Radical or Not-So-Radical Prostatectomy: Do Surgical Margins Matter?
Abstract
Simple Summary
Abstract
1. Introduction
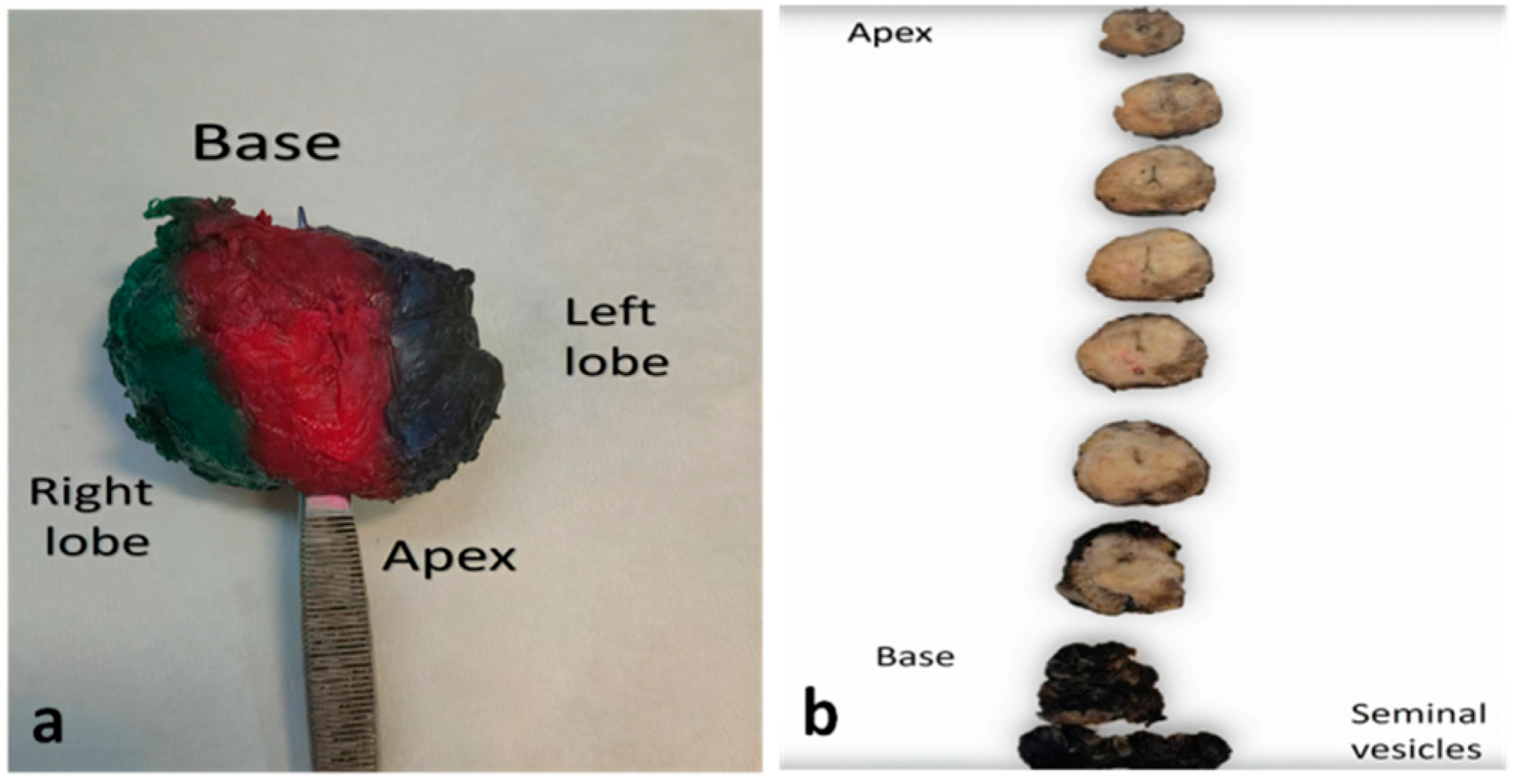
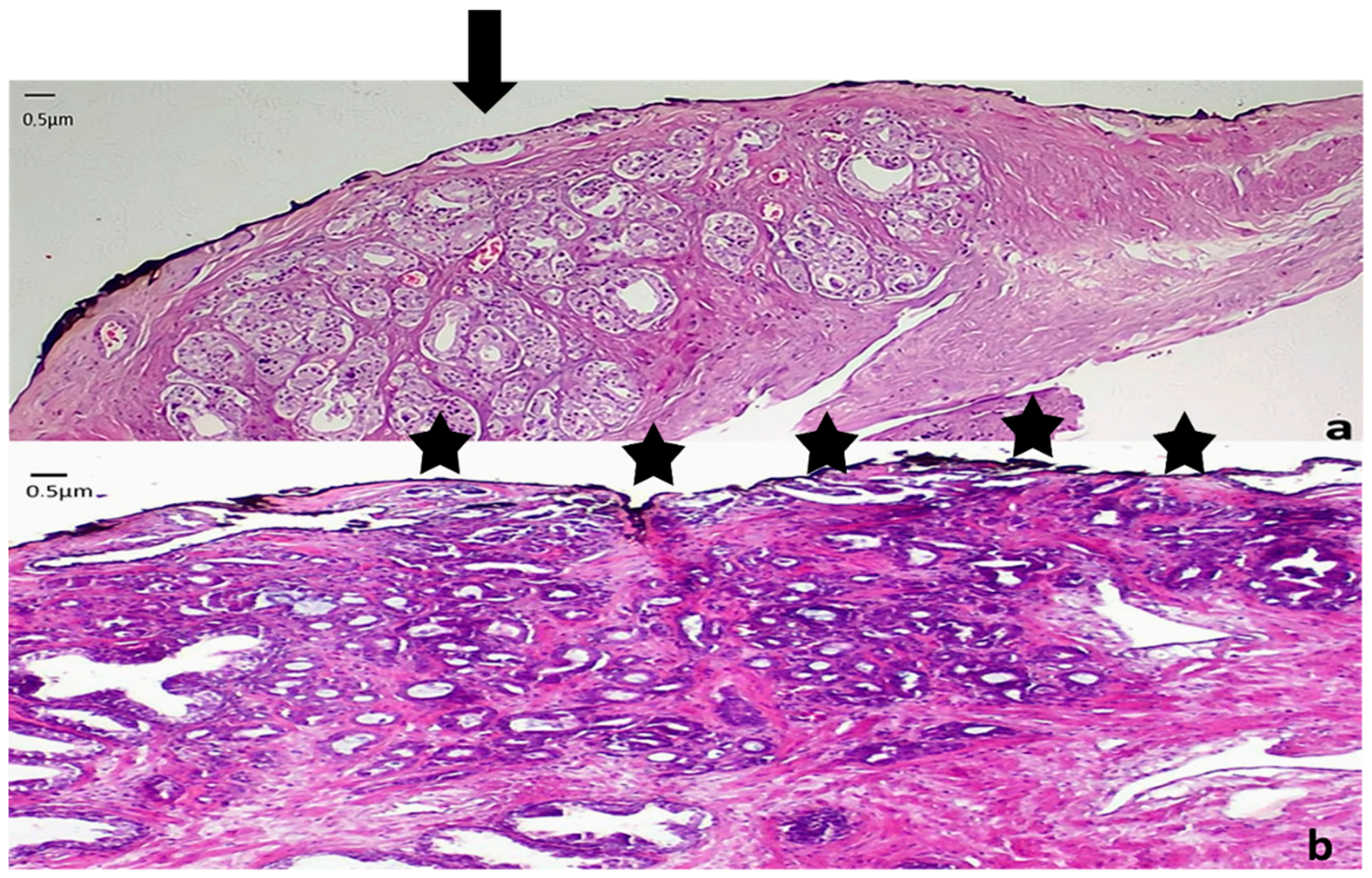
2. Pathology
3. Factors Affecting the Probability of Margin Positivity
4. Clinical Significance of Positive Margins
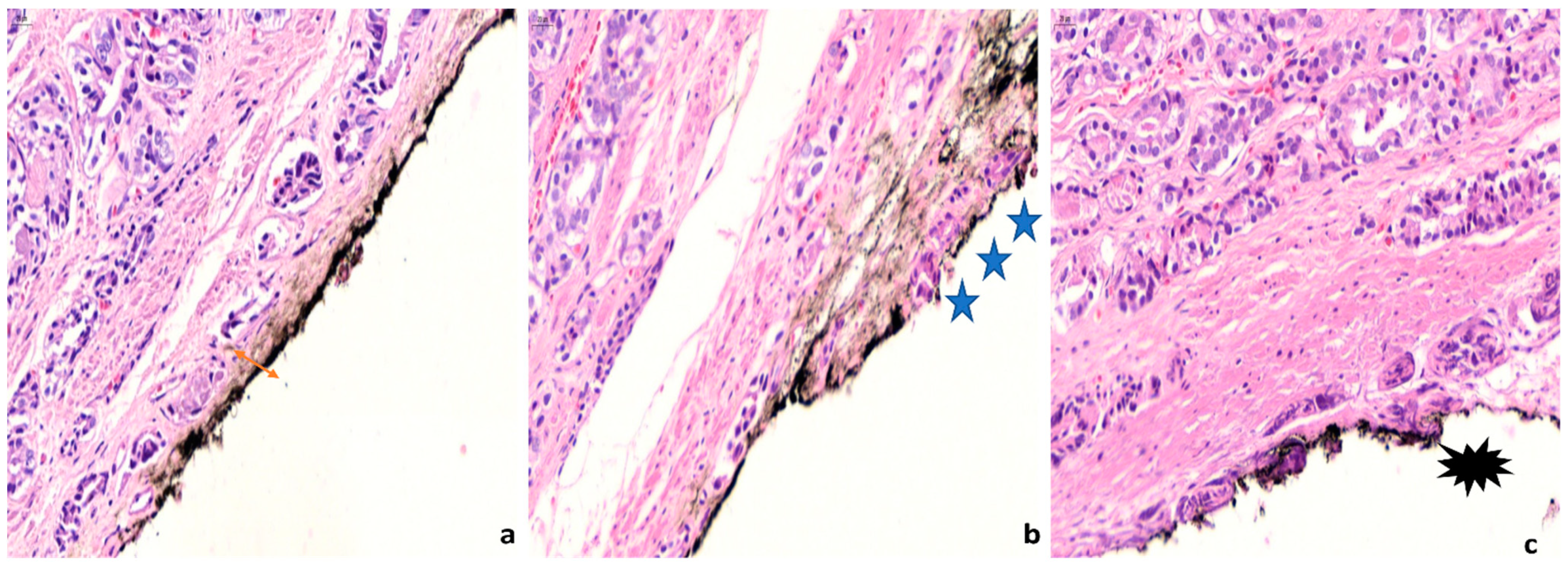
5. Pathologic Features of Positive Margins with Clinical Significance
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.J.; Mallin, K.; Graves, A.J.; Chang, S.S.; Penson, D.F.; Resnick, M.J.; Barocas, D.A. Recent Changes in Prostate Cancer Screening Practices and Epidemiology. J. Urol. 2017, 198, 1230–1240. [Google Scholar] [CrossRef]
- Johansson, J.; Andrén, O.; Andersson, S.-O.; Dickman, P.W.; Holmberg, L.; Magnuson, A.; Adami, H.-O. Natural history of early, localized prostate cancer. JAMA 2004, 291, 2713–2719. [Google Scholar] [CrossRef] [PubMed]
- Aparicio, A.; Tzelepi, V. Neuroendocrine (small-cell) carcinomas: Why they teach us essential lessons about prostate cancer. Oncology 2014, 28, 831–838. [Google Scholar]
- Epstein, J.I.; Egevad, L.; Amin, M.B.; Delahunt, B.; Srigley, J.R.; Humphrey, P.A. The 2014 international society of urological pathology (ISUP) consensus conference on gleason grading of prostatic carcinoma definition of grading patterns and proposal for a new grading system. Am. J. Surg. Pathol. 2016, 40, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, G.; Warde, P.; Pickles, T.; Crook, J.; Brundage, M.; Souhami, L.; Lukka, H. Pre-treatment risk stratification of prostate cancer patients: A critical review. Can. Urol. Assoc. J. 2012, 6, 121–127. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network, N. Prostate Cancer NCCN Guidelines (Version 2.2021). Available online: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1459 (accessed on 20 December 2021).
- Chade, D.C.; Eastham, J.; Graefen, M.; Hu, J.C.; Karnes, R.J.; Klotz, L.; Montorsi, F.; van Poppel, H.; Scardino, P.T.; Shariat, S.F. Cancer Control and Functional Outcomes of Salvage Radical Prostatectomy for Radiation-recurrent Prostate Cancer: A Systematic Review of the Literature. Eur. Urol. 2012, 61, 961–971. [Google Scholar] [CrossRef]
- May, D.N.; Parker, W.P. Narrative review of principles of salvage radical prostatectomy. AME Med. J. 2021, 6, 3. [Google Scholar] [CrossRef]
- McNeal, J.E. Prostate. In Histology for Histopathologists; Mills, S.E., Ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2007. [Google Scholar]
- Ayala, A.G.; Ro, J.Y.; Babaian, R.; Troncoso, P.; Grignon, D.J. The prostatic capsule: Does it exist? Its importance in the staging and treatment of prostatic carcinoma. Am. J. Surg. Pathol. 1989, 13, 21–27. [Google Scholar] [CrossRef]
- Paner, G.; Srigley, J.; Pettus, J.; Giannico, G.A.; Sirintrapun, J.; Harik, L.R. Protocol for the Examination of Radical Prostatectomy Specimens From Patients With Carcinoma of the Prostate Gland; College of American Pathologists (CAP): Northfield, IL, USA, 2021. [Google Scholar]
- Magi-Galluzzi, C.; Evans, A.J.; Delahunt, B.; Epstein, J.I.; Griffiths, D.F.; van der Kwast, T.H.; Montironi, R.; Wheeler, T.M.; Srigley, J.R.; Egevad, L.L.; et al. International Society of Urological Pathology (ISUP) Consensus Conference on Handling and Staging of Radical Prostatectomy Specimens. Working group 3: Extraprostatic extension, lymphovascular invasion and locally advanced disease. Mod. Pathol. 2011, 24, 26–38. [Google Scholar] [CrossRef]
- van der Kwast, T.H.; Amin, M.B.; Billis, A.; Epstein, J.I.; Griffiths, D.; Humphrey, P.A.; Montironi, R.; Wheeler, T.M.; Srigley, J.R.; Egevad, L.; et al. International Society of Urological Pathology (ISUP) Consensus Conference on Handling and Staging of Radical Prostatectomy Specimens. Working group 5: Surgical margins. Mod. Pathol. 2011, 24, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Silberstein, J.; Eastham, J. Significance and management of positive surgical margins at the time of radical prostatectomy. Indian J. Urol. 2014, 30, 423. [Google Scholar] [CrossRef]
- Samaratunga, H.; Montironi, R.; True, L.; Epstein, J.I.; Griffiths, D.F.; Humphrey, P.A.; van der Kwast, T.; Wheeler, T.M.; Srigley, J.R.; Delahunt, B.; et al. International Society of Urological Pathology (ISUP) Consensus Conference on Handling and Staging of Radical Prostatectomy Specimens. Working group 1: Specimen handling. Mod. Pathol. 2011, 24, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Epstein, J.I.; Sauvageot, J. Do close but negative margins in radical prostatectomy specimens increase the risk of postoperative progression? J. Urol. 1997, 157, 241–243. [Google Scholar] [CrossRef]
- Emerson, R.E.; Koch, M.O.; Daggy, J.K.; Cheng, L. Closest Distance Between Tumor and Resection Margin in Radical Prostatectomy Specimens. Am. J. Surg. Pathol. 2005, 29, 225–229. [Google Scholar] [CrossRef]
- Kang, Y.J.; Abalajon, M.J.; Jang, W.S.; Kwon, J.K.; Yoon, C.Y.; Lee, J.Y.; Cho, K.S.; Ham, W.S.; Choi, Y.D. Association of Anterior and Lateral Extraprostatic Extensions with Base-Positive Resection Margins in Prostate Cancer. PLoS ONE 2016, 11, e0158922. [Google Scholar] [CrossRef] [PubMed]
- Martini, A.; Gandaglia, G.; Fossati, N.; Scuderi, S.; Bravi, C.A.; Mazzone, E.; Stabile, A.; Scarcella, S.; Robesti, D.; Barletta, F.; et al. Defining Clinically Meaningful Positive Surgical Margins in Patients Undergoing Radical Prostatectomy for Localised Prostate Cancer. Eur. Urol. Oncol. 2021, 4, 42–48. [Google Scholar] [CrossRef]
- Meeks, J.J.; Eastham, J.A. Radical prostatectomy: Positive surgical margins matter. Urol. Oncol. Semin. Orig. Investig. 2013, 31, 974–979. [Google Scholar] [CrossRef]
- Lavallée, L.T.; Stokl, A.; Cnossen, S.; Mallick, R.; Morash, C.; Cagiannos, I.; Breau, R.H. The effect of wide resection during radical prostatectomy on surgical margins. Can. Urol. Assoc. J. 2016, 10, 14–17. [Google Scholar] [CrossRef]
- Porcaro, A.B.; Tafuri, A.; Sebben, M.; Amigoni, N.; Processali, T.; Pirozzi, M.; Rizzetto, R.; Shakir, A.; Corsi, P.; Tiso, L.; et al. High surgeon volume and positive surgical margins can predict the risk of biochemical recurrence after robot-assisted radical prostatectomy. Ther. Adv. Urol. 2019, 11, 1756287219878283. [Google Scholar] [CrossRef] [PubMed]
- Porcaro, A.B.; Tafuri, A.; Sebben, M.; Amigoni, N.; Shakir, A.; Corsi, P.; Processali, T.; Pirozzi, M.; Rizzetto, R.; Bernasconi, R.; et al. Linear extent of positive surgical margin impacts biochemical recurrence after robot-assisted radical prostatectomy in a high-volume center. J. Robot. Surg. 2020, 14, 663–675. [Google Scholar] [CrossRef]
- Lantz, A.; Bock, D.; Akre, O.; Angenete, E.; Bjartell, A.; Carlsson, S.; Modig, K.K.; Nyberg, M.; Kollberg, K.S.; Steineck, G.; et al. Functional and Oncological Outcomes after Open versus Robot-assisted Laparoscopic Radical Prostatectomy for Localised Prostate Cancer: 8-Year Follow-up. Eur. Urol. 2021, 80, 650–660. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.E.; Egger, S.; Böhm, M.; Siriwardana, A.R.; Haynes, A.-M.; Matthews, J.; Scheltema, M.J.; Stricker, P.D. Superior Biochemical Recurrence and Long-term Quality-of-life Outcomes Are Achievable with Robotic Radical Prostatectomy after a Long Learning Curve—Updated Analysis of a Prospective Single-surgeon Cohort of 2206 Consecutive Cases. Eur. Urol. 2018, 73, 664–671. [Google Scholar] [CrossRef]
- Parsons, J.K.; Bennett, J.L. Outcomes of Retropubic, Laparoscopic, and Robotic-Assisted Prostatectomy. Urology 2008, 72, 412–416. [Google Scholar] [CrossRef] [PubMed]
- Yaxley, J.W.; Coughlin, G.D.; Chambers, S.K.; Occhipinti, S.; Samaratunga, H.; Zajdlewicz, L.; Dunglison, N.; Carter, R.; Williams, S.; Payton, D.J.; et al. Robot-assisted laparoscopic prostatectomy versus open radical retropubic prostatectomy: Early outcomes from a randomised controlled phase 3 study. Lancet 2016, 388, 1057–1066. [Google Scholar] [CrossRef]
- Seisen, T.; Cole, A.P.; Sun, M.; Kibel, A.S.; Trinh, Q.-D. Assessing robot-assisted laparoscopic prostatectomy. Lancet 2017, 389, 799. [Google Scholar] [CrossRef]
- Suardi, N.; Moschini, M.; Gallina, A.; Gandaglia, G.; Abdollah, F.; Capitanio, U.; Bianchi, M.; Tutolo, M.; Passoni, N.; Salonia, A.; et al. Nerve-sparing approach during radical prostatectomy is strongly associated with the rate of postoperative urinary continence recovery. BJU Int. 2013, 111, 717–722. [Google Scholar] [CrossRef] [PubMed]
- Ayyathurai, R.; Manoharan, M.; Nieder, A.M.; Kava, B.; Soloway, M.S. Factors affecting erectile function after radical retropubic prostatectomy: Results from 1620 consecutive patients. BJU Int. 2008, 101, 833–836. [Google Scholar] [CrossRef]
- Matsuda, Y.; Narita, S.; Okubo, T.; Mitsuzuka, K.; Hatakeyama, S.; Koizumi, A.; Koie, T.; Kawamura, S.; Tochigi, T.; Ito, A.; et al. Impact of Nerve-Sparing Status on Positive Surgical Margin Location and Biochemical Recurrence in Patients with Prostate Cancer Post Radical Prostatectomy. Ann. Surg. Oncol. 2021, 28, 5341–5348. [Google Scholar] [CrossRef]
- Rosenberg, J.E.; Jung, J.H.; Edgerton, Z.; Lee, H.; Lee, S.; Bakker, C.J.; Dahm, P. Retzius-sparing versus standard robot-assisted laparoscopic prostatectomy for the treatment of clinically localized prostate cancer. BJU Int. 2021, 128, 12–20. [Google Scholar] [CrossRef]
- Qiu, X.; Li, Y.; Chen, M.; Xu, L.; Guo, S.; Marra, G.; Elliot Rosenberg, J.; Ma, H.; Li, X.; Guo, H. Retzius-sparing robot-assisted radical prostatectomy improves early recovery of urinary continence: A randomized, controlled, single-blind trial with a 1-year follow-up. BJU Int. 2020, 126, 633–640. [Google Scholar] [CrossRef]
- Porcaro, A.B.; Sebben, M.; Corsi, P.; Tafuri, A.; Processali, T.; Pirozzi, M.; Amigoni, N.; Rizzetto, R.; Cacciamani, G.; Mariotto, A.; et al. Risk factors of positive surgical margins after robot-assisted radical prostatectomy in high-volume center: Results in 732 cases. J. Robot. Surg. 2020, 14, 167–175. [Google Scholar] [CrossRef]
- Wang, S.; Du, P.; Cao, Y.; Yang, X.; Yang, Y. Tumor Biological Feature and Its Association with Positive Surgical Margins and Apical Margins after Radical Prostatectomy in Non-Metastasis Prostate Cancer. Curr. Oncol. 2021, 28, 1528–1536. [Google Scholar] [CrossRef]
- Gurung, P.M.S.; Wang, B.; Hassig, S.; Wood, J.; Ellis, E.; Feng, C.; Ghazi, A.E.; Joseph, J.V. Oncological and functional outcomes in patients over 70 years of age treated with robotic radical prostatectomy: A propensity-matched analysis. World J. Urol. 2021, 39, 1131–1140. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.; Jiang, K.; Chen, H.; Chen, Z.; Xu, H.; Ye, Z. Robotic vs. Retropubic radical prostatectomy in prostate cancer: A systematic review and a meta-analysis update. Oncotarget 2017, 8, 32237–32257. [Google Scholar] [CrossRef]
- Lee, W.R.; Dignam, J.J.; Amin, M.B.; Bruner, D.W.; Low, D.; Swanson, G.P.; Shah, A.B.; D’Souza, D.P.; Michalski, J.M.; Dayes, I.S.; et al. Randomized Phase III Noninferiority Study Comparing Two Radiotherapy Fractionation Schedules in Patients with Low-Risk Prostate Cancer. J. Clin. Oncol. 2016, 34, 2325–2332. [Google Scholar] [CrossRef] [PubMed]
- Koskas, Y.; Lannes, F.; Branger, N.; Giusiano, S.; Guibert, N.; Pignot, G.; Walz, J.; Rossi, D.; Bastide, C. Extent of positive surgical margins following radical prostatectomy: Impact on biochemical recurrence with long-term follow-up. BMC Urol. 2019, 19, 37. [Google Scholar] [CrossRef]
- Ploussard, G.; Agamy, M.A.; Alenda, O.; Allory, Y.; Mouracade, P.; Vordos, D.; Hoznek, A.; Abbou, C.-C.; de la Taille, A.; Salomon, L. Impact of positive surgical margins on prostate-specific antigen failure after radical prostatectomy in adjuvant treatment-naïve patients. BJU Int. 2011, 107, 1748–1754. [Google Scholar] [CrossRef]
- Alkhateeb, S.; Alibhai, S.; Fleshner, N.; Finelli, A.; Jewett, M.; Zlotta, A.; Nesbitt, M.; Lockwood, G.; Trachtenberg, J. Impact of Positive Surgical Margins after Radical Prostatectomy Differs by Disease Risk Group. J. Urol. 2010, 183, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhao, H.; Wu, B.; Zha, Z.; Yuan, J.; Feng, Y. Predictive Factors for Positive Surgical Margins in Patients with Prostate Cancer after Radical Prostatectomy: A Systematic Review and Meta-Analysis. Front. Oncol. 2021, 10, 539592. [Google Scholar] [CrossRef]
- Ouzzane, A.; Rozet, F.; Salas, R.S.; Galiano, M.; Barret, E.; Prapotnich, D.; Cathelineau, X. Positive surgical margins after minimally invasive radical prostatectomy in patients with pT2 and pT3a disease could be considered pathological upstaging. BJU Int. 2014, 113, 586–591. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, B.; Zhou, J.; Wu, S.; Guo, M.; Zhang, Y.; Liu, R. The impact of surgical margin status on prostate cancer-specific mortality after radical prostatectomy: A systematic review and meta-analysis. Clin. Transl. Oncol. 2020, 22, 2087–2096. [Google Scholar] [CrossRef]
- Chalfin, H.J.; Dinizo, M.; Trock, B.J.; Feng, Z.; Partin, A.W.; Walsh, P.C.; Humphreys, E.; Han, M. Impact of surgical margin status on prostate-cancer-specific mortality. BJU Int. 2012, 110, 1684–1689. [Google Scholar] [CrossRef]
- Preisser, F.; Coxilha, G.; Heinze, A.; Oh, S.; Chun, F.K.; Sauter, G.; Pompe, R.S.; Huland, H.; Graefen, M.; Tilki, D. Impact of positive surgical margin length and Gleason grade at the margin on biochemical recurrence in patients with organ-confined prostate cancer. Prostate 2019, 79, 1832–1836. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wu, B.; Zha, Z.; Zhao, H.; Jiang, Y.; Yuan, J. Positive surgical margin is associated with biochemical recurrence risk following radical prostatectomy: A meta-analysis from high-quality retrospective cohort studies. World J. Surg. Oncol. 2018, 16, 124. [Google Scholar] [CrossRef] [PubMed]
- Seo, W.I.; Kang, P.M.; Yoon, J.H.; Kim, W.; Chung, J. Il Correlation between postoperative prostate-specific antigen and biochemical recurrence in positive surgical margin patients: Single surgeon series. Prostate Int. 2017, 5, 53–58. [Google Scholar] [CrossRef]
- Van der Kwast, T.H.; Bolla, M.; Van Poppel, H.; Van Cangh, P.; Vekemans, K.; Da Pozzo, L.; Bosset, J.-F.; Kurth, K.H.; Schröder, F.H.; Collette, L. Identification of Patients with Prostate Cancer Who Benefit from Immediate Postoperative Radiotherapy: EORTC 22911. J. Clin. Oncol. 2007, 25, 4178–4186. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.J.; Hong, S.K.; Byun, S.-S.; Choe, G.; Lee, S.E. Prognostic significance of positive surgical margins after radical prostatectomy among pT2 and pT3a prostate cancer. Urol. Oncol. Semin. Orig. Investig. 2013, 31, 595–600. [Google Scholar] [CrossRef]
- Lake, A.M.; He, C.; Wood, D.P. Focal Positive Surgical Margins Decrease Disease-free Survival After Radical Prostatectomy Even in Organ-confined Disease. Urology 2010, 76, 1212–1216. [Google Scholar] [CrossRef]
- Meeks, J.J.; Kern, S.Q.; Dalbagni, G.; Eastham, J.A.; Sandhu, J.S. The Prevalence of Persistent Prostate Cancer after Radiotherapy Detected at Radical Cystoprostatectomy for Bladder Cancer. J. Urol. 2014, 191, 1760–1763. [Google Scholar] [CrossRef]
- Swindle, P.; Eastham, J.A.; Ohori, M.; Kattan, M.W.; Wheeler, T.; Maru, N.; Slawin, K.; Scardino, P.T. Do margins matter? The prognostic significance of positive surgical margins in radical prostatectomy specimens. J. Urol. 2005, 174, 903–907. [Google Scholar] [CrossRef]
- Sooriakumaran, P.; Ploumidis, A.; Nyberg, T.; Olsson, M.; Akre, O.; Haendler, L.; Egevad, L.; Nilsson, A.; Carlsson, S.; Jonsson, M.; et al. The impact of length and location of positive margins in predicting biochemical recurrence after robot-assisted radical prostatectomy with a minimum follow-up of 5 years. BJU Int. 2015, 115, 106–113. [Google Scholar] [CrossRef]
- Blute, M.L.; Bostwick, D.G.; Bergstralh, E.J.; Slezak, J.M.; Martin, S.K.; Amling, C.L.; Zincke, H. Anatomic site-specific positive margins in organconfined prostate cancer and its impact on outcome after radical prostatectomy. Urology 1997, 50, 733–739. [Google Scholar] [CrossRef]
- Lee, S.; Kim, K.B.; Jo, J.K.; Ho, J.-N.; Oh, J.J.; Jeong, S.J.; Hong, S.K.; Byun, S.-S.; Choe, G.; Lee, S.E. Prognostic Value of Focal Positive Surgical Margins After Radical Prostatectomy. Clin. Genitourin. Cancer 2016, 14, e313–e319. [Google Scholar] [CrossRef] [PubMed]
- Kates, M.; Sopko, N.A.; Han, M.; Partin, A.W.; Epstein, J.I. Importance of Reporting the Gleason Score at the Positive Surgical Margin Site: Analysis of 4,082 Consecutive Radical Prostatectomy Cases. J. Urol. 2016, 195, 337–342. [Google Scholar] [CrossRef]
- Cao, D.; Kibel, A.S.; Gao, F.; Tao, Y.; Humphrey, P.A. The Gleason Score of Tumor at the Margin in Radical Prostatectomy is Predictive of Biochemical Recurrence. Am. J. Surg. Pathol. 2010, 34, 994–1001. [Google Scholar] [CrossRef] [PubMed]
- Savdie, R.; Horvath, L.G.; Benito, R.P.; Rasiah, K.K.; Haynes, A.-M.; Chatfield, M.; Stricker, P.D.; Turner, J.J.; Delprado, W.; Henshall, S.M.; et al. High Gleason grade carcinoma at a positive surgical margin predicts biochemical failure after radical prostatectomy and may guide adjuvant radiotherapy. BJU Int. 2012, 109, 1794–1800. [Google Scholar] [CrossRef] [PubMed]
- Viers, B.R.; Sukov, W.R.; Gettman, M.T.; Rangel, L.J.; Bergstralh, E.J.; Frank, I.; Tollefson, M.K.; Thompson, R.H.; Boorjian, S.A.; Karnes, R.J. Primary Gleason Grade 4 at the Positive Margin Is Associated with Metastasis and Death among Patients with Gleason 7 Prostate Cancer Undergoing Radical Prostatectomy. Eur. Urol. 2014, 66, 1116–1124. [Google Scholar] [CrossRef]
- John, A.; John, H.; Catterwell, R.; Selth, L.A.; Callaghan, M.O. Primary Gleason grade and Gleason grade group at positive surgical margins: A systematic review and meta-analysis. BJU Int. 2021, 127, 13–22. [Google Scholar] [CrossRef]
- Kulac, I.; Haffner, M.C.; Yegnasubramanian, S.; Epstein, J.I.; De Marzo, A.M. Should Gleason 6 be labeled as cancer? Curr. Opin. Urol. 2015, 25, 238–245. [Google Scholar] [CrossRef]
- Jamaspishvili, T.; Berman, D.M.; Ross, A.E.; Scher, H.I.; De Marzo, A.M.; Squire, J.A.; Lotan, T.L. Clinical implications of PTEN loss in prostate cancer. Nat. Rev. Urol. 2018, 15, 222–234. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yoshimoto, M.; Trpkov, K.; Duan, Q.; Firszt, M.; Corcos, J.; Squire, J.A.; Bismar, T.A. Detection of ERG gene rearrangements and PTEN deletions in unsuspected prostate cancer of the transition zone. Cancer Biol. Ther. 2011, 11, 562–566. [Google Scholar] [CrossRef] [PubMed]
- Koppie, T.M.; Bianco, F.J.; Kuroiwa, K.; Reuter, V.E.; Guillonneau, B.; Eastham, J.A.; Scardino, P.T. The clinical features of anterior prostate cancers. BJU Int. 2006, 98, 1167–1171. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grypari, I.M.; Zolota, V.; Tzelepi, V. Radical or Not-So-Radical Prostatectomy: Do Surgical Margins Matter? Cancers 2022, 14, 13. https://doi.org/10.3390/cancers14010013
Grypari IM, Zolota V, Tzelepi V. Radical or Not-So-Radical Prostatectomy: Do Surgical Margins Matter? Cancers. 2022; 14(1):13. https://doi.org/10.3390/cancers14010013
Chicago/Turabian StyleGrypari, Ioanna Maria, Vasiliki Zolota, and Vasiliki Tzelepi. 2022. "Radical or Not-So-Radical Prostatectomy: Do Surgical Margins Matter?" Cancers 14, no. 1: 13. https://doi.org/10.3390/cancers14010013
APA StyleGrypari, I. M., Zolota, V., & Tzelepi, V. (2022). Radical or Not-So-Radical Prostatectomy: Do Surgical Margins Matter? Cancers, 14(1), 13. https://doi.org/10.3390/cancers14010013






