Prognostic Impact of PD-L1 Expression in pN1 NSCLC: A Retrospective Single-Center Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Tissue Processing and Immunohistochemistry
2.3. Statistical Analysis
3. Results
3.1. Clinical Patient Characteristics
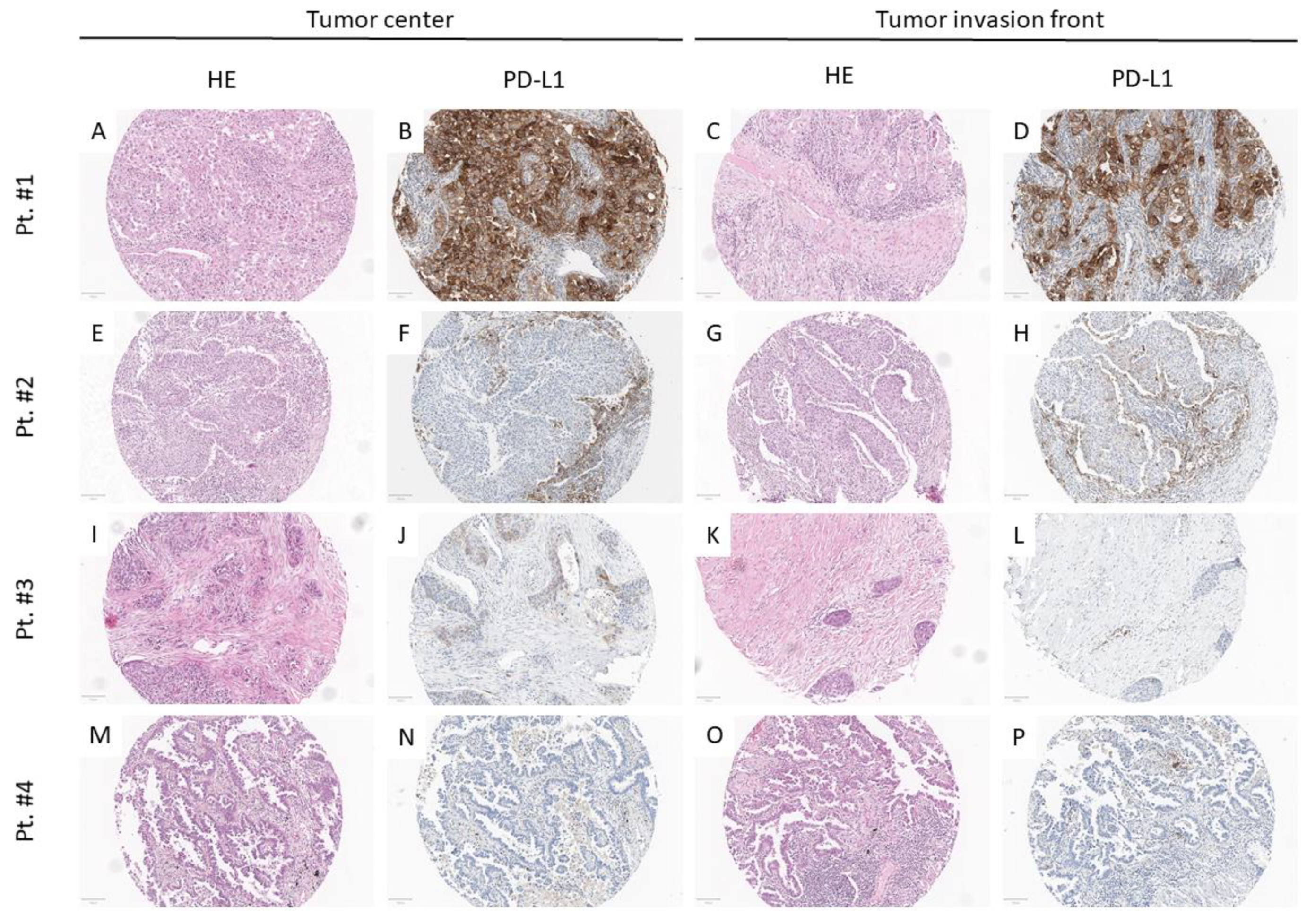
3.2. PD-L1 Assessment
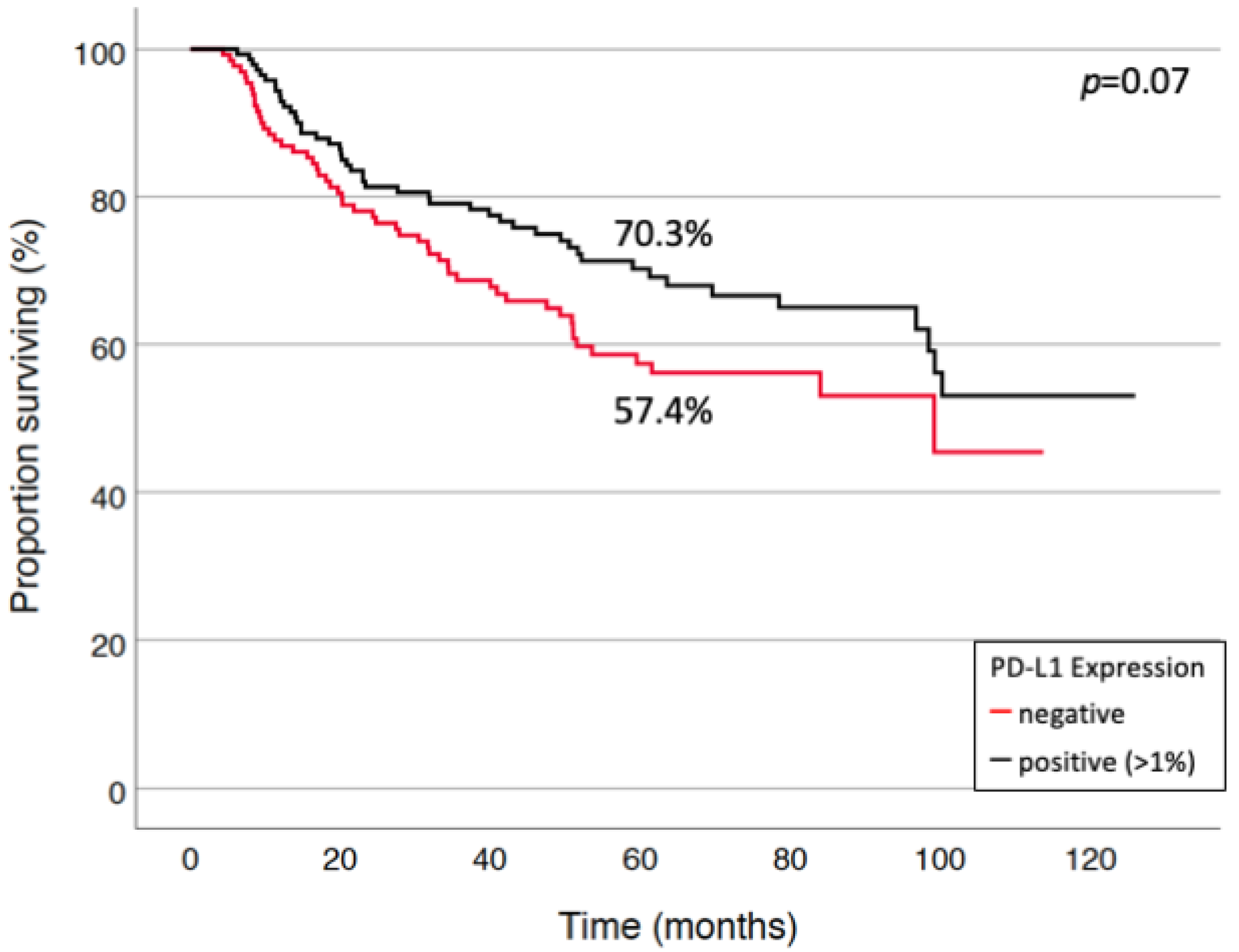
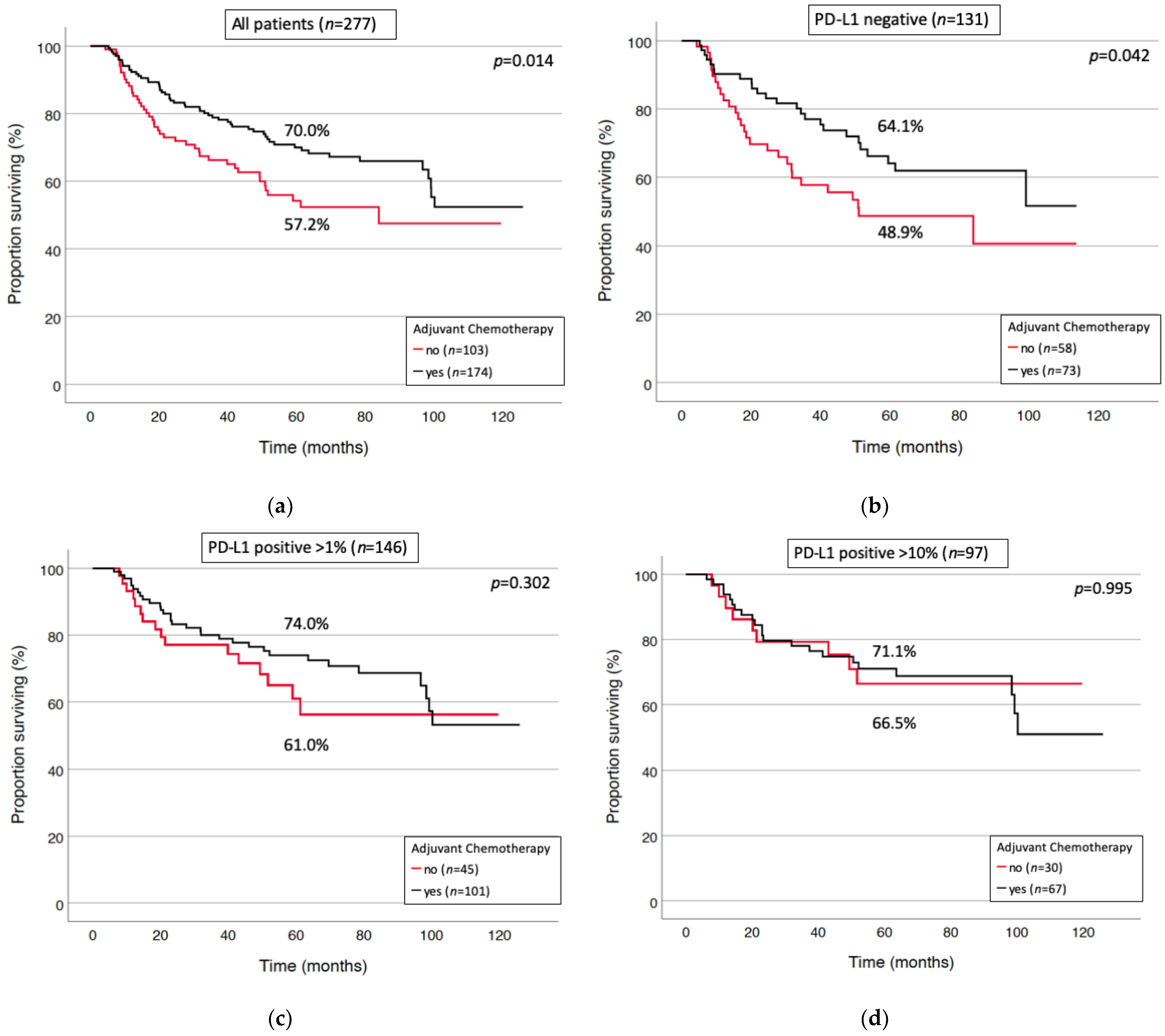
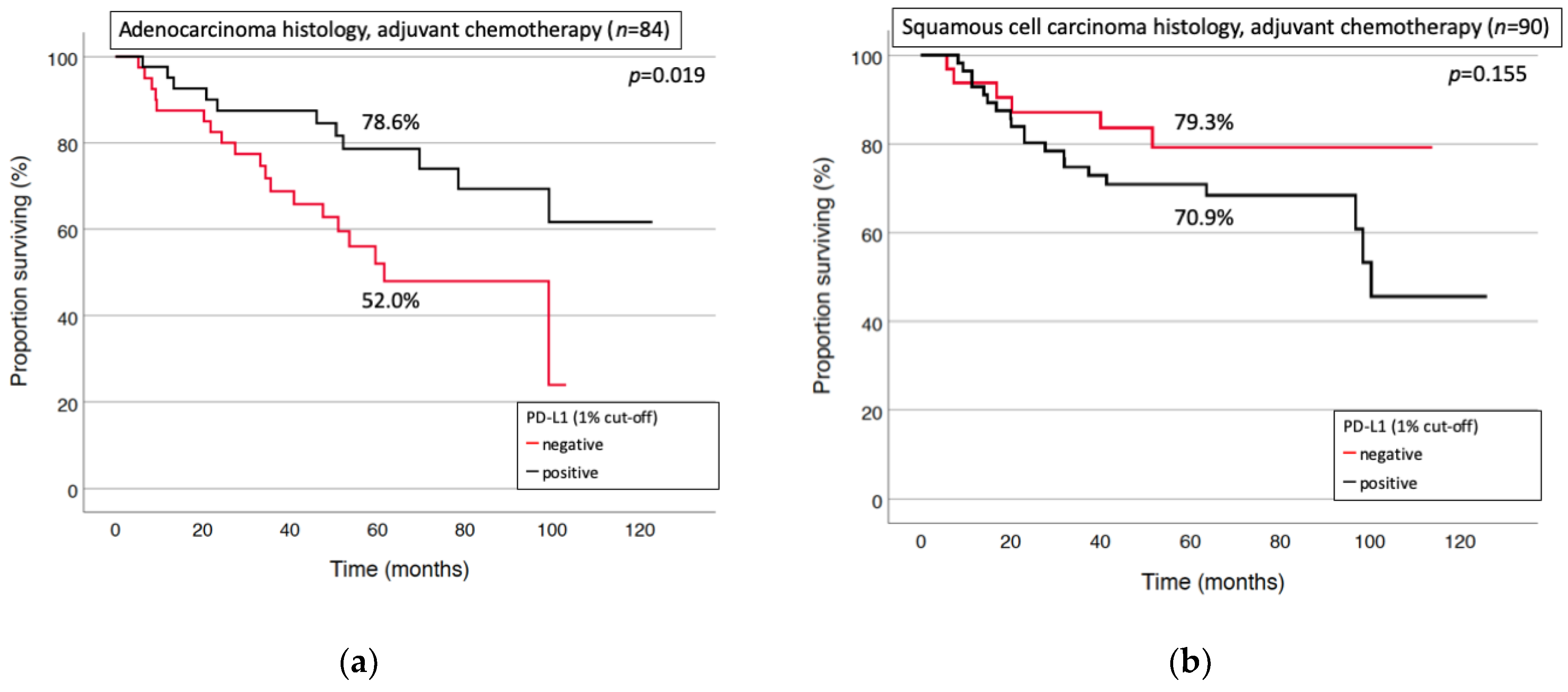
3.3. Prognostic Factors for Long-Term Survival and Relapse
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Cruz, C.; Afonso, M.; Oliveiros, B.; Pego, A. Recurrence and Risk Factors for Relapse in Patients with Non-Small Cell Lung Cancer Treated by Surgery with Curative Intent. Oncology 2017, 92, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Asamura, H.; Chansky, K.; Crowley, J.; Goldstraw, P.; Rusch, V.W.; Vansteenkiste, J.F.; Watanabe, H.; Wu, Y.L.; Zielinski, M.; Ball, D.; et al. The International Association for the Study of Lung Cancer Lung Cancer Staging Project: Proposals for the Revision of the N Descriptors in the Forthcoming 8th Edition of the TNM Classification for Lung Cancer. J. Thorac. Oncol. 2015, 10, 1675–1684. [Google Scholar] [CrossRef]
- Rusch, V.W.; Asamura, H.; Watanabe, H.; Giroux, D.J.; Rami-Porta, R.; Goldstraw, P. The IASLC lung cancer staging project: A proposal for a new international lymph node map in the forthcoming seventh edition of the TNM classification for lung cancer. J. Thorac. Oncol. 2009, 4, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Arriagada, R.; Bergman, B.; Dunant, A.; Le Chevalier, T.; Pignon, J.P.; Vansteenkiste, J.; International Adjuvant Lung Cancer Trial Collaborative Group. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N. Engl. J. Med. 2004, 350, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Arriagada, R.; Dunant, A.; Pignon, J.P.; Bergman, B.; Chabowski, M.; Grunenwald, D.; Kozlowski, M.; Le Pechoux, C.; Pirker, R.; Pinel, M.I.; et al. Long-term results of the international adjuvant lung cancer trial evaluating adjuvant Cisplatin-based chemotherapy in resected lung cancer. J. Clin. Oncol. 2010, 28, 35–42. [Google Scholar] [CrossRef]
- Herbst, R.S.; Baas, P.; Kim, D.W.; Felip, E.; Perez-Gracia, J.L.; Han, J.Y.; Molina, J.; Kim, J.H.; Arvis, C.D.; Ahn, M.J.; et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): A randomised controlled trial. Lancet 2016, 387, 1540–1550. [Google Scholar] [CrossRef]
- Reck, M.; Rodriguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csoszi, T.; Fulop, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef] [PubMed]
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Yokoi, T.; Chiappori, A.; Lee, K.H.; de Wit, M.; et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2017, 377, 1919–1929. [Google Scholar] [CrossRef]
- Deslypere, G.; Gullentops, D.; Wauters, E.; Vansteenkiste, J. Immunotherapy in non-metastatic non-small cell lung cancer: Can the benefits of stage IV therapy be translated into earlier stages? Ther. Adv. Med. Oncol. 2018, 10, 1758835918772810. [Google Scholar] [CrossRef]
- Postmus, P.E.; Kerr, K.M.; Oudkerk, M.; Senan, S.; Waller, D.A.; Vansteenkiste, J.; Escriu, C.; Peters, S. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28, iv1–iv21. [Google Scholar] [CrossRef]
- Forde, P.M.; Chaft, J.E.; Smith, K.N.; Anagnostou, V.; Cottrell, T.R.; Hellmann, M.D.; Zahurak, M.; Yang, S.C.; Jones, D.R.; Broderick, S.; et al. Neoadjuvant PD-1 Blockade in Resectable Lung Cancer. N. Engl. J. Med. 2018, 378, 1976–1986. [Google Scholar] [CrossRef] [PubMed]
- Provencio, M.; Nadal, E.; Insa, A.; Garcia-Campelo, M.R.; Casal-Rubio, J.; Domine, M.; Majem, M.; Rodriguez-Abreu, D.; Martinez-Marti, A.; De Castro Carpeno, J.; et al. Neoadjuvant chemotherapy and nivolumab in resectable non-small-cell lung cancer (NADIM): An open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol. 2020, 21, 1413–1422. [Google Scholar] [CrossRef]
- Pirker, R.; Filipits, M. Adjuvant Therapy in Patients With Completely Resected Non-small-cell Lung Cancer: Current Status and Perspectives. Clin. Lung Cancer 2019, 20, 1–6. [Google Scholar] [CrossRef]
- Bai, R.; Li, L.; Chen, X.; Chen, N.; Song, W.; Cui, J. Neoadjuvant and Adjuvant Immunotherapy: Opening New Horizons for Patients With Early-Stage Non-small Cell Lung Cancer. Front. Oncol. 2020, 10, 575472. [Google Scholar] [CrossRef] [PubMed]
- Bodor, J.N.; Boumber, Y.; Borghaei, H. Biomarkers for immune checkpoint inhibition in non-small cell lung cancer (NSCLC). Cancer 2020, 126, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Travis, W.D.; Brambilla, E.; Nicholson, A.G.; Yatabe, Y.; Austin, J.H.; Beasley, M.B.; Chirieac, L.R.; Dacic, S.; Duhig, E.; Flieder, D.B.; et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J. Thorac. Oncol. 2015, 10, 1243–1260. [Google Scholar] [CrossRef] [PubMed]
- Lisenko, K.; Leichsenring, J.; Zgorzelski, C.; Longuespee, R.; Casadonte, R.; Harms, A.; Kazdal, D.; Stenzinger, A.; Warth, A.; Kriegsmann, M. Qualitative Comparison Between Carrier-based and Classical Tissue Microarrays. Appl. Immunohistochem. Mol. Morphol. 2017, 25, e74–e79. [Google Scholar] [CrossRef] [PubMed]
- Casadonte, R.; Longuespee, R.; Kriegsmann, J.; Kriegsmann, M. MALDI IMS and Cancer Tissue Microarrays. Adv. Cancer Res. 2017, 134, 173–200. [Google Scholar] [CrossRef]
- Lantuejoul, S.; Sound-Tsao, M.; Cooper, W.A.; Girard, N.; Hirsch, F.R.; Roden, A.C.; Lopez-Rios, F.; Jain, D.; Chou, T.Y.; Motoi, N.; et al. PD-L1 Testing for Lung Cancer in 2019: Perspective From the IASLC Pathology Committee. J. Thorac. Oncol. 2020, 15, 499–519. [Google Scholar] [CrossRef]
- Wei, S.; Asamura, H.; Kawachi, R.; Sakurai, H.; Watanabe, S. Which is the better prognostic factor for resected non-small cell lung cancer: The number of metastatic lymph nodes or the currently used nodal stage classification? J. Thorac. Oncol. 2011, 6, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Eichhorn, F.; Klotz, L.V.; Muley, T.; Kobinger, S.; Winter, H.; Eichhorn, M.E. Prognostic relevance of regional lymph-node distribution in patients with N1-positive non-small cell lung cancer: A retrospective single-center analysis. Lung Cancer 2019, 138, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, L.; Rodriguez-Abreu, D.; Gadgeel, S.; Esteban, E.; Felip, E.; De Angelis, F.; Domine, M.; Clingan, P.; Hochmair, M.J.; Powell, S.F.; et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 2078–2092. [Google Scholar] [CrossRef] [PubMed]
- Mok, T.S.K.; Wu, Y.L.; Kudaba, I.; Kowalski, D.M.; Cho, B.C.; Turna, H.Z.; Castro, G., Jr.; Srimuninnimit, V.; Laktionov, K.K.; Bondarenko, I.; et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): A randomised, open-label, controlled, phase 3 trial. Lancet 2019, 393, 1819–1830. [Google Scholar] [CrossRef]
- Velcheti, V.; Schalper, K.A.; Carvajal, D.E.; Anagnostou, V.K.; Syrigos, K.N.; Sznol, M.; Herbst, R.S.; Gettinger, S.N.; Chen, L.; Rimm, D.L. Programmed death ligand-1 expression in non-small cell lung cancer. Lab. Investig. 2014, 94, 107–116. [Google Scholar] [CrossRef]
- Cooper, W.A.; Tran, T.; Vilain, R.E.; Madore, J.; Selinger, C.I.; Kohonen-Corish, M.; Yip, P.; Yu, B.; O’Toole, S.A.; McCaughan, B.C.; et al. PD-L1 expression is a favorable prognostic factor in early stage non-small cell carcinoma. Lung Cancer 2015, 89, 181–188. [Google Scholar] [CrossRef]
- Schmidt, L.H.; Kummel, A.; Gorlich, D.; Mohr, M.; Brockling, S.; Mikesch, J.H.; Grunewald, I.; Marra, A.; Schultheis, A.M.; Wardelmann, E.; et al. PD-1 and PD-L1 Expression in NSCLC Indicate a Favorable Prognosis in Defined Subgroups. PLoS ONE 2015, 10, e0136023. [Google Scholar] [CrossRef]
- Ameratunga, M.; Asadi, K.; Lin, X.; Walkiewicz, M.; Murone, C.; Knight, S.; Mitchell, P.; Boutros, P.; John, T. PD-L1 and Tumor Infiltrating Lymphocytes as Prognostic Markers in Resected NSCLC. PLoS ONE 2016, 11, e0153954. [Google Scholar] [CrossRef] [PubMed]
- Takada, K.; Okamoto, T.; Toyokawa, G.; Kozuma, Y.; Matsubara, T.; Haratake, N.; Akamine, T.; Takamori, S.; Katsura, M.; Shoji, F.; et al. The expression of PD-L1 protein as a prognostic factor in lung squamous cell carcinoma. Lung Cancer 2017, 104, 7–15. [Google Scholar] [CrossRef]
- Takada, K.; Okamoto, T.; Shoji, F.; Shimokawa, M.; Akamine, T.; Takamori, S.; Katsura, M.; Suzuki, Y.; Fujishita, T.; Toyokawa, G.; et al. Clinical Significance of PD-L1 Protein Expression in Surgically Resected Primary Lung Adenocarcinoma. J. Thorac. Oncol. 2016, 11, 1879–1890. [Google Scholar] [CrossRef] [PubMed]
- Cha, Y.J.; Kim, H.R.; Lee, C.Y.; Cho, B.C.; Shim, H.S. Clinicopathological and prognostic significance of programmed cell death ligand-1 expression in lung adenocarcinoma and its relationship with p53 status. Lung Cancer 2016, 97, 73–80. [Google Scholar] [CrossRef]
- Tuminello, S.; Sikavi, D.; Veluswamy, R.; Gamarra, C.; Lieberman-Cribbin, W.; Flores, R.; Taioli, E. PD-L1 as a prognostic biomarker in surgically resectable non-small cell lung cancer: A meta-analysis. Transl. Lung Cancer Res. 2020, 9, 1343–1360. [Google Scholar] [CrossRef]
- Yang, C.Y.; Lin, M.W.; Chang, Y.L.; Wu, C.T.; Yang, P.C. Programmed cell death-ligand 1 expression in surgically resected stage I pulmonary adenocarcinoma and its correlation with driver mutations and clinical outcomes. Eur. J. Cancer 2014, 50, 1361–1369. [Google Scholar] [CrossRef]
- Mu, C.Y.; Huang, J.A.; Chen, Y.; Chen, C.; Zhang, X.G. High expression of PD-L1 in lung cancer may contribute to poor prognosis and tumor cells immune escape through suppressing tumor infiltrating dendritic cells maturation. Med. Oncol. 2011, 28, 682–688. [Google Scholar] [CrossRef]
- Lin, G.; Fan, X.; Zhu, W.; Huang, C.; Zhuang, W.; Xu, H.; Lin, X.; Hu, D.; Huang, Y.; Jiang, K.; et al. Prognostic significance of PD-L1 expression and tumor infiltrating lymphocyte in surgically resectable non-small cell lung cancer. Oncotarget 2017, 8, 83986–83994. [Google Scholar] [CrossRef] [PubMed]
- Calles, A.; Liao, X.; Sholl, L.M.; Rodig, S.J.; Freeman, G.J.; Butaney, M.; Lydon, C.; Dahlberg, S.E.; Hodi, F.S.; Oxnard, G.R.; et al. Expression of PD-1 and Its Ligands, PD-L1 and PD-L2, in Smokers and Never Smokers with KRAS-Mutant Lung Cancer. J. Thorac. Oncol. 2015, 10, 1726–1735. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.Y.; Koh, J.; Kim, S.; Go, H.; Jeon, Y.K.; Chung, D.H. Clinicopathological analysis of PD-L1 and PD-L2 expression in pulmonary squamous cell carcinoma: Comparison with tumor-infiltrating T cells and the status of oncogenic drivers. Lung Cancer 2015, 88, 24–33. [Google Scholar] [CrossRef]
- Rizvi, N.A.; Hellmann, M.D.; Snyder, A.; Kvistborg, P.; Makarov, V.; Havel, J.J.; Lee, W.; Yuan, J.; Wong, P.; Ho, T.S.; et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015, 348, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Brahmer, J.; Reckamp, K.L.; Baas, P.; Crino, L.; Eberhardt, W.E.; Poddubskaya, E.; Antonia, S.; Pluzanski, A.; Vokes, E.E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 123–135. [Google Scholar] [CrossRef]
- Alexander, A.C.; Ali, J.; McDevitt-Murphy, M.E.; Forde, D.R.; Stockton, M.; Read, M.; Ward, K.D. Racial Differences in Posttraumatic Stress Disorder Vulnerability Following Hurricane Katrina Among a Sample of Adult Cigarette Smokers from New Orleans. J. Racial Ethn. Health Disparities 2017, 4, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, C.; Wang, Q.; Li, Z.; Lin, J.; Wang, H. Differences in Stage of Cancer at Diagnosis, Treatment, and Survival by Race and Ethnicity Among Leading Cancer Types. JAMA Netw. Open 2020, 3, e202950. [Google Scholar] [CrossRef] [PubMed]
- Yatabe, Y.; Kerr, K.M.; Utomo, A.; Rajadurai, P.; Tran, V.K.; Du, X.; Chou, T.Y.; Enriquez, M.L.; Lee, G.K.; Iqbal, J.; et al. EGFR mutation testing practices within the Asia Pacific region: Results of a multicenter diagnostic survey. J. Thorac. Oncol. 2015, 10, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Pao, W.; Miller, V.A. Epidermal growth factor receptor mutations, small-molecule kinase inhibitors, and non-small-cell lung cancer: Current knowledge and future directions. J. Clin. Oncol. 2005, 23, 2556–2568. [Google Scholar] [CrossRef]
- Pan, Z.K.; Ye, F.; Wu, X.; An, H.X.; Wu, J.X. Clinicopathological and prognostic significance of programmed cell death ligand1 (PD-L1) expression in patients with non-small cell lung cancer: A meta-analysis. J. Thorac. Dis. 2015, 7, 462–470. [Google Scholar] [CrossRef]
- Zaric, B.; Brcic, L.; Buder, A.; Brandstetter, A.; Buresch, J.O.; Traint, S.; Kovacevic, T.; Stojsic, V.; Perin, B.; Pirker, R.; et al. PD-1 and PD-L1 Protein Expression Predict Survival in Completely Resected Lung Adenocarcinoma. Clin. Lung Cancer 2018, 19, e957–e963. [Google Scholar] [CrossRef]
- Heath, E.I.; Lynce, F.; Xiu, J.; Ellerbrock, A.; Reddy, S.K.; Obeid, E.; Liu, S.V.; Bollig-Fischer, A.; Separovic, D.; Vanderwalde, A. Racial Disparities in the Molecular Landscape of Cancer. Anticancer Res. 2018, 38, 2235–2240. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, A.; Ganti, A.K. Lung cancer-A global perspective. J. Surg. Oncol. 2017, 115, 550–554. [Google Scholar] [CrossRef]
- Scheel, A.H.; Baenfer, G.; Baretton, G.; Dietel, M.; Diezko, R.; Henkel, T.; Heukamp, L.C.; Jasani, B.; Johrens, K.; Kirchner, T.; et al. Interlaboratory concordance of PD-L1 immunohistochemistry for non-small-cell lung cancer. Histopathology 2018, 72, 449–459. [Google Scholar] [CrossRef]
- Williams, G.H.; Nicholson, A.G.; Snead, D.R.J.; Thunnissen, E.; Lantuejoul, S.; Cane, P.; Kerr, K.M.; Loddo, M.; Scott, M.L.J.; Scorer, P.W.; et al. Interobserver Reliability of Programmed Cell Death Ligand-1 Scoring Using the VENTANA PD-L1 (SP263) Assay in NSCLC. J. Thorac. Oncol. 2020, 15, 550–555. [Google Scholar] [CrossRef]
- Kerr, K.M.; Tsao, M.S.; Nicholson, A.G.; Yatabe, Y.; Wistuba, I.I.; Hirsch, F.R. Programmed Death-Ligand 1 Immunohistochemistry in Lung Cancer: In what state is this art? J. Thorac. Oncol. 2015, 10, 985–989. [Google Scholar] [CrossRef]
- Ilie, M.; Long-Mira, E.; Bence, C.; Butori, C.; Lassalle, S.; Bouhlel, L.; Fazzalari, L.; Zahaf, K.; Lalvee, S.; Washetine, K.; et al. Comparative study of the PD-L1 status between surgically resected specimens and matched biopsies of NSCLC patients reveal major discordances: A potential issue for anti-PD-L1 therapeutic strategies. Ann. Oncol. 2016, 27, 147–153. [Google Scholar] [CrossRef] [PubMed]
| Variable | n (%) |
|---|---|
| No. of patients | 277 (100%) |
| Gender | |
| Male | 186 (67.1%) |
| Female | 91 (32.9%) |
| Age, years: mean (range) | 65.1 (40–89) |
| <65 years | 142 (51.3%) |
| >65 years | 135 (48.7%) |
| Performance status | |
| ECOG * 0 | 213 (76.9%) |
| ECOG 1 | 64 (23.1%) |
| Histology | |
| Squamous cell carcinoma | 151 (54.5%) |
| Adenocarcinoma | 126 (45.5%) |
| Surgical procedure | |
| Lobectomy | 178 (64.3%) |
| Bilobectomy | 18 (6.5%) |
| Pneumonectomy | 81 (29.2%) |
| Tumor stage | |
| pT1a/b | 25 (9.0%) |
| pT2a/b | 131 (47.3%) |
| pT3 | 79 (28.5%) |
| pT4 | 42 (15.2%) |
| Adjuvant chemotherapy | |
| Yes | 174 (62.8%) |
| No | 103 (37.2%) |
| PD-L1 assessment | |
| Positive at any threshold | 146 (52.7%) |
| >1% of Tumor cells positive | 146 (52.7%) |
| >10% of tumor cells positive | 97 (35.0%) |
| >50% of tumor cells positive | 53 (19.1%) |
| negative | 131 (47.3%) |
| First site of recurrence at Follow up | |
| No recurrence | 163 (58.8%) |
| Local | 31 of 114 (27.2%) |
| Single distant | 61 of 114 (53.5%) |
| Multiple | 22 of 114 (19.3%) |
| Tumor-specific survival (5-year; %) | 64% |
| Disease free survival (5-year; %) | 58% |
| Variable | Tumor-Specific Survival | ||
|---|---|---|---|
| HR | 95% CI | p-Value | |
| Histology SCC vs. AC | 0.67 | [0.43–1.02] | 0.063 |
| Adjuvant chemotherapy No vs. Yes | 1.56 | [1.03–2.37] | 0.036 |
| Tumor Stage IIA/IIB vs. IIIA | 0.43 | [0.28–0.64] | <0.0001 |
| Sex Male vs. female | 1.14 | [0.74–1.78] | 0.55 |
| PD-L1—status Negative vs. positive (at any cut-off) | 1.29 | [0.86–1.93] | 0.22 |
| Age >65 years vs. <65 years | 1.26 | [0.84–1.94] | 0.25 |
| Variable | Tumor-Specific Survival | ||
|---|---|---|---|
| HR | 95% CI | p-Value | |
| Tumor Stage IIA/IIB vs. IIIA | 0.36 | [0.17–0.77] | 0.008 |
| Sex Male vs. female | 1.31 | [0.61–2.80] | 0.49 |
| PD-L1—status Negative vs. positive (at any cut-off) | 2.92 | [1.33–6.39] | 0.007 |
| Age >65 years vs. <65 years | 0.75 | [0.34–1.66] | 0.48 |
| Performance status ECOG 0 vs. ECOG 1 | 1.66 | [0.38–7.29] | 0.50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eichhorn, F.; Kriegsmann, M.; Klotz, L.V.; Kriegsmann, K.; Muley, T.; Zgorzelski, C.; Christopoulos, P.; Winter, H.; Eichhorn, M.E. Prognostic Impact of PD-L1 Expression in pN1 NSCLC: A Retrospective Single-Center Analysis. Cancers 2021, 13, 2046. https://doi.org/10.3390/cancers13092046
Eichhorn F, Kriegsmann M, Klotz LV, Kriegsmann K, Muley T, Zgorzelski C, Christopoulos P, Winter H, Eichhorn ME. Prognostic Impact of PD-L1 Expression in pN1 NSCLC: A Retrospective Single-Center Analysis. Cancers. 2021; 13(9):2046. https://doi.org/10.3390/cancers13092046
Chicago/Turabian StyleEichhorn, Florian, Mark Kriegsmann, Laura V. Klotz, Katharina Kriegsmann, Thomas Muley, Christiane Zgorzelski, Petros Christopoulos, Hauke Winter, and Martin E. Eichhorn. 2021. "Prognostic Impact of PD-L1 Expression in pN1 NSCLC: A Retrospective Single-Center Analysis" Cancers 13, no. 9: 2046. https://doi.org/10.3390/cancers13092046
APA StyleEichhorn, F., Kriegsmann, M., Klotz, L. V., Kriegsmann, K., Muley, T., Zgorzelski, C., Christopoulos, P., Winter, H., & Eichhorn, M. E. (2021). Prognostic Impact of PD-L1 Expression in pN1 NSCLC: A Retrospective Single-Center Analysis. Cancers, 13(9), 2046. https://doi.org/10.3390/cancers13092046







