Immune Checkpoint Inhibitors in Prostate Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Biological Rationale and Barriers to Immune Checkpoint Blockade in Prostate Cancer
3. Predictors of Response to Immune Checkpoint Blockade
4. Studies Evaluating Single Agent CTLA-4 Inhibitors in mCRPC
5. Studies Evaluating Single Agent PD-1/L1 Inhibitors in mCRPC
6. PD-L1 Blockade in Combination with Androgen Inhibitors
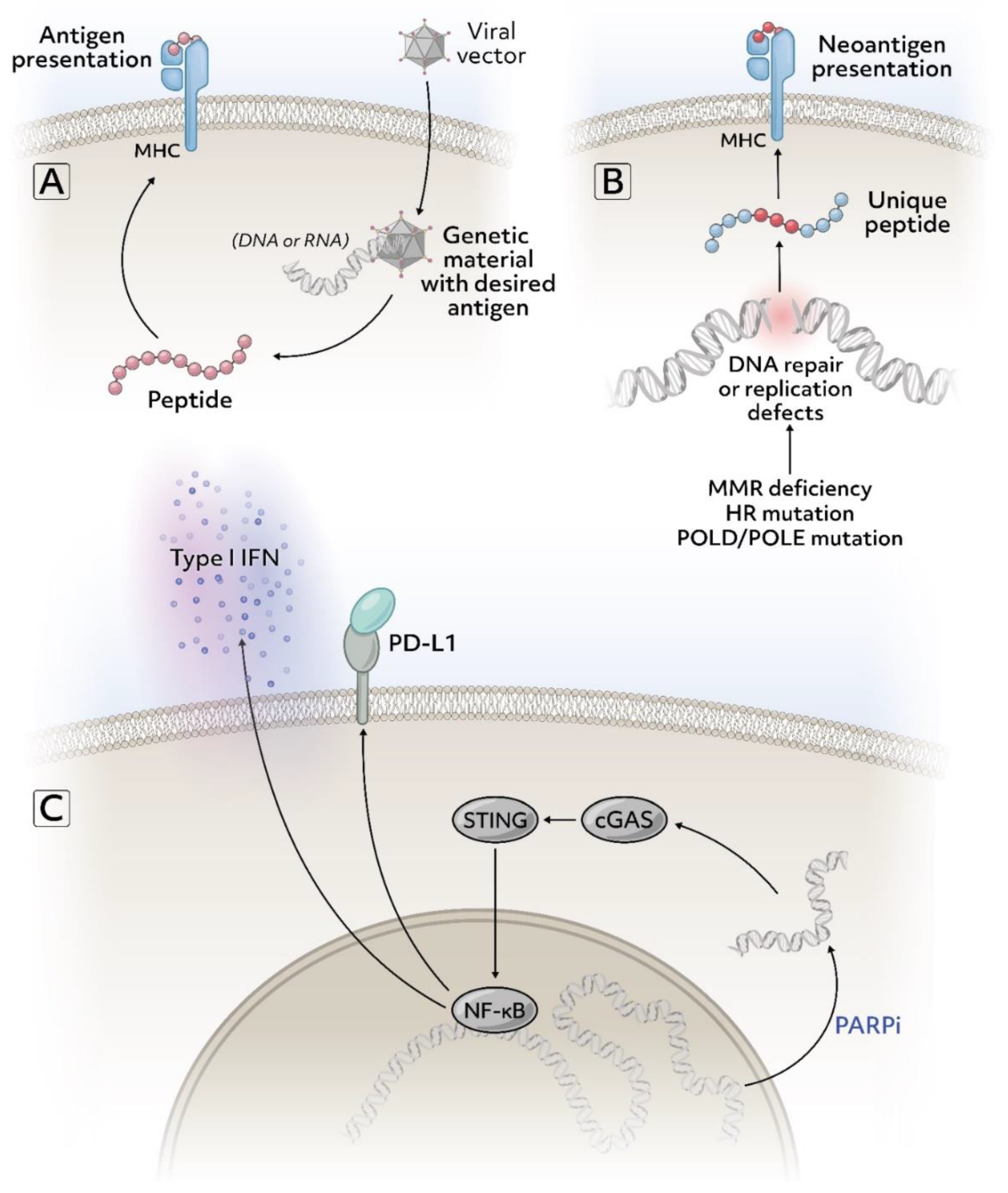
7. Immune Checkpoint Blockade with PARP Inhibitors
8. Immune Checkpoint Blockade with Radiotherapeutic Agents, Radiotherapy, or Cryotherapy
9. Immune Checkpoint Blockade with Tumor Vaccines
10. Immune Checkpoint Blockade with Chemotherapy
11. CTLA-4 and PD-1/PD-L1 Combination Therapy
12. Tyrosine Kinase Inhibitors with Immune Checkpoint Blockade
13. Other Combinations with Immunecheck Point Blockade
14. Future Directions
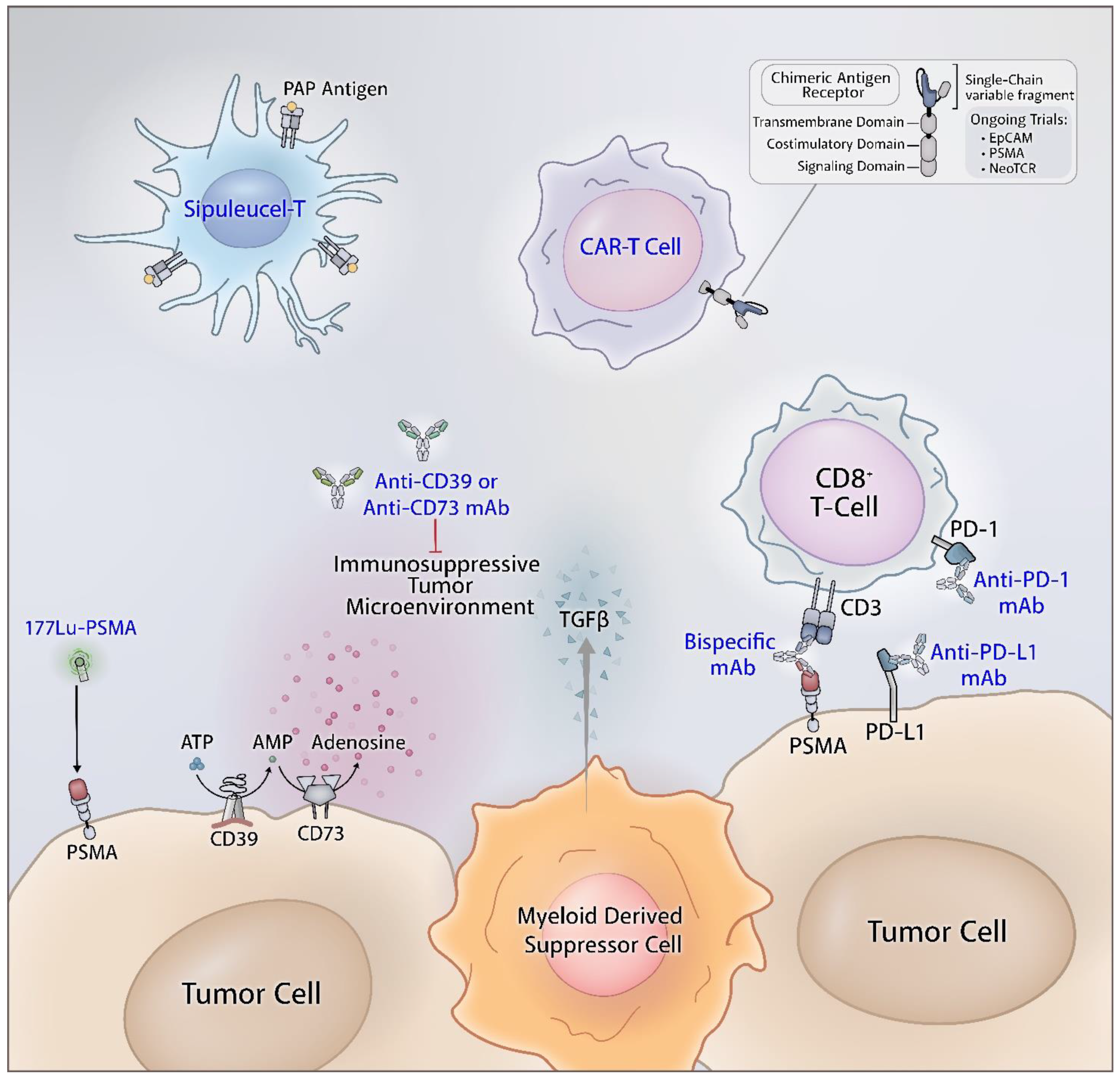
14.1. Combination Immune Checkpoint and Adenosine Axis Blockade
14.2. Bispecific T Cell Engager and Immune Check Point Blockade
14.3. Lu-PSMA-617 and Immune Checkpoint Blockade
14.4. Adoptive T Cell Therapy and Immune Checkpoint Blockade
14.5. Miscellaneous Agents
15. Conclusions
Funding
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021. [Google Scholar] [CrossRef] [PubMed]
- Swami, U.; McFarland, T.R.; Nussenzveig, R.; Agarwal, N. Advanced Prostate Cancer: Treatment Advances and Future Directions. Trends Cancer 2020, 6, 702–715. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network. Prostate Cancer (Version 2.2021). Available online: http://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf (accessed on 23 February 2021).
- Wolchok, J.D.; Kluger, H.; Callahan, M.K.; Postow, M.A.; Rizvi, N.A.; Lesokhin, A.M.; Segal, N.H.; Ariyan, C.E.; Gordon, R.A.; Reed, K.; et al. Nivolumab plus ipilimumab in advanced melanoma. N. Engl. J. Med. 2013, 369, 122–133. [Google Scholar] [CrossRef]
- Ellis, P.M.; Vella, E.T.; Ung, Y.C. Immune Checkpoint Inhibitors for Patients With Advanced Non–Small-Cell Lung Cancer: A Systematic Review. Clin. Lung Cancer 2017, 18, 444–459.e1. [Google Scholar] [CrossRef]
- Sun, C.; Mezzadra, R.; Schumacher, T.N. Regulation and Function of the PD-L1 Checkpoint. Immunity 2018, 48, 434–452. [Google Scholar] [CrossRef]
- Francisco, L.M.; Sage, P.T.; Sharpe, A.H. The PD-1 pathway in tolerance and autoimmunity. Immunol. Rev. 2010, 236, 219–242. [Google Scholar] [CrossRef]
- Francisco, L.M.; Salinas, V.H.; Brown, K.E.; Vanguri, V.K.; Freeman, G.J.; Kuchroo, V.K.; Sharpe, A.H. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J. Exp. Med. 2009, 206, 3015–3029. [Google Scholar] [CrossRef]
- Muenst, S.; Laubli, H.; Soysal, S.D.; Zippelius, A.; Tzankov, A.; Hoeller, S. The immune system and cancer evasion strategies: Therapeutic concepts. J. Intern. Med. 2016, 279, 541–562. [Google Scholar] [CrossRef]
- Ness, N.; Andersen, S.; Khanehkenari, M.R.; Nordbakken, C.V.; Valkov, A.; Paulsen, E.-E.; Nordby, Y.; Bremnes, R.M.; Donnem, T.; Busund, L.-T.; et al. The prognostic role of immune checkpoint markers programmed cell death protein 1 (PD-1) and programmed death ligand 1 (PD-L1) in a large, multicenter prostate cancer cohort. Oncotarget 2017, 8, 26789–26801. [Google Scholar] [CrossRef]
- Gevensleben, H.; Dietrich, D.; Golletz, C.; Steiner, S.; Jung, M.; Thiesler, T.; Majores, M.; Stein, J.; Uhl, B.; Müller, S.; et al. The Immune Checkpoint Regulator PD-L1 Is Highly Expressed in Aggressive Primary Prostate Cancer. Clin. Cancer Res. 2016, 22, 1969–1977. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.M.; Nirschl, T.R.; Nirschl, C.J.; Francica, B.J.; Kochel, C.M.; van Bokhoven, A.; Meeker, A.K.; Lucia, M.S.; Anders, R.A.; DeMarzo, A.M.; et al. Paucity of PD-L1 expression in prostate cancer: Innate and adaptive immune resistance. Prostate Cancer Prostatic Dis. 2015, 18, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Massari, F.; Ciccarese, C.; Calio, A.; Munari, E.; Cima, L.; Porcaro, A.B.; Novella, G.; Artibani, W.; Sava, T.; Eccher, A.; et al. Magnitude of PD-1, PD-L1 and T Lymphocyte Expression on Tissue from Castration-Resistant Prostate Adenocarcinoma: An Exploratory Analysis. Target Oncol. 2016, 11, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Baas, W.; Gershburg, S.; Dynda, D.; Delfino, K.; Robinson, K.; Nie, D.; Yearley, J.H.; Alanee, S. Immune Characterization of the Programmed Death Receptor Pathway in High Risk Prostate Cancer. Clin Genitourin. Cancer 2017, 15, 577–581. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.F.; Lawrence, M.S.; Demichelis, F.; Drier, Y.; Cibulskis, K.; Sivachenko, A.Y.; Sboner, A.; Esgueva, R.; Pflueger, D.; Sougnez, C.; et al. The genomic complexity of primary human prostate cancer. Nature 2011, 470, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Chouaib, S.; Noman, M.Z.; Kosmatopoulos, K.; Curran, M.A. Hypoxic stress: Obstacles and opportunities for innovative immunotherapy of cancer. Oncogene 2017, 36, 439–445. [Google Scholar] [CrossRef]
- Jayaprakash, P.; Ai, M.; Liu, A.; Budhani, P.; Bartkowiak, T.; Sheng, J.; Ager, C.; Nicholas, C.; Jaiswal, A.R.; Sun, Y.; et al. Targeted hypoxia reduction restores T cell infiltration and sensitizes prostate cancer to immunotherapy. J. Clin. Investig. 2018, 128, 5137–5149. [Google Scholar] [CrossRef] [PubMed]
- Topalian, S.L.; Taube, J.M.; Anders, R.A.; Pardoll, D.M. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat. Rev. Cancer 2016, 16, 275–287. [Google Scholar] [CrossRef]
- Miller, A.M.; Lundberg, K.; Özenci, V.; Banham, A.H.; Hellström, M.; Egevad, L.; Pisa, P. CD4+ CD25high T cells are enriched in the tumor and peripheral blood of prostate cancer patients. J. Immunol. 2006, 177, 7398–7405. [Google Scholar] [CrossRef]
- Kiniwa, Y.; Miyahara, Y.; Wang, H.Y.; Peng, W.; Peng, G.; Wheeler, T.M.; Thompson, T.C.; Old, L.J.; Wang, R.-F. CD8+ Foxp3+ regulatory T cells mediate immunosuppression in prostate cancer. Clin. Cancer Res. 2007, 13, 6947–6958. [Google Scholar] [CrossRef]
- Sanda, M.G.; Restifo, N.P.; Walsh, J.C.; Kawakami, Y.; Nelson, W.G.; Pardoll, D.M.; Simons, J.W. Molecular characterization of defective antigen processing in human prostate cancer. J. Clin. Oncol. 1995, 87, 280–285. [Google Scholar] [CrossRef]
- Bander, N.H.; Yao, D.; Liu, H.; Chen, Y.T.; Steiner, M.; Zuccaro, W.; Moy, P. MHC class I and II expression in prostate carcinoma and modulation by interferon-alpha and -gamma. Prostate 1997, 33, 233–239. [Google Scholar] [CrossRef]
- Jamaspishvili, T.; Berman, D.M.; Ross, A.E.; Scher, H.I.; De Marzo, A.M.; Squire, J.A.; Lotan, T.L. Clinical implications of PTEN loss in prostate cancer. Nat. Rev. Urol. 2018, 15, 222–234. [Google Scholar] [CrossRef]
- Vitkin, N.; Nersesian, S.; Siemens, D.R.; Koti, M. The Tumor Immune Contexture of Prostate Cancer. Front. Immunol. 2019, 10. [Google Scholar] [CrossRef]
- Karzai, F.; VanderWeele, D.; Madan, R.A.; Owens, H.; Cordes, L.M.; Hankin, A.; Couvillon, A.; Nichols, E.; Bilusic, M.; Beshiri, M.L.; et al. Activity of durvalumab plus olaparib in metastatic castration-resistant prostate cancer in men with and without DNA damage repair mutations. J. Immunother. Cancer 2018, 6, 141. [Google Scholar] [CrossRef]
- Hotte1, S.J.; Winquist, E.; Chi, K.N.; Ellard, S.L.; Sridhar, S.; Emmenegger, U.; Salim, M.; Iqbal, N.N.; C. Canil, C.K.; Kollmannsberger, A.R.; et al. 1085—CCTG IND 232: A Phase II Study of Durvalumab With or Without Tremelimumab in Patients with Metastatic Castration Resistant Prostate Cancer (mCRPC). Ann. Oncol. 2019, 30, v851–v934. [Google Scholar] [CrossRef]
- Kim, J.W.; Shaffer, D.R.; Massard, C.; Powles, T.; Harshman, L.C.; Braiteh, F.S.; Conkling, P.R.; Sarkar, I.; Kadel, E.E.; Mariathasan, S.; et al. A phase Ia study of safety and clinical activity of atezolizumab (atezo) in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC). J. Clin. Oncol. 2018, 36, 187. [Google Scholar] [CrossRef]
- Sweeney, C.J.; Gillessen, S.; Rathkopf, D.; Matsubara, N.; Drake, C.; Fizazi, K.; Piulats, J.M.; Wysocki, P.J.; Buchschacher, G.L.; Doss, J.; et al. Abstract CT014: IMbassador250: A phase III trial comparing atezolizumab with enzalutamide vs enzalutamide alone in patients with metastatic castration-resistant prostate cancer (mCRPC). Cancer Res. 2020, 80, CT014. [Google Scholar] [CrossRef]
- Agarwal, N.; Loriot, Y.; McGregor, B.A.; Dreicer, R.; Dorff, T.B.; Maughan, B.L.; Kelly, W.K.; Pagliaro, L.C.; Srinivas, S.; Squillante, C.M.; et al. Cabozantinib in combination with atezolizumab in patients with metastatic castration-resistant prostate cancer: Results of cohort 6 of the COSMIC-021 study. J. Clin. Oncol. 2020, 38, 5564. [Google Scholar] [CrossRef]
- Rosser, C.J.; Hirasawa, Y.; Acoba, J.D.; Tamura, D.J.; Pal, S.K.; Huang, J.; Scholz, M.C.; Dorff, T.B. Phase Ib study assessing different sequencing regimens of atezolizumab (anti-PD-L1) and sipuleucel-T (SipT)in patients who have asymptomatic or minimally symptomatic metastatic castrate resistant prostate cancer. J. Clin. Oncol. 2020, 38, e17564. [Google Scholar] [CrossRef]
- Boudadi, K.; Suzman, D.L.; Anagnostou, V.; Fu, W.; Luber, B.; Wang, H.; Niknafs, N.; White, J.R.; Silberstein, J.L.; Sullivan, R.; et al. Ipilimumab plus nivolumab and DNA-repair defects in AR-V7-expressing metastatic prostate cancer. Oncotarget 2018, 9, 28561–28571. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Pachynski, R.K.; Narayan, V.; Flechon, A.; Gravis, G.; Galsky, M.D.; Mahammedi, H.; Patnaik, A.; Subudhi, S.K.; Ciprotti, M.; et al. Nivolumab Plus Ipilimumab for Metastatic Castration-Resistant Prostate Cancer: Preliminary Analysis of Patients in the CheckMate 650 Trial. Cancer Cell 2020, 38, 489–499. [Google Scholar] [CrossRef]
- Fizazi, K.; Drake, C.G.; Shaffer, D.R.; Pachynski, R.; Saad, F.; Ciprotti, M.; Kong, G.; Ryan, C.J.; Petrylak, D.P. An open-label, phase 2 study of nivolumab in combination with either rucaparib, docetaxel, or enzalutamide in men with castration-resistant metastatic prostate cancer (mCRPC; CheckMate 9KD). J. Clin. Oncol. 2018, 36, TPS3126. [Google Scholar] [CrossRef]
- Tuthill, M.; Cappuccini, F.; Carter, L.; Pollock, E.; Poulton, I.; Verrill, C.; Evans, T.; Gillessen, S.; Attard, G.; Protheroe, A.; et al. 682P Results from ADVANCE: A phase I/II open-label non-randomised safety and efficacy study of the viral vectored ChAdOx1-MVA 5T4 (VTP-800) vaccine in combination with PD-1 checkpoint blockade in metastatic prostate cancer. Ann.Oncol. 2020, 31, S543. [Google Scholar] [CrossRef]
- Ross, A.E.; Hurley, P.J.; Tran, P.T.; Rowe, S.P.; Benzon, B.; Neal, T.O.; Chapman, C.; Harb, R.; Milman, Y.; Trock, B.J.; et al. A pilot trial of pembrolizumab plus prostatic cryotherapy for men with newly diagnosed oligometastatic hormone-sensitive prostate cancer. Prostate Cancer Prostatic Dis. 2020, 23, 184–193. [Google Scholar] [CrossRef]
- Hansen, A.R.; Massard, C.; Ott, P.A.; Haas, N.B.; Lopez, J.S.; Ejadi, S.; Wallmark, J.M.; Keam, B.; Delord, J.P.; Aggarwal, R.; et al. Pembrolizumab for advanced prostate adenocarcinoma: Findings of the KEYNOTE-028 study. Ann. Oncol. 2018, 29, 1807–1813. [Google Scholar] [CrossRef]
- Yu, E.Y.; Piulats, J.M.; Gravis, G.; Laguerre, B.; Arija, J.A.A.; Oudard, S.; Fong, P.C.C.; Kolinsky, M.P.; Augustin, M.; Feyerabend, S.; et al. KEYNOTE-365 cohort A updated results: Pembrolizumab (pembro) plus olaparib in docetaxel-pretreated patients (pts) with metastatic castration-resistant prostate cancer (mCRPC). J. Clin. Oncol. 2020, 38, 100. [Google Scholar] [CrossRef]
- Kolinsky, M.P.; Gravis, G.; Mourey, L.; Piulats, J.M.; Sridhar, S.S.; Romano, E.; Berry, W.R.; Gurney, H.; Retz, M.; Appleman, L.J.; et al. KEYNOTE-365 cohort B updated results: Pembrolizumab (pembro) plus docetaxel and prednisone in abiraterone (abi) or enzalutamide (enza)-pretreated patients (pts) with metastatic castrate-resistant prostate cancer (mCRPC). J. Clin. Oncol. 2020, 38, 103. [Google Scholar] [CrossRef]
- Yu, E.Y.; Fong, P.; Piulats, J.M.; Appleman, L.; Conter, H.; Feyerabend, S.; Shore, N.; Gravis, G.; Laguerre, B.; Gurney, H.; et al. PD16-12–PEMBROLIZUMAB PLUS ENZALUTAMIDE IN ABIRATERONE-PRETREATED PATIENTS WITH METASTATIC CASTRATION-RESISTANT PROSTATE CANCER: UPDATED RESULTS FROM KEYNOTE-365 COHORT C. J. Urol. 2020, 203, e368. [Google Scholar] [CrossRef]
- Antonarakis, E.S.; Piulats, J.M.; Gross-Goupil, M.; Goh, J.; Ojamaa, K.; Hoimes, C.J.; Vaishampayan, U.; Berger, R.; Sezer, A.; Alanko, T.; et al. Pembrolizumab for Treatment-Refractory Metastatic Castration-Resistant Prostate Cancer: Multicohort, Open-Label Phase II KEYNOTE-199 Study. J. Clin. Oncol. 2020, 38, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Hoimes, C.J.; Graff, J.N.; Tagawa, S.T.; Hwang, C.; Kilari, D.; Ten Tije, A.J.; Omlin, A.; McDermott, R.S.; Vaishampayan, U.N.; Elliott, T.; et al. KEYNOTE-199 cohorts (C) 4 and 5: Phase II study of pembrolizumab (pembro) plus enzalutamide (enza) for enza-resistant metastatic castration-resistant prostate cancer (mCRPC). J. Clin. Oncol. 2020, 38, 5543. [Google Scholar] [CrossRef]
- Fong, L.; Kwek, S.S.; O’Brien, S.; Kavanagh, B.; McNeel, D.G.; Weinberg, V.; Lin, A.M.; Rosenberg, J.; Ryan, C.J.; Rini, B.I.; et al. Potentiating endogenous antitumor immunity to prostate cancer through combination immunotherapy with CTLA4 blockade and GM-CSF. Cancer Res. 2009, 69, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Small, E.J.; Tchekmedyian, N.S.; Rini, B.I.; Fong, L.; Lowy, I.; Allison, J.P. A Pilot Trial of CTLA-4 Blockade with Human Anti-CTLA-4 in Patients with Hormone-Refractory Prostate Cancer. Clin. Cancer Res. 2007, 13, 1810–1815. [Google Scholar] [CrossRef] [PubMed]
- Madan, R.A.; Mohebtash, M.; Arlen, P.M.; Vergati, M.; Rauckhorst, M.; Steinberg, S.M.; Tsang, K.Y.; Poole, D.J.; Parnes, H.L.; Wright, J.J.; et al. Ipilimumab and a poxviral vaccine targeting prostate-specific antigen in metastatic castration-resistant prostate cancer: A phase 1 dose-escalation trial. Lancet Oncol. 2012, 13, 501–508. [Google Scholar] [CrossRef]
- Slovin, S.F.; Higano, C.S.; Hamid, O.; Tejwani, S.; Harzstark, A.; Alumkal, J.J.; Scher, H.I.; Chin, K.; Gagnier, P.; McHenry, M.B.; et al. Ipilimumab alone or in combination with radiotherapy in metastatic castration-resistant prostate cancer: Results from an open-label, multicenter phase I/II study. Ann. Oncol. Off. J. Eur. Soc. Med Oncol. 2013, 24, 1813–1821. [Google Scholar] [CrossRef]
- Beer, T.M.; Kwon, E.D.; Drake, C.G.; Fizazi, K.; Logothetis, C.; Gravis, G.; Ganju, V.; Polikoff, J.; Saad, F.; Humanski, P.; et al. Randomized, Double-Blind, Phase III Trial of Ipilimumab Versus Placebo in Asymptomatic or Minimally Symptomatic Patients With Metastatic Chemotherapy-Naive Castration-Resistant Prostate Cancer. J. Clin. Oncol. 2017, 35, 40–47. [Google Scholar] [CrossRef]
- Kwon, E.D.; Drake, C.G.; Scher, H.I.; Fizazi, K.; Bossi, A.; van den Eertwegh, A.J.M.; Krainer, M.; Houede, N.; Santos, R.; Mahammedi, H.; et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): A multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2014, 15, 700–712. [Google Scholar] [CrossRef]
- Morris, M.J.; Fong, L.; Petrylak, D.P.; Sartor, A.O.; Higano, C.S.; Pagliaro, L.C.; Alva, A.S.; Appleman, L.J.; Tan, W.; Vaishampayan, U.N.; et al. Safety and clinical activity of atezolizumab (atezo) + radium-223 dichloride (r-223) in 2L metastatic castration-resistant prostate cancer (mCRPC): Results from a phase Ib clinical trial. J. Clin. Oncol. 2020, 38, 5565. [Google Scholar] [CrossRef]
- Carbognin, L.; Pilotto, S.; Milella, M.; Vaccaro, V.; Brunelli, M.; Calio, A.; Cuppone, F.; Sperduti, I.; Giannarelli, D.; Chilosi, M.; et al. Differential Activity of Nivolumab, Pembrolizumab and MPDL3280A according to the Tumor Expression of Programmed Death-Ligand-1 (PD-L1): Sensitivity Analysis of Trials in Melanoma, Lung and Genitourinary Cancers. PLoS ONE 2015, 10, e0130142. [Google Scholar] [CrossRef] [PubMed]
- Geng, L.; Huang, D.; Liu, J.; Qian, Y.; Deng, J.; Li, D.; Hu, Z.; Zhang, J.; Jiang, G.; Zheng, S. B7-H1 up-regulated expression in human pancreatic carcinoma tissue associates with tumor progression. J. Cancer Res. Clin. Oncol. 2008, 134, 1021–1027. [Google Scholar] [CrossRef]
- Kordbacheh, T.; Honeychurch, J.; Blackhall, F.; Faivre-Finn, C.; Illidge, T. Radiotherapy and anti-PD-1/PD-L1 combinations in lung cancer: Building better translational research platforms. Ann. Oncol. 2018, 29, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Sakai, H.; Takeda, M.; Sakai, K.; Nakamura, Y.; Ito, A.; Hayashi, H.; Tanaka, K.; Nishio, K.; Nakagawa, K. Impact of cytotoxic chemotherapy on PD-L1 expression in patients with non-small cell lung cancer negative for EGFR mutation and ALK fusion. Lung Cancer 2019, 127, 59–65. [Google Scholar] [CrossRef]
- Deng, L.; Liang, H.; Burnette, B.; Beckett, M.; Darga, T.; Weichselbaum, R.R.; Fu, Y.X. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J. Clin. Invest. 2014, 124, 687–695. [Google Scholar] [CrossRef]
- Langer, C.J.; Gadgeel, S.M.; Borghaei, H.; Papadimitrakopoulou, V.A.; Patnaik, A.; Powell, S.F.; Gentzler, R.D.; Martins, R.G.; Stevenson, J.P.; Jalal, S.I.; et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: A randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol. 2016, 17, 1497–1508. [Google Scholar] [CrossRef]
- Sato, H.; Niimi, A.; Yasuhara, T.; Permata, T.B.M.; Hagiwara, Y.; Isono, M.; Nuryadi, E.; Sekine, R.; Oike, T.; Kakoti, S.; et al. DNA double-strand break repair pathway regulates PD-L1 expression in cancer cells. Nat. Commun. 2017, 8, 1751. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M.J.; Bose, R. Genomic Alteration Burden in Advanced Prostate Cancer and Therapeutic Implications. Front. Oncol. 2019, 9, 1287. [Google Scholar] [CrossRef]
- Pritchard, C.C.; Mateo, J.; Walsh, M.F.; De Sarkar, N.; Abida, W.; Beltran, H.; Garofalo, A.; Gulati, R.; Carreira, S.; Eeles, R.; et al. Inherited DNA-Repair Gene Mutations in Men with Metastatic Prostate Cancer. N. Engl. J. Med. 2016, 375, 443–453. [Google Scholar] [CrossRef]
- Wang, F.; Zhao, Q.; Wang, Y.-N.; Jin, Y.; He, M.-M.; Liu, Z.-X.; Xu, R.-H. Evaluation of POLE and POLD1 Mutations as Biomarkers for Immunotherapy Outcomes Across Multiple Cancer Types. JAMA Oncol. 2019, 5, 1504–1506. [Google Scholar] [CrossRef] [PubMed]
- Blazek, D.; Kohoutek, J.; Bartholomeeusen, K.; Johansen, E.; Hulinkova, P.; Luo, Z.; Cimermancic, P.; Ule, J.; Peterlin, B.M. The Cyclin K/Cdk12 complex maintains genomic stability via regulation of expression of DNA damage response genes. Genes Dev. 2011, 25, 2158–2172. [Google Scholar] [CrossRef]
- Wu, Y.-M.; Cieślik, M.; Lonigro, R.J.; Vats, P.; Reimers, M.A.; Cao, X.; Ning, Y.; Wang, L.; Kunju, L.P.; de Sarkar, N.; et al. Inactivation of CDK12 Delineates a Distinct Immunogenic Class of Advanced Prostate Cancer. Cell 2018, 173, 1770–1782.e14. [Google Scholar] [CrossRef]
- Abida, W.; Cheng, M.L.; Armenia, J.; Middha, S.; Autio, K.A.; Vargas, H.A.; Rathkopf, D.; Morris, M.J.; Danila, D.C.; Slovin, S.F.; et al. Analysis of the Prevalence of Microsatellite Instability in Prostate Cancer and Response to Immune Checkpoint Blockade. JAMA Oncol. 2019, 5, 471–478. [Google Scholar] [CrossRef]
- Bishop, J.L.; Sio, A.; Angeles, A.; Roberts, M.E.; Azad, A.A.; Chi, K.N.; Zoubeidi, A. PD-L1 is highly expressed in Enzalutamide resistant prostate cancer. Oncotarget 2015, 6, 234–242. [Google Scholar] [CrossRef]
- Morse, M.D.; McNeel, D.G. Prostate cancer patients on androgen deprivation therapy develop persistent changes in adaptive immune responses. Hum. Immunol. 2010, 71, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Graff, J.N.; Beer, T.M.; Alumkal, J.J.; Slottke, R.E.; Redmond, W.L.; Thomas, G.V.; Thompson, R.F.; Wood, M.A.; Koguchi, Y.; Chen, Y.; et al. A phase II single-arm study of pembrolizumab with enzalutamide in men with metastatic castration-resistant prostate cancer progressing on enzalutamide alone. J. Immunother. Cancer 2020, 8. [Google Scholar] [CrossRef] [PubMed]
- Donahue, R.N.; Madan, R.A.; Richards, J.; Grenga, I.; Lepone, L.M.; Heery, C.R.; Gulley, J.L.; Schlom, J. Abstract 4901: Short-course enzalutamide reveals immune activating properties in patients with biochemically recurrent prostate cancer. Cancer Res. 2016, 76, 4901. [Google Scholar] [CrossRef]
- Mateo, J.; Carreira, S.; Sandhu, S.; Miranda, S.; Mossop, H.; Perez-Lopez, R.; Nava Rodrigues, D.; Robinson, D.; Omlin, A.; Tunariu, N.; et al. DNA-Repair Defects and Olaparib in Metastatic Prostate Cancer. N. Engl. J. Med. 2015, 373, 1697–1708. [Google Scholar] [CrossRef] [PubMed]
- Peyraud, F.; Italiano, A. Combined PARP Inhibition and Immune Checkpoint Therapy in Solid Tumors. Cancers 2020, 12, 1502. [Google Scholar] [CrossRef]
- Parker, C.; Nilsson, S.; Heinrich, D.; Helle, S.I.; O’Sullivan, J.M.; Fosså, S.D.; Chodacki, A.; Wiechno, P.; Logue, J.; Seke, M.; et al. Alpha Emitter Radium-223 and Survival in Metastatic Prostate Cancer. N. Engl. J. Med. 2013, 369, 213–223. [Google Scholar] [CrossRef]
- Demaria, S.; Ng, B.; Devitt, M.L.; Babb, J.S.; Kawashima, N.; Liebes, L.; Formenti, S.C. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int. J. Radiat. Oncol. Biol. Phys. 2004, 58, 862–870. [Google Scholar] [CrossRef]
- Dewan, M.Z.; Galloway, A.E.; Kawashima, N.; Dewyngaert, J.K.; Babb, J.S.; Formenti, S.C.; Demaria, S. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin. Cancer Res. 2009, 15, 5379–5388. [Google Scholar] [CrossRef]
- Fizazi, K.; Drake, C.G.; Beer, T.M.; Kwon, E.D.; Scher, H.I.; Gerritsen, W.R.; Bossi, A.; den Eertwegh, A.J.M.v.; Krainer, M.; Houede, N.; et al. Final Analysis of the Ipilimumab Versus Placebo Following Radiotherapy Phase III Trial in Postdocetaxel Metastatic Castration-resistant Prostate Cancer Identifies an Excess of Long-term Survivors. Eur. Urol. 2020, 78, 822–830. [Google Scholar] [CrossRef]
- Abdo, J.; Cornell, D.L.; Mittal, S.K.; Agrawal, D.K. Immunotherapy Plus Cryotherapy: Potential Augmented Abscopal Effect for Advanced Cancers. Front. Oncol. 2018, 8, 85. [Google Scholar] [CrossRef]
- Cappuccini, F.; Bryant, R.; Pollock, E.; Carter, L.; Verrill, C.; Hollidge, J.; Poulton, I.; Baker, M.; Mitton, C.; Baines, A.; et al. Safety and immunogenicity of novel 5T4 viral vectored vaccination regimens in early stage prostate cancer: A phase I clinical trial. J. J. Immunother. Cancer 2020, 8, e000928. [Google Scholar] [CrossRef]
- Galluzzi, L.; Buque, A.; Kepp, O.; Zitvogel, L.; Kroemer, G. Immunological Effects of Conventional Chemotherapy and Targeted Anticancer Agents. Cancer Cell 2015, 28, 690–714. [Google Scholar] [CrossRef]
- Apetoh, L.; Ladoire, S.; Coukos, G.; Ghiringhelli, F. Combining immunotherapy and anticancer agents: The right path to achieve cancer cure? Ann. Oncol. 2015, 26, 1813–1823. [Google Scholar] [CrossRef] [PubMed]
- Zitvogel, L.; Galluzzi, L.; Smyth, M.J.; Kroemer, G. Mechanism of action of conventional and targeted anticancer therapies: Reinstating immunosurveillance. Immunity 2013, 39, 74–88. [Google Scholar] [CrossRef]
- Intlekofer, A.M.; Thompson, C.B. At the bench: Preclinical rationale for CTLA-4 and PD-1 blockade as cancer immunotherapy. J Leukoc Biol 2013, 94, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Ward, J.F.; Pettaway, C.A.; Shi, L.Z.; Subudhi, S.K.; Vence, L.M.; Zhao, H.; Chen, J.; Chen, H.; Efstathiou, E.; et al. VISTA is an inhibitory immune checkpoint that is increased after ipilimumab therapy in patients with prostate cancer. Nat. Med. 2017, 23, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Palapattu, G.S. Commentary on “AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer.” Antonarakis ES, Lu C, Wang H, Luber B, Nakazawa M, Roeser JC, Chen Y, Mohammad TA, Chen Y, Fedor HL, Lotan TL, Zheng Q, De Marzo AM, Isaacs JT, Isaacs WB, Nadal R, Paller CJ, Denmeade SR, Carducci MA, Eisenberger MA, Luo J, Division of Urologic Oncology, Department of Urology, University of Michigan, MI. N. Engl. J. Med. 2014; 371(11):1028-38. Urol. Oncol. 2016, 34, 520. [Google Scholar] [CrossRef]
- Antonarakis, E.S.; Lu, C.; Luber, B.; Wang, H.; Chen, Y.; Nakazawa, M.; Nadal, R.; Paller, C.J.; Denmeade, S.R.; Carducci, M.A.; et al. Androgen Receptor Splice Variant 7 and Efficacy of Taxane Chemotherapy in Patients With Metastatic Castration-Resistant Prostate Cancer. JAMA Oncol. 2015, 1, 582–591. [Google Scholar] [CrossRef]
- Joshi, H.; Pinski, J.K. Association of ARV7 expression with molecular and clinical characteristics in prostate cancer. J. Clin. Oncol. 2016, 34, 109. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Meyer, T.; Cheng, A.-L.; El-Khoueiry, A.B.; Rimassa, L.; Ryoo, B.-Y.; Cicin, I.; Merle, P.; Chen, Y.; Park, J.-W.; et al. Cabozantinib in Patients with Advanced and Progressing Hepatocellular Carcinoma. N. Engl. J. Med. 2018, 379, 54–63. [Google Scholar] [CrossRef]
- Choueiri, T.K.; Halabi, S.; Sanford, B.L.; Hahn, O.; Michaelson, M.D.; Walsh, M.K.; Feldman, D.R.; Olencki, T.; Picus, J.; Small, E.J.; et al. Cabozantinib Versus Sunitinib As Initial Targeted Therapy for Patients With Metastatic Renal Cell Carcinoma of Poor or Intermediate Risk: The Alliance A031203 CABOSUN Trial. J. Clin. Oncol. 2017, 35, 591–597. [Google Scholar] [CrossRef]
- Cartron, G.; Zhao-Yang, L.; Baudard, M.; Kanouni, T.; Rouille, V.; Quittet, P.; Klein, B.; Rossi, J.F. Granulocyte-macrophage colony-stimulating factor potentiates rituximab in patients with relapsed follicular lymphoma: Results of a phase II study. J. Clin. Oncol. 2008, 26, 2725–2731. [Google Scholar] [CrossRef]
- Vigano, S.; Alatzoglou, D.; Irving, M.; Ménétrier-Caux, C.; Caux, C.; Romero, P.; Coukos, G. Targeting Adenosine in Cancer Immunotherapy to Enhance T-Cell Function. Front. Immunol. 2019, 10. [Google Scholar] [CrossRef]
- Wei, Q.; Costanzi, S.; Balasubramanian, R.; Gao, Z.-G.; Jacobson, K.A. A2B adenosine receptor blockade inhibits growth of prostate cancer cells. Purinergic Signal. 2013, 9, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Diao, L.; Yang, Y.; Yi, X.; Rodriguez, B.L.; Li, Y.; Villalobos, P.A.; Cascone, T.; Liu, X.; Tan, L. CD38-mediated immunosuppression as a mechanism of tumor cell escape from PD-1/PD-L1 blockade. Cancer Discov. 2018, 8, 1156–1175. [Google Scholar] [CrossRef] [PubMed]
- Allard, B.; Pommey, S.; Smyth, M.J.; Stagg, J. Targeting CD73 enhances the antitumor activity of anti-PD-1 and anti-CTLA-4 mAbs. Clin. Cancer Res. 2013, 19, 5626–5635. [Google Scholar] [CrossRef] [PubMed]
- Hay, C.M.; Sult, E.; Huang, Q.; Mulgrew, K.; Fuhrmann, S.R.; McGlinchey, K.A.; Hammond, S.A.; Rothstein, R.; Rios-Doria, J.; Poon, E. Targeting CD73 in the tumor microenvironment with MEDI9447. Oncoimmunology 2016, 5, e1208875. [Google Scholar] [CrossRef]
- Huehls, A.M.; Coupet, T.A.; Sentman, C.L. Bispecific T-cell engagers for cancer immunotherapy. Immunol. Cell Biol. 2015, 93, 290–296. [Google Scholar] [CrossRef]
- Feucht, J.; Kayser, S.; Gorodezki, D.; Hamieh, M.; Doring, M.; Blaeschke, F.; Schlegel, P.; Bosmuller, H.; Quintanilla-Fend, L.; Ebinger, M.; et al. T-cell responses against CD19+ pediatric acute lymphoblastic leukemia mediated by bispecific T-cell engager (BiTE) are regulated contrarily by PD-L1 and CD80/CD86 on leukemic blasts. Oncotarget 2016, 7, 76902–76919. [Google Scholar] [CrossRef]
- Krupka, C.; Kufer, P.; Kischel, R.; Zugmaier, G.; Lichtenegger, F.S.; Kohnke, T.; Vick, B.; Jeremias, I.; Metzeler, K.H.; Altmann, T.; et al. Blockade of the PD-1/PD-L1 axis augments lysis of AML cells by the CD33/CD3 BiTE antibody construct AMG 330: Reversing a T-cell-induced immune escape mechanism. Leukemia 2016, 30, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Hummel, H.D.; Kufer, P.; Grüllich, C.; Seggewiss-Bernhardt, R.; Deschler-Baier, B.; Chatterjee, M.; Goebeler, M.E.; Miller, K.; de Santis, M.; Loidl, W.; et al. Pasotuxizumab, a BiTE(®) immune therapy for castration-resistant prostate cancer: Phase I, dose-escalation study findings. Immunotherapy 2021, 13, 125–141. [Google Scholar] [CrossRef] [PubMed]
- Alok Tewari, M.D. AMG 160, a Half-Life Extended, PSMA-Targeted, Bispecific T-cell Engager (BiTE®) immune Therapy for mCRPC—Interim Results From a Phase I Study. Available online: https://www.urotoday.com/conference-highlights/esmo-2020/prostate-cancer/124632-esmo-virtual-congress-2020-amg-160-a-half-life-extended-psma-targeted-bispecific-t-cell-engager-bite-immune-therapy-for-mcrpc-interim-results-from-a-phase-i-study.html (accessed on 30 April 2021).
- Rahbar, K.; Ahmadzadehfar, H.; Kratochwil, C.; Haberkorn, U.; Schafers, M.; Essler, M.; Baum, R.P.; Kulkarni, H.R.; Schmidt, M.; Drzezga, A.; et al. German Multicenter Study Investigating 177Lu-PSMA-617 Radioligand Therapy in Advanced Prostate Cancer Patients. J. Nucl. Med. 2017, 58, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Silver, D.A.; Pellicer, I.; Fair, W.R.; Heston, W.D.; Cordon-Cardo, C. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin. Cancer Res. 1997, 3, 81–85. [Google Scholar]
- Santoni, M.; Scarpelli, M.; Mazzucchelli, R.; Lopez-Beltran, A.; Cheng, L.; Cascinu, S.; Montironi, R. Targeting prostate-specific membrane antigen for personalized therapies in prostate cancer: Morphologic and molecular backgrounds and future promises. J. Biol. Regul. Homeost. Agents 2014, 28, 555–563. [Google Scholar]
- Hofman, M.S.; Violet, J.; Hicks, R.J.; Ferdinandus, J.; Thang, S.P.; Akhurst, T.; Iravani, A.; Kong, G.; Ravi Kumar, A.; Murphy, D.G.; et al. [(177)Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): A single-centre, single-arm, phase 2 study. Lancet Oncol. 2018, 19, 825–833. [Google Scholar] [CrossRef]
- Czernin, J.; Current, K.; Mona, C.E.; Nyiranshuti, L.; Hikmat, F.; Radu, C.G.; Lueckerath, K. Immune-Checkpoint Blockade Enhances 225Ac-PSMA617 Efficacy in a Mouse Model of Prostate Cancer. J. Nucl. Med. 2020. [Google Scholar] [CrossRef]
- Perica, K.; Varela, J.C.; Oelke, M.; Schneck, J. Adoptive T cell immunotherapy for cancer. Rambam Maimonides Med. J. 2015, 6, e0004. [Google Scholar] [CrossRef]
- Kverneland, A.H.; Pedersen, M.; Westergaard, M.C.W.; Nielsen, M.; Borch, T.H.; Olsen, L.R.; Aasbjerg, G.; Santegoets, S.J.; van der Burg, S.H.; Milne, K.; et al. Adoptive cell therapy in combination with checkpoint inhibitors in ovarian cancer. Oncotarget 2020, 11, 2092–2105. [Google Scholar] [CrossRef]
- Shi, L.Z.; Gao, J.; Allison, J.P.; Sharma, P. Combination therapy of adoptive T cell therapy and immune checkpoint blockades engages distinct mechanisms in CD4+ and CD8+ T cells. J. Immunol. 2018, 200, 122.21. [Google Scholar]
| Specimen Type | Number of Patients | Cut Off for Positivity | Antibody/Clone Used to Detect PD-L1 | PD-L1 Expression |
|---|---|---|---|---|
| Primary prostate cancer [10] | 402 | No staining = 0, weak staining = 1, moderate staining = 2, and strong staining = 3. PD-L1+ stromal cells and PD-1+ lymphocytes were scored as number of positive stained cells per 0.6 mm diameter core as follows: 0 = 0–3, 1 = 4–10, 2 = 11–15, and 3 ≥ 15 | Rabbit monoclonal PD-L1 antibody (Cat#13684, clone: E1L3N, Cell signaling technology, Danvers, MA, USA) | 92% (371/402) of patients were positive for PD-L1 staining in tumor epithelial (TE) cells and 59% (236/402) had high PD-L1 intensity score. Also, 66% (267/402) of patients had PD-L1+ stromal cells. |
| Primary prostate cancer [11] | Training cohort (n = 209) Test cohort (n = 611) | Semi-quantitative scoring as negative (0), weak (1), moderate (2), or strong (3) | Monoclonal rabbit PD-L1 antibody (clone EPR1161) | Moderate to high PD-L1 levels in 52.2% in the training cohort and 61.7% in the test cohort |
| Primary prostate cancer [12] | 20 | >5% membrane staining of malignant epithelial cells | 5H1 clone of the mouse anti-human CD274 monoclonal PD-L1 antibody | PD-L1 positivity in 15% (3/20) of samples |
| Primary prostate cancer [13] | 16 | PD-1 positivity: negative (0), <5%; low (1+), 5–30%; high (2+), >30% of CD3+ T cells. PD-L1 staining intensity: 0 (no signal), 1+ (light signal), 2+ (high signal) in >50% of neoplastic cells. | Clone 015, Sino biological | Eight of 16 (50%) were PD-L1 positive and 19% were strongly (2+) positive |
| Primary prostate cancer [14] | 25 | “High” expression- 3 to 5 on the semiquantitative 0 to 5 score. “Low” expression- 0 to 2 on the semiquantitative 0 to 5 score | Anti-PD-L1 clone 22C3; Merck research laboratories | Low: 92% (23/25) High: 8% (2/25) |
| NCT ID/Trial Name | Phase and Status | Disease Cohort | Number of Patients (with Prostate Cancer) Enrolled | Name of Investigational Agent | Primary Endpoint | Outcome |
|---|---|---|---|---|---|---|
| NCT02484404 [25] | Phase I/II Study Recruiting | mCRPC previously treated with enzalutamide and/or abiraterone | 17 | Durvalumab plus olaparib | Improved PFS (70% PFS vs. an estimated 50% PFS at 4 months) | rPFS of 51.5% at 12 months with a median rPFS of 16.1 months |
| NCT02788773 [26] | Phase II Study, active, not recruiting | mCRPC patients after prior abiraterone and/or enzalutamide, and no more than one taxane | 52 | Durvalumab with or without tremelimumab | ORR measured by RECIST 1.1 and iRECIST | ORR 0% (0/13) vs. 16% (6/37) and PSA response rate 0% (0/13) vs. 16% (6/37) in the durvalumab arm vs. durvalumab plus tremelimumab arm |
| NCT01375842 [27] | Phase I, completed | mCRPC after progression on enzalutamide and/or sipuleucel-T | 15 | Atezolizumab | Safety and activity | Any TRAEs 60%, one grade 3 hyponatremia, and no grade 4–5 TRAEs 12-month OS 55.6% |
| NCT03016312 IMbassador250 [28] | Phase III, active, not recruiting | mCRPC after the failure of an androgen synthesis inhibitor and failure of, ineligibility for, or refusal of a taxane regimen | 759 | Atezolizumab with enzalutamide vs. enzalutamide only | OS | Median OS 15.2 vs. 16.6 months respectively |
| NCT03170960 COSMIC-021 [29] | Phase 1b, recruiting | mCRPC after progression on enzalutamide and/or abiraterone | 44 | Cabozantinib with and without atezolizumab | ORR per RECIST 1.1 | ORR per RECIST 1.1–32% |
| NCT03024216 [30] | Phase 1/1b, recruiting | Asymptomatic or minimally symptomatic progressive mCRPC | 37 | Atezolizumab and sipuleucel-T in 2 different arms (depending on the dosing schedules) | Safety and tolerability | OR by RECIST at 6 months-SD 41% (10/24) and PR 8% (2/24) Grade 3 TRAEs 12/37 (events/number of patients), Grade 4 TRAEs 2/37 (events/number of patients), no Grade 5 TRAEs or grade 3 or 4 irAEs |
| NCT02601014 STARVE-PC [31] | Phase 2, active not recruiting | mCRPC expressing AR-V7 | 15 | Nivolumab plus ipilimumab | Change in PSA response (>50% PSA decline) | PSA reponse-13.3% (2/15) |
| NCT02985957, CheckMate 650 Trial [32] | Phase 2, recruiting | mCRPC Cohort 1 (pre-chemotherapy), cohort 2 (post-chemotherapy) | 45 in cohort 1 and 45 in cohort 2 | Nivolumab Plus ipilimumab | ORR at 24 weeks and Radiographic Progression-Free Survival (rPFS) at 12 months | ORR–25% and 10%, median PFS-5.5 and 3.8 months in cohort 1 and 2 respectively |
| NCT03338790 CheckMate 9KD, ARM B [33] | Phase II study, active, not recruiting | Chemotherapy naïve metastatic adenocarcinoma of the prostate | 41 | Nivolumab plus docetaxel | ORR and prostate-specific antigen (PSA) response rate (≥50% PSA reduction from baseline) | ORR–36.8% with one CR and six PRs. PSA response rate 46.3% |
| NCT03815942 ADVANCE [34] | Phase I/II, active, not recruiting | mCRPC patients with disease progression on enzalutamide or abiraterone | 23 | Viral vectored ChAd-MVA 5T4 vaccine plus nivolumab | Composite response rate measured as 50% reduction of circulating tumor DNA or 50% PSA decrease at 24-weeks | PSA (>50% PSA decrease) response at any time point 22% |
| NCT02489357 [35] | Pilot phase II, completed | Newly Diagnosed Oligo-metastatic Prostate Cancer | 12 | Pembrolizumab plus cryosurgery | Number of patients with a PSA level of <0.6 ng/mL at one year and the frequency of AEs | PSAs of <0.6 ng/mL at one year 42% (5/12) All AEs were grade ≤2 |
| NCT02054806/KEYNOTE-28 [36] | Phase IB, active, not recruiting | PD-L1–positive heavily pretreated advanced mCRPC | 23 | Pembrolizumab | ORR, CR, or PR per RECIST v1.1 at any point during the study | ORR 17.4%, all 4/23 responses were PR |
| NCT02861573 KEYNOTE-365 COHORT A [37] | Phase 1b/2, recruiting | Docetaxel-pretreated, molecularly unselected pts with mCRPC | 84 | Pembrolizumab + olaparib | PSA response (>50% decline), ORR based on RECIST 1.1, number of AEs, and number of drug discontinuations due to AE’s | PSA response rate 7/82 (9%) ORR based on RECIST 1.1–2/24 (8); 2 PRs. TRAEs 83% of patients |
| NCT02861573 KEYNOTE-365 COHORT B [38] | Phase 1b/2, recruiting | mCRPC pts who failed or were intolerant to ≥4 wk of abiraterone or enzalutamide in the prechemotherapy setting | 104 | Pembrolizumab + docetaxel + prednisone | PSA response (>50% decline), ORR based on RECIST 1.1, number of AEs, and number of drug discontinuations due to AE’s | PSA response rate 29/103 (28%) ORR based on RECIST 1.1–7/39 (18%); 7 PRs TRAEs 100 (96%) of patients Grade 3–5 TRAEs 29/104 (35%) including 2 deaths from TRAEs |
| NCT02861573 KEYNOTE-365 COHORT C [39] | Phase 1b/2, recruiting | Chemotherapy naïve mCRPC with progression or intolerance to abiraterone | 102 | Pembrolizumab plus enzalutamide | PSA response (>50% decline), ORR based on RECIST 1.1, number of AEs, and number of drug discontinuations due to AE’s | PSA response rate 22% ORR based on RECIST 1.1 in patients with measurable disease 12 TRAEs 92 (90%) Grade 3–4 TRAEs 39% One treatment-related death |
| NCT02787005KEYNOTE-199 (cohort 1,2 &3) [40] | Phase II, active, not recruiting | mCRPC previously treated with docetaxel and targeted endocrine therapy. Cohorts 1 and 2- RECIST-measurable PD-L1–positive and PD-L1–negative disease, respectively. Cohort 3-bone-predominant disease, regardless of PD-L1 expression | 258 cohort 1-133 cohort 2-66 and cohort 3-59 | Pembrolizumab | ORR by RECIST 1.1 | ORR was 5% in cohort 1, 3% in cohort 2 |
| NCT02787005 KEYNOTE-199, (cohort 4&5) [41] | Phase II, active, not recruiting | Chemotherapy naive mCRPC after progression on enzalutamide, cohort 4 (RECIST-measurable disease) and cohort 5 (bone predominant disease) | 126 Cohort 4-81, cohort 5-54 | Pembrolizumab plus enzalutamide | ORR per RECIST v1.1 (C4) | The ORR 12% (in cohort 4), 2 CR’s and 8 PR’s |
| PMID: 19,147,575 [42] | Phase I, completed | CRPC with disease progression as defined by the PSA Working Group Consensus Criteria | 24 | Ipilimumab plus GM-CSF | AEs graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0 | irAE in the higher dose cohorts-pan-hypopituitarism, mild rash, diarrhea, temporal arteritis |
| PMID: 17363537 [43] | Pilot trial | mCRPC | 14 | Ipilimumab | AEs, graded by the Common Toxicity Criteria, version 2.0 | TRAEs Grade 3-asthenia, fatigue, limb pain, rash, and pruritus. No deaths or treatment discontinuation due to toxicity |
| NCT00113984 [44] | Phase 1, completed | mCRPC with no bone pain requiring narcotics | 30 | Vaccine plus GM-CSF plus ipilimumab | Safety and tolerability using NCI 3.0 | The range of toxic effects exceeded those in single-agent studies especially with higher doses IrAEs were not associated with clinical responses in this study |
| NCT00323882 [45] | Phase I/II, completed | mCRPC | 71 | Ipilimumab with and without radiotherapy | AEs, prostate-specific antigen (PSA) decline, and tumor response. | 8/50 patients in the 10 mg ± radiotherapy arm had PSA response (≥50% decline) and 1/28 of the tumor evaluable patients had a complete response. irAEs Grade 3–4 colitis and hepatitis and one treatment-related death |
| NCT01057810/(CA184-095) [46] | Phase 3, completed | Asymptomatic or minimally symptomatic patients with chemotherapy-naive mCRPC without visceral metastases | 837 | Ipilimumab vs. placebo | OS | Median OS 28.7 months versus 29.7 months. No improvement in OS with ipilimumab |
| NCT00861614/CA184-043 [47] | Phase 3, completed | mCRPC patients with progression after treatment with docetaxel | 799 | Ipilimumab vs. placebo following radiotherapy | OS and OS rate | Median OS 11, 2 months vs. 10, 0 months. |
| NCT02814669 [48] | Phase Ib, completed | mCRPC patients after progression on an androgen pathway inhibitors | 45 | Atezolizumab + radium-223 dichloride (r-223) | Frequency of dose-limiting toxicities and AEs. ORR per RECIST v1.1 | Grade 3–4 AE’s 52.3%, 4 treatment-related deaths ORR 6.8%, no clinical benefit from combination treatment |
| NCT Number | Phase | Number of Patients | Intervention(s) | Randomized vs. Non-Randomized | Notes |
|---|---|---|---|---|---|
| NCT03525652 | Phase 1/2, recruiting | 30 | Therapeutic vaccine PD-1 knockout T cells | Randomized | Therapeutic vaccine—patient’s mononuclear cells are treated ex vivo with a recombinant fusion protein (PAP-GM-CSF) to induce antigen expression to activate the immune system PD-1 knockout T cells—prepared ex vivo from patient’s white cells and the maturated PD-1 knockout T cells will be infused back |
| NCT03658447 PRINCE | Phase Ib/II, active, not recruiting | 37 | 177Lu-PSMA Pembrolizumab | Non-randomized | 177Lu-PSMA—a compound that binds to the extracellular domain of the prostate-specific membrane antigen |
| NCT04631601 | Phase I, not yet recruiting | 105 | AMG 160 Enzalutamide Abiraterone AMG 404 | Non-randomized | AMG 160—BITE binds PSMA on tumor cells and CD3 on T cells AMG 404—PD-1 monoclonal antibody |
| NCT03689699 | Phase 1b/2, recruiting | 60 | Nivolumab Degarelix BMS-986253 | Randomized | BMS-986253—anti-IL-8 monoclonal antibody Degarelix—gonadotropin releasing hormone (GnRH) receptor antagonist |
| NCT03792841 | Phase I, recruiting | 288 | AMG 160 Pembrolizumab Etanercept Immunomodulating Agent | Non-randomized | AMG 160—BITE binds PSMA on tumor cells and CD3 on T cells Etanercept—TNF-alpha inhibitor Immunomodulating Agent—prophylaxis for AMG 160-related cytokine release syndrome |
| NCT03910660 | Phase 1b/2, recruiting | 40 | Talabostat Mesylate (BXCL701) plus Pembrolizumab | Non-randomized | Talabostat Mesylate (BXCL701)—a small molecule inhibitor of dipeptidyl peptidases involved in cancer progression |
| NCT03367819 | Phase 1/2, active not recruiting | 134 | Isatuximab (SAR650984) Cemiplimab (REGN2810) | Randomized | Isatuximab (SAR650984)—anti-CD38 monoclonal antibody Cemiplimab (REGN2810)—anti-PD-1 monoclonal antibody |
| NCT02861573, (cohort G and cohort H) | Phase Ib/II, recruiting | 1000 (total 10 cohorts) | MK-7684A (coformulation of pembrolizumab + vibostolimab) | Non-randomized | Vibostolimab—monoclonal antibody, that binds to the T-cell immunoreceptor with Ig and ITIM domains (TIGIT) and blocks its interaction with its ligands, CD112 and CD155, thereby activating T lymphocytes. |
| NCT04060342 | Phase 1, recruiting | 242 | GB1275 nab-paclitaxel and gemcitabine pembrolizumab | Non-randomized | GB1275—CD11b modulator that reduces MDSCs and tumor-associated macrophages (TAMs), repolarizes immunosuppressive M2 tumor-associated macrophages to an M1 phenotype and increases tumor infiltration of activated CD8+ T cells |
| NCT04381832 | Phase 1b/2, recruiting | 140 | Etrumadenant (AB928) Zimberelimab AB680 Enzalutamide Docetaxel | Randomized | Zimberelimab—anti-PD-1 antibody Etrumadenant(AB928)—adenosine receptor antagonist AB680- CD73 inhibitor, blocks adenosine production |
| NCT03493945 | Phase I/II, recruiting | 113 | ALT-803 MVA-BN-Brachyury FPV-Brachyury Epacadostat | Randomized | M7824—bifunctional fusion protein composed of anti-PD-L1 monoclonal antibody fused with 2 extracellular domains of TGF-βRII (a TGF-β “trap”). ALT-803—a recombinant IL15 complex that delivers stimulatory signals to NK and CD8+ T cells and enhances antitumor responses Epacadostat- inhibitor of indoleamine 2,3-dioxygenase (IDO1), with immunomodulating and antineoplastic activities MVA-BN-Brachyury—priming vaccine FPV-Brachyury—boosting vaccine |
| NCT03629756 | Phase 1, active not recruiting | 44 | Etrumadenant Zimberelimab | Non-randomized | Zimberelimab—anti-PD-1 antibody Etrumadenant(AB928)—adenosine receptor antagonist AB680-CD73 inhibitor, blocks adenosine production |
| NCT03970382 | Phase 1a/1b, recruiting | 148 | NeoTCR-P1 adoptive cell therapy nivolumab IL-2 | Non-randomized | NeoTCR-P1 adoptive cell therapy—apheresis derived CD8 and CD4 T cells that are engineered to express one autologous TCR of native sequence that targets a neoepitope presented by human leukocyte antigen (HLA) receptors exclusively on the surface of that patient’s tumor cells and not on other cells in the body. |
| NCT03454451 | Phase 1/1b recruiting | 378 | CPI-006 ciforadenant pembrolizumab | Randomized | CPI-006—a humanized monoclonal antibody against CD73 cell-surface ectonucleotidase (blocks adenosine production) ciforadenant—an oral adenosine 2A receptor antagonist |
| NCT03829436 | Phase 1/1b, recruiting | 138 | TPST-1120 nivolumab | Non-randomized | TPST-1120—a small molecule selective antagonist of PPARα (peroxisome proliferator-activated receptor alpha) |
| NCT04306900 | Phase 1/1b, recruiting | 152 | budigalimab docetaxel mFOLFOX6 TTX-030 | Randomized | Budigalimab—anti-PD-1 monoclonal antibody TTX-030-anti-CD39 monoclonal antibody that inhibits the production of adenosine |
| NCT04423029 | Phase 1/2, recruiting | 260 | DF6002 Nivolumab | Non-randomized | DF6002—monovalent IL-12 immunoglobulin Fc fusion protein that establishes an inflammatory tumor microenvironment for productive anti-tumor responses |
| NCT03549000 | Phase I/Ib, recruiting | 344 | NZV930 PDR001 NIR178 | Non-randomized | NZV930—anti-CD73 antibody, CD73 plays a key role in the generation of extracellular adenosine PDR001-anti-PD-1 antibody NIR178-adenosine A2a receptor antagonist |
| NCT03849469 DUET-4 | Phase 1, recruiting | 242 | XmAb®22841 Pembrolizumab | Non-randomized | XmAb®22841—a bispecific antibody that simultaneously targets immune checkpoint receptors CTLA-4 and LAG-3 to promote tumor-selective T-cell activation |
| NCT04388852 | Phase Ib, recruiting | 80 | Ipilimumab Valemetostat | Non-randomized | Valemetostat—EZH1/2 Dual Inhibitor (stops tumor growth by blocking enzymes needed for cell growth) |
| NCT02643303 | Phase 1/2, recruiting | 102 | Durvalumab Tremelimumab Poly ICLC | Non-randomized | Poly ICLC—a synthetic double-stranded RNA complex (which is a ligand for toll-like receptor-3 and MDA-5) that can activate immune cells, such as dendritic cells, and trigger natural killer cells to kill tumor cells. |
| NCT02655822 | Phase 1/1b, recruiting | 336 | Ciforadenant atezolizumab | Randomized | ciforadenant—an oral adenosine 2A receptor antagonist |
| NCT02484404 | Phase I/II, recruiting | 384 | Olaparib Cediranib Durvalumab (MEDI4736) | Non-randomized | Cediranib—inhibitor of vascular endothelial growth factor (VEGF) receptor tyrosine kinases |
| NCT04116775 | Phase II, recruiting | 32 | Fecal microbiota transplant Pembrolizumab Enzalutamide | Non-randomized | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Venkatachalam, S.; McFarland, T.R.; Agarwal, N.; Swami, U. Immune Checkpoint Inhibitors in Prostate Cancer. Cancers 2021, 13, 2187. https://doi.org/10.3390/cancers13092187
Venkatachalam S, McFarland TR, Agarwal N, Swami U. Immune Checkpoint Inhibitors in Prostate Cancer. Cancers. 2021; 13(9):2187. https://doi.org/10.3390/cancers13092187
Chicago/Turabian StyleVenkatachalam, Shobi, Taylor R. McFarland, Neeraj Agarwal, and Umang Swami. 2021. "Immune Checkpoint Inhibitors in Prostate Cancer" Cancers 13, no. 9: 2187. https://doi.org/10.3390/cancers13092187
APA StyleVenkatachalam, S., McFarland, T. R., Agarwal, N., & Swami, U. (2021). Immune Checkpoint Inhibitors in Prostate Cancer. Cancers, 13(9), 2187. https://doi.org/10.3390/cancers13092187







