The Lymph-Sparing Quotient: A Retrospective Risk Analysis on Extremity Radiation for Soft Tissue Sarcoma Treatment
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
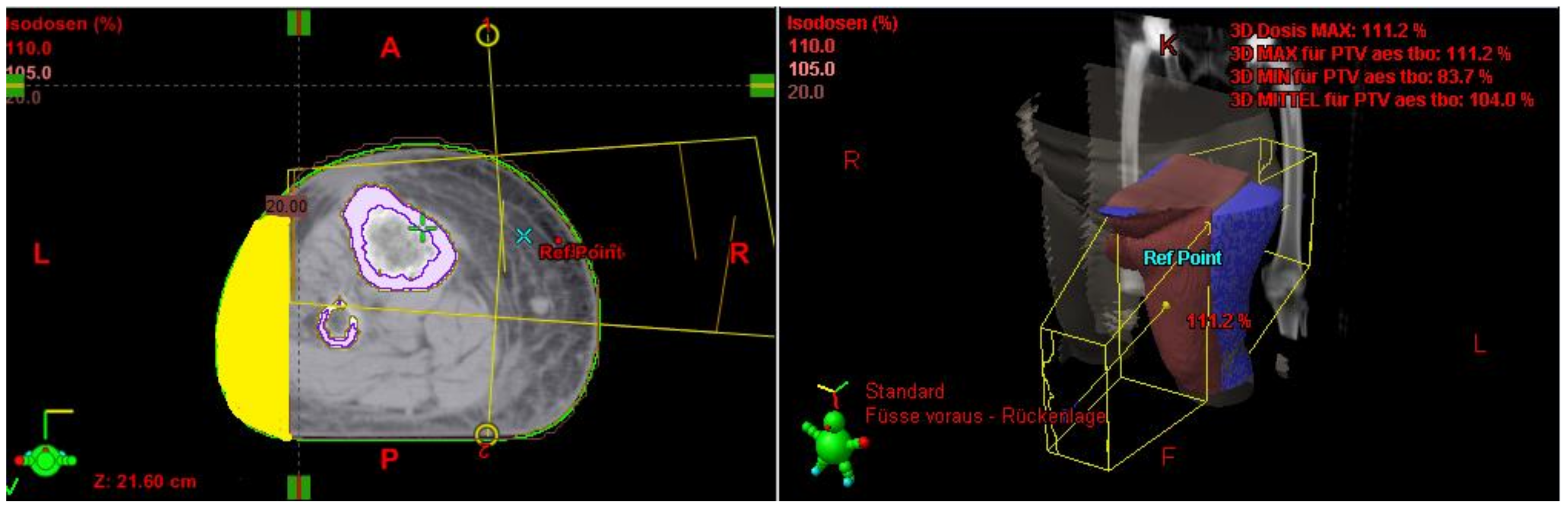
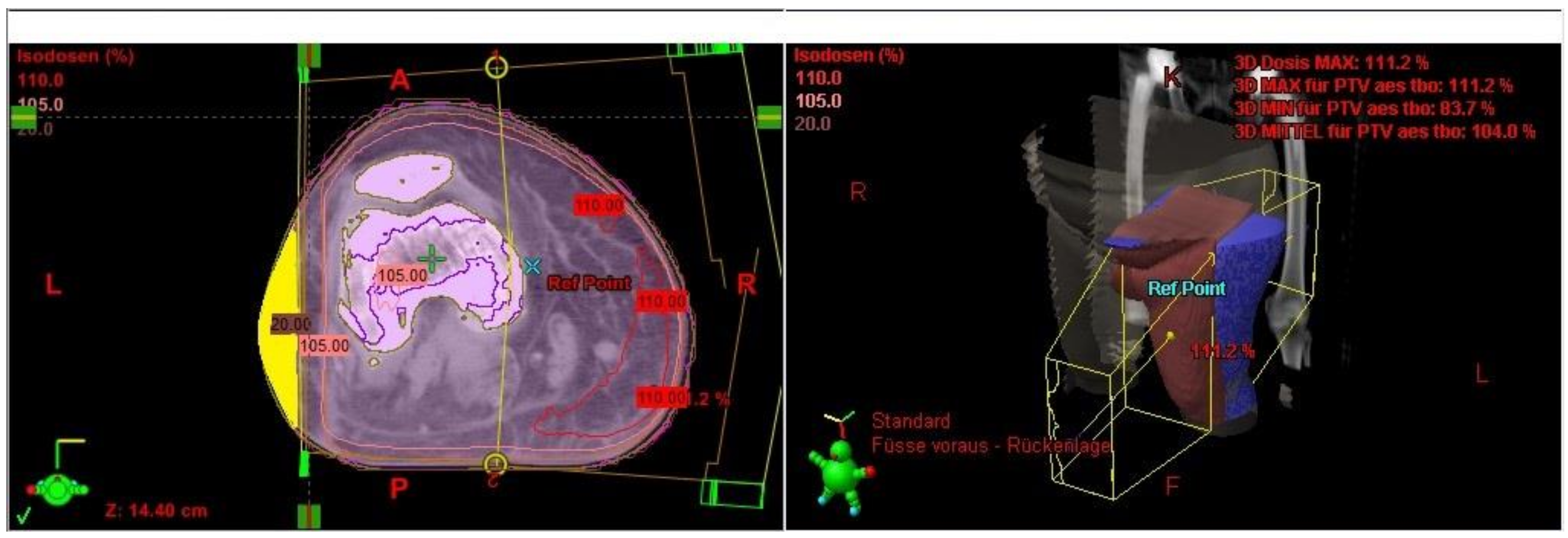
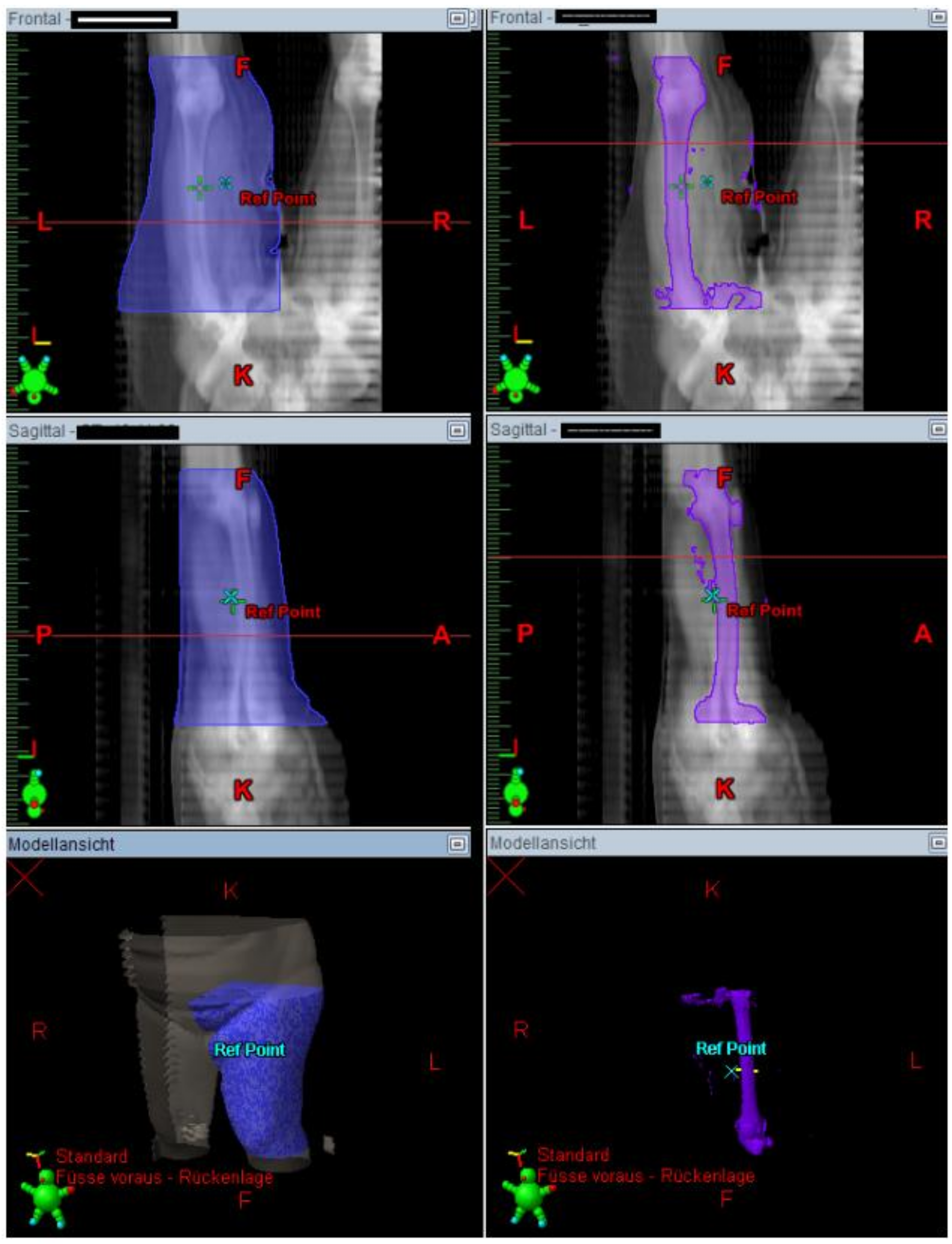
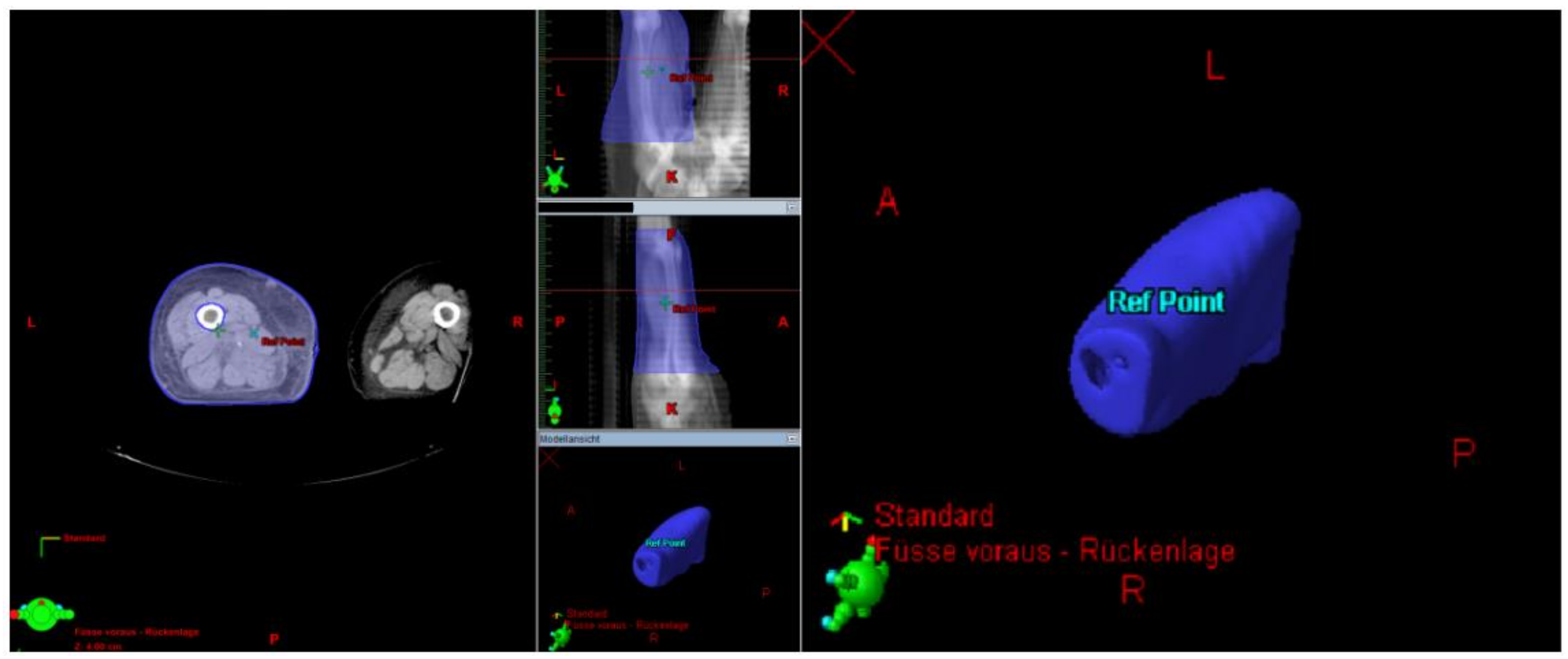
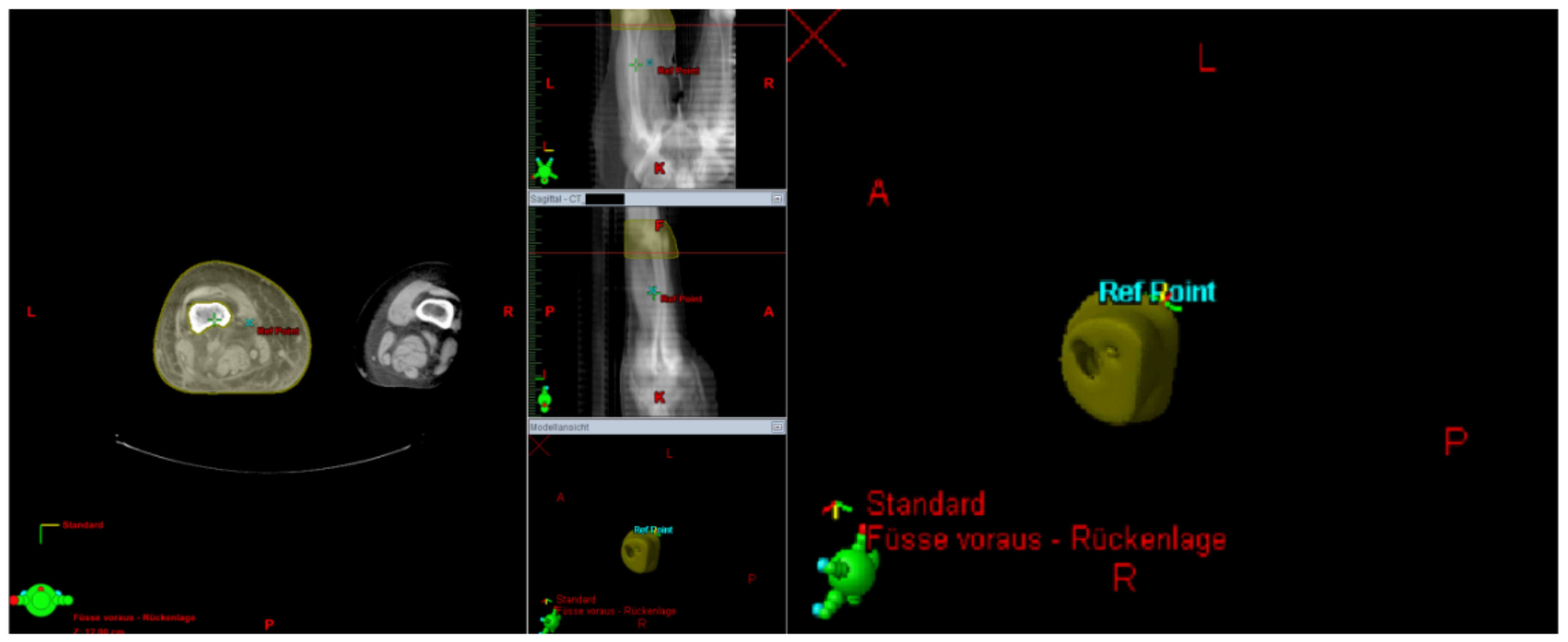
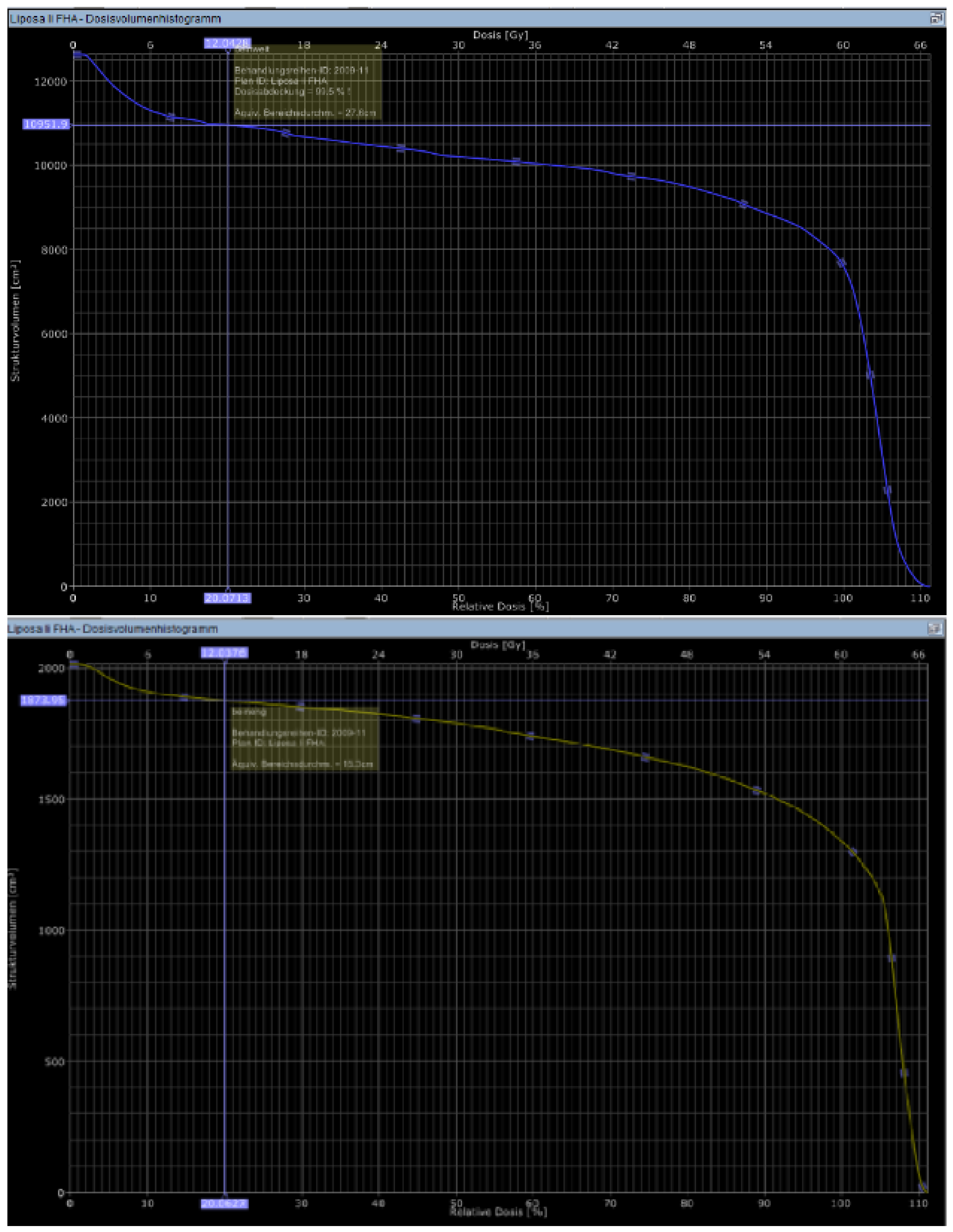
The Lymph-Sparing Quotient
3. Results
Use of the Lymph-Sparing Quotient
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoefkens, F.; Dehandschutter, C.; Somville, J.; Meijnders, P.; van Gestel, D. Soft tissue sarcoma of the extremities: Pending questions on surgery and radiotherapy. Radiat. Oncol. 2016, 11, 136. [Google Scholar] [CrossRef]
- Jebsen, N.L.; Trovik, C.S.; Bauer, H.C.F.; Rydholm, A.; Monge, O.R.; Hall, K.S.; Alvegård, T.; Bruland, O.S. Radiotherapy to improve local control regardless of surgical margin and malignancy grade in extremity and trunk wall soft tissue sarcoma: A Scandinavian sarcoma group study. Int. J. Radiat. Oncol. Biol. Phys. 2008, 71, 1196–1203. [Google Scholar] [CrossRef] [PubMed]
- Kachare, S.D.; Brinkley, J.; Vohra, N.A.; Zervos, E.E.; Wong, J.H.; Fitzgerald, T.L. Radiotherapy associated with improved survival for high-grade sarcoma of the extremity. J. Surg. Oncol. 2015, 112, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Alektiar, K.M.; Velasco, J.; Zelefsky, M.J.; Woodruff, J.M.; Lewis, J.J.; Brennan, M.F. Adjuvant radiotherapy for margin-positive high-grade soft tissue sarcoma of the extremity. Int. J. Radiat. Oncol. Biol. Phys. 2000, 48, 1051–1058. [Google Scholar] [CrossRef]
- Levay, J.; O’Sullivan, B.; Catton, C.; Bell, R.; Fornasier, V.; Cummings, B.; Hao, D.; Warr, D.; Quirt, I. Outcome and prognostic factors in soft tissue sarcoma in the adult. Int. J. Radiat. Oncol. Biol. Phys. 1993, 27, 1091–1099. [Google Scholar] [CrossRef]
- Pisters, P.W.; Harrison, L.B.; Leung, D.H.; Woodruff, J.M.; Casper, E.S.; Brennan, M.F. Long-term results of a prospective randomized trial of adjuvant brachytherapy in soft tissue sarcoma. J. Clin. Oncol. 1996, 14, 859–868. [Google Scholar] [CrossRef]
- Yang, J.C.; Chang, A.E.; Baker, A.R.; Sindelar, W.F.; Danforth, D.N.; Topalian, S.L.; DeLaney, T.; Glatstein, E.; Steinberg, S.M.; Merino, M.J.; et al. Randomized prospective study of the benefit of adjuvant radiation therapy in the treatment of soft tissue sarcomas of the extremity. J. Clin. Oncol. 1998, 16, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Ratan, R.; Patel, S.R. Chemotherapy for soft tissue sarcoma. Cancer 2016, 122, 2952–2960. [Google Scholar] [CrossRef]
- Friedmann, D.; Wunder, J.S.; Ferguson, P.; O’Sullivan, B.; Roberge, D.; Catton, C.; Freeman, C.; Saran, N.; Turcotte, R.E. Incidence and Severity of Lymphoedema following Limb Salvage of Extremity Soft Tissue Sarcoma. Sarcoma 2011, 2011, 289673. [Google Scholar] [CrossRef] [PubMed]
- Pedrosa, B.C.d.S.; Maia, J.N.; Ferreira, A.P.d.L.; de Araújo, M.d.G.R.; Montenegro, E.J.N.; da Silva, F.L.; de Castro, C.M.M.B.; Andrade, M.d.A. Functionality and quality of life of patients with unilateral lymphedema of a lower limb: A cross-sectional study. J. Vasc. Bras. 2019, 18, e20180066. [Google Scholar] [CrossRef]
- Földi, M.; Földi, E.; Kubik, S. Lehrbuch der Lymphologie für Mediziner, Masseure und Physiotherapeuten, 6th ed.; Elsevier Urban und Fischer: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Azhar, S.H.; Lim, H.Y.; Tan, B.-K.; Angeli, V. The Unresolved Pathophysiology of Lymphedema. Front. Physiol. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Coindre, J.M.; Trojani, M.; Contesso, G.; David, M.; Rouesse, J.; Bui, N.B.; Bodaert, A.; De Mascarel, I.; De Mascarel, A.; Goussot, J.-F. Reproducibility of a histopathologic grading system for adult soft tissue sarcoma. Cancer 1986, 58, 306–309. [Google Scholar] [CrossRef]
- Bell, R.S.; O’Sullivan, B.; Davis, A.; Langer, F.; Cummings, B.; Fornasier, V.L. Functional outcome in patients treated with surgery and irradiation for soft tissue tumours. J. Surg. Oncol. 1991, 48, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Stinson, S.F.; Delaney, T.F.; Greenberg, J.; Yang, J.C.; Lampert, M.H.; Hicks, J.E.; Venzon, D.; White, D.E.; Rosenberg, S.A.; Glatstein, E.J. Acute and long-term effects on limb function of combined modality limb sparing therapy for extremity soft tissue sarcoma. Int. J. Radiat. Oncol. Biol. Phys. 1991, 21, 1493–1499. [Google Scholar] [CrossRef]
- Lampert, M.H.; Gerber, L.H.; Glatstein, E.; Rosenberg, S.A.; Danoff, J.V. Soft tissue sarcoma: Functional outcome after wide local excision and radiation therapy. Arch. Phys. Med. Rehabil. 1984, 65, 477–480. [Google Scholar]
- Robinson, M.H.; Spruce, L.; Eeles, R.; Fryatt, I.; Harmer, C.L.; Thomas, J.M.; Westbury, G. Limb function following conservation treatment of adult soft tissue sarcoma. Eur. J. Cancer Clin. Oncol. 1991, 27, 1567–1574. [Google Scholar] [CrossRef]
- Bedi, M.; King, D.M.; Whitfield, R.; Hackbarth, D.A.; Neilson, J.C.; Charlson, J.A.; Wang, D. The effect of smoking and major vein resection on post-therapy lymphedema in soft tissue sarcomas treated with neoadjuvant radiation and limb-salvage surgery. Am. J. Clin. Oncol. 2015, 38, 184–188. [Google Scholar] [CrossRef]
- The Diagnosis and Treatment of Peripheral Lymphedema: 2016 Consensus Document of the International Society of Lymphology. Lymphology 2016, 49, 170–184.
- Wu, P.; Elswick, S.M.; Arkhavan, A.; Molinar, V.E.; Mohan, A.T.; Curiel, D.; Sim, F.H.; Martinez-Jorge, J.; Saint-Cyr, M. Risk Factors for Lymphedema after Thigh Sarcoma Resection and Reconstruction. Plast. Reconstr. Surg. Glob. Open 2020, 8, e2912. [Google Scholar] [CrossRef]
- Allam, O.; Park, K.E.; Chandler, L.; Mozaffari, M.A.; Ahmad, M.; Lu, X.; Alperovich, M. The impact of radiation on lymphedema: A review of the literature. Gland Surg. 2020, 9, 596–602. [Google Scholar] [CrossRef]
- Moseley, A.; Piller, N. Reliability of bioimpedance spectroscopy and tonometry after breast conserving cancer treatment. Lymphat. Res. Biol. 2008, 6, 85–87. [Google Scholar] [CrossRef]
- Bagheri, S.; Ohlin, K.; Olsson, G.; Brorson, H. Tissue tonometry before and after liposuction of arm lymphedema following breast cancer. Lymphat. Res. Biol. 2005, 3, 66–80. [Google Scholar] [CrossRef] [PubMed]
- Cornish, B.H.; Chapman, M.; Hirst, C.; Mirolo, B.; Bunce, I.H.; Ward, L.C.; Thomas, B.J. Early diagnosis of lymphedema using multiple frequency bioimpedance. Lymphology 2001, 34, 2–11. [Google Scholar] [PubMed]
- Cambria, R.A.; Gloviczki, P.; Naessens, J.M.; Wahner, H.W. Noninvasive evaluation of the lymphatic system with lymphoscintigraphy: A prospective, semiquantitative analysis in 386 extremities. J. Vasc. Surg. 1993, 18, 773–782. [Google Scholar] [CrossRef]
- Hinrichs, C.S.; Gibbs, J.F.; Driscoll, D.; Kepner, J.L.; Wilkinson, N.W.; Edge, S.B.; Fassl, K.A.; Muir, R.; Kraybill, W.G. The effectiveness of complete decongestive physiotherapy for the treatment of lymphedema following groin dissection for melanoma. J. Surg. Oncol. 2004, 85, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, R.; Yamamoto, T. Effectiveness of the treatment-phase of two-phase complex decongestive physiotherapy for the treatment of extremity lymphedema. Int. J. Clin. Oncol. 2007, 12, 463–468. [Google Scholar] [CrossRef]
- Yoshida, S.; Koshima, I.; Imai, H.; Uchiki, T.; Sasaki, A.; Fujioka, Y.; Nagamatsu, S.; Yokota, K.; Harima, M.; Yamashita, S. Localized lymphedema after treatment for soft tissue sarcoma in the lower limbs: Comparison of improvement according to duration before lymphaticovenular anastomosis. Clin. Case Rep. 2019, 7, 1534–1538. [Google Scholar] [CrossRef] [PubMed]
- Raju, A.; Chang, D.W. Vascularized lymph node transfer for treatment of lymphedema: A comprehensive literature review. Ann. Surg. 2015, 261, 1013–1023. [Google Scholar] [CrossRef] [PubMed]
- Boccardo, F.; Valenzano, M.; Costantini, S.; Casabona, F.; Morotti, M.; Sala, P.; Cian, F.de; Molinari, L.; Spinaci, S.; Dessalvi, S.; et al. LYMPHA Technique to Prevent Secondary Lower Limb Lymphedema. Ann. Surg. Oncol. 2016, 23, 3558–3563. [Google Scholar] [CrossRef]
- Felmerer, G.; Dowlatshahi, A.S.; Stark, G.B.; Földi, E.; Földi, M.; Ahls, M.G.; Ströbel, P.; Aung, T. Lymphangiosarcoma: Is Stewart-Treves Syndrome a Preventable Condition? Lymphat. Res. Biol. 2016, 14, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, S.E.; Iclozan, C.; Bui, M.M.; Cotter, M.J.; Ramakrishnan, R.; Ahmed, J.; Noyes, D.R.; Cheong, D.; Gonzalez, R.J.; Heysek, R.V.; et al. Combination of external beam radiotherapy (EBRT) with intratumoral injection of dendritic cells as neo-adjuvant treatment of high-risk soft tissue sarcoma patients. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 924–932. [Google Scholar] [CrossRef] [PubMed]
- Tawbi, H.A.; Burgess, M.; Bolejack, V.; van Tine, B.A.; Schuetze, S.M.; Hu, J.; D’Angelo, S.; Attia, S.; Riedel, R.F.; Priebat, D.A.; et al. Pembrolizumab in advanced soft-tissue sarcoma and bone sarcoma (SARC028): A multicentre, two-cohort, single-arm, open-label, phase 2 trial. Lancet Oncol. 2017, 18, 1493–1501. [Google Scholar] [CrossRef]
| Characteristic | Value | Percentage/Range |
|---|---|---|
| Gender: | ||
| Male | 16 | 47% |
| Female | 18 | 53% |
| Age: | ||
| Median | 53 y | |
| Range | 21–80 y | |
| Tumor location: | ||
| Lower extremity | 32 | 94% |
| Upper extremity | 2 | 6% |
| Histology: | ||
| Liposarcoma | 9 | 26% |
| Spindle cell soft tissue sarcoma | 5 | 15% |
| Synovial sarcoma | 4 | 12% |
| Leiomyosarcoma | 4 | 12% |
| Fibrosarcoma | 1 | 3% |
| Myxofibrosacoma | 4 | 12% |
| Alveolar sarcoma | 1 | 3% |
| Soft tissue sarcoma (not otherwise specified) | 1 | 3% |
| Pleomorphic sarcoma | 5 | 15% |
| Histologic differentiation FNCLCC = Fédération Nationale des Centres de Lutte Contre Le Cancer [13]: | ||
| Grade 1 | 6 | 18% |
| Grade 2 | 4 | 12% |
| Grade 3 | 24 | 70 |
| Stage: | ||
| Stage I | 5 | 15% |
| Stage II | 9 | 26% |
| Stage III | 20 | 59% |
| Resection: | ||
| Intralesional | 1 | 3% |
| Marginal | 15 | 44% |
| Wide | 18 | 53% |
| Treatment parameters: | ||
| Med. radiation dose (range), Gy | 66.3 Gy (50.4–70.2 Gy) | |
| Med. fraction dose (range), Gy | 1.8 Gy (1.8–2 Gy) | |
| Boost | 27/34 | 79% |
| Med. follow up, months (range) | 40 (3–223) | |
| Lymphedema ≥ Grade 2: | ||
| Acute | 5 | 15% |
| Chronic | 17 | 50% |
| Acute or chronic | 24 | 71% |
| Lymph-sparing quotient: | 2YLEFS | p |
| ≤0.2 | 0% | 0.006 |
| >0.2 | 39% | |
| Lymph-sparing quotient: | HR (95% CI) | p |
| /% increase | 0.964 (0.93–1.0) | 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarif, I.; Elsayad, K.; Rolf, D.; Kittel, C.; Gosheger, G.; Wardelmann, E.; Haverkamp, U.; Eich, H.T. The Lymph-Sparing Quotient: A Retrospective Risk Analysis on Extremity Radiation for Soft Tissue Sarcoma Treatment. Cancers 2021, 13, 2113. https://doi.org/10.3390/cancers13092113
Sarif I, Elsayad K, Rolf D, Kittel C, Gosheger G, Wardelmann E, Haverkamp U, Eich HT. The Lymph-Sparing Quotient: A Retrospective Risk Analysis on Extremity Radiation for Soft Tissue Sarcoma Treatment. Cancers. 2021; 13(9):2113. https://doi.org/10.3390/cancers13092113
Chicago/Turabian StyleSarif, Iqbal, Khaled Elsayad, Daniel Rolf, Christopher Kittel, Georg Gosheger, Eva Wardelmann, Uwe Haverkamp, and Hans Theodor Eich. 2021. "The Lymph-Sparing Quotient: A Retrospective Risk Analysis on Extremity Radiation for Soft Tissue Sarcoma Treatment" Cancers 13, no. 9: 2113. https://doi.org/10.3390/cancers13092113
APA StyleSarif, I., Elsayad, K., Rolf, D., Kittel, C., Gosheger, G., Wardelmann, E., Haverkamp, U., & Eich, H. T. (2021). The Lymph-Sparing Quotient: A Retrospective Risk Analysis on Extremity Radiation for Soft Tissue Sarcoma Treatment. Cancers, 13(9), 2113. https://doi.org/10.3390/cancers13092113








