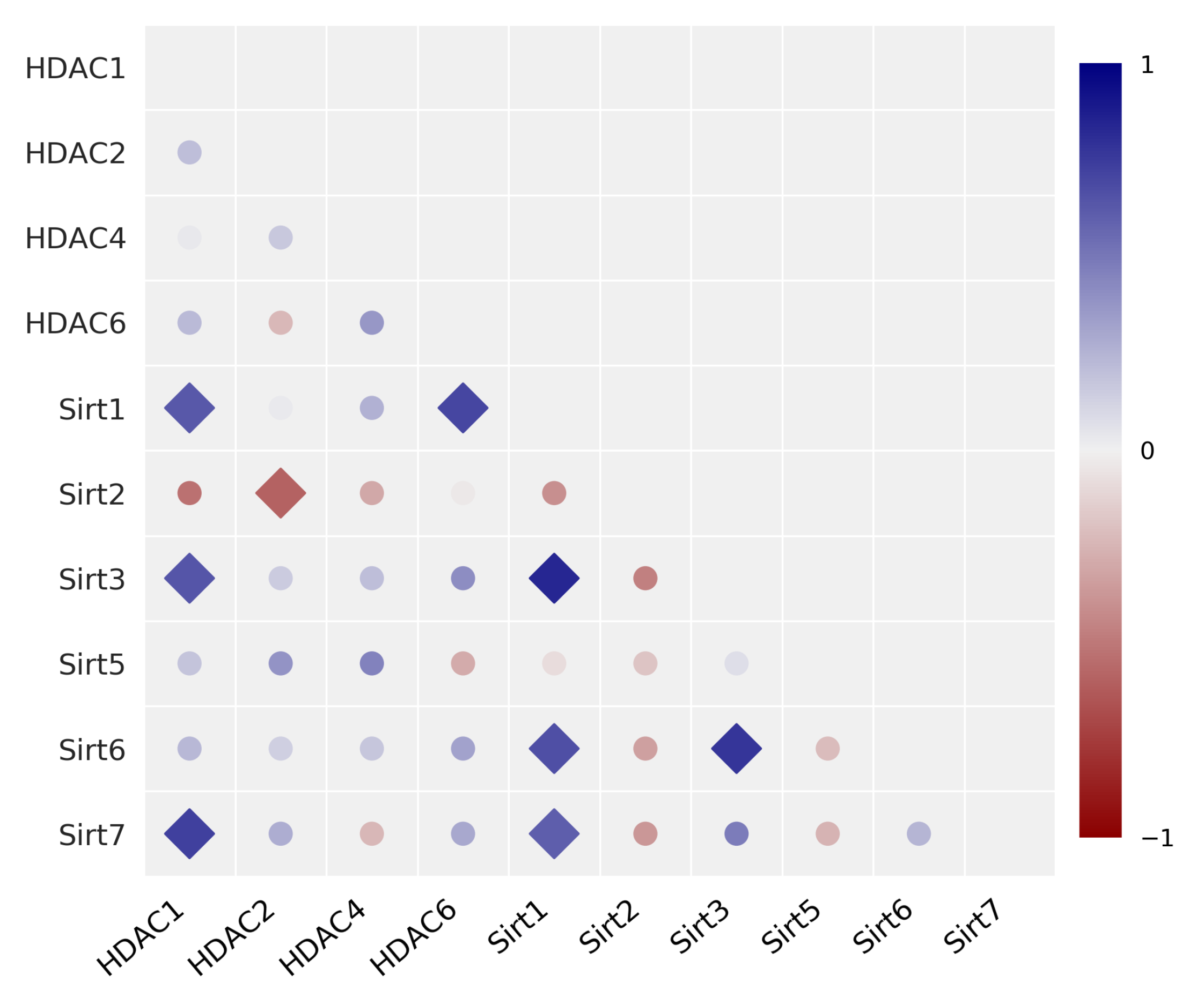
3.1. Expression of HDAC/Sirt Isoenzymes and Correlation Analysis
3.2. Correlation Analysis with Data from the NCI 60 Cancer Cell Line Program
3.3. Antiproliferative Activity of SAHA and TSA—Correlations with HDAC/Sirt Expression
3.4. Correlation of Anticancer Drug Potency and the Doubling Time of Cancer Cells with HDAC/Sirt Expression
3.5. Correlation Analysis of Anticancer Drugs Potency and the mRNA Expression with Data from the NCI 60 Cancer Cell Line Program
3.6. Combination Effects of HDAC Inhibition on Anticancer Drug Potency
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BSA | bovine serum albumin |
| CGI | relative combination growth inhibition at 50% |
| FDR | false discovery rate |
| GI50 | growth inhibition at 50% |
| HAT | histone acetyltransferase |
| HDAC | histone deacetylase |
| NCI | National Cancer Institute |
| OLS | ordinary least squares |
| SAHA | suberanilohydroxamic acid (vorinostat) |
| SD | standard deviation |
| Sirt | sirtuin |
| TBST | Tris buffered saline/tween buffer |
| TSA | trichostatin A |
References
- Timp, W.; Feinberg, A.P. Cancer as a dysregulated epigenome allowing cellular growth advantage at the expense of the host. Nat. Rev. Cancer 2013, 13, 497–510. [Google Scholar] [CrossRef] [Green Version]
- Vilcinskas, A. The role of epigenetics in host–Parasite coevolution: Lessons from the model host insects Galleria mellonella and Tribolium castaneum. Zoology 2016, 119, 273–280. [Google Scholar] [CrossRef]
- Zhao, Z.; Shilatifard, A. Epigenetic modifications of histones in cancer. Genome Biol. 2019, 20, 245. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.K.; Workman, J.L. Histone acetyltransferase complexes: One size doesn’t fit all. Nat. Rev. Mol. Cell Biol. 2007, 8, 284–295. [Google Scholar] [CrossRef]
- Seto, E.; Yoshida, M. Erasers of histone acetylation: The histone deacetylase enzymes. Cold Spring Harb. Perspect. Biol. 2014, 6, a018713. [Google Scholar] [CrossRef] [Green Version]
- Yoon, S.; Eom, G.H. HDAC and HDAC Inhibitor: From Cancer to Cardiovascular Diseases. Chonnam Med. J. 2016, 52, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Eckschlager, T.; Plch, J.; Stiborova, M.; Hrabeta, J. Histone Deacetylase Inhibitors as Anticancer Drugs. Int. J. Mol. Sci. 2017, 18, 1414. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Jing, H.; Lin, H. Sirtuin inhibitors as anticancer agents. Future Med. Chem. 2014, 6, 945–966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomes, I.D.; Pflum, M.K.H. Optimal Substrate-Trapping Mutants to Discover Substrates of HDAC1. ChemBioChem 2019, 20, 1444–1449. [Google Scholar] [CrossRef]
- Schiedel, M.; Robaa, D.; Rumpf, T.; Sippl, W.; Jung, M. The Current State of NAD+-Dependent Histone Deacetylases (Sirtuins) as Novel Therapeutic Targets. Med. Res. Rev. 2018, 38, 147–200. [Google Scholar] [CrossRef] [PubMed]
- Schemies, J.; Uciechowska, U.; Sippl, W.; Jung, M. NAD+-dependent histone deacetylases (sirtuins) as novel therapeutic targets. Med. Res. Rev. 2010, 30, 861–889. [Google Scholar] [CrossRef] [PubMed]
- Kazantsev, A.G.; Thompson, L.M. Therapeutic application of histone deacetylase inhibitors for central nervous system disorders. Nat. Rev. Drug Discov. 2008, 7, 854–868. [Google Scholar] [CrossRef] [PubMed]
- Grathwol, C.W.; Wössner, N.; Swyter, S.; Smith, A.C.; Tapavicza, E.; Hofstetter, R.K.; Bodtke, A.; Jung, M.; Link, A. Azologization and repurposing of a hetero-stilbene-based kinase inhibitor: Towards the design of photoswitchable sirtuin inhibitors. Beilstein J. Org. Chem. 2019, 15, 2170–2183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grathwol, C.W.; Wössner, N.; Behnisch-Cornwell, S.; Schulig, L.; Zhang, L.; Einsle, O.; Jung, M.; Link, A. Activation of sirtuin 2 inhibitors employing photoswitchable geometry and aqueous solubility. ChemMedChem 2020, 15, 1480–1489. [Google Scholar] [CrossRef]
- Rumpf, T.; Schiedel, M.; Karaman, B.; Roessler, C.; North, B.J.; Lehotzky, A.; Oláh, J.; Ladwein, K.I.; Schmidtkunz, K.; Gajer, M.; et al. Selective Sirt2 inhibition by ligand-induced rearrangement of the active site. Nat. Commun. 2015, 6, 6263. [Google Scholar] [CrossRef] [PubMed]
- Swyter, S.; Schiedel, M.; Monaldi, D.; Szunyogh, S.; Lehotzky, A.; Rumpf, T.; Ovadi, J.; Sippl, W.; Jung, M. New chemical tools for probing activity and inhibition of the NAD+-dependent lysine deacylase sirtuin 2. Philos. Trans. R Soc. Lond. B Biol. Sci. 2018, 373, 20170083. [Google Scholar] [CrossRef] [Green Version]
- Bracht, K.; Boubakari; Grunert, R.; Bednarski, P.J. Correlations between the activities of 19 anti-tumor agents and the intracellular glutathione concentrations in a panel of 14 human cancer cell lines: Comparisons with the National Cancer Institute data. Anticancer Drugs 2006, 17, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Gürtler, A.; Kunz, N.; Gomolka, M.; Hornhardt, S.; Friedl, A.A.; McDonald, K.; Kohn, J.E.; Posch, A. Stain-Free technology as a normalization tool in Western blot analysis. Anal. Biochem. 2013, 433, 105–111. [Google Scholar] [CrossRef]
- Gilda, J.E.; Gomes, A.V. Western Blotting Using In-Gel Protein Labeling as a Normalization Control: Stain-Free Technology. In Methods in Molecular Biology; Springer: New York, NY, USA, 2015; pp. 381–391. [Google Scholar]
- Seabold, S.; Perktold, J. Statsmodels: Econometric and Statistical Modeling with Python. Available online: https://pdfs.semanticscholar.org/3a27/6417e5350e29cb6bf04ea5a4785601d5a215.pdf (accessed on 29 November 2021).
- Hunter, J. Matplotlib: A 2D graphics environment. Comput. Sci. Eng. 2007, 9, 90–95. [Google Scholar] [CrossRef]
- Bracht, K.; Liebeke, M.; Ritter, C.A.; Grunert, R.; Bednarski, P.J. Correlations between the activities of 19 standard anticancer agents, antioxidative enzyme activities and the expression of ATP-binding cassette transporters: Comparison with the National Cancer Institute data. Anticancer Drugs 2007, 18, 389–404. [Google Scholar] [CrossRef]
- Benjamini, Y.; Yekutieli, D. The control of the false discovery rate in multiple testing under dependency. Ann. Stat. 2001, 29, 1165–1188. [Google Scholar] [CrossRef]
- Miteva, Y.V.; Cristea, I.M. A Proteomic Perspective of Sirtuin 6 (SIRT6) Phosphorylation and Interactions and Their Dependence on Its Catalytic Activity. Mol. Cell. Proteom. 2014, 13, 168–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaharevitz, D.W.; Holbeck, S.L.; Bowerman, C.; Svetlik, P.A. COMPARE: A web accessible tool for investigating mechanisms of cell growth inhibition. J. Mol. Graph. Model. 2002, 20, 297–303. [Google Scholar] [CrossRef]
- Staberg, M.; Michaelsen, S.R.; Rasmussen, R.D.; Villingshoj, M.; Poulsen, H.S.; Hamerlik, P. Inhibition of histone deacetylases sensitizes glioblastoma cells to lomustine. Cell Oncol. (Dordr.) 2017, 40, 21–32. [Google Scholar] [CrossRef]
- Weichert, W. HDAC expression and clinical prognosis in human malignancies. Cancer Lett. 2009, 280, 168–176. [Google Scholar] [CrossRef]
- Li, Y.; Seto, E. HDACs and HDAC Inhibitors in Cancer Development and Therapy. Cold Spring Harb. Perspect. Med. 2016, 6, a026831. [Google Scholar] [CrossRef] [Green Version]
- Zhao, E.; Hou, J.; Ke, X.; Abbas, M.N.; Kausar, S.; Zhang, L.; Cui, H. The Roles of Sirtuin Family Proteins in Cancer Progression. Cancers 2019, 11, 1949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hosokawa, M.; Tanaka, S.; Ueda, K.; Iwakawa, S. Different Schedule-Dependent Effects of Epigenetic Modifiers on Cytotoxicity by Anticancer Drugs in Colorectal Cancer Cells. Biol. Pharm. Bull. 2017, 40, 2199–2204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, Y.J.; Lee, Y.T.; Petersen, S.H.; Kaur, G.; Kono, K.; Tan, S.C.; Majid, A.; Oon, C.E. BZD9L1 sirtuin inhibitor as a potential adjuvant for sensitization of colorectal cancer cells to 5-fluorouracil. Ther. Adv. Med. Oncol. 2019, 11, 1758835919878977. [Google Scholar] [CrossRef] [Green Version]
- Gray, J.; Cubitt, C.L.; Zhang, S.; Chiappori, A. Combination of HDAC and topoisomerase inhibitors in small cell lung cancer. Cancer Biol. Ther. 2012, 13, 614–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Unland, R.; Clemens, D.; Heinicke, U.; Potratz, J.C.; Hotfilder, M.; Fulda, S.; Wardelmann, E.; Fruhwald, M.C.; Dirksen, U. Suberoylanilide hydroxamic acid synergistically enhances the antitumor activity of etoposide in Ewing sarcoma cell lines. Anticancer Drugs 2015, 26, 843–851. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.C.; Liu, Y.M.; Lin, M.H.; Liao, Y.H.; Lai, M.J.; Chuang, H.Y.; Hung, T.Y.; Chen, C.H.; Liou, J.P. Design, synthesis, and evaluation of N-phenyl-4-(2-phenylsulfonamido)-benzamides as microtubule-targeting agents in drug-resistant cancer cells, displaying HDAC inhibitory response. Eur. J. Med. Chem. 2020, 192, 112158. [Google Scholar] [CrossRef] [PubMed]
- Grohmann, T.; Penke, M.; Petzold-Quinque, S.; Schuster, S.; Richter, S.; Kiess, W.; Garten, A. Inhibition of NAMPT sensitizes MOLT4 leukemia cells for etoposide treatment through the SIRT2-p53 pathway. Leuk Res. 2018, 69, 39–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoffmann, G.; Breitenbucher, F.; Schuler, M.; Ehrenhofer-Murray, A.E. A novel sirtuin 2 (SIRT2) inhibitor with p53-dependent pro-apoptotic activity in non-small cell lung cancer. J. Biol. Chem. 2014, 289, 5208–5216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tu, H.J.; Lin, Y.J.; Chao, M.W.; Sung, T.Y.; Wu, Y.W.; Chen, Y.Y.; Lin, M.H.; Liou, J.P.; Pan, S.L.; Yang, C.R. The anticancer effects of MPT0G211, a novel HDAC6 inhibitor, combined with chemotherapeutic agents in human acute leukemia cells. Clin. Epigenetics 2018, 10, 162. [Google Scholar] [CrossRef] [Green Version]
- Chao, M.W.; Lai, M.J.; Liou, J.P.; Chang, Y.L.; Wang, J.C.; Pan, S.L.; Teng, C.M. The synergic effect of vincristine and vorinostat in leukemia in vitro and in vivo. J. Hematol. Oncol. 2015, 8, 82. [Google Scholar] [CrossRef] [Green Version]
- Mutze, K.; Langer, R.; Becker, K.; Ott, K.; Novotny, A.; Luber, B.; Hapfelmeier, A.; Gottlicher, M.; Hofler, H.; Keller, G. Histone deacetylase (HDAC) 1 and 2 expression and chemotherapy in gastric cancer. Ann. Surg. Oncol. 2010, 17, 3336–3343. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.; Eberherr, C.; Hagemann, M.; Cairo, S.; Haberle, B.; Vokuhl, C.; von Schweinitz, D.; Kappler, R. Connectivity map identifies HDAC inhibition as a treatment option of high-risk hepatoblastoma. Cancer Biol. Ther. 2016, 17, 1168–1176. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.-H.; Zheng, Y.-L.; Xu, C.-Q.; Gu, L.-Z.; Ding, Z.-L.; Qin, L.; Wang, Y.; Fu, R.; Wan, Y.-F.; Hu, C.-P. Valproic acid (VPA) enhances cisplatin sensitivity of non-small cell lung cancer cells via HDAC2 mediated down regulation of ABCA1. Biol. Chem. 2017, 398, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Pflieger, M.; Hamacher, A.; Oz, T.; Horstick-Muche, N.; Boesen, B.; Schrenk, C.; Kassack, M.U.; Kurz, T. Novel alpha,beta-unsaturated hydroxamic acid derivatives overcome cisplatin resistance. Bioorg. Med. Chem. 2019, 27, 115036. [Google Scholar] [CrossRef] [PubMed]
- Asfaha, Y.; Schrenk, C.; Alves Avelar, L.A.; Lange, F.; Wang, C.; Bandolik, J.J.; Hamacher, A.; Kassack, M.U.; Kurz, T. Novel alkoxyamide-based histone deacetylase inhibitors reverse cisplatin resistance in chemoresistant cancer cells. Bioorg. Med. Chem. 2020, 28, 115108. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.Y.; Ding, W.; Li, T.Q.; Zhang, Y.X.; Zhao, S.C. Histone Deacetylase (HDAC) Inhibitor, Suberoylanilide Hydroxamic Acid (SAHA), Induces Apoptosis in Prostate Cancer Cell Lines via the Akt/FOXO3a Signaling Pathway. Med. Sci. Monit. 2017, 23, 5793–5802. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, U.; Venkatesan, T.; Radhakrishnan, V.; Samuel, S.; Rathinavelu, A. Differential Mechanisms of Cell Death Induced by HDAC Inhibitor SAHA and MDM2 Inhibitor RG7388 in MCF-7 Cells. Cells 2018, 8, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilardini Montani, M.S.; Granato, M.; Santoni, C.; Del Porto, P.; Merendino, N.; D’Orazi, G.; Faggioni, A.; Cirone, M. Histone deacetylase inhibitors VPA and TSA induce apoptosis and autophagy in pancreatic cancer cells. Cell Oncol. (Dordr.) 2017, 40, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Ocker, M. Deacetylase inhibitors—Focus on non-histone targets and effects. World J. Biol. Chem. 2010, 1, 55. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Lan, P.; Hou, Z.; Guan, Y.; Zhang, J.; Xu, W.; Tian, Z.; Zhang, C. Histone deacetylase inhibitor SAHA epigenetically regulates miR-17-92 cluster and MCM7 to upregulate MICA expression in hepatoma. Br. J. Cancer 2014, 112, 112–121. [Google Scholar] [CrossRef] [Green Version]
- Glozak, M.A.; Sengupta, N.; Zhang, X.; Seto, E. Acetylation and deacetylation of non-histone proteins. Gene 2005, 363, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Bisson, W.H.; Löhr, C.V.; Williams, D.E.; Ho, E.; Dashwood, R.H.; Rajendran, P. Histone and Non-Histone Targets of Dietary Deacetylase Inhibitors. Curr. Top. Med. Chem. 2015, 16, 714–731. [Google Scholar] [CrossRef]
- Bruzzese, F.; Rocco, M.; Castelli, S.; Di Gennaro, E.; Desideri, A.; Budillon, A. Synergistic antitumor effect between vorinostat and topotecan in small cell lung cancer cells is mediated by generation of reactive oxygen species and DNA damage-induced apoptosis. Mol. Cancer Ther. 2009, 8, 3075–3087. [Google Scholar] [CrossRef] [Green Version]
- Sato, A.; Asano, T.; Horiguchi, A.; Ito, K.; Sumitomo, M.; Asano, T. Antitumor effect of suberoylanilide hydroxamic acid and topotecan in renal cancer cells. Oncol. Res. 2011, 19, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Blake, M.; Baek, J.H.; Kohlhagen, G.; Pommier, Y.; Carrier, F. Inhibition of Histone Deacetylase Increases Cytotoxicity to Anticancer Drugs Targeting DNA. Cancer Res. 2003, 63, 7291–7300. [Google Scholar]
- Karagiannis, T.; El-Osta, A. Clinical Potential of Histone Deacetylase Inhibitors as Stand Alone Therapeutics and in Combination with other Chemotherapeutics or Radiotherapy for Cancer. Epigenetics 2006, 1, 121–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]










| Cell Line | SAHA | TSA | SAHA | TSA | |||||
|---|---|---|---|---|---|---|---|---|---|
| GI50 (nm) | rel. GI50 | GI50 (nm) | rel. GI50 | Cell Line | GI50 (nm) | rel. GI50 | GI50 (nm) | rel. GI50 | |
| MCF-7 | 527.8 | 0.64 | 33.55 | 0.45 | BHY | 598.7 | 0.73 | 52.36 | 0.71 |
| 79.4 | 14.41 | 136.3 | 7.20 | ||||||
| MT-3 | 689.6 | 0.84 | 77.74 | 1.05 | DanG | 586.1 | 0.72 | 71.90 | 0.97 |
| 114.9 | 14.48 | 35.7 | 11.33 | ||||||
| SiSo | 909.3 | 1.11 | 60.13 | 0.81 | Pa-Tu-8902 | 1536.6 | 1.88 | 123.92 | 1.67 |
| 126.6 | 3.56 | 248.5 | 9.36 | ||||||
| Kyse-70 | 602.0 | 0.74 | 50.56 | 0.68 | YAPC | 1210.4 | 1.48 | 72.83 | 0.98 |
| 67.5 | 7.69 | 228.9 | 38.57 | ||||||
| Kyse-510 | 1862.6 | 2.28 | 174.83 | 2.36 | 5637 | 667.2 | 0.82 | 78.71 | 1.06 |
| 210.3 | 40.01 | 239.4 | 38.98 | ||||||
| Kyse-520 | 501.5 | 0.61 | 78.14 | 1.06 | RT-4 | 873.6 | 1.07 | 98.75 | 1.33 |
| 45.4 | 29.97 | 115.5 | 22.38 | ||||||
| A427 | 228.3 | 0.28 | 24.89 | 0.34 | RT-112 | 825.9 | 1.01 | 92.68 | 1.25 |
| 158.0 | 24.26 | 106.6 | 16.12 | ||||||
| LCLC-103H | 511.8 | 0.63 | 38.72 | 0.52 | A2780 | 964.9 | 1.18 | 54.47 | 0.74 |
| 74.2 | 13.13 | 165.0 | 6.98 | ||||||
| MCF-7 | MT-3 | Kyse-510 | Kyse-520 | A427 | LCLC-103H | BHY | DanG | Pa-Tu-8902 | YAPC | 5637 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Imatinib () | 11.61 | 11.49 | 13.38 | 9.24 | 6.47 | 9.29 | 13.20 | 6.78 | 16.56 | 11.77 | 12.98 |
| 3.75 | 0.27 | 1.16 | 1.30 | 5.63 | 3.97 | 0.91 | 1.21 | 0.71 | 1.14 | 1.66 | |
| Lomustine () | 55.83 | 3.51 | 23.30 | 16.03 | 11.47 | 22.29 | 41.00 | 39.40 | 37.45 | 46.04 | 15.14 |
| 8.63 | 0.35 | 1.16 | 4.91 | 3.06 | 6.24 | 4.65 | 10.80 | 1.97 | 5.86 | 3.75 | |
| Temozolomide () | >400 | >400 | 22.82 | >400 | 6.07 | 71.27 | >400 | >400 | 211.92 | >400 | >400 |
| 2.23 | 0.52 | 7.40 | 35.32 | ||||||||
| Bortezomib () | 4.51 | 6.16 | 9.00 | 8.69 | 7.79 | 5.69 | 4.89 | 6.24 | 4.10 | 6.34 | 8.65 |
| 2.03 | 1.46 | 4.21 | 3.39 | 0.78 | 1.37 | 1.58 | 0.51 | 0.28 | 1.09 | 1.22 | |
| Paclitaxel () | 1.22 | 3.61 | 1.68 | 1.19 | 1.42 | 1.17 | 1.24 | 1.01 | 1.46 | 1.63 | 1.23 |
| 0.33 | 0.70 | 0.31 | 0.18 | 0.36 | 0.04 | 0.11 | 0.26 | 0.17 | 0.08 | 0.04 | |
| Topotecan () | 18.71 | 10.24 | 56.30 | 11.70 | 21.98 | 15.41 | 21.02 | 52.95 | 12.12 | 20.67 | 13.04 |
| 2.82 | 4.15 | 1.36 | 1.66 | 7.20 | 0.88 | 1.33 | 2.08 | 0.98 | 1.12 | 0.81 |
| Cisplatin | Lomustine | Topotecan | ||||
|---|---|---|---|---|---|---|
| GI50 (μM) | CGI | GI50 (μM) | CGI | GI50 (μM) | CGI | |
| W/O | 237.9 | 1.000 | 4.93 | 1.000 | 20.15 | 1.000 |
| 60.8 | 0.61 | 2.03 | ||||
| SAHA | 156.3 | 0.805 | 4.90 | 0.948 | 16.31 | 0.634 |
| 18.2 | 0.070 | 0.36 | 0.023 | 1.26 | 0.318 | |
| TSA | 177.5 | 0.914 | 5.15 | 0.995 | 16.52 | 0.804 |
| 25.6 | 0.103 | 0.50 | 0.045 | 0.94 | 0.032 | |
| 1a | 134.9 | 0.428 | 2.40 | 0.495 | 13.54 | 0.700 |
| 50.9 | 0.072 | 0.28 | 0.074 | 0.58 | 0.050 | |
| 2a | 184.2 | 0.875 | 4.10 | 0.853 | 10.03 | 0.518 |
| 13.7 | 0.066 | 0.99 | 0.069 | 0.59 | 0.029 | |
| 3a | 189.7 | 0.606 | 2.31 | 0.473 | 11.20 | 0.578 |
| 70.8 | 0.112 | 0.40 | 0.055 | 0.80 | 0.029 | |
| 4a | 265.3 | 0.855 | 4.05 | 0.828 | 14.64 | 0.754 |
| 64.4 | 0.053 | 0.70 | 0.092 | 1.44 | 0.041 | |
| 5a | 120.6 | 0.566 | 2.85 | 0.591 | 12.83 | 0.659 |
| 31.8 | 0.089 | 0.72 | 0.042 | 1.68 | 0.035 | |
| 5b | 308.0 | 1.002 | 5.18 | 1.068 | 18.84 | 0.967 |
| 47.5 | 0.025 | 0.76 | 0.164 | 2.68 | 0.039 | |
| 5c | 275.3 | 0.890 | 4.56 | 0.929 | 15.44 | 0.790 |
| 55.0 | 0.048 | 0.72 | 0.039 | 3.12 | 0.074 | |

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Behnisch-Cornwell, S.; Grathwol, C.W.; Schulig, L.; Voigt, A.; Baecker, D.; Link, A.; Bednarski, P.J. Correlation Analysis of Protein Expression of 10 HDAC/Sirtuin Isoenzymes with Sensitivities of 23 Anticancer Drugs in 17 Cancer Cell Lines and Potentiation of Drug Activity by Co-Treatment with HDAC Inhibitors. Cancers 2022, 14, 187. https://doi.org/10.3390/cancers14010187
Behnisch-Cornwell S, Grathwol CW, Schulig L, Voigt A, Baecker D, Link A, Bednarski PJ. Correlation Analysis of Protein Expression of 10 HDAC/Sirtuin Isoenzymes with Sensitivities of 23 Anticancer Drugs in 17 Cancer Cell Lines and Potentiation of Drug Activity by Co-Treatment with HDAC Inhibitors. Cancers. 2022; 14(1):187. https://doi.org/10.3390/cancers14010187
Chicago/Turabian StyleBehnisch-Cornwell, Steven, Christoph W. Grathwol, Lukas Schulig, Anika Voigt, Daniel Baecker, Andreas Link, and Patrick J. Bednarski. 2022. "Correlation Analysis of Protein Expression of 10 HDAC/Sirtuin Isoenzymes with Sensitivities of 23 Anticancer Drugs in 17 Cancer Cell Lines and Potentiation of Drug Activity by Co-Treatment with HDAC Inhibitors" Cancers 14, no. 1: 187. https://doi.org/10.3390/cancers14010187
APA StyleBehnisch-Cornwell, S., Grathwol, C. W., Schulig, L., Voigt, A., Baecker, D., Link, A., & Bednarski, P. J. (2022). Correlation Analysis of Protein Expression of 10 HDAC/Sirtuin Isoenzymes with Sensitivities of 23 Anticancer Drugs in 17 Cancer Cell Lines and Potentiation of Drug Activity by Co-Treatment with HDAC Inhibitors. Cancers, 14(1), 187. https://doi.org/10.3390/cancers14010187







