Esophageal Cancer in Elderly Patients, Current Treatment Options and Outcomes; A Systematic Review and Pooled Analysis
Simple Summary
Abstract
1. Introduction
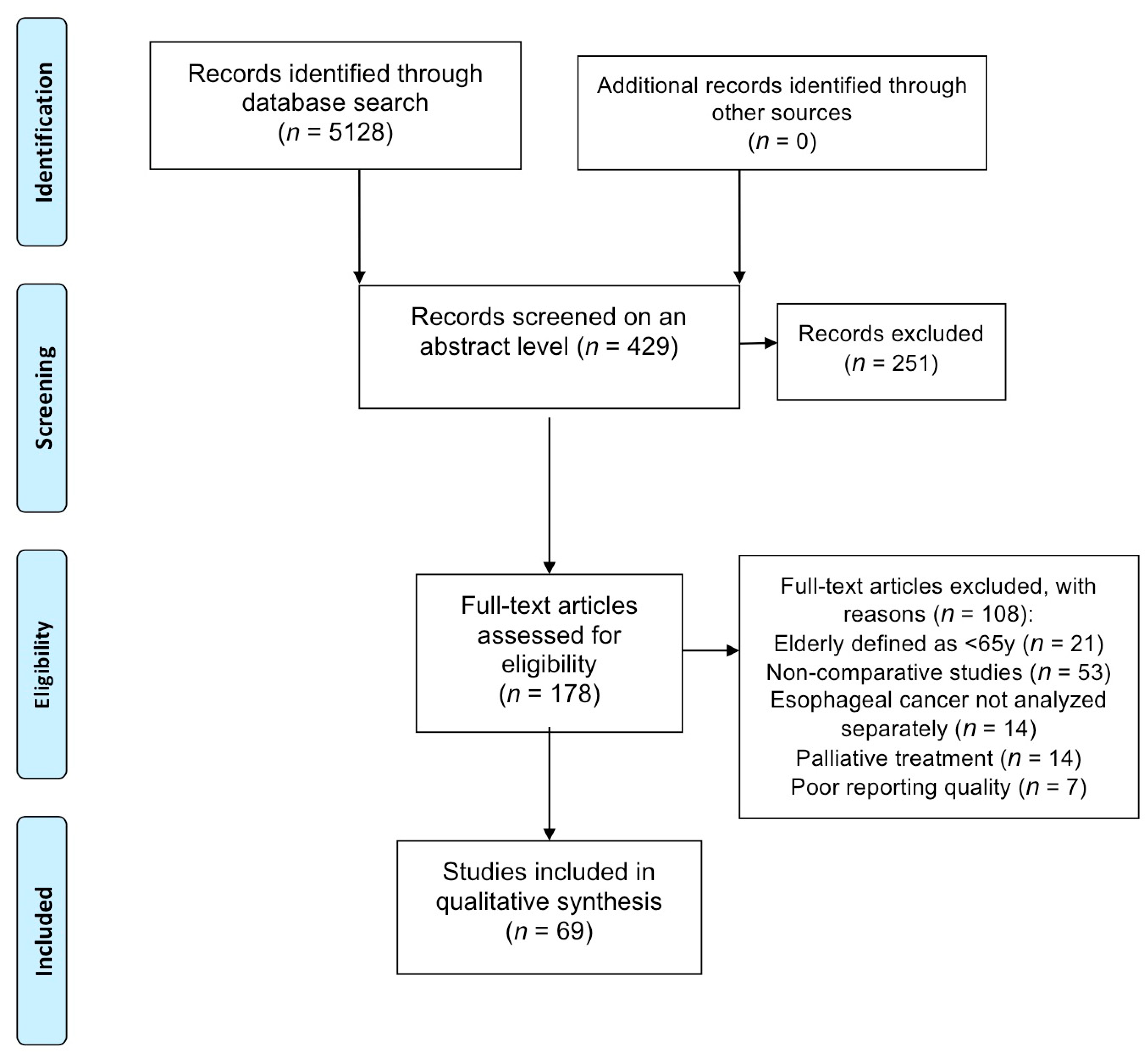
2. Methods
2.1. Search Strategy and Selection Criteria
2.2. Data Management, Risk of Bias Assessment
3. Results
3.1. Access to Curative Treatment for Elderly Patients (EP)
3.2. Comparison of Different Treatment Strategies Offered to EP
3.2.1. Surgery Alone Versus Neoadjuvant Treatment and Surgery (NATS) in EP
3.2.2. Surgery Versus Definitive Chemoradiotherapy (DCRT) in EP
3.3. Surgery for Esophageal Cancer in EP
3.3.1. Anastomotic Leakage
3.3.2. Pulmonary Complications
3.3.3. Cardiovascular Complications
3.3.4. Postoperative Mortality
| Author, Year [Reference] | EP Definition(Years) | No of EP Patients (% to All) | Anastomotic Leakage (%) EP/YP | Pulmonary Complications (%) EP/YP | Cardiovascular Complications (%) EP/YP | Postoperative Mortality (%) EP/YP | |
|---|---|---|---|---|---|---|---|
| Aoyama, 2020 [43] | 75 | 24 (24.5) | 37.5/33.7 | 33.3/27.5 | 12.6/6.1 | 4.2/0 * | 30-day |
| Song, 2020 [58] | 79 | 35 (3.5) | - | - | - | 5.8/5.7 * | 90-day |
| Chen, 2019 [44] | 70 | 142 (25.8) | 17.6/10 | 4.2/7.6 | - | 2.1/2.9 | In-hospital |
| Chen, 2019 [53] | 80 | 34 (21.7) | - | 29/27 | 3/2 | 5/2 * | 90-day |
| Kanda, 2019 [45] | 75 | 59 (15.8) | 4/3 | 20/16 | 12.5/7.5 | - | - |
| Baranov, 2019 [46] | 75 | 89 (24.9) | 19.1/17.1 | 62.9/62.2 | 24.7/14 * | 9/5 | 90-day |
| Klevebro, 2018 [47] | 75 | 62 (16.9) | 8.3/10.7 | 20/19.7 | 6.7/5.7 | 18.6/7.6 * | 90-day |
| Schlottmann, 2018 [48] | 70 | 1544 (29.5) | - | 25/20 * | 9/4 * | 8.9/4.3 * | In-hospital |
| Paulus, 2017 [36] | 80 | 33 (50) | 18/12 | 18/6 | 24/21 | 18/9 | 90-day |
| Miyata, 2015 [49] | 70 | 213 (30) | - | 17/14 | - | 0/0.4 | In-hospital |
| Liu, 2015 [50] | 70 | 39 (21) | - | 30.8/6.8 * (♯) | 7.7/3.4 | - | |
| Stahl, 2014 [56] | 80 | 289 (-) | - | - | - | 8/2.1 * | - |
| Morita, 2013 [51] | 80 | 104 (10) | - | 13/17.7 | - | 13/4.8 | In-hospital |
| Mirza, 2013 [59] | 70 | 46 (22) | - | - | - | 11/9 | 30-day |
| Schweigert, 2013 [37] | 75 | 45 (15) | 13/13 | 53/30 * | - | 24/9 * | In-hospital |
| Tapias, 2013 [38] | 80 | 16 (3.4) | 0/4.8 | 31.3/10.8 * | 37.5/14.4 * | - | - |
| Markar, 2013 [52] | 80 | 32 (6.4) | - | 18.8/8.3 * | 31.3/16.7 * | 0/0.6 | 90-day |
| Cijs, 2010 [29] | 70 | 564 (29) | - | - | - | 11/5.4 * | In-hospital |
| Elsayed, 2010 [39] | 70 | 108 (33.1) | 5/9 | 32.5/37 | - | 12/5 * | In-hospital |
| Yang, 2010 [40] | 70 | 136 (50) | 6.6/4.4 | 17.6/8.1 * | 24.2/14 * | 5.9/0.7 * | - |
| Pultrum, 2010 [41] | 70 | 64 (27.4) | 13/18 | 56/42 | 36/16 * | - | - |
| Davies, 2010 [14] | 70 | 59 (21) | 5/6 | - | 3.4/1.4 | 7/2.4 | - |
| Alibakshi, 2009 [42] | 70 | 165 (34.4) | 7.2/7.9 | 10.3/9.8 | 6/4.4 | 3/2.8 | In-hospital |
| Takagawa, 2008 [25] | 75 | 19 (8.6) | - | - | - | 15.8/3 * | In-hospital |
| Morita, 2008 [57] | 80 | 16 (2.4) | - | - | - | 6/2 * | In-hospital |
| Ruol, 2007 [30] | 70 | 159 (21.5) | 7.5/10.2 | 17/15.3 | - | 1.9/2.7 | In-hospital |
| Finlayson, 2007 [54] | 80 | 3150 (11.3) | - | - | - | 19.9/8.8 * | In-hospital |
| Moskovitz, 2006 [32] | 80 | 31 (3.6) | 6.5/8.8 | 9.7/4.7 | 0/0.8 | 19.4/7.3 * | In-hospital |
| Ma, 2006 [33] | 70 | 60 (3.3) | 3.3/2 | 43.4/28.1 * | 38.3/19.8 * | 3.3/1.1 | In-hospital |
| Di Martino, 2005 [55] | 70 | 51 (46.8) | - | - | - | 12.1/4.1 * | In-hospital |
| Rahamim, 2003 [34] | 70 | 199 (33.4) | 4.5/6.1 | 10.1/5.1 * | 15.6/7 * | 12.1/5 * | 30-day |
| Sabel, 2002 [18] | 70 | 147 (36) | - | - | 23/13 (♯♯)8/0 *(†) | 4/2 | In-hospital |
| Fang, 2001 [35] | 70 | 79 (17.9) | 26.6/35.1 | - | - | 7.6/3.3 | In-hospital |
| Kinugasa, 2001 [31] | 70 | 55 (26.9) | 18.2/12.8 | 45.5/19.5 * | 20/8.1 | 10.9/5.4 | 60-day |
| Johansson, 2000 [60] | 70 | 48 (40) | - | - | - | 0/2.8 | In-hospital |
3.3.5. Surgical Approach, Quality of Surgery
3.3.6. Long-Term Survival after Surgery
| Author, Year [Reference] | EP Definition (Years) | Disease-Related Survival EP/YP | Overall Survival EP/YP | ||
|---|---|---|---|---|---|
| Aoyama, 2020 [43] | 75 | 15.1/20.7 * | Median DFS, mo | 16.4/29.8 * | Median (mo) |
| Song, 2020 [58] | 79 | - | - | 18/62 * | Median (mo) |
| Chen, 2019 [44] | 80 | 38.5/40.4 | Median (mo) | ||
| Bakhos, 2019 [65] | 80 | - | - | 23/29.3 * | Median (mo) |
| Kanda, 2019 [45] | 75 | 12/33 | 5-year DFS (%) | 12/35 | 5-year (%) |
| Baranov, 2019 [46] | 75 | - | - | 57.3/54.5 | 2-years (%) |
| Paulus, 2017 [36] | 80 | - | - | 40/62 * | 5-year (%) |
| Miyata, 2015 [49] | 70 | 42/58* | 5-year DFS (%) | 29.3/52.4 * | 5-year (%) |
| Liu, 2015 [50] | 70 | - | 15.8/13.7 | Median (mo) | |
| Morita, 2013 [51] | 80 | 38/40 | 5-year DFS (%) | 10/35 | 5-year (%) |
| Mirza, 2013 [59] | 70 | - | 10.8/15.6 | Median (mo) | |
| Tapias, 2013 [38] | 80 | 49.2/72.4 * | 5-year DSS (%) | 49.2/64.8 * | 5-year (%) |
| Markar, 2013 [52] | 80 | - | 53.2/77.6 | Median (mo) | |
| Cijs, 2010 [29] | 70 | 27/34 * | 5-year DSS (%) | 22/29 * | 5-year (%) |
| Zehetner, 2010 [64] | 80 | 48.9/57.3 | Median, DSS (mo) | 19.7/42.5 * | Median (mo) |
| Elsayed, 2010 [39] | 70 | - | 20/27.6 | Median (mo) | |
| Yang, 2010 [40] | 70 | 24/35.5 | 5-year DFS (%) | 30/41.8 * | 5-year (%) |
| Pultrum, 2010 [41] | 70 | - | 33/33 | 5-year (%) | |
| Davies, 2010 [14] | 70 | - | 20/28 | Median (mo) | |
| Takagawa, 2008 [25] | 75 | 40/46 | Median, DFS (mo) | 22/38 * | Median (mo) |
| Morita, 2008 [57] | 80 | 22/46 | Median, DFS (mo) | 9/39 * | 5-year (%) |
| Ruol, 2007 [30] | 70 | - | 35.4/33.6 | 5-year (%) | |
| Finlayson, 2007 [54] | 80 | - | 17.6/31.4 * | 5-year (%) | |
| Moskovitz, 2006 [32] | 80 | - | 16.8/48 * | Median (mo) | |
| Di Martino, 2005 [55] | 70 | - | 17.8/35.1 | 5-year (%) | |
| Rahamim, 2003 [34] | 70 | - | 13/21 * | 5-year (%) | |
| Sabel, 2002 [18] | 70 | - | 24/27 | Median (mo) | |
| Fang, 2001 [35] | 70 | 55.4/59.1 | 5-year (%), DSS | 40.9/48.1 | 5-year (%) |
| Kinugasa, 2001 [31] | 70 | - | 32.9/35.3 | 5-year (%) | |
| Johansson, 2000 [60] | 70 | - | 24/24 | Median (mo) | |
3.4. Neoadjuvant Treatment Followed by Surgery (NATS)
3.4.1. Postoperative Complications and Mortality Following NATS
3.4.2. Histologic Response to Neoadjuvant Treatment
3.4.3. Long-Term Survival after NATS
| Author, Year [Reference] | EP Definition(years) | No of Patients | NAT Details | pCR | Anastomotic LeakAge (%) | Pulmonary Complications (%) | Cardiovascular Complications (%) | Postop Mortality (%) | Disease-Free Survival | Overal Survival |
|---|---|---|---|---|---|---|---|---|---|---|
| EP (%) | EP/YP | EP/YP | EP/YP | EP/YP | EP/YP | EP/YP | EP/YP | |||
| Vöncken, 2018 [27] | 70 | 76 (30) | 5FU/Cisplatin +50 Gy or Carbo/Taxol + 41 Gy | 50/25 * | - | - | - | - | 22/29 median (mo) | 26/38 Median (mo) |
| Blom, 2013 [63] | 75 y | 17 (8) | Carbo/Taxol | - | 12/11 | 18/25 | 41/14 * | 0/2 | - | 31/59 3-year (%) |
| Camerlo, 2012 [21] | 70 y | 52 (44) | 5FU/Cisplatin + 45 Gy | 10/21 | 10/4 | 35/27 | 10/7 | 10/7 | 22/29 Median (mo) | 23/44 Median (mo) |
| Fogh, 2011 [70] | 70 y | 57 (22) | 5FU/Cisplatin or 5FU/Taxol + 45–61 Gy | - | 14/12 | 18/17 | 25/15 | 7/5 | - | - |
| Braiteh, 2009 [68] | 70 y | 341 (52) | - | - | 11/6 * | 32/27 * | 21/12 * | - | - | 36/42 * Median (mo) |
| Vallböhmer, 2008 [67] | 70 y | 52 (23) | 5FU/Cisplatin + 36 Gy | 33/19 | - | - | - | - | - | 27/61 * 5-year (%) |
| Ruol, 2007 [69] | 70 y | 31 (4) | 5FU/Platin+ 45–50 Gy | 17/22 | 7/9 | 23/15 | 23/5 * | 7/2 | 24/23 Median (mo) | |
| Rice, 2005 [22] | 70 y | 35 (11) | 5FU-cisplatin + 45–50 Gy | - | 20/9 | 20/24 | 34/15 * | 3/4 | - | 34/42 Median (mo) |
3.5. Definitive Chemoradiation (DCRT)
3.5.1. Treatment Toxicity
3.5.2. Clinical Response to Treatment
3.5.3. Comparison of DCRT Modalities in EP
3.5.4. Long-Term Survival
| Author, Year [Reference] | EP Definition (Years) | No of EP (%)% of SCC | DRCT Details | cCR% | Hematologic Toxicity, % (>Grade II) | Pulmonary Toxicity, % (>Grade II) | Esophagitis% (>Grade II) | Progression-Free Survival | Survival Overall |
|---|---|---|---|---|---|---|---|---|---|
| EP/YP | EP/YP | EP/YP | EP/YP | EP/YP | EP/YP | ||||
| Jingu, 2020 [77] | 80 | 358 (15.3) 96.1 | - | - | - | - | - | - | 13/52 * 5-year, (%) |
| Vöncken, 2017 [27] | 70 | 76 (30) 33 | Carbo-Taxol + 50 Gy | 48/31 | - | - | - | 20.5/7.4 * | 23.6/13.1 * |
| Münch, 2017 [19] | 75 | 32 (45) 81 | 5FU/Cisplatin + 7–60 Gy | - | 13/29 | - | - | 10/9 median, (mo) | 16/20 median, (mo) |
| Xu, 2017 [72] | 80 | 56 (20) 29 | 5FU/Taxane + 45–50.4 Gy | 78/56 * | 4/12 | 11/0 * | 16/14 | 58/56 5-year (%) | 28/23 median, (mo) |
| Davies, 2010 [14] | 70 | 106 (45) 51 | 5FU/Cisplatin + 50 Gy | 22/19 | - | 7/7 | - | 22/21 median, (mo) | |
| Takagawa, 2008 [25] | 75 | 19 (9) 84 | 5FU + 60 Gy | 66/67 | - | - | - | - | 15/10 median, (mo) |
| Takeuchi, 2007 [73] | 70 | 33 (19) 100 | 5FU/Cisplatin + 60 Gy | 64/63 | 70/50 * | - | 3/9 | - | 15/35 * median, (mo) |
| Tanisada, 2000 [74] | 75 | 123 (22) NA | - | - | - | - | - | - | 9/11 5-year, (%) |
3.6. Endoscopic Treatment of Early Stage Cancer
3.7. Quality of Life
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Lagergren, J.; Smyth, E.; Cunningham, D.; Lagergren, P. Oesophageal cancer. Lancet 2017, 390, 2383–2396. [Google Scholar] [CrossRef]
- Global Burden of Disease Cancer Collaboration; Fitzmaurice, C.; Allen, C.; Barber, R.M.; Barregard, L.; Bhutta, Z.A.; Brenner, H.; Dicker, D.J.; Chimed-Orchir, O.; Dandona, R.; et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2017, 3, 524–548. [Google Scholar] [CrossRef]
- De Angelis, R.; Sant, M.; Coleman, M.P.; Francisci, S.; Baili, P.; Pierannunzio, D.; Trama, A.; Visser, O.; Brenner, H.; Ardanaz, E.; et al. Cancer survival in Europe 1999-2007 by country and age: Results of EUROCARE--5-a population-based study. Lancet Oncol. 2014, 15, 23–34. [Google Scholar] [CrossRef]
- Van Blankenstein, M.; Looman, C.W.; Siersema, P.D.; Kuipers, E.J.; Coebergh, J.W. Trends in the incidence of adenocarcinoma of the oesophagus and cardia in the Netherlands 1989–2003. Br. J. Cancer 2007, 96, 1767–1771. [Google Scholar] [CrossRef] [PubMed]
- Rustgi, A.K.; El-Serag, H.B. Esophageal carcinoma. N. Engl. J. Med. 2014, 371, 2499–2509. [Google Scholar] [CrossRef]
- Markar, S.R.; Karthikesalingam, A.; Thrumurthy, S.; Ho, A.; Muallem, G.; Low, D.E. Systematic review and pooled analysis assessing the association between elderly age and outcome following surgical resection of esophageal malignancy. Dis. Esophagus 2013, 26, 250–262. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Liu, S.; Guo, W.; Zhang, Y.; Li, H. Clinical outcomes of oesophagectomy in elderly versus relatively younger patients: A meta-analysis. Interact. Cardiovasc. Thorac. Surg. 2019, 29, 897–905. [Google Scholar] [CrossRef] [PubMed]
- Bouras, G.; Markar, S.R.; Burns, E.M.; Huddy, J.R.; Bottle, A.; Athanasiou, T.; Darzi, A.; Hanna, G.B. The psychological impact of symptoms related to esophagogastric cancer resection presenting in primary care: A national linked database study. Eur. J. Surg. Oncol. 2017, 43, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef]
- Cochrane Effective Practice and Organisation of Care (EPOC). EPOC Resources for Review Authors; Norwegian Knowledge Centre for the Health Services: Oslo, Norway, 2017; Available online: https://epoc.cochrane.org/resources/epoc-resources-review-authors (accessed on 26 April 2021).
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses; Ottawa Hospital Research Institute: Ottawa, ON, Canada, 2019; Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 26 April 2021).
- Vlacich, G.; Samson, P.P.; Perkins, S.M.; Roach, M.C.; Parikh, P.J.; Bradley, J.D.; Lockhart, A.C.; Puri, V.; Meyers, B.F.; Kozower, B.; et al. Treatment utilization and outcomes in elderly patients with locally advanced esophageal carcinoma: A review of the National Cancer Database. Cancer Med. 2017, 6, 2886–2896. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Biagi, J.; Banashkevich, A.; Mercer, C.D.; Tremblay, L.; Mahmud, A. Management and outcomes of localized esophageal and gastroesophageal junction cancer in older patients. Curr Oncol. 2015, 22, e435–e442. [Google Scholar] [CrossRef][Green Version]
- Davies, L.; Lewis, W.G.; Arnold, D.T.; Escofet, X.; Blackshaw, G.; Gwynne, S.; Evans, M.; Roberts, S.A.; Appadurai, I.; Crosby, T.D. Prognostic significance of age in the radical treatment of oesophageal cancer with surgery or chemoradiotherapy: A prospective observational cohort study. Clin. Oncol. 2010, 22, 578–585. [Google Scholar] [CrossRef]
- Faiz, Z.; Lemmens, V.E.; Siersema, P.D.; Nieuwenhuijzen, G.A.; Wouters, M.W.; Rozema, T.; Coebergh, J.W.; Wijnhoven, B.P. Increased resection rates and survival among patients aged 75 years and older with esophageal cancer: A Dutch nationwide population-based study. World J. Surg. 2012, 36, 2872–2878. [Google Scholar] [CrossRef]
- Tougeron, D.; Hamidou, H.; Scotte, M.; Di Fiore, F.; Antonietti, M.; Paillot, B.; Michel, P. Esophageal cancer in the elderly: An analysis of the factors associated with treatment decisions and outcomes. BMC Cancer 2010, 10, 510. [Google Scholar] [CrossRef] [PubMed]
- Ruol, A.; Portale, G.; Castoro, C.; Merigliano, S.; Cavallin, F.; Battaglia, G.; Michieletto, S.; Ancona, E. Management of esophageal cancer in patients aged over 80 years. Eur. J. Cardiothorac. Surg. 2007, 32, 445–448. [Google Scholar] [CrossRef]
- Sabel, M.S.; Smith, J.L.; Nava, H.R.; Mollen, K.; Douglass, H.O.; Gibbs, J.F. Esophageal resection for carcinoma in patients older than 70 years. Ann. Surg. Oncol. 2002, 9, 210–214. [Google Scholar] [CrossRef] [PubMed]
- Munch, S.; Heinrich, C.; Habermehl, D.; Oechsner, M.; Combs, S.E.; Duma, M.N. Primary radio(chemo)therapy for esophageal cancer in elderly patients: Are efficiency and toxicity comparable with younger patients? Eur. J. Med. Res. 2017, 22, 24. [Google Scholar] [CrossRef] [PubMed]
- Guttmann, D.M.; Mitra, N.; Metz, J.M.; Plastaras, J.; Feng, W.; Swisher-McClure, S. Neoadjuvant chemoradiation is associated with improved overall survival in older patients with esophageal cancer. J. Geriatr. Oncol. 2018, 9, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Camerlo, A.; D’Journo, X.B.; Ouattara, M.; Trousse, D.; Doddoli, C.; Thomas, P.A. Adenocarcinoma of the esophagus and esophagogastric junction in patients older than 70 years: Results of neoadjuvant radiochemotherapy followed by transthoracic esophagectomy. J. Visc. Surg. 2012, 149, e203–e210. [Google Scholar] [CrossRef]
- Rice, D.C.; Correa, A.M.; Vaporciyan, A.A.; Sodhi, N.; Smythe, W.R.; Swisher, S.G.; Walsh, G.L.; Putnam, J.B., Jr.; Komaki, R.; Ajani, J.A.; et al. Preoperative chemoradiotherapy prior to esophagectomy in elderly patients is not associated with increased morbidity. Ann. Thorac. Surg. 2005, 79, 391–397. [Google Scholar] [CrossRef]
- Jing, W.; Guo, H.; Kong, L.; Zhang, Y.; Wang, H.; An, C.; Zhu, H.; Yu, J. Clinical outcomes of elderly patients (>/=70 years) with resectable esophageal squamous cell carcinoma who underwent esophagectomy or chemoradiotherapy: A retrospective analysis from a single cancer institute. Medicine 2016, 95, e5630. [Google Scholar] [CrossRef] [PubMed]
- Kosugi, S.; Sasamoto, R.; Kanda, T.; Matsuki, A.; Hatakeyama, K. Retrospective review of surgery and definitive chemoradiotherapy in patients with squamous cell carcinoma of the thoracic esophagus aged 75 years or older. Jpn. J. Clin. Oncol. 2009, 39, 360–366. [Google Scholar] [CrossRef][Green Version]
- Takagawa, R.; Kunisaki, C.; Makino, H.; Oshima, T.; Nagano, Y.; Fujii, S.; Kosaka, T.; Ono, H.A.; Akiyama, H.; Shimada, H. Therapeutic management of elderly patients with esophageal cancer. Esophagus 2008, 5, 133–139. [Google Scholar] [CrossRef]
- Zeng, Y.; Liang, W.; Liu, J.; He, J.; Ng, C.S.H.; Liu, C.C.; Petersen, R.H.; Rocco, G.; D’Amico, T.; Brunelli, A.; et al. Esophageal cancer in elderly patients: A population-based study. J. Thorac. Dis. 2018, 10, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Voncken, F.E.M.; van der Kaaij, R.T.; Sikorska, K.; van Werkhoven, E.; van Dieren, J.M.; Grootscholten, C.; Snaebjornsson, P.; van Sandick, J.W.; Aleman, B.M.P. Advanced Age is Not a Contraindication for Treatment With Curative Intent in Esophageal Cancer. Am. J. Clin. Oncol. 2018, 41, 919–926. [Google Scholar] [CrossRef]
- Schlesinger-Raab, A.; Werner, J.; Friess, H.; Holzel, D.; Engel, J. Age and Outcome in Gastrointestinal Cancers: A Population-Based Evaluation of Oesophageal, Gastric and Colorectal Cancer. Visc. Med. 2017, 33, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Cijs, T.M.; Verhoef, C.; Steyerberg, E.W.; Koppert, L.B.; Tran, T.C.; Wijnhoven, B.P.; Tilanus, H.W.; de Jonge, J. Outcome of esophagectomy for cancer in elderly patients. Ann. Thorac. Surg. 2010, 90, 900–907. [Google Scholar] [CrossRef] [PubMed]
- Ruol, A.; Portale, G.; Zaninotto, G.; Cagol, M.; Cavallin, F.; Castoro, C.; Sileni, V.C.; Alfieri, R.; Rampado, S.; Ancona, E. Results of esophagectomy for esophageal cancer in elderly patients: Age has little influence on outcome and survival. J. Thorac. Cardiovasc. Surg. 2007, 133, 1186–1192. [Google Scholar] [CrossRef]
- Kinugasa, S.; Tachibana, M.; Yoshimura, H.; Dhar, D.K.; Shibakita, M.; Ohno, S.; Kubota, H.; Masunaga, R.; Nagasue, N. Esophageal resection in elderly esophageal carcinoma patients: Improvement in postoperative complications. Ann. Thorac. Surg. 2001, 71, 414–418. [Google Scholar] [CrossRef]
- Moskovitz, A.H.; Rizk, N.P.; Venkatraman, E.; Bains, M.S.; Flores, R.M.; Park, B.J.; Rusch, V.W. Mortality increases for octogenarians undergoing esophagogastrectomy for esophageal cancer. Ann. Thorac. Surg. 2006, 82, 2031–2036. [Google Scholar] [CrossRef]
- Ma, J.Y.; Wu, Z.; Wang, Y.; Zhao, Y.F.; Liu, L.X.; Kou, Y.L.; Zhou, Q.H. Clinicopathologic characteristics of esophagectomy for esophageal carcinoma in elderly patients. World J. Gastroenterol. 2006, 12, 1296–1299. [Google Scholar] [CrossRef] [PubMed]
- Rahamim, J.S.; Murphy, G.J.; Awan, Y.; Junemann-Ramirez, M. The effect of age on the outcome of surgical treatment for carcinoma of the oesophagus and gastric cardia. Eur. J. Cardiothorac. Surg. 2003, 23, 805–810. [Google Scholar] [CrossRef]
- Fang, W.; Igaki, H.; Tachimori, Y.; Sato, H.; Daiko, H.; Kato, H. Three-field lymph node dissection for esophageal cancer in elderly patients over 70 years of age. Ann. Thorac. Surg. 2001, 72, 867–871. [Google Scholar] [CrossRef]
- Paulus, E.; Ripat, C.; Koshenkov, V.; Prescott, A.T.; Sethi, K.; Stuart, H.; Tiesi, G.; Livingstone, A.S.; Yakoub, D. Esophagectomy for cancer in octogenarians: Should we do it? Langenbecks Arch. Surg. 2017, 402, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Schweigert, M.; Solymosi, N.; Dubecz, A.; Stadlhuber, R.J.; Ofner, D.; Stein, H.J. Current outcome of esophagectomy in the very elderly: Experience of a German high-volume center. Am. Surg. 2013, 79, 754–763. [Google Scholar] [CrossRef]
- Tapias, L.F.; Muniappan, A.; Wright, C.D.; Gaissert, H.A.; Wain, J.C.; Morse, C.R.; Donahue, D.M.; Mathisen, D.J.; Lanuti, M. Short and long-term outcomes after esophagectomy for cancer in elderly patients. Ann. Thorac. Surg. 2013, 95, 1741–1748. [Google Scholar] [CrossRef]
- Elsayed, H.; Whittle, I.; McShane, J.; Howes, N.; Hartley, M.; Shackcloth, M.; Page, R. The influence of age on mortality and survival in patients undergoing oesophagogastrectomies. A seven-year experience in a tertiary centre. Interact. Cardiovasc. Thorac. Surg. 2010, 11, 65–69. [Google Scholar] [CrossRef]
- Yang, H.X.; Ling, L.; Zhang, X.; Lin, P.; Rong, T.H.; Fu, J.H. Outcome of elderly patients with oesophageal squamous cell carcinoma after surgery. Br. J. Surg. 2010, 97, 862–867. [Google Scholar] [CrossRef]
- Pultrum, B.B.; Bosch, D.J.; Nijsten, M.W.; Rodgers, M.G.; Groen, H.; Slaets, J.P.; Plukker, J.T. Extended esophagectomy in elderly patients with esophageal cancer: Minor effect of age alone in determining the postoperative course and survival. Ann. Surg. Oncol. 2010, 17, 1572–1580. [Google Scholar] [CrossRef]
- Alibakhshi, A.; Aminian, A.; Mirsharifi, R.; Jahangiri, Y.; Dashti, H.; Karimian, F. The effect of age on the outcome of esophageal cancer surgery. Ann. Thorac. Med. 2009, 4, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, T.; Hara, K.; Kazama, K.; Atsumi, Y.; Tamagawa, H.; Tamagawa, A.; Machida, D.; Komori, K.; Maezawa, Y.; Kano, K.; et al. The Short- and Long-term Outcomes of Esophagectomy for Esophageal Cancer in Patients Older than 75 Years. Anticancer Res. 2020, 40, 1087–1093. [Google Scholar] [CrossRef]
- Chen, D.; Hu, Y.; Chen, Y.; Hu, J.; Wen, Z. Comparison of Outcomes Between McKeown and Sweet Esophagectomy in the Elderly Patients for Esophageal Squamous Cell Carcinoma: A Propensity Score-Matched Analysis. Cancer Control. 2020, 27, 1073274820904700. [Google Scholar] [CrossRef]
- Kanda, M.; Koike, M.; Tanaka, C.; Kobayashi, D.; Hayashi, M.; Yamada, S.; Nakayama, G.; Omae, K.; Kodera, Y. Feasibility of subtotal esophagectomy with systematic lymphadenectomy in selected elderly patients with esophageal cancer; a propensity score matching analysis. BMC Surg. 2019, 19, 143. [Google Scholar] [CrossRef]
- Baranov, N.S.; van Workum, F.; van der Maas, J.; Kouwenhoven, E.; van Det, M.; van den Wildenberg, F.J.H.; Polat, F.; Nieuwenhuijzen, G.A.P.; Luyer, M.D.P.; Rosman, C. The Influence of Age on Complications and Overall Survival After Ivor Lewis Totally Minimally Invasive Esophagectomy. J. Gastrointest. Surg. 2019, 23, 1293–1300. [Google Scholar] [CrossRef] [PubMed]
- Klevebro, F.; Garritano, S.; Scandavini, C.M.; Shetye, A.; Coppola, A.; Kamiya, S.; Nilsson, M.; Lundell, L.; Rouvelas, I. Surgical outcomes of oesophagectomy or gastrectomy due to cancer for patients >/=75 years of age: A single-centre cohort study. ANZ J. Surg. 2019, 89, 228–233. [Google Scholar] [CrossRef]
- Schlottmann, F.; Strassle, P.D.; Nayyar, A.; Herbella, F.A.M.; Cairns, B.A.; Patti, M.G. Postoperative outcomes of esophagectomy for cancer in elderly patients. J. Surg. Res. 2018, 229, 9–14. [Google Scholar] [CrossRef]
- Miyata, H.; Yamasaki, M.; Makino, T.; Miyazaki, Y.; Takahashi, T.; Kurokawa, Y.; Nakajima, K.; Takiguchi, S.; Mori, M.; Doki, Y. Clinical Outcome of Esophagectomy in Elderly Patients With and Without Neoadjuvant Therapy for Thoracic Esophageal Cancer. Ann. Surg. Oncol. 2015, 22 (Suppl. S3), S794–S801. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.C.; Huang, W.C.; Chen, C.H.; Chan, M.L. Radical esophagectomy in elderly patients with esophageal cancer. Formos. J. Surg. 2015, 48, 121–127. [Google Scholar] [CrossRef]
- Morita, M.; Otsu, H.; Kawano, H.; Kumashiro, R.; Taketani, K.; Kimura, Y.; Saeki, H.; Ando, K.; Ida, S.; Oki, E.; et al. Advances in esophageal surgery in elderly patients with thoracic esophageal cancer. Anticancer Res. 2013, 33, 1641–1647. [Google Scholar] [PubMed]
- Markar, S.R.; Low, D.E. Physiology, not chronology, dictates outcomes after esophagectomy for esophageal cancer: Outcomes in patients 80 years and older. Ann. Surg. Oncol. 2013, 20, 1020–1026. [Google Scholar] [CrossRef]
- Chen, E.; Senders, Z.J.; Hardacre, J.; Kim, J.; Ammori, J. Perioperative outcomes and survival of octogenarians undergoing curative resection for esophagogastric adenocarcinoma. J. Surg. Oncol. 2020, 121, 1015–1021. [Google Scholar] [CrossRef]
- Finlayson, E.; Fan, Z.; Birkmeyer, J.D. Outcomes in octogenarians undergoing high-risk cancer operation: A national study. J. Am. Coll Surg. 2007, 205, 729–734. [Google Scholar] [CrossRef]
- Di Martino, N.; Izzo, G.; Cosenza, A.; Cerullo, G.; Torelli, F.; Brillantino, A.; del Genio, A. Adenocarcinoma of gastric cardia in the elderly: Surgical problems and prognostic factors. World J. Gastroenterol. 2005, 11, 5123–5128. [Google Scholar] [CrossRef]
- Stahl, C.C.; Hanseman, D.J.; Wima, K.; Sutton, J.M.; Wilson, G.C.; Hohmann, S.F.; Shah, S.A.; Abbott, D.E. Increasing age is a predictor of short-term outcomes in esophagectomy: A propensity score adjusted analysis. J. Gastrointest. Surg. 2014, 18, 1423–1428. [Google Scholar] [CrossRef]
- Morita, M.; Egashira, A.; Yoshida, R.; Ikeda, K.; Ohgaki, K.; Shibahara, K.; Oki, E.; Sadanaga, N.; Kakeji, Y.; Maehara, Y. Esophagectomy in patients 80 years of age and older with carcinoma of the thoracic esophagus. J. Gastroenterol. 2008, 43, 345–351. [Google Scholar] [CrossRef]
- Song, E.Y.; Frakes, J.M.; Extermann, M.; Klocksieben, F.; Mehta, R.; Saeed, S.; Hoffe, S.E.; Pimiento, J.M. Clinical Factors and Outcomes of Octogenarians Receiving Curative Surgery for Esophageal Cancer. J. Surg. Res. 2020, 251, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Mirza, A.; Pritchard, S.; Welch, I. Is surgery in the elderly for oesophageal cancer justifiable? Results from a single centre. ISRN Surg. 2013, 2013, 609252. [Google Scholar] [CrossRef] [PubMed]
- Johansson, J.; Walther, B. Clinical outcome and long-term survival rates after esophagectomy are not determined by age over 70 years. J. Gastrointest. Surg. 2000, 4, 55–62. [Google Scholar] [CrossRef]
- Li, X.; Zhao, L.J.; Liu, N.B.; Zhang, W.C.; Pang, Q.S.; Wang, P.; Yuan, Z.Y. Feasibility and efficacy of concurrent chemoradiotherapy in elderly patients with esophageal squamous cell carcinoma: A respective study of 116 cases from a single institution. Asian Pac. J. Cancer Prev 2015, 16, 1463–1469. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, Q.; Chen, J.; Wen, J.; Yang, H.; Hu, Y.; Luo, K.; Tan, Z.; Fu, J. Comparison of right- and left-approach esophagectomy for elderly patients with operable thoracic esophageal squamous cell carcinoma: A propensity matched study. J. Thorac. Dis. 2017, 9, 1883–1890. [Google Scholar] [CrossRef]
- Blom, R.L.; van Heijl, M.; Klinkenbijl, J.H.; Bergman, J.J.; Wilmink, J.W.; Richel, D.J.; Hulshof, M.C.; Reitsma, J.B.; Busch, O.R.; van Berge Henegouwen, M.I. Neoadjuvant chemoradiotherapy followed by esophagectomy does not increase morbidity in patients over 70. Dis. Esophagus 2013, 26, 510–516. [Google Scholar] [CrossRef]
- Zehetner, J.; Lipham, J.C.; Ayazi, S.; Banki, F.; Oezcelik, A.; DeMeester, S.R.; Hagen, J.A.; DeMeester, T.R. Esophagectomy for cancer in octogenarians. Dis. Esophagus 2010, 23, 666–669. [Google Scholar] [CrossRef]
- Bakhos, C.T.; Salami, A.C.; Kaiser, L.R.; Petrov, R.V.; Abbas, A.E. Outcomes of octogenarians with esophageal cancer: An analysis of the National Cancer Database. Dis. Esophagus 2019, 32, 1–8. [Google Scholar] [CrossRef]
- Cavallin, F.; Pinto, E.; Saadeh, L.M.; Alfieri, R.; Cagol, M.; Castoro, C.; Scarpa, M. Health related quality of life after oesophagectomy: Elderly patients refer similar eating and swallowing difficulties than younger patients. BMC Cancer 2015, 15, 640. [Google Scholar] [CrossRef]
- Vallbohmer, D.; Holscher, A.H.; Brabender, J.; Prenzel, K.; Gutschow, C.; Schroder, W.; Metzger, R.; Bollschweiler, E. Clinicopathologic and prognostic factors of young and elderly patients with esophageal adenocarcinoma: Is there really a difference? Dis. Esophagus 2008, 21, 596–600. [Google Scholar] [CrossRef]
- Braiteh, F.; Correa, A.M.; Hofstetter, W.L.; Rice, D.C.; Vaporciyan, A.A.; Walsh, G.L.; Roth, J.A.; Mehran, R.J.; Swisher, S.G.; Ajani, J.A. Association of age and survival in patients with gastroesophageal cancer undergoing surgery with or without preoperative therapy. Cancer 2009, 115, 4450–4458. [Google Scholar] [CrossRef] [PubMed]
- Ruol, A.; Portale, G.; Castoro, C.; Merigliano, S.; Cagol, M.; Cavallin, F.; Chiarion Sileni, V.; Corti, L.; Rampado, S.; Costantini, M.; et al. Effects of neoadjuvant therapy on perioperative morbidity in elderly patients undergoing esophagectomy for esophageal cancer. Ann. Surg. Oncol. 2007, 14, 3243–3250. [Google Scholar] [CrossRef]
- Fogh, S.E.; Yu, A.; Kubicek, G.J.; Scott, W.; Mitchell, E.; Rosato, E.L.; Berger, A.C. Do elderly patients experience increased perioperative or postoperative morbidity or mortality when given neoadjuvant chemoradiation before esophagectomy? Int. J. Radiat Oncol. Biol. Phys. 2011, 80, 1372–1376. [Google Scholar] [CrossRef] [PubMed]
- Furlong, H.; Bass, G.; Breathnach, O.; O’Neill, B.; Leen, E.; Walsh, T.N. Targeting therapy for esophageal cancer in patients aged 70 and over. J. Geriatr. Oncol. 2013, 4, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Xi, M.; Moreno, A.; Shiraishi, Y.; Hobbs, B.P.; Huang, M.; Komaki, R.; Lin, S.H. Definitive Chemoradiation Therapy for Esophageal Cancer in the Elderly: Clinical Outcomes for Patients Exceeding 80 Years Old. Int. J. Radiat Oncol. Biol. Phys. 2017, 98, 811–819. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, S.; Ohtsu, A.; Doi, T.; Kojima, T.; Minashi, K.; Mera, K.; Yano, T.; Tahara, M.; Muto, M.; Nihei, K. A retrospective study of definitive chemoradiotherapy for elderly patients with esophageal cancer. Am. J. Clin. Oncol. 2007, 30, 607–611. [Google Scholar] [CrossRef]
- Tanisada, K.; Teshima, T.; Ikeda, H.; Abe, M.; Owen, J.B.; Hanks, G.E.; Yamashita, T.; Nishio, M.; Yamada, S.; Sakai, K.; et al. A preliminary outcome analysis of the Patterns of Care Study in Japan for esophageal cancer patients with special reference to age: Non surgery group. Int. J. Radiat Oncol. Biol. Phys. 2000, 46, 1223–1233. [Google Scholar] [CrossRef]
- Zhao, Q.; Hu, G.; Xiao, W.; Chen, Y.; Shen, M.; Tang, Q.; Ning, X. Comparison of definitive chemoradiotherapy and radiotherapy alone in patients older than 75 years with locally advanced esophageal carcinoma: A retrospective cohort study. Medicine 2017, 96, e7920. [Google Scholar] [CrossRef]
- Lu, X.; Wu, H.; Wang, J.; Xu, J. Short- and long-term outcomes of definitive chemoradiotherapy in patients with esophageal carcinoma aged >/=75 years. Mol. Clin. Oncol. 2014, 2, 297–301. [Google Scholar] [CrossRef][Green Version]
- Jingu, K.; Numasaki, H.; Toh, Y.; Nemoto, K.; Uno, T.; Doki, Y.; Matsubara, H. Chemoradiotherapy and radiotherapy alone in patients with esophageal cancer aged 80 years or older based on the Comprehensive Registry of Esophageal Cancer in Japan. Esophagus 2020. [Google Scholar] [CrossRef]
- Kikuchi, O.; Mouri, H.; Matsueda, K.; Yamamoto, H. Endoscopic Submucosal Dissection for Treatment of Patients Aged 75 Years and over with Esophageal Cancer. ISRN Gastroenterol. 2012, 2012, 671324. [Google Scholar] [CrossRef] [PubMed]
- Ishii, N.; Uchida, S.; Itoh, T.; Horiki, N.; Matsuda, M.; Setoyama, T.; Suzuki, S.; Uemura, M.; Iizuka, Y.; Fukuda, K.; et al. Endoscopic submucosal dissection with a combination of small-caliber-tip transparent hood and flex knife for superficial esophageal neoplasia. Is it safe for elderly patients? Surg. Endosc. 2010, 24, 2110–2119. [Google Scholar] [CrossRef]
- Song, T.; Du, D.; Zhang, X.; Fang, M.; Wu, S. Comparative study of radiotherapy plus erlotinib versus chemoradio-therapy for elderly patients with esophageal cancer: A propensity score-matched analysis. Dis. Esophagus 2017, 30, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Cao, X.F.; Zhu, B.; Ji, L.; Tao, L.; Wang, D.D. Long-term efficacy of perioperative chemoradiotherapy on esophageal squamous cell carcinoma. World J. Gastroenterol. 2010, 16, 1649–1654. [Google Scholar] [CrossRef]
- Naughton, C.; Feneck, R.O. The impact of age on 6-month survival in patients with cardiovascular risk factors undergoing elective non-cardiac surgery. Int. J. Clin. Pract. 2007, 61, 768–776. [Google Scholar] [CrossRef] [PubMed]
- Lagarde, S.M.; Reitsma, J.B.; Maris, A.K.; van Berge Henegouwen, M.I.; Busch, O.R.; Obertop, H.; Zwinderman, A.H.; van Lanschot, J.J. Preoperative prediction of the occurrence and severity of complications after esophagectomy for cancer with use of a nomogram. Ann. Thorac. Surg. 2008, 85, 1938–1945. [Google Scholar] [CrossRef]
- Raymond, D.P.; Seder, C.W.; Wright, C.D.; Magee, M.J.; Kosinski, A.S.; Cassivi, S.D.; Grogan, E.L.; Blackmon, S.H.; Allen, M.S.; Park, B.J.; et al. Predictors of Major Morbidity or Mortality After Resection for Esophageal Cancer: A Society of Thoracic Surgeons General Thoracic Surgery Database Risk Adjustment Model. Ann. Thorac. Surg. 2016, 102, 207–214. [Google Scholar] [CrossRef]
- Low, D.E.; Kuppusamy, M.K.; Alderson, D.; Cecconello, I.; Chang, A.C.; Darling, G.; Davies, A.; D’Journo, X.B.; Gisbertz, S.S.; Griffin, S.M.; et al. Benchmarking Complications Associated with Esophagectomy. Ann. Surg. 2019, 269, 291–298. [Google Scholar] [CrossRef]
- Liou, D.Z.; Serna-Gallegos, D.; Mirocha, J.; Bairamian, V.; Alban, R.F.; Soukiasian, H.J. Predictors of Failure to Rescue After Esophagectomy. Ann. Thorac. Surg. 2018, 105, 871–878. [Google Scholar] [CrossRef]
- Sheetz, K.H.; Krell, R.W.; Englesbe, M.J.; Birkmeyer, J.D.; Campbell, D.A., Jr.; Ghaferi, A.A. The importance of the first complication: Understanding failure to rescue after emergent surgery in the elderly. J. Am. Coll Surg. 2014, 219, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Verma, V.; Haque, W.; Zheng, D.; Osayande, F.; Lin, C. Patterns of Care and Outcomes of Elderly Esophageal Cancer Patients Not Meeting Age-based Criteria of the CROSS Trial. Am. J. Clin. Oncol. 2019, 42, 67–74. [Google Scholar] [CrossRef]
- Vincent, J.; Mariette, C.; Pezet, D.; Huet, E.; Bonnetain, F.; Bouche, O.; Conroy, T.; Roullet, B.; Seitz, J.F.; Herr, J.P.; et al. Early surgery for failure after chemoradiation in operable thoracic oesophageal cancer. Analysis of the non-randomised patients in FFCD 9102 phase III trial: Chemoradiation followed by surgery versus chemoradiation alone. Eur. J. Cancer 2015, 51, 1683–1693. [Google Scholar] [CrossRef] [PubMed]
- Lorenzen, S.; Pauligk, C.; Homann, N.; Schmalenberg, H.; Jager, E.; Al-Batran, S.E. Feasibility of perioperative chemotherapy with infusional 5-FU, leucovorin, and oxaliplatin with (FLOT) or without (FLO) docetaxel in elderly patients with locally advanced esophagogastric cancer. Br. J. Cancer 2013, 108, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Van Hagen, P.; Hülshof, M.C.; van Lanschot, J.J.; Steyerberg, E.W.; van Berge Henegouwen, M.I.; Wijnhoven, B.P.; Richel, D.J.; Nieuwenhuijzen, G.A.; Hospers, G.A.; Bonenkamp, J.J.; et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N. Engl. J. Med. 2012, 366, 2074–2084. [Google Scholar] [CrossRef]
- Medical Research Council Oesophageal Cancer Working Group. Surgical resection with or without preoperative chemotherapy in oesophageal cancer: A randomised controlled trial. Lancet 2002, 359, 1727–1733. [Google Scholar] [CrossRef]
- Cunningham, D.; Allum, W.H.; Stenning, S.P.; Thompson, J.N.; Van de Velde, C.J.; Nicolson, M.; Scarffe, J.H.; Lofts, F.J.; Falk, S.J.; Iveson, T.J.; et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N. Engl. J. Med. 2006, 355, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Klevebro, F.; von Dobeln, G.A.; Wang, N.; Johnsen, G.; Jacobsen, A.B.; Friesland, S.; Hatlevoll, I.; Glenjen, N.I.; Lind, P.; Tsai, J.A.; et al. A randomized clinical trial of neoadjuvant chemotherapy versus neoadjuvant chemoradiotherapy for cancer of the oesophagus or gastro-oesophageal junction. Ann. Oncol. 2016, 27, 660–667. [Google Scholar] [CrossRef]
- Mariette, C.; Markar, S.R.; Dabakuyo-Yonli, T.S.; Meunier, B.; Pezet, D.; Collet, D.; D’Journo, X.B.; Brigand, C.; Perniceni, T.; Carrère, N.; et al. Fédération de Recherche en Chirurgie (FRENCH) and French Eso-Gastric Tumors (FREGAT) Working Group. Hybrid Minimally Invasive Esophagectomy for Esophageal Cancer. N. Engl. J. Med. 2019, 380, 152–162. [Google Scholar] [CrossRef]
- Mariette, C.; Markar, S.; Dabakuyo-Yonli, T.S.; Meunier, B.; Pezet, D.; Collet, D.; D’Journo, X.B.; Brigand, C.; Perniceni, T.; Carrere, N.; et al. FRENCH, FREGAT. Health-related Quality of Life Following Hybrid Minimally Invasive Versus Open Esophagectomy for Patients With Esophageal Cancer, Analysis of a Multicenter, Open-label, Randomized Phase III Controlled Trial: The MIRO Trial. Ann. Surg. 2020, 271, 1023–1029. [Google Scholar] [CrossRef] [PubMed]
- Sugita, Y.; Nakamura, T.; Sawada, R.; Takiguchi, G.; Uragawa, N.; Hasegawa, H.; Yamamoto, M.; Kanaji, S.; Matsuda, Y.; Yamashita, K.; et al. Safety and feasibility of minimally invasive esophagectomy for elderly esophageal cancer patients. Dis. Esophagus 2021, 34, doaa083. [Google Scholar] [CrossRef]
- Voron, T.; Gronnier, C.; Pasquer, A.; Thereaux, J.; Gagniere, J.; Lebreton, G.; Meunier, B.; Collet, D.; Piessen, G.; Paye, F.; et al. Adenocarcinoma of the oesophagogastric junction Siewert II: An oesophageal cancer better cured with total gastrectomy. Eur. J. Surg. Oncol. 2019, 45, 2473–2481. [Google Scholar] [CrossRef]
- Deldycke, A.; Van Daele, E.; Ceelen, W.; Van Nieuwenhove, Y.; Pattyn, P. Functional outcome after Ivor Lewis esophagectomy for cancer. J. Surg. Oncol. 2016, 113, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Decoster, L.; Van Puyvelde, K.; Mohile, S.; Wedding, U.; Basso, U.; Colloca, G.; Rostoft, S.; Overcash, J.; Wildiers, H.; Steer, C.; et al. Screening tools for multidimensional health problems warranting a geriatric assessment in older cancer patients: An update on SIOG recommendations dagger. Ann. Oncol. 2015, 26, 288–300. [Google Scholar] [CrossRef] [PubMed]
- Montroni, I.; Rostoft, S.; Spinelli, A.; Van Leeuwen, B.L.; Ercolani, G.; Saur, N.M.; Jaklitsch, M.T.; Somasundar, P.S.; de Liguori Carino, N.; Ghignone, F.; et al. For the SIOG surgical task force/ESSO GOSAFE study group. GO-SAFE—Geriatric Oncology Surgical Assessment and Functional rEcovery after Surgery: Early analysis on 977 patients. J. Geriatr. Oncol. 2020, 11, 244–255. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mantziari, S.; Teixeira Farinha, H.; Bouygues, V.; Vignal, J.-C.; Deswysen, Y.; Demartines, N.; Schäfer, M.; Piessen, G. Esophageal Cancer in Elderly Patients, Current Treatment Options and Outcomes; A Systematic Review and Pooled Analysis. Cancers 2021, 13, 2104. https://doi.org/10.3390/cancers13092104
Mantziari S, Teixeira Farinha H, Bouygues V, Vignal J-C, Deswysen Y, Demartines N, Schäfer M, Piessen G. Esophageal Cancer in Elderly Patients, Current Treatment Options and Outcomes; A Systematic Review and Pooled Analysis. Cancers. 2021; 13(9):2104. https://doi.org/10.3390/cancers13092104
Chicago/Turabian StyleMantziari, Styliani, Hugo Teixeira Farinha, Vianney Bouygues, Jean-Charles Vignal, Yannick Deswysen, Nicolas Demartines, Markus Schäfer, and Guillaume Piessen. 2021. "Esophageal Cancer in Elderly Patients, Current Treatment Options and Outcomes; A Systematic Review and Pooled Analysis" Cancers 13, no. 9: 2104. https://doi.org/10.3390/cancers13092104
APA StyleMantziari, S., Teixeira Farinha, H., Bouygues, V., Vignal, J.-C., Deswysen, Y., Demartines, N., Schäfer, M., & Piessen, G. (2021). Esophageal Cancer in Elderly Patients, Current Treatment Options and Outcomes; A Systematic Review and Pooled Analysis. Cancers, 13(9), 2104. https://doi.org/10.3390/cancers13092104







