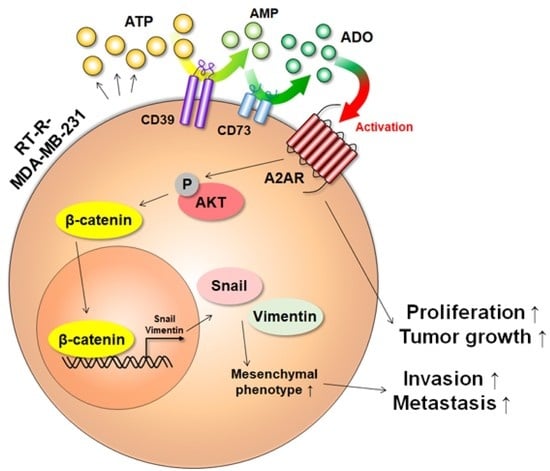
Increased Extracellular Adenosine in Radiotherapy-Resistant Breast Cancer Cells Enhances Tumor Progression through A2AR-Akt-β-Catenin Signaling
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Case Selection
2.2. Tissue Microarray and Immunohistochemistry (IHC)
2.3. Evaluation of IHC Data
2.4. Reagents and Cell Lines
2.5. Establishment of RT-R-BC Cells
2.6. Total RNA Extraction and Reverse Transcription Polymerase Chain Reaction (RT-PCR)
2.7. Extracellular ADO and ATP Measurements
2.8. Cell Proliferation Assay
2.9. Gene Silencing by siRNA Transfection
2.10. Colony Formation Assay
2.11. Matrigel Invasion Assay
2.12. Protein Extraction and Western Blot Analysis
2.13. Animal Experiments
2.14. Statistical Analysis
3. Results
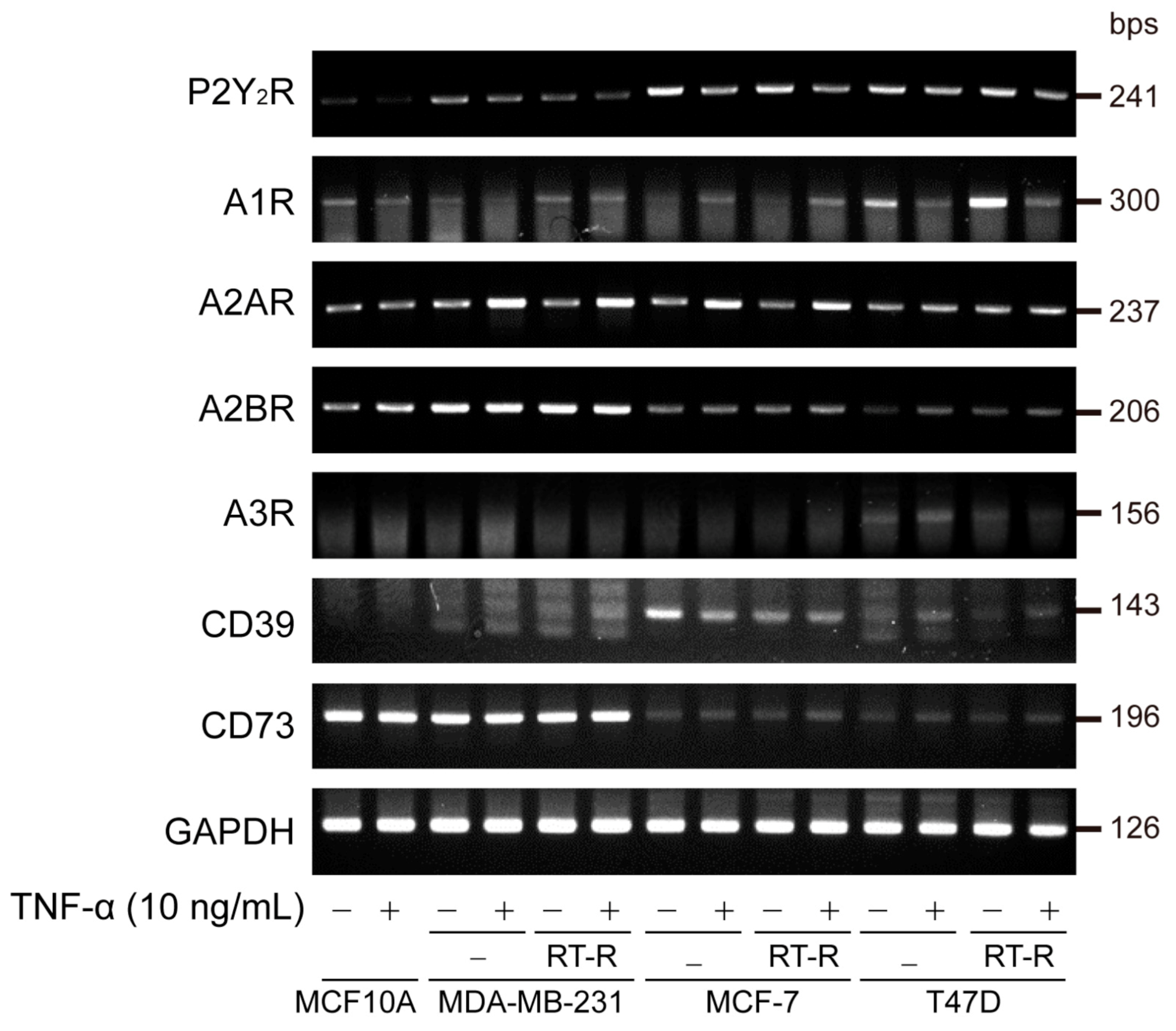
3.1. The Expression of A2AR, A2BR, and CD73 Is Increased in TNBC and Further Increased in RT-R-TNBC Cells
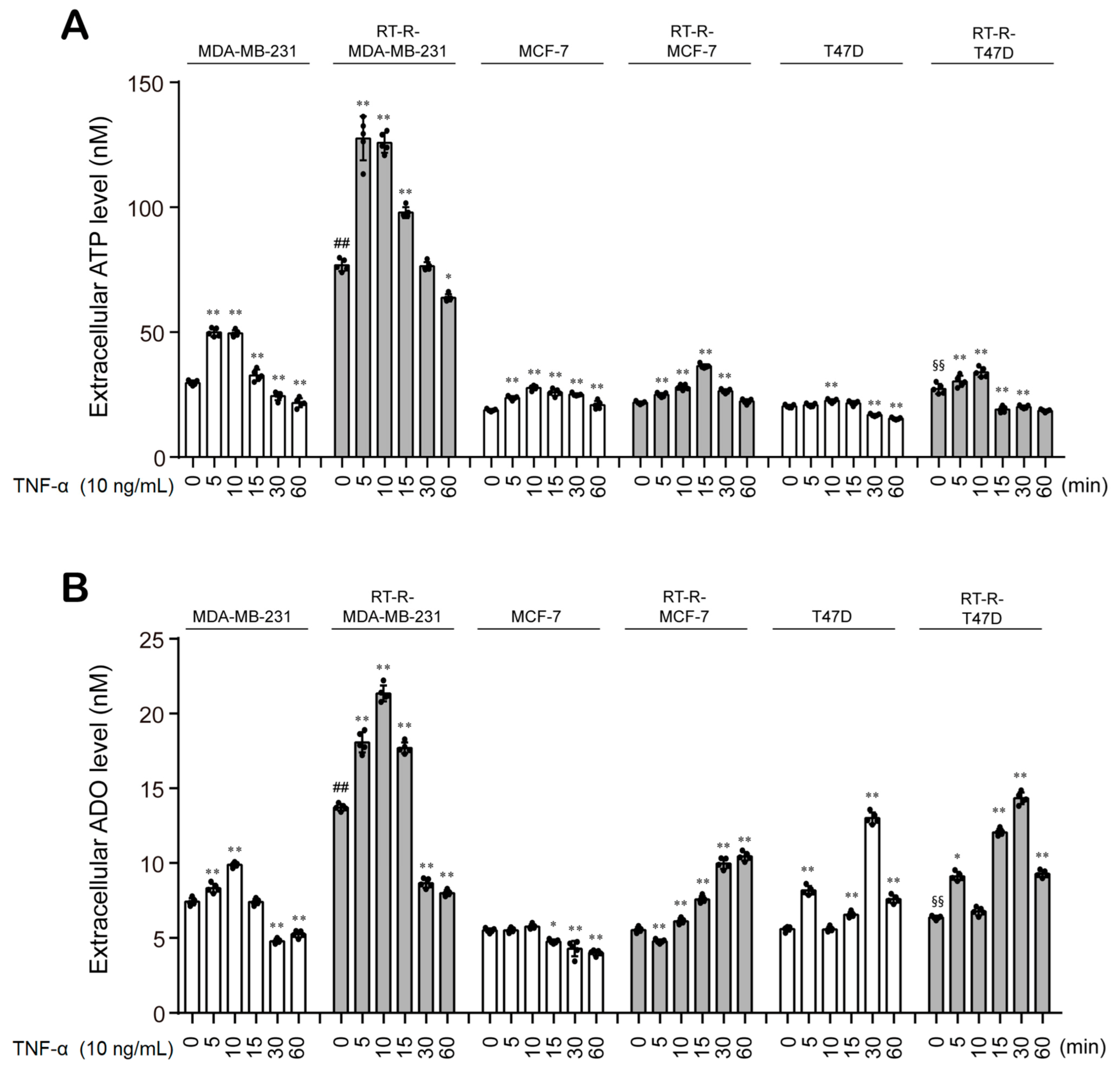
3.2. Control- and TNF-α-Treated MDA-MB-231 Cells Show Higher Extracellular ATP and ADO Levels Than Non-TNBC Cells, and This Effect Is Further Enhanced in RT-R-MDA-MB-231 Cells
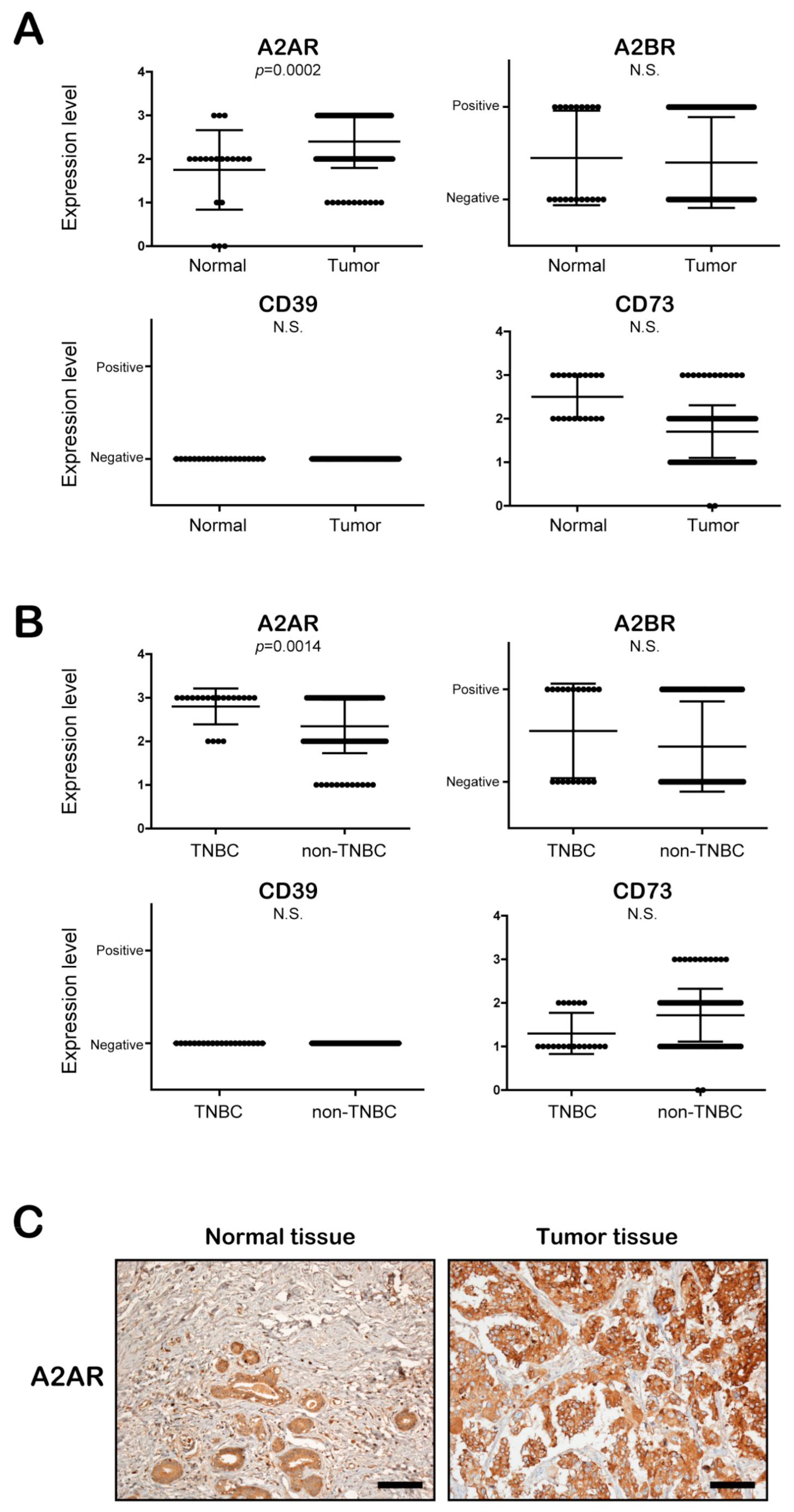
3.3. The Expression of A2AR, but Not A2BR, CD39, and CD73, Is Significantly Increased in BC Patient Tissues, Especially TNBC Patient Tissues, Compared to Normal Epithelial Tissues
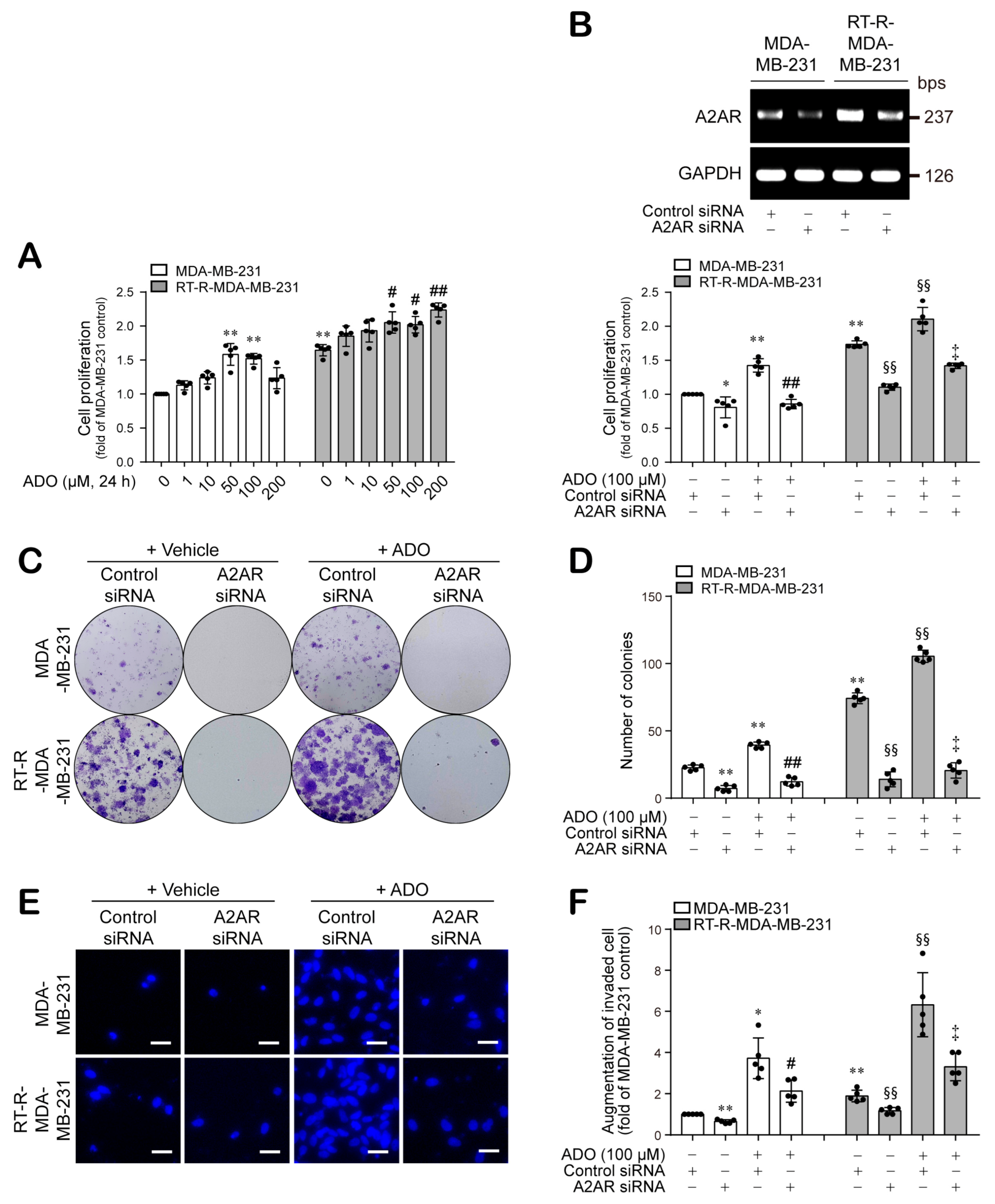
3.4. Extracellular ADO Enhances the Proliferation, Colony Formation, and Invasion of MDA-MB-231 and RT-R-MDA-MB-231 Cells through A2AR Activation
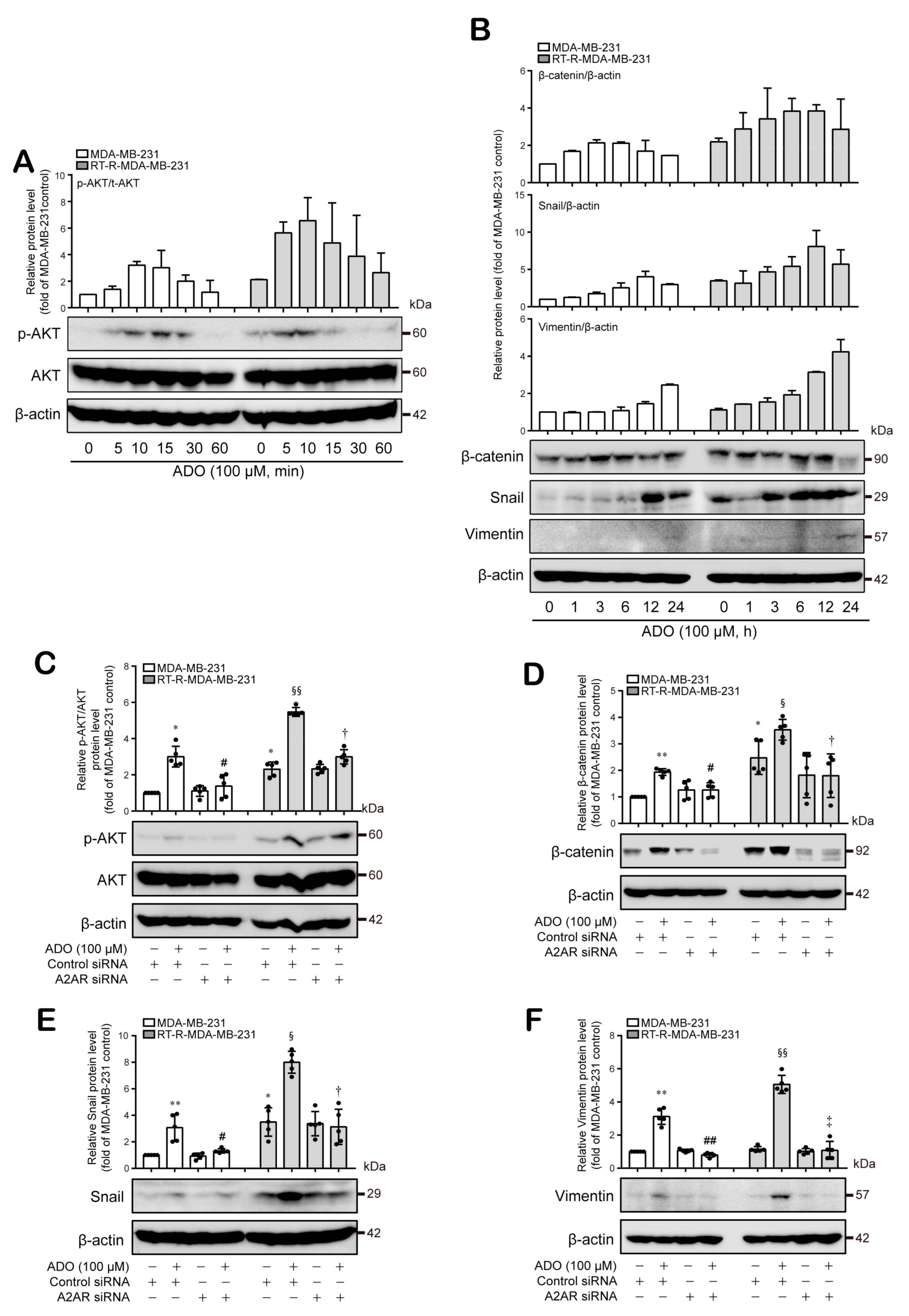
3.5. Extracellular ADO-Mediated Activation of A2AR Stimulates AKT Activation and Induces β-Catenin, Snail, and Vimentin Expression, Which Are Involved in Tumor Invasion and Metastasis
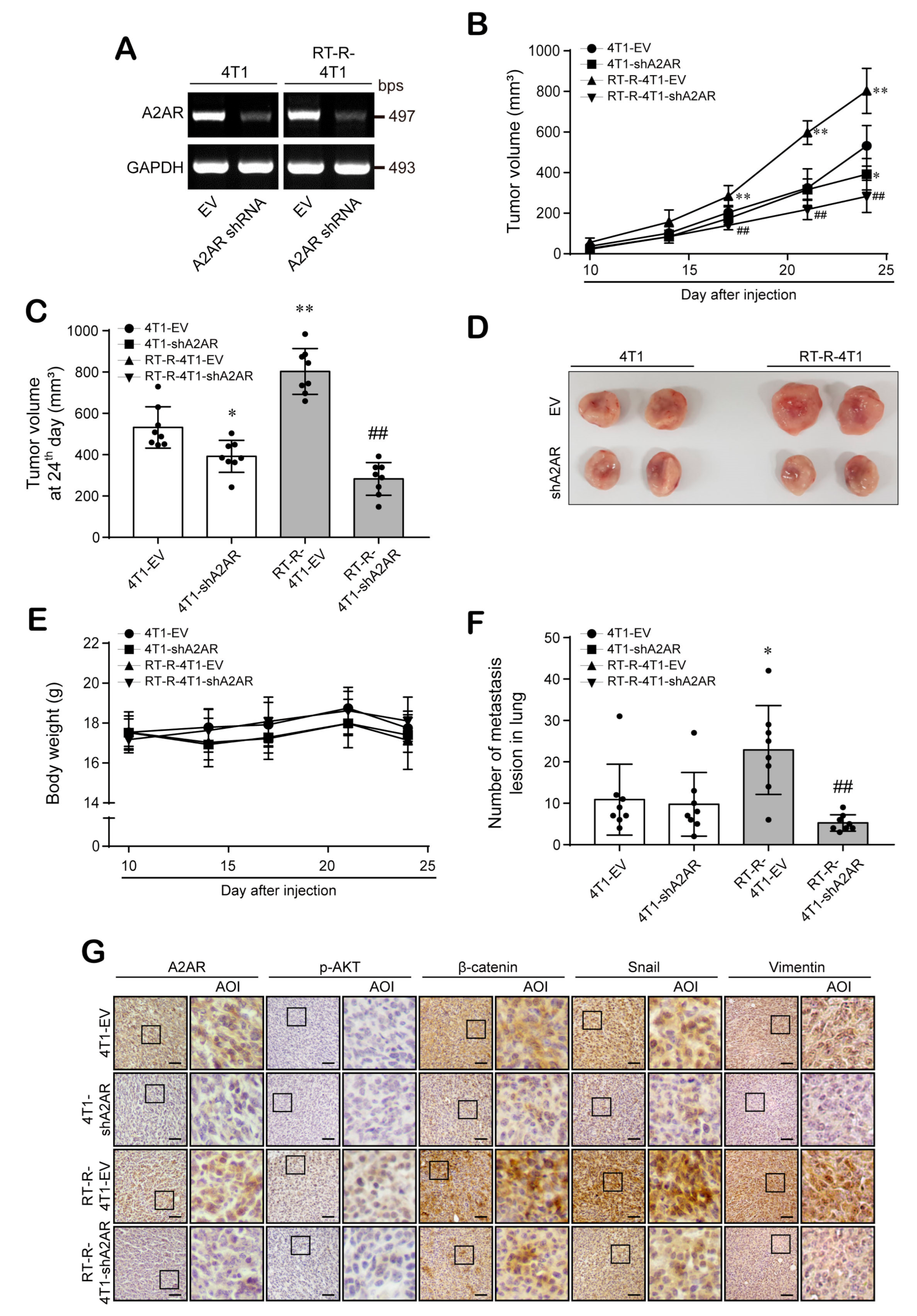
3.6. A2AR Is Involved in the Progression and Metastasis of BC In Vivo
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| A2AR | adenosine receptor type 2A |
| A2BR | adenosine receptor type 2B |
| AC | adenyl cyclase |
| ADO | adenosine |
| AJCC | American Joint Committee on Cancer |
| BC | breast cancer |
| CCK-8 | Cell Counting Kit-8 |
| CTRL | control |
| ECL | enhanced chemiluminescence |
| ECs | endothelial cells |
| EMT | epithelial–mesenchymal transition |
| EV | empty vectors |
| HRP | horseradish peroxidase |
| IHC | immunohistochemistry |
| NK | natural killer |
| OD | optical density |
| P2Y2R | P2Y purinoceptor 2 |
| RT-PCR | reverse transcription polymerase chain reaction |
| RT-R | radiotherapy-resistant |
| SD | standard deviation |
| TMA | tissue microarray |
| TME | tumor microenvironment |
| TNBC | triple negative breast cancer |
| WHO | World Health Organization |
References
- Pollard, J.W. Macrophages define the invasive microenvironment in breast cancer. J. Leukoc. Biol. 2008, 84, 623–630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kepp, O.; Loos, F.; Liu, P.; Kroemer, G. Extracellular nucleosides and nucleotides as immunomodulators. Immunol. Rev. 2017, 280, 83–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Virgilio, F.; Adinolfi, E. Extracellular purines, purinergic receptors and tumor growth. Oncogene 2017, 36, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Eun, S.Y.; Lee, J.S.; Park, S.W.; Lee, J.H.; Chang, K.C.; Kim, H.J. P2Y2 receptor activation by nucleotides released from highly metastatic breast cancer cells increases tumor growth and invasion via crosstalk with endothelial cells. Breast Cancer Res. 2014, 16, R77. [Google Scholar] [CrossRef] [Green Version]
- Eltzschig, H.K. Extracellular adenosine signaling in molecular medicine. J. Mol. Med. 2013, 91, 141–146. [Google Scholar] [CrossRef]
- Zimmermann, H. Extracellular metabolism of ATP and other nucleotides. Naunyn. Schmiedebergs Arch. Pharmacol. 2000, 362, 299–309. [Google Scholar] [CrossRef]
- Kumar, V. Adenosine as an endogenous immunoregulator in cancer pathogenesis: Where to go? Purinergic. Signal 2013, 9, 145–165. [Google Scholar] [CrossRef] [Green Version]
- Cronstein, B.N. Adenosine, an endogenous anti-inflammatory agent. J. Appl. Physiol. 1994, 76, 5–13. [Google Scholar] [CrossRef] [Green Version]
- Tsuchiya, A.; Nishizaki, T. Anticancer effect of adenosine on gastric cancer via diverse signaling pathways. World J. Gastroenterol. 2015, 21, 10931–10935. [Google Scholar] [CrossRef]
- Chen, J.F.; Eltzschig, H.K.; Fredholm, B.B. Adenosine receptors as drug targets what are the challenges? Nat. Rev. Drug Discov. 2013, 12, 265–286. [Google Scholar] [CrossRef] [Green Version]
- Fredholm, B.B.; Ijzerman, A.P.; Jacobson, K.A.; Linden, J.; Müller, C.E. International union of basic and clinical pharmacology. LXXXI. Nomenclature and classification of adenosine receptors an update. Pharmacol. Rev. 2011, 63, 1–34. [Google Scholar] [CrossRef]
- Mølck, C.; Ryall, J.; Failla, L.M.; Coates, J.L.; Pascussi, J.M.; Heath, J.K.; Hollande, F. The A2b adenosine receptor antagonist PSB-603 promotes oxidative phosphorylation and ROS production in colorectal cancer cells via adenosine receptor-independent mechanism. Cancer Lett. 2016, 383, 135–143. [Google Scholar] [CrossRef]
- Lynge, J.; Schulte, G.; Nordsborg, N.; Fredholm, B.B.; Hellsten, Y. Adenosine A 2B receptors modulate cAMP levels and induce CREB but not ERK1/2 and p38 phosphorylation in rat skeletal muscle cells. Biochem. Biophys. Res. Commun. 2003, 307, 180–187. [Google Scholar] [CrossRef]
- Schulte, G.; Fredholm, B.B. Signalling from adenosine receptors to mitogen-activated protein kinases. Cell Signal 2003, 15, 813–827. [Google Scholar] [CrossRef]
- Fredholm, B.B.; Ijzerman, A.P.; Jacobson, K.A.; Klotz, K.N.; Linden, J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol. Rev. 2001, 53, 527–552. [Google Scholar]
- Raskovalova, T.; Lokshin, A.; Huang, X.; Su, Y.; Mandic, M.; Zarour, H.M.; Jackson, E.K.; Gorelik, E. Inhibition of cytokine production and cytotoxic activity of human antimelanoma specific CD8+ and CD4+ T lymphocytes by adenosine-protein kinase A type I signaling. Cancer Res. 2007, 67, 5949–5956. [Google Scholar] [CrossRef] [Green Version]
- Zarek, P.E.; Huang, C.T.; Lutz, E.R.; Kowalski, J.; Horton, M.R.; Linden, J.; Drake, C.G.; Powell, J.D. A2A receptor signaling promotes peripheral tolerance by inducing T-cell anergy and the generation of adaptive regulatory T cells. Blood 2008, 111, 251–259. [Google Scholar] [CrossRef]
- Lappas, C.M.; Rieger, J.M.; Linden, J. A2A adenosine receptor induction inhibits IFN-gamma production in murine CD4+ T cells. J. Immunol. 2005, 174, 1073–1080. [Google Scholar] [CrossRef] [Green Version]
- Etique, N.; Grillier-Vuissoz, I.; Lecomte, J.; Flament, S. Crosstalk between adenosine receptor (A2A isoform) and ER alpha mediates ethanol action in MCF-7 breast cancer cells. Oncol. Rep. 2009, 21, 977–981. [Google Scholar]
- Merighi, S.; Mirandola, P.; Milani, D.; Varani, K.; Gessi, S.; Klotz, K.N.; Leung, E.; Baraldi, P.G.; Borea, P.A. Adenosine receptors as mediators of both cell proliferation and cell death of cultured human melanoma cells. J. Invest. Dermatol. 2002, 119, 923–933. [Google Scholar] [CrossRef] [Green Version]
- Yasuda, Y.; Saito, M.; Yamamura, T.; Yaguchi, T.; Nishizaki, T. Extracellular adenosine induces apoptosis in Caco-2 human colonic cancer cells by activating caspase-9/-3 via A(2a) adenosine receptors. J. Gastroenterol. 2009, 44, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Cekic, C.; Sag, D.; Li, Y.; Theodorescu, D.; Strieter, R.M.; Linden, J. Adenosine A2B receptor blockade slows growth of bladder and breast tumors. J. Immunol. 2012, 118, 198–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desmet, C.J.; Gallenne, T.; Prieur, A.; Reyal, F.; Visser, N.L.; Wittner, B.S.; Smit, M.A.; Geiger, T.R.; Laoukili, J.; Iskit, S.; et al. Identification of a pharmacologically tractable Fra-1/ADORA2B axis promoting breast cancer metastasis. Proc. Natl. Acad. Sci. USA 2013, 110, 5139–5144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ko, Y.S.; Jin, H.; Lee, J.S.; Park, S.W.; Chang, K.C.; Kang, K.M.; Jeong, B.K.; Kim, H.J. Radioresistant breast cancer cells exhibit increased resistance to chemotherapy and enhanced invasive properties due to cancer stem cells. Oncol. Rep. 2018, 40, 3752–3762. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Ko, Y.S.; Kim, H.J. P2Y2R-mediated inflammasome activation is involved in tumor progression in breast cancer cells and in radiotherapy-resistant breast cancer. Int. J. Oncol. 2018, 53, 1953–1966. [Google Scholar] [CrossRef] [Green Version]
- Sarrio, D.; Rodriguez-Pinilla, S.M.; Hardisson, D.; Cano, A.; Moreno-Bueno, G.; Palacios, J. Epithelial-mesenchymal transition in breast cancer relates to the basal-like phenotype. Cancer Res. 2008, 68, 989–997. [Google Scholar] [CrossRef] [Green Version]
- Lamouille, S.; Xu, J.; Derynck, R. Molecular mechanisms of epithelial-mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2014, 15, 178–196. [Google Scholar] [CrossRef] [Green Version]
- Shi, L.; Wu, Z.; Miao, J.; Du, S.; Ai, S.; Xu, E.; Feng, M.; Song, J.; Guan, W. Adenosine interaction with adenosine receptor A2a promotes gastric cancer metastasis by enhancing PI3K-AKT-mTOR signaling. Mol. Biol. Cell 2019, 30, 2527–2534. [Google Scholar] [CrossRef]
- Ham, B.; Fernandez, M.C.; D’Costa, Z.; Brodt, P. The diverse roles of the TNF axis in cancer progression and metastasis. Trends Cancer Res. 2016, 11, 1–27. [Google Scholar]
- Schmiegel, W.; Roeder, C.; Schmielau, J.; Rodeck, U.; Kalthoff, H. Tumor necrosis factor alpha induces the expression of transforming growth factor alpha and the epidermal growth factor receptor in human pancreatic cancer cells. Proc. Natl. Acad. Sci. USA 1993, 90, 863–867. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Boyer, C.M.; Whitaker, R.S.; Berchuck, A.; Wiener, J.R.; Weinberg, J.B.; Bast, R.C., Jr. Tumor necrosis factor alpha as an autocrine and paracrine growth factor for ovarian cancer: Monokine induction of tumor cell proliferation and tumor necrosis factor alpha expression. Cancer Res. 1993, 53, 1939–1944. [Google Scholar]
- Duncombe, A.S.; Heslop, H.E.; Turner, M.; Meager, A.; Priest, R.; Exley, T.; Brenner, M.K. Tumor necrosis factor mediates autocrine growth inhibition in a chronic leukemia. J. Immunol. 1989, 143, 3828–3834. [Google Scholar]
- Elbaz, O.; Mahmoud, L.A. Tumor necrosis factor and human acute leukemia. Leuk Lymphoma 1994, 12, 191–195. [Google Scholar] [CrossRef]
- Ardizzoia, A.; Lissoni, P.; Brivio, F.; Tisi, E.; Perego, M.S.; Grassi, M.G.; Pittalis, S.; Crispino, S.; Barni, S.; Tancini, G. Tumor necrosis factor in solid tumors: Increased blood levels in the metastatic disease. J. Biol. Regul. Homeost. Agents 1992, 6, 103–107. [Google Scholar]
- Joo, Y.N.; Jin, H.; Eun, S.Y.; Park, S.W.; Chang, K.C.; Kim, H.J. P2Y2R activation by nucleotides released from the highly metastatic breast cancer cell MDA-MB-231 contributes to pre-metastatic niche formation by mediating lysyl oxidase secretion, collagen crosslinking, and monocyte recruitment. Oncotarget 2014, 5, 9322–9334. [Google Scholar] [CrossRef] [Green Version]
- Leone, R.D.; Emens, L.A. Targeting adenosine for cancer immunotherapy. J. Immunother. Cancer 2018, 6, 57. [Google Scholar] [CrossRef] [Green Version]
- Sepúlveda, C.; Palomo, I.; Fuentes, E. Role of adenosine A2b receptor overexpression in tumor progression. Life Sci. 2016, 166, 92–99. [Google Scholar] [CrossRef]
- Fernandez-Gallardo, M.; González-Ramírez, R.; Sandoval, A.; Felix, R.; Monjaraz, E. Adenosine Stimulate Proliferation and Migration in Triple Negative Breast Cancer Cells. PLoS ONE 2016, 11, e0167445. [Google Scholar] [CrossRef] [Green Version]
- Roh, M.; Wainwright, D.A.; Wu, J.D.; Wan, Y.; Zhang, B. Targeting CD73 to augment cancer immunotherapy. Curr. Opin. Pharmacol. 2020, 53, 66–76. [Google Scholar] [CrossRef]
- Quezada, C.; Garrido, W.; Oyarzún, C.; Fernández, K.; Segura, R.; Melo, R.; Casanello, P.; Sobrevia, L.; San Martín, R. 5′-ectonucleotidase mediates multiple-drug resistance in glioblastoma multiforme cells. J. Cell Physiol. 2013, 228, 602–608. [Google Scholar] [CrossRef]
- Yang, Q.; Du, J.; Zu, L. Overexpression of CD73 in prostate cancer is associated with lymph node metastasis. Pathol. Oncol. Res. 2013, 19, 811–814. [Google Scholar] [CrossRef] [PubMed]
- Leclerc, B.G.; Charlebois, R.; Chouinard, G.; Allard, B.; Pommey, S.; Saad, F.; Stagg, J. CD73 expression is an independent prognostic factor in prostate cancer. Clin. Cancer Res. 2016, 22, 158–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Häusler, S.F.; Montalbán del Barrio, I.; Strohschein, J.; Chandran, P.A.; Engel, J.B.; Hönig, A.; Ossadnik, M.; Horn, E.; Fischer, B.; Krockenberger, M.; et al. Ectonucleotidases CD39 and CD73 on OvCA cells are potent adenosine-generating enzymes responsible for adenosine receptor 2A-dependent suppression of T cell function and NK cell cytotoxicity. Cancer Immunol. Immunother. 2011, 60, 1405–1418. [Google Scholar] [CrossRef] [PubMed]
- Turcotte, M.; Spring, K.; Pommey, S.; Chouinard, G.; Cousineau, I.; George, J.; Chen, G.M.; Gendoo, D.M.; Haibe-Kains, B.; Karn, T.; et al. CD73 is associated with poor prognosis in high-grade serous ovarian cancer. Cancer Res. 2015, 75, 4494–4503. [Google Scholar] [CrossRef] [Green Version]
- Loi, S.; Pommey, S.; Haibe-Kains, B.; Beavis, P.A.; Darcy, P.K.; Smyth, M.J.; Stagg, J. CD73 promotes anthracycline resistance and poor prognosis in triple negative breast cancer. Proc. Natl. Acad. Sci. USA 2013, 110, 11091–11096. [Google Scholar] [CrossRef] [Green Version]
- Coustan-Smith, E.; Song, G.; Clark, C.; Key, L.; Liu, P.; Mehrpooya, M.; Stow, P.; Su, X.; Shurtleff, S.; Pui, C.H.; et al. New markers for minimal residual disease detection in acute lymphoblastic leukemia. Blood 2011, 117, 6267–6276. [Google Scholar] [CrossRef]
- Serra, S.; Horenstein, A.L.; Vaisitti, T.; Brusa, D.; Rossi, D.; Laurenti, L.; D’Arena, G.; Coscia, M.; Tripodo, C.; Inghirami, G.; et al. CD73-generated extracellular adenosine in chronic lymphocytic leukemia creates local conditions counteracting drug-induced cell death. Blood 2011, 118, 6141–6152. [Google Scholar] [CrossRef]
- Martin, T.A.; Goyal, A.; Watkins, G.; Jiang, W.G. Expression of the transcription factors snail, slug, and twist and their clinical significance in human breast cancer. Ann. Surg. Oncol. 2005, 12, 488–496. [Google Scholar] [CrossRef]
- Come, C.; Magnino, F.; Bibeau, F.; De Santa, B.P.; Becker, K.F.; Theillet, C.; Savagner, P. Snail and slug play distinct roles during breast carcinoma progression. Clin. Cancer Res. 2006, 12, 5395–5402. [Google Scholar] [CrossRef] [Green Version]
- Karihtala, P.; Auvinen, P.; Kauppila, S.; Haapasaari, K.M.; Jukkola-Vuorinen, A.; Soini, Y. Vimentin, zeb1 and Sip1 are up-regulated in triple-negative and basal-like breast cancers: Association with an aggressive tumour phenotype. Breast Cancer Res. Treat. 2013, 138, 81–90. [Google Scholar] [CrossRef]
- Zhou, B.P.; Deng, J.; Xia, W.; Xu, J.; Li, Y.M.; Gunduz, M.; Hung, M.C. Dual regulation of snail by GSK-3beta-mediated phosphorylation in control of epithelial-mesenchymal transition. Nat. Cell Biol. 2004, 6, 931–940. [Google Scholar] [CrossRef]
- Yook, J.I.; Li, X.Y.; Ota, I.; Hu, C.; Kim, H.S.; Kim, N.H.; Cha, S.Y.; Ryu, J.K.; Choi, Y.J.; Kim, J.; et al. A Wnt-Axin2-GSK3beta cascade regulates Snail1 activity in breast cancer cells. Nat. Cell Biol. 2006, 8, 1398–1406. [Google Scholar] [CrossRef]
- Vara, J.; Casado, E.; de Castro, J.; Cejas, P.; Belda-Iniesta, C.; Gonzalez-Baron, M. PI3K/AKT signaling pathway and cancer. Cancer Treat. Rev. 2004, 2, 193–204. [Google Scholar] [CrossRef]
- Manning, B.D.; Cantley, L.C. AKT/PKB signaling: Navigating downstream. Cell 2007, 7, 1261–1274. [Google Scholar] [CrossRef] [Green Version]
- Majumder, P.K.; Febbo, P.G.; Bikoff, R.; Berger, R.; Xue, Q.; McMahon, L.M.; Manola, J.; Brugarolas, J.; McDonnell, T.J.; Golub, T.R.; et al. mTOR inhibition reverses Akt-dependent prostate intraepithelial neoplasia through regulation of apoptotic and HIF-1-dependent pathways. Nat. Med. 2004, 10, 594–601. [Google Scholar] [CrossRef]
- West, K.A.; Linnoila, I.R.; Belinsky, S.A.; Harris, C.C.; Dennis, P.A. Tobacco carcinogen-induced cellular transformation increases activation of the phosphatidylinositol 3′-kinase/AKT pathway in vitro and in vivo. Cancer Res. 2004, 64, 446–451. [Google Scholar] [CrossRef] [Green Version]
- Bose, S.; Chandran, S.; Mirocha, J.M.; Bose, N. The AKT pathway in human breast cancer: A tissue-array-based analysis. Mod. Pathol. 2006, 19, 238–245. [Google Scholar] [CrossRef]
- Dai, D.L.; Martinka, M.; Li, G. Prognostic significance of activated AKT expression in melanoma: A clinicopathologic study of 292 cases. J. Clin. Oncol. 2005, 23, 1473–1482. [Google Scholar] [CrossRef]
- Li, W.; Jia, G.; Qu, Y.; Du, Q.; Liu, B.; Liu, B. Long non-coding RNA (LncRNA) HOXA11-AS promotes breast cancer invasion and metastasis by regulating epithelial–mesenchymal transition. Med. Sci. Monit. 2017, 23, 3393–3403. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Han, Q.; Cui, Y. Decreased long non-coding RNA SPRY4-IT1 contributes to ovarian cancer cell metastasis partly via affecting epithelial–mesenchymal transition. Tumour. Biol. 2017, 39, 1010428317709129. [Google Scholar] [CrossRef] [Green Version]
- Sowa, T.; Menju, T.; Sonobe, M.; Nakanishi, T.; Shikuma, K.; Imamura, N.; Motoyama, H.; Hijiya, K.; Aoyama, A.; Chen, F.; et al. Association between epithelial–mesenchymal transition and cancer stemness and their effect on the prognosis of lung adenocarcinoma. Cancer Med. 2015, 4, 1853–1862. [Google Scholar] [CrossRef]
- Zheng, X.; Carstens, J.L.; Kim, J.; Scheible, M.; Kaye, J.; Sugimoto, H.; Wu, C.C.; LeBleu, V.S.; Kalluri, R. Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature 2015, 527, 525–530. [Google Scholar] [CrossRef] [Green Version]
- Mir, N.; Jayachandran, A.; Dhungel, B.; Shrestha, R.; Steel, J.C. Epithelial-to-mesenchymal transition: A mediator of sorafenib resistance in advanced hepatocellular carcinoma. Curr. Cancer Drug Targets 2017, 17, 698–706. [Google Scholar] [CrossRef]
- Marie-Egyptienne, D.T.; Lohse, I.; Hill, R.P. Cancer stem cells, the epithelial to mesenchymal transition (EMT) and radioresistance: Potential role of hypoxia. Cancer Lett. 2013, 341, 63–72. [Google Scholar] [CrossRef]
- Shintani, Y.; Okimura, A.; Sato, K.; Nakagiri, T.; Kadota, Y.; Inoue, M.; Sawabata, N.; Minami, M.; Ikeda, N.; Kawahara, K.; et al. Epithelial to mesenchymal transition is a determinant of sensitivity to chemoradiotherapy in non-small cell lung cancer. Ann. Thorac. Surg. 2011, 92, 1794–1804. [Google Scholar] [CrossRef]
- Stark, T.W.; Hensley, P.J.; Spear, A.; Pu, H.; Strup, S.S.; Kyprianou, N. Predictive value of epithelial–mesenchymal-transition (EMT) signature and PARP-1 in prostate cancer radioresistance. Prostate 2017, 77, 1583–1591. [Google Scholar] [CrossRef]
- Chang, X.; Xue, X.; Zhang, Y.; Zhang, G.; Zhou, H.; Yang, Y.; Ran, Y.; Xiao, Z.; Ge, X.; Liu, H. The role of NRAGE subcellular location and epithelial–mesenchymal transition on radiation resistance of esophageal carcinoma cell. J. Cancer Res. Ther. 2018, 14, 46–51. [Google Scholar]
| Characteristics | A2AR | p | A2BR | p | ||
|---|---|---|---|---|---|---|
| Low (n = 97) | High (n = 83) | Negative (n = 108) | Positive (n = 72) | |||
| Age (years) | 51 (30–81) | 51 (25–82) | 0.637 | 51 (30–81) | 51 (25–82) | 0.699 |
| Sex | 0.461 | 0.400 | ||||
| Female | 97 (100.0) | 82 (99.8) | 108 (100.0) | 71 (98.6) | ||
| Menopausal status | 0.651 | >0.990 | ||||
| Pre | 44 (45.4) | 34 (41.0) | 47 (43.5) | 31 (43.1) | ||
| Post | 53 (54.6) | 48 (57.8) | 61 (56.6) | 40 (55.6) | ||
| Histology | 0.055 | 0.785 | ||||
| Ductal | 93 (95.9) | 73 (88.0) | 99 (91.7) | 67 (93.1) | ||
| Others | 4 (4.1) | 10 (12.0) | 9 (8.3) | 5 (6.9) | ||
| ER status | <0.001 | 0.093 | ||||
| Negative | 14 (14.4) | 36 (43.4) | 25 (23.1) | 25 (34.7) | ||
| Positive | 83 (85.6) | 47 (56.6) | 83 (76.9) | 47 (65.3) | ||
| PR status | 0.133 | 0.234 | ||||
| Negative | 22 (22.7) | 28 (33.7) | 34 (31.5) | 16 (22.2) | ||
| Positive | 75(77.3) | 55 (66.3) | 74 (68.5) | 56 (77.8) | ||
| HER-2 status | 0.685 | 0.543 | ||||
| Negative | 80 (82.5) | 71 (85.5) | 89 (82.4) | 62 (86.1) | ||
| Positive | 17 (17.5) | 12 (14.5) | 19 (17.6) | 10 (13.9) | ||
| TNBC | 0.002 | 0.155 | ||||
| Yes | 4 (4.1) | 16 (19.3) | 9 (8.3) | 11 (15.3) | ||
| No | 93 (95.9) | 67 (80.7) | 99 (91.7) | 61 (84.7) | ||
| AJCC 8th stage | 0.135 | 0.916 | ||||
| I | 33 (34.0) | 37 (44.6) | 42 (38.9) | 28 (38.9) | ||
| II | 47 (48.5) | 28 (33.7) | 46 (42.6) | 29 (40.3) | ||
| III | 17 (17.5) | 18 (21.7) | 20 (18.5) | 15 (20.8) | ||
| Surgery | 0.532 | 0.341 | ||||
| MRM | 32 (33.0) | 32 (38.6) | 35 (32.4) | 29 (40.3) | ||
| BCS | 65 (67.0) | 51 (61.4) | 73 (67.6) | 43 (59.7) | ||
| Adjuvant chemotherapy | 0.806 | 0.801 | ||||
| Yes | 88 (90.7) | 74 (89.2) | 98 (90.7) | 64 (88.9) | ||
| No | 9 (9.3) | 9 (10.8) | 10 (9.3) | 8 (11.1) | ||
| Adjuvant radiotherapy | 0.608 | 0.119 | ||||
| Yes | 74 (76.3) | 60 (72.3) | 85 (78.7) | 49 (68.1) | ||
| No | 23 (23.7) | 23 (27.7) | 23 (21.3) | 23 (31.9) | ||
| Adjuvant hormone therapy | 0.018 | 0.521 | ||||
| Yes | 89 (91.8) | 65 (78.3) | 94 (87.0) | 60 (83.3) | ||
| No | 8 (8.2) | 18 (21.7) | 14 (13.0) | 12 (16.7) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, H.; Lee, J.-S.; Kim, D.-C.; Ko, Y.-S.; Lee, G.-W.; Kim, H.-J. Increased Extracellular Adenosine in Radiotherapy-Resistant Breast Cancer Cells Enhances Tumor Progression through A2AR-Akt-β-Catenin Signaling. Cancers 2021, 13, 2105. https://doi.org/10.3390/cancers13092105
Jin H, Lee J-S, Kim D-C, Ko Y-S, Lee G-W, Kim H-J. Increased Extracellular Adenosine in Radiotherapy-Resistant Breast Cancer Cells Enhances Tumor Progression through A2AR-Akt-β-Catenin Signaling. Cancers. 2021; 13(9):2105. https://doi.org/10.3390/cancers13092105
Chicago/Turabian StyleJin, Hana, Jong-Sil Lee, Dong-Chul Kim, Young-Shin Ko, Gyeong-Won Lee, and Hye-Jung Kim. 2021. "Increased Extracellular Adenosine in Radiotherapy-Resistant Breast Cancer Cells Enhances Tumor Progression through A2AR-Akt-β-Catenin Signaling" Cancers 13, no. 9: 2105. https://doi.org/10.3390/cancers13092105
APA StyleJin, H., Lee, J.-S., Kim, D.-C., Ko, Y.-S., Lee, G.-W., & Kim, H.-J. (2021). Increased Extracellular Adenosine in Radiotherapy-Resistant Breast Cancer Cells Enhances Tumor Progression through A2AR-Akt-β-Catenin Signaling. Cancers, 13(9), 2105. https://doi.org/10.3390/cancers13092105