Local Recurrence in Young Women with Breast Cancer: Breast Conserving Therapy vs. Mastectomy Alone
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Patient Characteristics
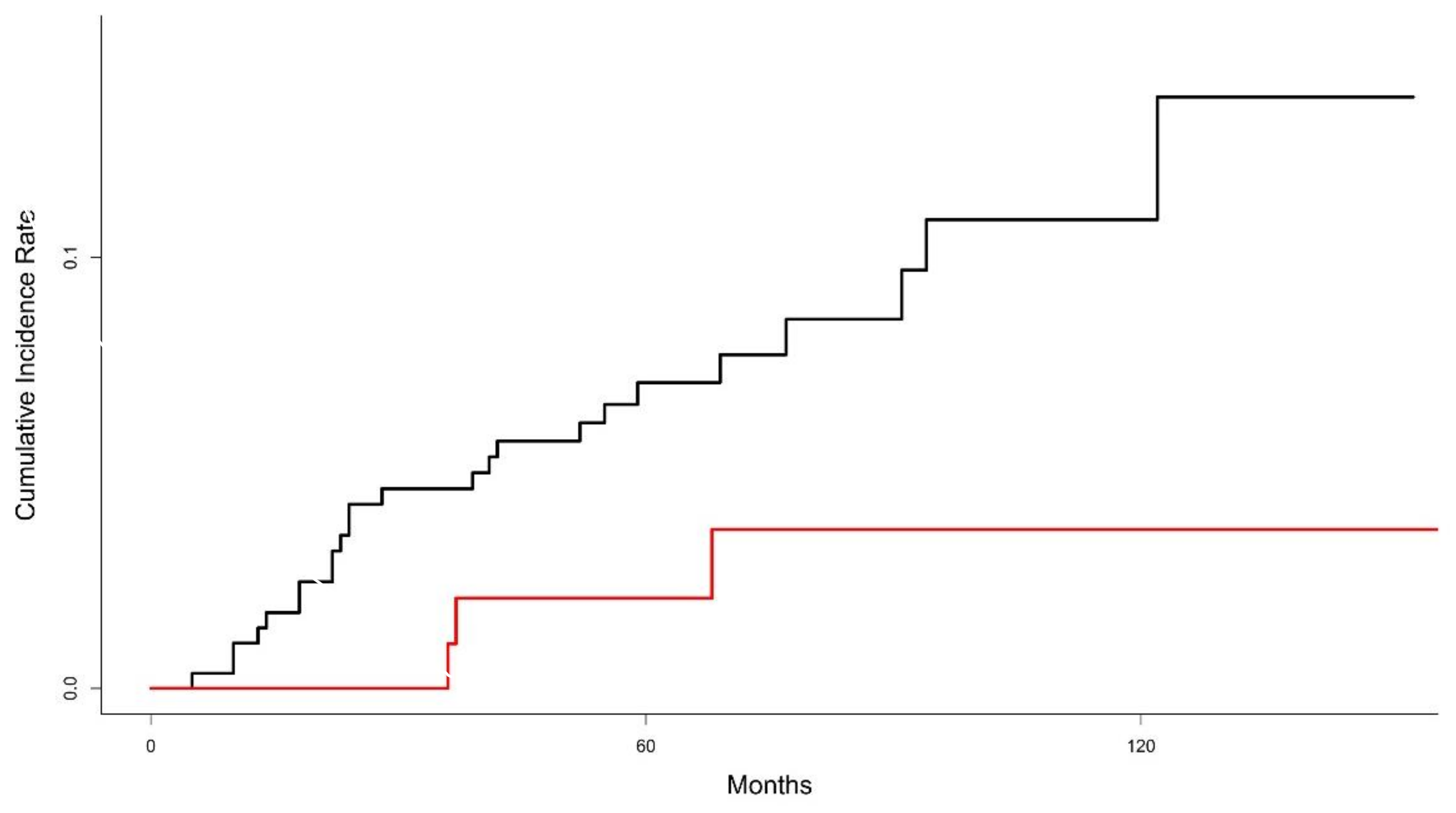
3.2. Local Recurrence
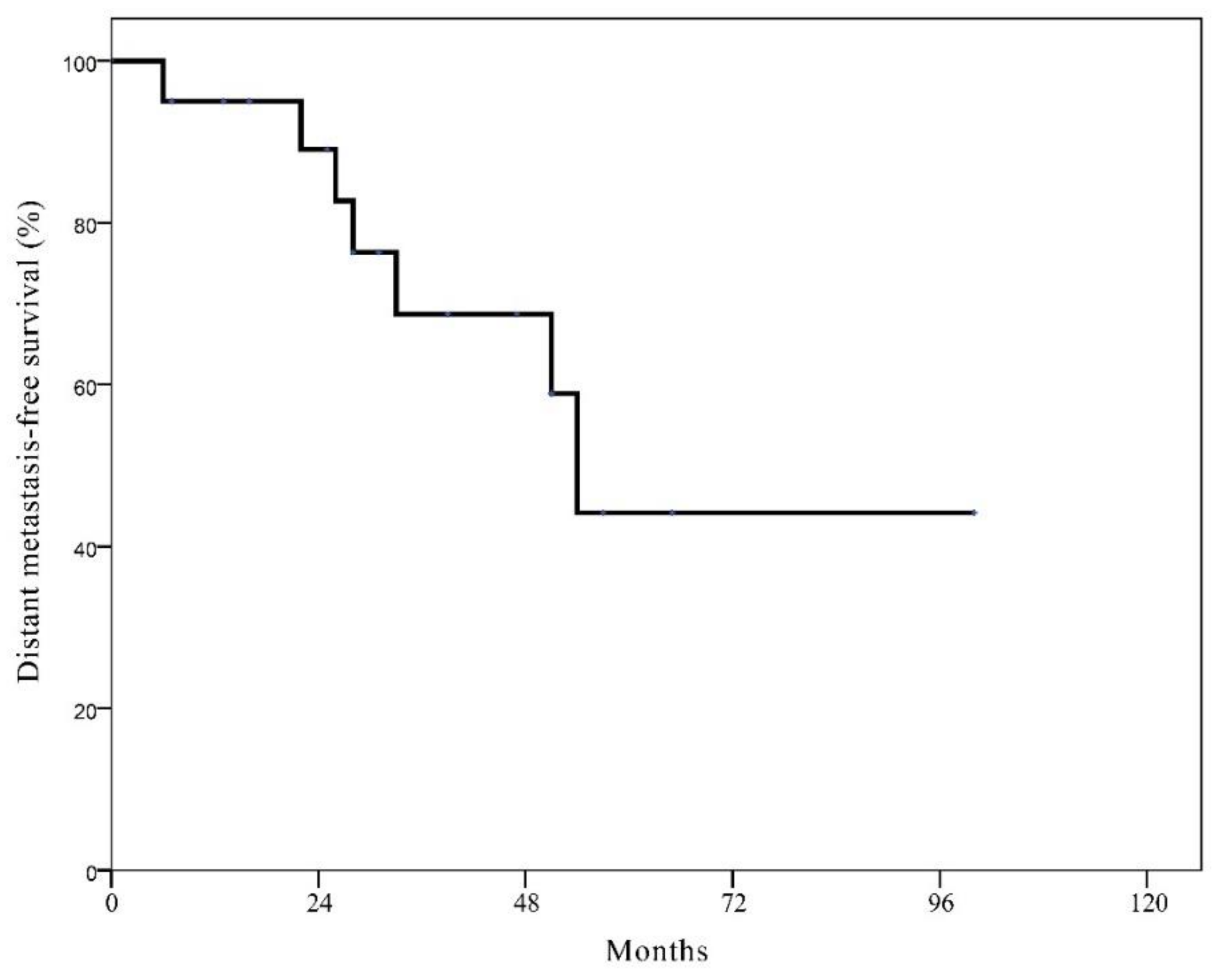
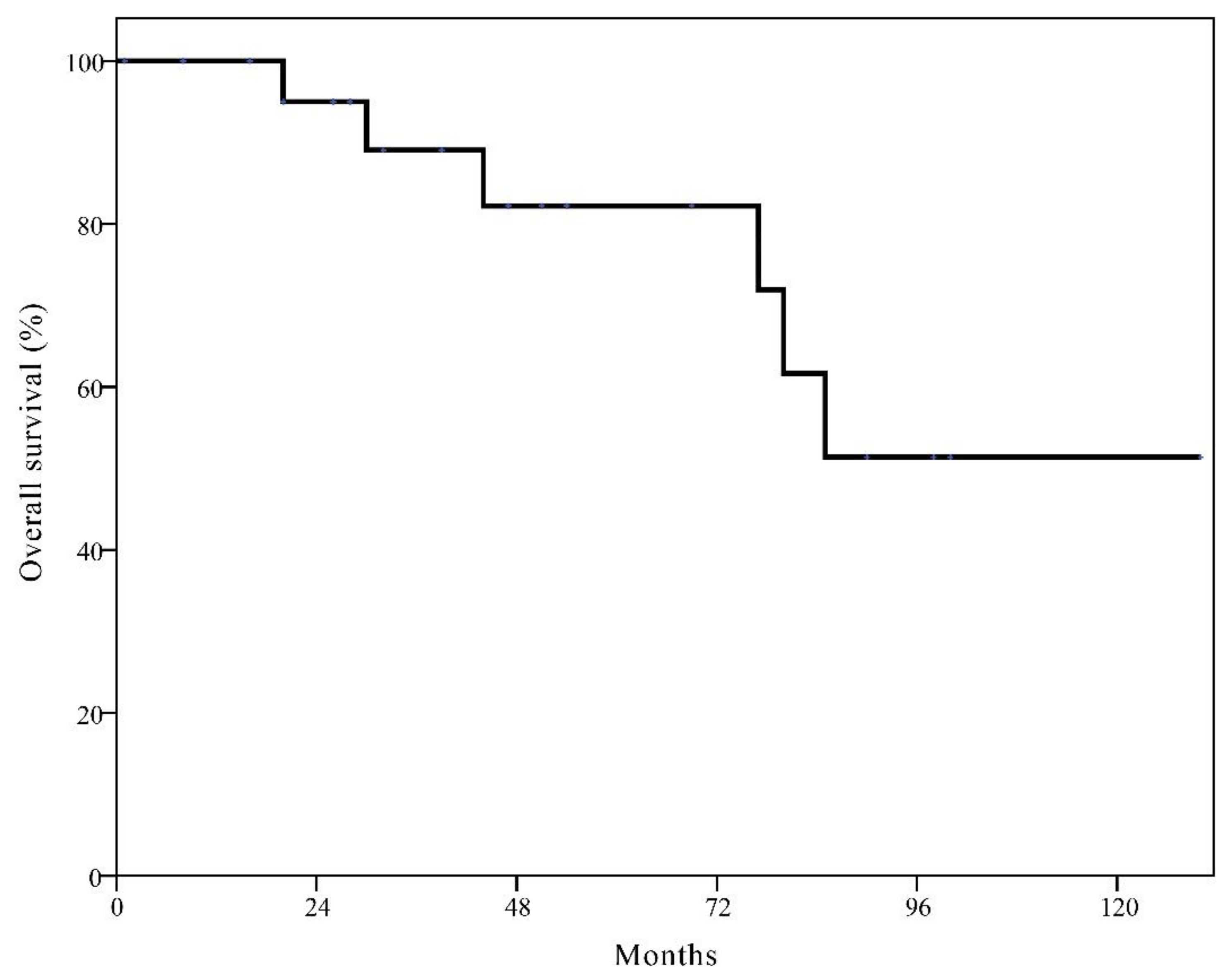
3.3. Survival Rates after Local Recurrence
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Early Breast Cancer Trialists’ Collaborative Group. Effects of Radiotherapy and Surgery in Early Breast Cancer—An Overview of the Randomized Trials. N. Engl. J. Med. 1995, 333, 1444–1455. [Google Scholar] [CrossRef] [PubMed]
- Early Breast Cancer Trialists’ Collaborative Group. Favourable and Unfavourable Effects on Long-Term Survival of Radiotherapy for Early Breast Cancer: An Overview of the Randomised Trials. Lancet 2000, 355, 1757–1770. [Google Scholar] [CrossRef]
- Elkhuizen, P.H.; Van De Vijver, M.J.; Hermans, J.; Zonderland, H.M.; Van De Velde, C.J.; Leer, J.W. Local Recurrence after Breast-Conserving Therapy for Invasive Breast Cancer: High Incidence in Young Patients and Association with Poor Survival. Int. J. Radiat. Oncol. Biol. Phys. 1998, 40, 859–867. [Google Scholar] [CrossRef]
- Vrieling, C.; Collette, L.; Fourquet, A.; Hoogenraad, W.; Horiot, J.-C.; Jager, J.; Oei, S.B.; Peterse, H.; Pierart, M.; Poortmans, P.; et al. Can Patient-, Treatment- and Pathology-Related Characteristics Explain the High Local Recurrence Rate Following Breast-Conserving Therapy in Young Patients? Eur. J. Cancer 2003, 39, 932–944. [Google Scholar] [CrossRef]
- Anders, C.K.; Hsu, D.S.; Broadwater, G.; Acharya, C.R.; Foekens, J.A.; Zhang, Y.; Wang, Y.; Marcom, P.K.; Marks, J.R.; Febbo, P.G.; et al. Young Age at Diagnosis Correlates with Worse Prognosis and Defines a Subset of Breast Cancers with Shared Patterns of Gene Expression. J. Clin. Oncol. 2008, 26, 3324–3330. [Google Scholar] [CrossRef] [PubMed]
- Fisher, B.; Anderson, S.; Bryant, J.; Margolese, R.G.; Deutsch, M.; Fisher, E.R.; Jeong, J.-H.; Wolmark, N. Twenty-Year Follow-up of a Randomized Trial Comparing Total Mastectomy, Lumpectomy, and Lumpectomy Plus Irradiation for the Treatment of Invasive Breast Cancer. N. Engl. J. Med. 2002, 347, 1233–1241. [Google Scholar] [CrossRef]
- Veronesi, U.; Cascinelli, N.; Mariani, L.; Greco, M.; Saccozzi, R.; Luini, A.; Aguilar, M.; Marubini, E. Twenty-Year Follow-up of a Randomized Study Comparing Breast-Conserving Surgery with Radical Mastectomy for Early Breast Cancer. N. Engl. J. Med. 2002, 347, 1227–1232. [Google Scholar] [CrossRef] [PubMed]
- Kroman, N.; Holtveg, H.; Wohlfahrt, J.; Jensen, M.-B.; Mouridsen, H.T.; Blichert-Toft, M.; Melbye, M. Effect of Breast-Conserving Therapy Versus Radical Mastectomy on Prognosis for Young Women with Breast Carcinoma. Cancer 2004, 100, 688–693. [Google Scholar] [CrossRef]
- Coulombe, G.; Tyldesley, S.; Speers, C.; Paltiel, C.; Aquino-Parsons, C.; Bernstein, V.; Truong, P.T.; Keyes, M.; Olivotto, I.A. Is Mastectomy Superior to Breast-Conserving Treatment for Young Women? Int. J. Radiat. Oncol. Biol. Phys. 2007, 67, 1282–1290. [Google Scholar] [CrossRef]
- Beadle, B.M.; Woodward, W.A.; Tucker, S.L.; Outlaw, E.D.; Allen, P.K.; Oh, J.L.; Strom, E.A.; Perkins, G.H.; Tereffe, W.; Yu, T.-K.; et al. Ten-Year Recurrence Rates in Young Women with Breast Cancer by Locoregional Treatment Approach. Int. J. Radiat. Oncol. Biol. Phys. 2009, 73, 734–744. [Google Scholar] [CrossRef]
- Bantema-Joppe, E.J.; De Munck, L.; Visser, O.; Willemse, P.H.; Langendijk, J.A.; Siesling, S.; Maduro, J.H. Early-Stage Young Breast Cancer Patients: Impact of Local Treatment on Survival. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, e553–e559. [Google Scholar] [CrossRef]
- Van Der Sangen, M.J.C.; Van De Wiel, F.M.M.; Poortmans, P.M.P.; Tjan-Heijnen, V.C.G.; Nieuwenhuijzen, G.A.P.; Roumen, R.M.H.; Ernst, M.F.; Nolthenius-Puylaert, M.C.B.J.E.T.; Voogd, A.C. Are Breast Conservation and Mastectomy Equally Effective in the Treatment of Young Women with Early Breast Cancer? Long-Term Results of a Population-Based Cohort of 1451 Patients Aged </= 40 Years. Breast Cancer Res. Treat. 2011, 127, 207–215. [Google Scholar] [CrossRef]
- Mahmood, U.; Morris, C.; Neuner, G.; Koshy, M.; Kesmodel, S.; Buras, R.; Chumsri, S.; Bao, T.; Tkaczuk, K.; Feigenberg, S. Similar Survival with Breast Conservation Therapy or Mastectomy in the Management of Young Women with Early-Stage Breast Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, 1387–1393. [Google Scholar] [CrossRef]
- Bantema-Joppe, E.J.; Heuvel, E.R.V.D.; De Munck, L.; De Bock, G.H.; Smit, W.G.J.M.; Timmer, P.R.; Dolsma, W.V.; Jansen, L.; Schröder, C.P.; Siesling, S.; et al. Impact of Primary Local Treatment on the Development of Distant Metastases or Death through Locoregional Recurrence in Young Breast Cancer Patients. Breast Cancer Res. Treat. 2013, 140, 577–585. [Google Scholar] [CrossRef]
- Jeon, Y.W.; Choi, J.E.; Park, H.K.; Kim, K.S.; Lee, J.Y.; Suh, Y.J. Impact of Local Surgical Treatment on Survival in Young Women with T1 Breast Cancer: Long-Term Results of a Population-Based Cohort. Breast Cancer Res. Treat. 2013, 138, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.Q.; Truong, P.T.; Olivotto, I.A.; Olson, R.; Coulombe, G.; Keyes, M.; Weir, L.; Gelmon, K.; Bernstein, V.; Woods, R.; et al. Should Women Younger Than 40 Years of Age with Invasive Breast Cancer Have a Mastectomy? 15-Year Outcomes in a Population-Based Cohort. Int. J. Radiat. Oncol. Biol. Phys. 2014, 90, 509–517. [Google Scholar] [CrossRef]
- Xie, Z.; Wang, X.; Lin, H.; Wei, W.; Liu, P.; Xiao, X.; Xie, X.; Guan, X.; Yang, M.; Tang, J. Breast-Conserving Therapy: A Viable Option for Young Women with Early Breast Cancer--Evidence from a Prospective Study. Ann. Surg. Oncol. 2014, 21, 2188–2196. [Google Scholar] [CrossRef]
- Frandsen, J.; Ly, D.; Cannon, G.; Suneja, G.; Matsen, C.; Gaffney, D.K.; Wright, M.; Kokeny, K.E.; Poppe, M.M. In the Modern Treatment Era, Is Breast Conservation Equivalent to Mastectomy in Women Younger Than 40 Years of Age? A Multi-Institution Study. Int. J. Radiat. Oncol. Biol. Phys. 2015, 93, 1096–1103. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.C.; Yan, W.; Christos, P.J.; Nori, D.; Ravi, A. Equivalent Survival with Mastectomy or Breast-Conserving Surgery Plus Radiation in Young Women Aged <40 Years with Early-Stage Breast Cancer: A National Registry-Based Stage-by-Stage Comparison. Clin. Breast Cancer 2015, 15, 390–397. [Google Scholar]
- Vila, J.; Gandini, S.; Gentilini, O. Overall Survival According to Type of Surgery in Young (</=40 Years) Early Breast Cancer Patients: A Systematic Meta-Analysis Comparing Breast-Conserving Surgery Versus Mastectomy. Breast 2015, 24, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Plichta, J.K.; Rai, U.; Tang, R.; Coopey, S.B.; Buckley, J.M.; Gadd, M.A.; Specht, M.C.; Hughes, K.S.; Taghian, A.G.; Smith, B.L. Factors Associated with Recurrence Rates and Long-Term Survival in Women Diagnosed with Breast Cancer Ages 40 and Younger. Ann. Surg. Oncol. 2016, 23, 3212–3220. [Google Scholar] [CrossRef] [PubMed]
- Quan, M.L.; Paszat, L.F.; Fernandes, K.A.; Sutradhar, R.; McCready, D.R.; Rakovitch, E.; Warner, E.; Wright, F.C.; Hodgson, N.; Brackstone, M.; et al. The Effect of Surgery Type on Survival and Recurrence in Very Young Women with Breast Cancer. J. Surg. Oncol. 2017, 115, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Maishman, T.; Cutress, R.I.; Hernandez, A.; Gerty, S.; Copson, E.R.; Durcan, L.; Eccles, D.M. Local Recurrence and Breast Oncological Surgery in Young Women with Breast Cancer: The Posh Observational Cohort Study. Ann. Surg. 2017, 266, 165–172. [Google Scholar] [CrossRef]
- Van Laar, C.; van der Sangen, M.; Poortmans, P.; Nieuwenhuijzen, G.; Roukema, J.; Roumen, R.; Tjan-Heijnen, V.; Voogd, A. Local Recurrence Following Breast-Conserving Treatment in Women Aged 40 Years or Younger: Trends in Risk and the Impact on Prognosis in a Population-Based Cohort of 1143 Patients. Eur. J. Cancer 2013, 49, 3093–3101. [Google Scholar] [CrossRef] [PubMed]
- Botteri, E.; Veronesi, P.; Vila, J.; Rotmensz, N.; Galimberti, V.; Thomazini, M.V.; Viale, G.; Orecchia, R.; Goldhirsch, A.; Gentilini, O. Improved Prognosis of Young Patients with Breast Cancer Undergoing Breast-Conserving Surgery. Br. J. Surg. 2017, 104, 1802–1810. [Google Scholar] [CrossRef]
- Cardoso, F.; Loibl, S.; Pagani, O.; Graziottin, A.; Panizza, P.; Martincich, L.; Gentilini, O.; Peccatori, F.; Fourquet, A.; Delaloge, S.; et al. The European Society of Breast Cancer Specialists Recommendations for the Management of Young Women with Breast Cancer. Eur. J. Cancer 2012, 48, 3355–3377. [Google Scholar] [CrossRef]
- Partridge, A.H.; Pagani, O.; Abulkhair, O.; Aebi, S.; Amant, F.; Azim, H.A.; Costa, A.; Delaloge, S.; Freilich, G.; Gentilini, O.D.; et al. First International Consensus Guidelines for Breast Cancer in Young Women (Bcy1). Breast 2014, 23, 209–220. [Google Scholar] [CrossRef]
- Kurian, A.W.; Lichtensztajn, D.Y.; Keegan, T.H.M.; Nelson, D.O.; Clarke, C.A.; Gomez, S.L. Use of and Mortality after Bilateral Mastectomy Compared with Other Surgical Treatments for Breast Cancer in California, 1998–2011. JAMA 2014, 312, 902–914. [Google Scholar] [CrossRef]
- Pesce, C.E.; Liederbach, E.; Czechura, T.; Winchester, D.J.; Yao, K. Changing Surgical Trends in Young Patients with Early Stage Breast Cancer, 2003 to 2010: A Report from the National Cancer Data Base. J. Am. Coll. Surg. 2014, 219, 19–28. [Google Scholar] [CrossRef]
- Rutter, C.E.; Park, H.S.; Killelea, B.K.; Evans, S.B. Growing Use of Mastectomy for Ductal Carcinoma-in Situ of the Breast among Young Women in the United States. Ann. Surg. Oncol. 2015, 22, 2378–2386. [Google Scholar] [CrossRef]
- Anderson, S.J.; Wapnir, I.; Dignam, J.J.; Fisher, B.; Mamounas, E.P.; Jeong, J.-H.; Geyer, C.E., Jr.; Wickerham, D.L.; Costantino, J.P.; Wolmark, N. Prognosis after Ipsilateral Breast Tumor Recurrence and Locoregional Recurrences in Patients Treated by Breast-Conserving Therapy in Five National Surgical Adjuvant Breast and Bowel Project Protocols of Node-Negative Breast Cancer. J. Clin. Oncol. 2009, 27, 2466–2473. [Google Scholar] [CrossRef] [PubMed]
- Voelkel, V.; Draeger, T.; Groothuis-Oudshoorn, C.G.M.; De Munck, L.; Hueting, T.; Gerken, M.; Klinkhammer-Schalke, M.; Lavric, M.; Siesling, S. Predicting the Risk of Locoregional Recurrence after Early Breast Cancer: An External Validation of the Dutch Influence-Nomogram with Clinical Cancer Registry Data from Germany. J. Cancer Res. Clin. Oncol. 2019, 145, 1823–1833. [Google Scholar] [CrossRef] [PubMed]
| Variables | BCT (n = 311) | Mastectomy (n = 117) | p Value |
|---|---|---|---|
| Age at diagnosis (years) Median IQR | 36 33 to 39 | 37 34 to 39 | 0.041 |
| Laterality Left Right | 169 (54.3%) 142 (45.7%) | 54 (46.2%) 63 (53.8%) | 0.161 |
| Histologic grade Low to intermediate High | 136 (46.7%) 155 (53.3%) | 53 (50.5%) 52 (49.5%) | 0.587 |
| Estrogen receptor Negative Positive | 104 (33.4%) 207 (66.6%) | 31 (26.7%) 85 (73.3%) | 0.226 |
| Progesterone receptor Negative Positive | 100 (32.2%) 211 (67.8%) | 36 (31.0%) 80 (69.0%) | 0.917 |
| HER2 Negative Positive | 258 (83.2%) 52 (16.8%) | 71 (61.7%) 44 (38.3%) | <0.001 |
| Intrinsic subtype Luminal A Luminal B HER2 Triple negative | 190 (61.3%) 36 (11.6%) 16 (5.2%) 68 (21.9%) | 63 (54.8%) 27 (23.5%) 17 (14.8%) 8 (7.0%) | <0.001 |
| Tumor size ≤2 cm >2 cm | 207 (66.6%) 104 (33.4%) | 68 (58.1%) 49 (41.9%) | 0.131 |
| No. of positive LNs 0 1–3 | 247 (79.4%) 64 (20.6%) | 74 (63.2%) 43 (36.8%) | 0.001 |
| AJCC stage I II | 176 (56.6%) 135 (43.4%) | 49 (41.9%) 68 (58.1%) | 0.009 |
| Adjuvant chemotherapy None CMF Anthracycline-based regimen Taxane-based regimen | 75 (24.1%) 18 (5.8%) 159 (51.1%) 59 (19.0%) | 30 (25.6%) 4 (3.4%) 45 (38.5%) 38 (32.5%) | 0.013 |
| Adjuvant endocrine therapy No oral regimen With LHRH analogues | 92 (29.6%) 134 (43.1%) 85 (27.3%) | 35 (29.9%) 56 (47.9%) 26 (22.2%) | 0.523 |
| Adjuvant trastuzumab Not indicated No Yes | 259 (83.3%) 46 (14.8%) 6 (1.9%) | 73 (62.4%) 39 (33.3%) 5 (4.3%) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, D.V.; Kim, S.-W.; Oh, Y.-T.; Noh, O.K.; Jung, Y.; Chun, M.; Yoon, D.S. Local Recurrence in Young Women with Breast Cancer: Breast Conserving Therapy vs. Mastectomy Alone. Cancers 2021, 13, 2150. https://doi.org/10.3390/cancers13092150
Nguyen DV, Kim S-W, Oh Y-T, Noh OK, Jung Y, Chun M, Yoon DS. Local Recurrence in Young Women with Breast Cancer: Breast Conserving Therapy vs. Mastectomy Alone. Cancers. 2021; 13(9):2150. https://doi.org/10.3390/cancers13092150
Chicago/Turabian StyleNguyen, Dang Van, Sang-Won Kim, Young-Taek Oh, O Kyu Noh, Yongsik Jung, Mison Chun, and Dae Sung Yoon. 2021. "Local Recurrence in Young Women with Breast Cancer: Breast Conserving Therapy vs. Mastectomy Alone" Cancers 13, no. 9: 2150. https://doi.org/10.3390/cancers13092150
APA StyleNguyen, D. V., Kim, S.-W., Oh, Y.-T., Noh, O. K., Jung, Y., Chun, M., & Yoon, D. S. (2021). Local Recurrence in Young Women with Breast Cancer: Breast Conserving Therapy vs. Mastectomy Alone. Cancers, 13(9), 2150. https://doi.org/10.3390/cancers13092150







