Dietary Modulation of Bacteriophages as an Additional Player in Inflammation and Cancer
Abstract
Simple Summary
Abstract
1. Introduction
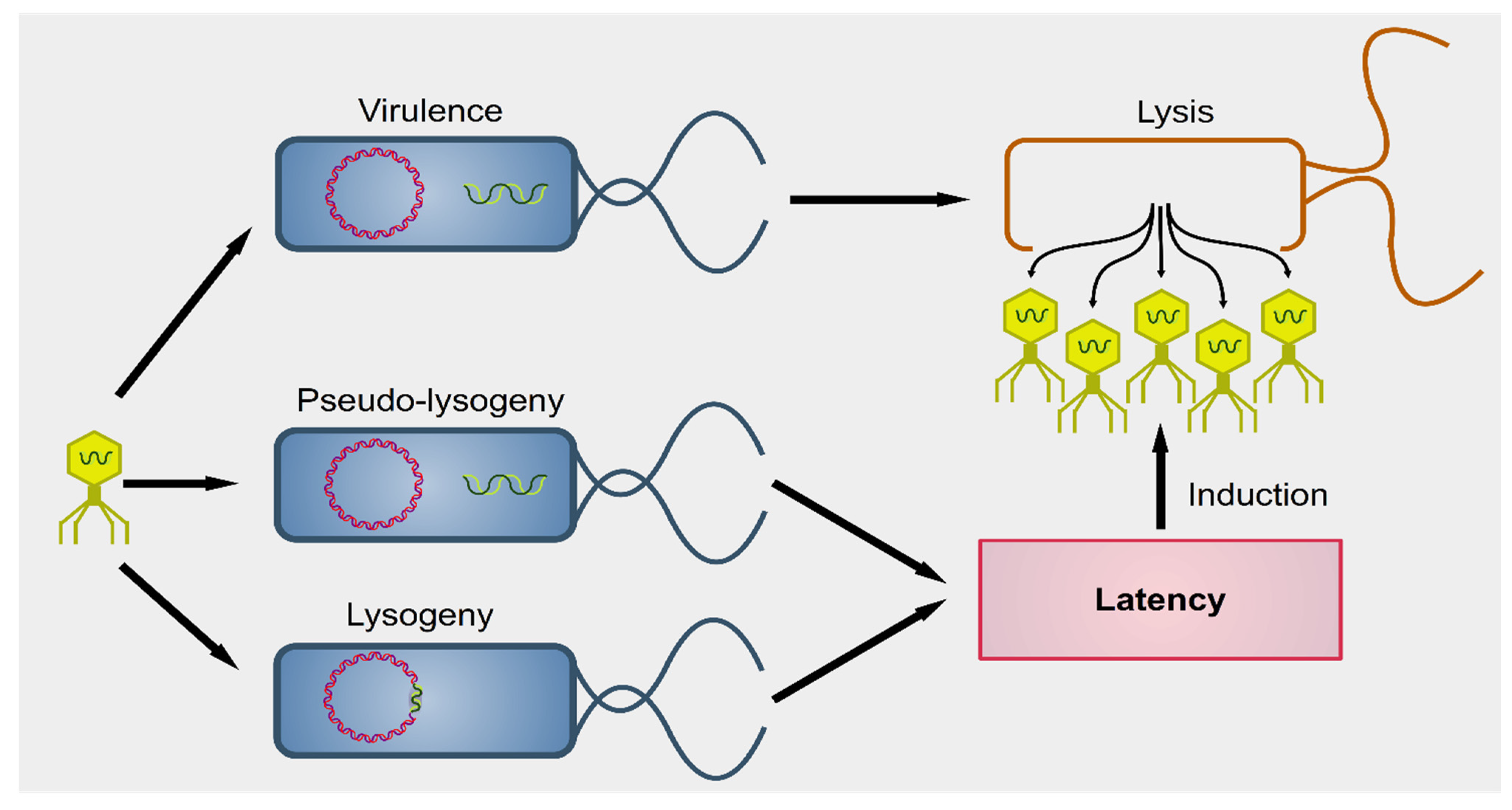
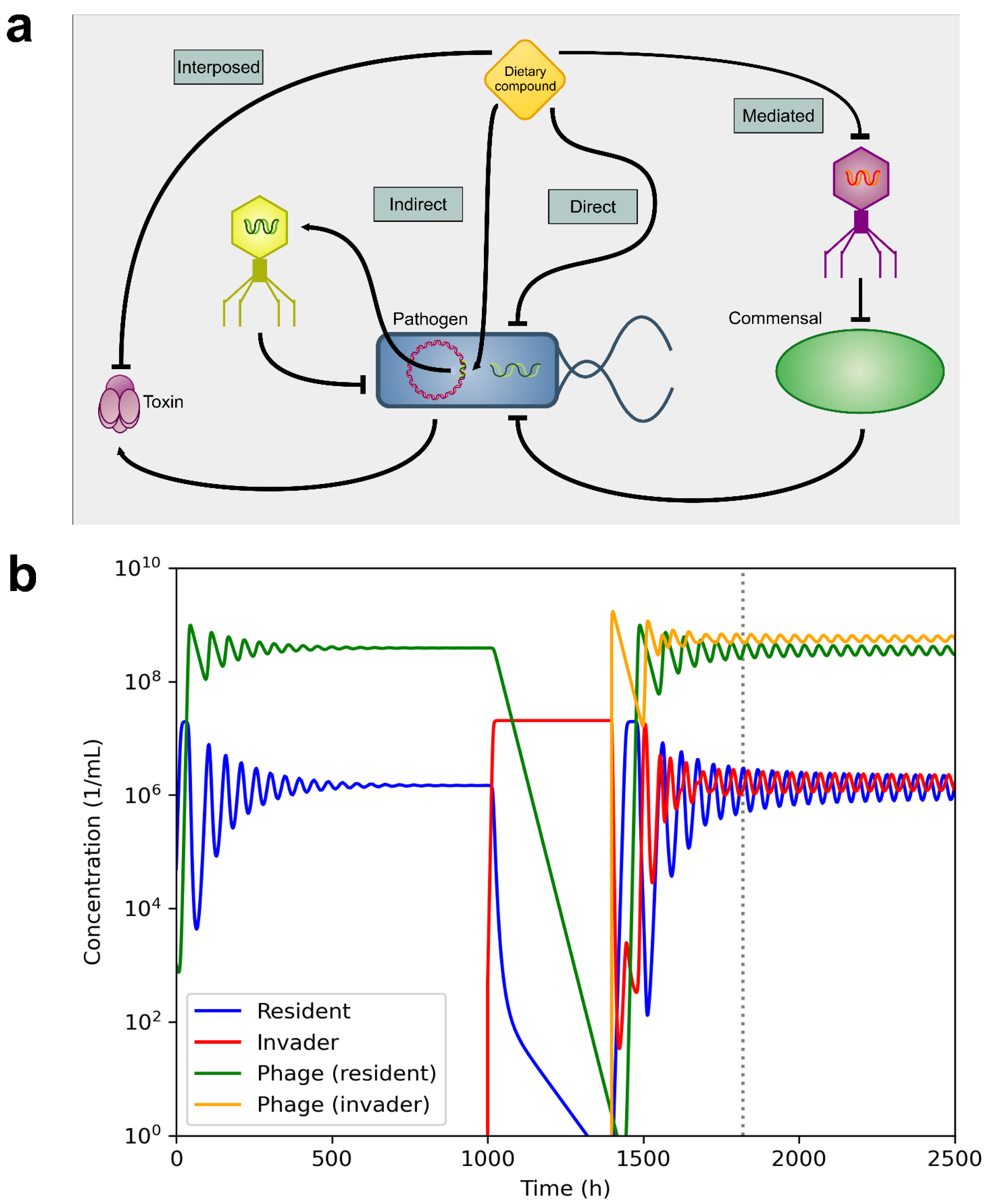
2. Interactions between Phages and Bacteria in the Gut Microbiome
3. Effect of Dietary Compounds on Phages
3.1. Phenolic Acids
3.2. Flavonoids
3.3. Saccharides
3.4. Essential Oils and Vitamins
3.5. Other Compounds
3.6. In Vivo Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, R.; Donahue, H.; Garcia, D.; Tan, J.; Shimizu, N.; Rice, A.P.; Ling, P.D. Epstein-barr virus BART9 miRNA modulates LMP1 levels and affects growth rate of nasal NK T cell lymphomas. PLoS ONE 2011, 6, e27271. [Google Scholar] [CrossRef] [PubMed]
- Bultman, S.J. Interplay between diet, gut microbiota, epigenetic events, and colorectal cancer. Mol. Nutr. Food Res. 2017, 61, 1500902. [Google Scholar] [CrossRef] [PubMed]
- Shirkey, T.W.; Siggers, R.H.; Goldade, B.G.; Marshall, J.K.; Drew, M.D.; Laarveld, B.; Kessel, A.G.V. Effects of commensal bacteria on intestinal morphology and expression of proinflammatory cytokines in the gnotobiotic pig. Exp. Biol. Med. 2006, 231, 1333–1345. [Google Scholar] [CrossRef]
- Pull, S.L.; Doherty, J.M.; Mills, J.C.; Gordon, J.I.; Stappenbeck, T.S. Activated macrophages are an adaptive element of the colonic epithelial progenitor niche necessary for regenerative responses to injury. Proc. Natl. Acad. Sci. USA 2005, 102, 99–104. [Google Scholar] [CrossRef]
- Ukena, S.N.; Singh, A.; Dringenberg, U.; Engelhardt, R.; Seidler, U.; Hansen, W.; Bleich, A.; Bruder, D.; Franzke, A.; Rogler, G.; et al. Probiotic escherichia coli Nissle 1917 inhibits leaky gut by enhancing mucosal integrity. PLoS ONE 2007, 2, e1308. [Google Scholar] [CrossRef]
- Mennigen, R.; Nolte, K.; Rijcken, E.; Utech, M.; Loeffler, B.; Senninger, N.; Bruewer, M. Probiotic mixture VSL # 3 protects the epithelial barrier by maintaining tight junction protein expression and preventing apoptosis in a murine model of colitis. Am. J. Physiol. 2009, 296, 1140–1149. [Google Scholar]
- Kelly, D.; Campbell, J.I.; King, T.P.; Grant, G.; Jansson, E.A.; Coutts, A.G.P.; Pettersson, S.; Conway, S. Commensal anaerobic gut bacteria attenuate inflammation by regulating nuclear-cytoplasmic shutting of PPAR and ReIA. Nat. Immunol. 2004, 5, 104–112. [Google Scholar] [CrossRef]
- Rakoff-Nahoum, S.; Paglino, J.; Eslami-Varzaneh, F.; Edberg, S.; Medzhitov, R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell 2004, 118, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, S.; Toh, H.; Hase, K.; Oshima, K.; Nakanishi, Y.; Yoshimura, K.; Tobe, T.; Clarke, J.M.; Topping, D.L.; Suzuki, T.; et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature 2011, 469, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Viaud, S.; Saccheri, F.; Mignot, G.; Yamazaki, T.; Daillre, R.; Hannani, D.; Enot, D.P.; Pfirschke, C.; Engblom, C.; Pittet, M.J.; et al. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science 2013, 342, 971–976. [Google Scholar] [CrossRef]
- Xu, J.; Gordon, J.I. Honor thy symbionts. Proc. Natl. Acad. Sci. USA 2003, 100, 10452–10459. [Google Scholar] [CrossRef]
- Maruvada, P.; Leone, V.; Kaplan, L.M.; Chang, E.B. The Human microbiome and obesity: Moving beyond associations. Cell Host Microbe 2017, 22, 589–599. [Google Scholar] [CrossRef]
- Beller, L.; Matthijnssens, J. What is (not) known about the dynamics of the human gut virome in health and disease. Curr. Opin. Virol. 2019, 37, 52–57. [Google Scholar] [CrossRef]
- Sutton, T.D.S.; Hill, C. Gut bacteriophage: Current understanding and challenges. Front. Endocrinol. 2019, 10, 784. [Google Scholar] [CrossRef]
- Nguyen, S.; Baker, K.; Padman, B.S.; Patwa, R.; Dunstan, R.A.; Weston, T.A.; Schlosser, K.; Bailey, B.; Lithgow, T.; Lazarou, M.; et al. Bacteriophage transcytosis provides a mechanism to cross epithelial cell layers. mBio 2017, 8. [Google Scholar] [CrossRef]
- Gogokhia, L.; Buhrke, K.; Bell, R.; Hoffman, B.; Brown, D.G.; Hanke-Gogokhia, C.; Ajami, N.J.; Wong, M.C.; Ghazaryan, A.; Valentine, J.F.; et al. Expansion of bacteriophages is linked to aggravated intestinal inflammation and colitis. Cell Host Microbe 2019, 25, 285–299. [Google Scholar] [CrossRef] [PubMed]
- Tetz, G.; Tetz, V. Bacteriophages as new human viral pathogens. Microorganisms 2018, 6, 54. [Google Scholar] [CrossRef] [PubMed]
- Levin, B.R.; Stewart, F.M.; Chao, L. Resource-limited growth, competition, and predation: A model and experimental studies with bacteria and bacteriophage. Math. Biosci. 1977, 111, 3–24. [Google Scholar] [CrossRef]
- Abedon, S.T.; Kuhl, S.J.; Blasdel, B.G.; Kutter, E.M. Phage treatment of human infections. Bacteriophage 2011, 1, 66–85. [Google Scholar] [CrossRef]
- Barr, J.J.; Auro, R.; Furlan, M.; Whiteson, K.L.; Erb, M.L.; Pogliano, J.; Stotland, A.; Wolkowicz, R.; Cutting, A.S.; Doran, K.S.; et al. Bacteriophage adhering to mucus provide a non-host-derived immunity. Proc. Natl. Acad. Sci. USA 2013, 110, 10771–10776. [Google Scholar] [CrossRef]
- Viertel, T.M.; Ritter, K.; Horz, H.-P. Viruses versus bacteria-novel approaches to phage therapy as a tool against multidrug-resistant pathogens. J. Antimicrob. Chemother. 2014, 69, 2326–2336. [Google Scholar] [CrossRef] [PubMed]
- Shlezinger, M.; Khalifa, L.; Houri-Haddad, Y.; Coppenhagen-Glazer, S.; Resch, G.; Que, Y.-A.; Beyth, S.; Dorfman, E.; Hazan, R.; Beyth, N. Phage therapy: A new horizon in the antibacterial treatment of oral pathogens. Curr. Top. Med. Chem. 2017, 17, 1199–1211. [Google Scholar] [CrossRef] [PubMed]
- Wernicki, A.; Nowaczek, A.; Urban-Chmiel, R. Bacteriophage therapy to combat bacterial infections in poultry. Virol. J. 2017, 14, 179. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.Y.K.; Wallin, M.; Lin, Y.; Leung, S.S.Y.; Wang, H.; Morales, S.; Chan, H.K. Phage therapy for respiratory infections. Adv. Drug Deliv. Rev. 2018, 133, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Gorski, A.; Midzybrodzki, R.; aczek, M.; Borysowski, J. Phages in the fight against COVID-19? Future Microbiol. 2020, 15, 1095–1100. [Google Scholar] [CrossRef]
- Dabrowska, K.; Opolski, A.; Wietrzyk, J.; Gorski, A. Anticancer activity of bacteriophage T4 and its mutant HAP1 in mouse experimental tumour models. Anticancer Res. 2004, 24, 3991–3995. [Google Scholar]
- Dabrowska, K.; Opolski, A.; Wietrzyk, J.; Switala-Jelen, K.; Boratynski, J.; Nasulewicz, A.; Lipinska, L.; Chybicka, A.; Kujawa, M.; Zabel, M.; et al. Antitumor activity of bacteriophages in murine experimental cancer models caused possibly by inhibition of beta3 integrin signaling pathway. Acta Virol. 2004, 48, 241–248. [Google Scholar]
- Voreades, N.; Kozil, A.; Weir, T.L. Diet and the development of the human intestinal microbiome. Front. Microbiol. 2014, 5, 494. [Google Scholar] [CrossRef]
- Leupold, J.H.; Yang, H.-S.; Colburn, N.H.; Asangani, I.; Post, S.; Allgayer, H. Tumor suppressor Pdcd4 inhibits invasion/intravasation and regulates urokinase receptor (u-PAR) gene expression via Sp-transcription factors. Oncogene 2007, 26, 4550–4562. [Google Scholar] [CrossRef]
- Asangani, I.A.; Rasheed, S.A.K.; Nikolova, D.A.; Leupold, J.H.; Colburn, N.H.; Post, S.; Allgayer, H. MicroRNA-21 (miR-21) post-transcriptionally downregulates tumor suppressor Pdcd4 and stimulates invasion, intravasation and metastasis in colorectal cancer. Oncogene 2008, 27, 2128–2136. [Google Scholar] [CrossRef]
- Rasheed, S.A.K.; Efferth, T.; Asangani, I.A.; Allgayer, H. First evidence that the antimalarial drug artesunate inhibits invasion and in vivo metastasis in lung cancer by targeting essential extracellular proteases. Int. J. Cancer 2010, 127, 1475–1485. [Google Scholar] [CrossRef]
- Mudduluru, G.; George-William, J.N.; Muppala, S.; Asangani, I.A.; Kumarswamy, R.; Nelson, L.D.; Allgayer, H. Curcumin regulates miR-21 expression and inhibits invasion and metastasis in colorectal cancer. Biosci. Rep. 2011, 31, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Fischer, G.; Gardell, S.; Jorpes, E. On the chemical nature of acerin and the virucidal and antiviral effects of some vegetable tannins. Experientia 1954, 10, 329–330. [Google Scholar] [CrossRef] [PubMed]
- Martinek, R.G.; Wolman, W. Xanthines, tannins, and sodium in coffee, tea, and cocoa. J. Am. Med. Assoc. 1955, 158, 1031–1051. [Google Scholar] [PubMed]
- Friedman, M. Overview of antibacterial, antitoxin, antiviral, and antifungal activities of tea flavonoids and teas. Mol. Nutr. Food Res. 2007, 51, 116–134. [Google Scholar] [CrossRef]
- Kon, K.V.; Rai, M.K. Plant essential oils and their constituents in coping with multidrug-resistant bacteria. Expert Rev. Anti-Infect. Ther. 2012, 10, 775–790. [Google Scholar] [CrossRef]
- Tarr, P.I. Escherichia coli O157:H7: Overview of clinical and epidemiological issues. J. Food Prot. 1994, 57, 632–636. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Brandt, L.J. Escherichia coli O157:H7 infection in humans. Ann. Intern. Med. 1995, 123, 698–714. [Google Scholar] [CrossRef]
- Johnson, K.E.; Thorpe, C.M.; Sears, C.L. The emerging clinical importance of non-O157 Shiga toxin-producing Escherichia coli. Clin. Infect. Dis. 2006, 43, 1587–1595. [Google Scholar]
- O’Brien, A.D.; Newland, J.W.; Miller, S.F.; Holmes, R.K.; Smith, H.W.; Formal, S.B. Shiga-like toxin-converting phages from Escherichia coli strains that cause hemorrhagic colitis or infantile diarrhea. Science 1984, 226, 694–696. [Google Scholar] [CrossRef] [PubMed]
- Strockbine, N.A.; Marques, L.R.M.; Newland, J.W.; Smith, H.W.; Holmes, R.K.; O’Brien, A.D. Two toxin-converting phages from Escherichia coli O157:H7 strain 933 encode antigenically distinct toxins with similar biologic activities. Infect. Immun. 1986, 53, 135–140. [Google Scholar] [CrossRef]
- Johansen, B.K.; Wasteson, Y.; Granum, P.E.; Brynestad, S. Mosaic structure of Shiga-toxin-2-encoding phages isolated from Escherichia coli O157:H7 indicates frequent gene exchange between lambdoid phage genomes. Microbiology 2001, 147, 1929–1936. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Canchaya, C.; Fournous, G.; Chibani-Chennoufi, S.; Dillmann, M.L.; Brssow, H. Phage as agents of lateral gene transfer. Curr. Opin. Microbiol. 2003, 6, 417–424. [Google Scholar] [CrossRef]
- Taieb, F.; Petit, C.; Nougayrde, J.-P.; Oswald, E. The enterobacterial genotoxins: Cytolethal distending toxin and colibactin. EcoSal Plus 2016, 7. [Google Scholar] [CrossRef]
- Friedman, M.; Rasooly, R. Review of the inhibition of biological activities of food-related selected toxins by natural compounds. Toxins 2013, 5, 743–775. [Google Scholar] [CrossRef]
- Oi, H.; Matsuura, D.; Miyake, M.; Ueno, M.; Takai, I.; Yamamoto, T.; Kubo, M.; Moss, J.; Noda, M. Identification in traditional herbal medications and confirmation by synthesis of factors that inhibit cholera toxin-induced fluid accumulation. Proc. Natl. Acad. Sci. USA 2002, 99, 3042–3046. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Miyake, M.; Toba, M.; Okamatsu, H.; Shimizu, S.; Noda, M. Inhibition by apple polyphenols of ADP-ribosyltransferase activity of cholera toxin and toxin-induced fluid accumulation in mice. Microbiol. Immunol. 2002, 46, 249–255. [Google Scholar] [CrossRef]
- Morinaga, N.; Iwamaru, Y.; Yahiro, K.; Tagashira, M.; Moss, J.; Noda, M. Differential activities of plant polyphenols on the binding and internalization of cholera toxin in vero cells. J. Biol. Chem. 2005, 280, 23303–23309. [Google Scholar] [CrossRef] [PubMed]
- Becker, P.M.; van der Meulen, J.; Jansman, A.J.M.; van Wikselaar, P.G. In vitro inhibition of ETEC K88 adhesion by pea hulls and of LT enterotoxin binding by faba bean hulls. J. Anim. Physiol. Anim. Nutr. 2012, 96, 1121–1126. [Google Scholar] [CrossRef]
- D’Herelle, F. On an invisible microbe antagonistic toward dysenteric bacilli: Brief note by Mr. F. D’Herelle, presented by Mr. Roux. Res. Microbiol. 2007, 158, 553–554. [Google Scholar]
- Chanishvili, N. Phage therapy-history from twort and d’herelle through soviet experience to current approaches. Adv. Vir. Res. 2012, 83, 3–40. [Google Scholar]
- Salmond, G.P.C.; Fineran, P.C. A century of the phage: Past, present and future. Nat. Rev. Microbiol. 2015, 13, 777–786. [Google Scholar] [CrossRef] [PubMed]
- Davies, E.V.; Winstanley, C.; Fothergill, J.L.; James, C.E. The role of temperate bacteriophages in bacterial infection. FEMS Microbiol. Lett. 2016, 363, 15. [Google Scholar] [CrossRef] [PubMed]
- Erez, Z.; Steinberger-Levy, I.; Shamir, M.; Doron, S.; Stokar-Avihail, A.; Peleg, Y.; Melamed, S.; Leavitt, A.; Savidor, A.; Albeck, S.; et al. Communication between viruses guides lysis-lysogeny decisions. Nature 2017, 541, 488–493. [Google Scholar] [CrossRef]
- Sharma, A.K.; Dhasmana, N.; Dubey, N.; Kumar, N.; Gangwal, A.; Gupta, M.; Singh, Y. Bacterial virulence factors: Secreted for survival. Indian J. Microbiol. 2017, 57, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mogna, L.; Del Piano, M.; Deidda, F.; Nicola, S.; Soattini, L.; Debiaggi, R.; Sforza, F.; Strozzi, G.; Mogna, G. Assessment of the in vitro inhibitory activity of specific probiotic bacteria against different Escherichia coli strains. J. Clin. Gastroenterol. 2012, 46, S29–S32. [Google Scholar] [CrossRef]
- Hsu, B.B.; Gibson, T.E.; Yeliseyev, V.; Liu, Q.; Lyon, L.; Bry, L.; Silver, P.A.; Gerber, G.K. Dynamic modulation of the gut microbiota and metabolome by bacteriophages in a mouse model. Cell Host Microbe 2019, 25, 803–814. [Google Scholar] [CrossRef] [PubMed]
- Kakisu, E.; Abraham, A.G.; Farinati, C.T.; Ibarra, C.; De Antoni, G.L. Lactobacillus plantarum isolated from kefir protects vero cells from cytotoxicity by type-II shiga toxin from Escherichia coli O157:H7. J. Dairy Res. 2013, 80, 64–71. [Google Scholar] [CrossRef]
- Barr, J.J. A bacteriophages journey through the human body. Immunol. Rev. 2017, 279, 106–122. [Google Scholar] [CrossRef]
- Caldwell, J.A. Bacteriologic and bacteriophagic study of infected urines. J. Infect. Dis. 1928, 43, 353–362. [Google Scholar] [CrossRef]
- Weber-Dabrowska, B.; Dabrowski, M.; Slopek, S. Studies on bacteriophage penetration in patients subjected to phage therapy. Arch. Immunol. Ther. Exp. 1987, 35, 563–568. [Google Scholar]
- Moustafa, A.; Xie, C.; Kirkness, E.; Biggs, W.; Wong, E.; Turpaz, Y.; Bloom, K.; Delwart, E.; Nelson, K.E.; Venter, J.C.; et al. The blood DNA virome in 8,000 humans. PLoS Pathog. 2017, 13, 1–20. [Google Scholar] [CrossRef]
- Ghose, C.; Ly, M.; Schwanemann, L.K.; Shin, J.H.; Atab, K.; Barr, J.J.; Little, M.; Schooley, R.T.; Chopyk, J.; Pride, D.T. The virome of cerebrospinal fluid: Viruses where we once thought there were none. Front. Microbiol. 2019, 10, 1–14. [Google Scholar] [CrossRef]
- Gorski, A.; Wazna, E.; Dąbrowska, B.W.; Dąbrowska, K.; Świtała-Jeleń, K.; Międzybrodzki, R. Bacteriophage translocation. FEMS Immunol. Med. Microbiol. 2006, 46, 313–319. [Google Scholar] [CrossRef]
- Heinemann, B.; Howard, A.J. Induction of lambda-bacteriophage in Escherichia coli as a screening test for potential antitumor agents. Appl. Environ. Microbiol. 1964, 12, 234–239. [Google Scholar] [CrossRef]
- Chao, S.C.; Young, D.G.; Oberg, C.J. Screening for inhibitory activity of essential oils on selected bacteria, fungi and viruses. J. Essent. Oil Res. 2000, 12, 639–649. [Google Scholar] [CrossRef]
- Boling, L.; Cuevas, D.A.; Grasis, J.A.; Kang, H.S.; Knowles, B.; Levi, K.; Maughan, H.; McNair, K.; Rojas, M.I.; Sanchez, S.E.; et al. Dietary prophage inducers and antimicrobials: Toward landscaping the human gut microbiome. Gut Microbes 2020, 1, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Goyal, S.K.; Samsher; Goyal, R.K. Stevia (Stevia rebaudiana) a bio-sweetener: A review. Int. J. Food Sci. Nutr. 2010, 61, 1–10. [Google Scholar] [CrossRef]
- Durazzo, A.; Lucarini, M.; Souto, E.B.; Cicala, C.; Caiazzo, E.; Izzo, A.A.; Novellino, E.; Santini, A. Polyphenols: A concise overview on the chemistry, occurrence, and human health. Phytother. Res. 2019, 33, 2221–2243. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rmsy, C.; Jimnez, L. Polyphenols: Food sources and bioavailability. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef]
- Kim, H.-S.; Quon, M.J.; Kim, J.-A. New insights into the mechanisms of polyphenols beyond antioxidant properties; lessons from the green tea polyphenol, epigallocatechin 3-gallate. Redox Biol. 2014, 2, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Nehlig, A.; Debry, G. Potential genotoxic, mutagenic and antimutagenic effects of coffee: A review. Mutat. Res. 1994, 317, 145–162. [Google Scholar] [CrossRef]
- Setchell, K.D.R.; Welsh, M.B.; Lim, C.K. High-performance liquid chromatographic analysis of phytoestrogens in soy protein preparations with ultraviolet, electrochemical and thermospray mass spectrometric detection. J. Chromatogr. A 1987, 386, 315–323. [Google Scholar] [CrossRef]
- Jiang, T.A. Health benefits of culinary herbs and spices. J. AOAC Int. 2019, 102, 395–411. [Google Scholar] [CrossRef] [PubMed]
- Busch, C.; Burkard, M.; Leischner, C.; Lauer, U.M.; Frank, J.; Venturelli, S. Epigenetic activities of flavonoids in the prevention and treatment of cancer. Clin. Epigenetics 2015, 7, 64. [Google Scholar] [CrossRef]
- Venturelli, S.; Burkard, M.; Biendl, M.; Lauer, U.M.; Frank, J.; Busch, C. Prenylated chalcones and flavonoids for the prevention and treatment of cancer. Nutrition 2016, 32, 1171–1178. [Google Scholar] [CrossRef] [PubMed]
- Burkard, M.; Leischner, C.; Lauer, U.M.; Busch, C.; Venturelli, S.; Frank, J. Dietary flavonoids and modulation of natural killer cells: Implications in malignant and viral diseases. J. Nutrit. Biochem. 2017, 46, 1–12. [Google Scholar] [CrossRef]
- Kosugi, A.; Nagao, M.; Suwa, Y.; Wakabayashi, K.; Sugimura, T. Roasting coffee beans produces compounds that induce prophage lambda in E. coli and are mutagenic in E. coli and S. typhimurium. Mutat. Res. 1983, 116, 179–184. [Google Scholar] [CrossRef]
- Silva-Beltárn, N.P.; Ruiz-Cruz, S.; Chaidez, C.; Ornelas-Paz, J.D.J.; Lpez-Mata, M.A.; Mrquez-Ris, E.; Estrada, M.I. Chemical constitution and effect of extracts of tomato plants byproducts on the enteric viral surrogates. Int. J. Environ. Health Res. 2015, 25, 299–311. [Google Scholar] [CrossRef]
- Lee, A.; Eschenbruch, R.; Waller, J. Effect of phenolic compounds, ethyl alcohol, and sodium metabisulphite on the lytic activity of phage PL-1 on a Lactobacillus casei S strain. Can. J. Microbiol. 1985, 31, 873–875. [Google Scholar] [CrossRef]
- Su, X.; D’Souza, D.H. Inactivation of human norovirus surrogates by benzalkonium chloride, potassium peroxymonosulfate, tannic acid, and gallic acid. Foodborne Pathog. Dis. 2012, 9, 829–834. [Google Scholar] [CrossRef]
- Khatibi, S.A.; Misaghi, A.; Moosavy, M.H. Effect of nanoliposomes containing Zataria multiflora Boiss. essential oil on gene expression of Shiga toxin 2 in Escherichia coli O157:H7. J. Appl. Microbiol. 2018, 124, 389–397. [Google Scholar] [CrossRef] [PubMed]
- De Siqueira, R.S.; Dodd, C.E.R.; Rees, C.E.D. Evaluation of the natural virucidal activity of teas for use in the phage amplification assay. Int. J. Food Microbiol. 2006, 111, 259–262. [Google Scholar] [CrossRef]
- Su, X.; Sangster, M.Y.; D’Souza, D.H. In vitro effects of pomegranate juice and pomegranate polyphenols on foodborne viral surrogates. Foodborne Pathog. Dis. 2010, 7, 1473–1479. [Google Scholar] [CrossRef] [PubMed]
- Catel-Ferreira, M.; Tnani, H.; Hellio, C.; Cosette, P.; Lebrun, L. Antiviral effects of polyphenols: Development of bio-based cleaning wipes and filters. J. Virol. Methods 2015, 212, 1–7. [Google Scholar] [CrossRef]
- Yang, J.; Tang, C.B.; Xiao, J.; Du, W.F.; Li, R. Influences of epigallocatechin gallate and citric acid on Escherichia coli O157:H7 toxin gene expression and virulence-associated stress response. Lett. Appl. Microbiol. 2018, 67, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Richter, H.E.; Loewen, P.C. Rapid inactivation of bacteriophage T7 by ascorbic acid is repairable. BBA Gene Struct. Expr. 1982, 697, 25–30. [Google Scholar] [CrossRef]
- Yen, G.-C.; Lai, H.-H. Inhibitory effects of isoflavones on nitric oxide-or peroxynitrite-mediated DNA damage in RAW 264.7 cells and fX174 DNA. Food Chem. Toxicol. 2002, 40, 1433–1440. [Google Scholar] [CrossRef]
- Su, X.; Howell, A.B.; D’Souza, D.H. The effect of cranberry juice and cranberry proanthocyanidins on the infectivity of human enteric viral surrogates. Food Microbiol. 2010, 27, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Howell, A.B.; D’Souza, D.H. Antiviral effects of cranberry juice and cranberry proanthocyanidins on foodborne viral surrogates—A time dependence study in vitro. Food Microbiol. 2010, 27, 985–991. [Google Scholar] [CrossRef]
- Su, X.; Sangster, M.Y.; D’Souza, D.H. Time-dependent effects of pomegranate juice and pomegranate polyphenols on foodborne viral reduction. Foodborne Pathog. Dis. 2011, 8, 1177–1183. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; D’Souza, D.H. Grape seed extract for control of human enteric viruses. Appl. Environ. Microbiol. 2011, 77, 3982–3987. [Google Scholar] [CrossRef]
- Sheng, L.; Rasco, B.; Zhu, M.J. Cinnamon oil inhibits Shiga toxin type 2 phage induction and Shiga toxin type 2 production in Escherichia coli O157:H7. Appl. Environ. Microbiol. 2016, 82, 6531–6540. [Google Scholar] [CrossRef] [PubMed]
- Lipson, S.M.; Sethi, L.; Cohen, P.; Gordon, R.E.; Tan, I.P.; Burdowski, A.; Stotzky, G. Antiviral effects on bacteriophages and rotavirus by cranberry juice. Phytomedicine 2007, 14, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Silva-Beltrán, N.P. Antiviral effects of Brazilian green and red propolis extracts on Enterovirus surrogates. Environ. Sci. Pollut. Res. Int. 2020, 23, 28510–28517. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-G.; Lee, J.-H.; Kim, S.-I.; Baek, K.-H.; Lee, J. Cinnamon bark oil and its components inhibit biofilm formation and toxin production. Int. J. Food Microbiol. 2015, 195, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.; Zivanovic, S. Enteric viral surrogate reduction by chitosan. Food Environ. Virol. 2015, 7, 359–365. [Google Scholar] [CrossRef]
- Kochkina, Z.M.; Chirkov, S.N. Influence of Chitosan Derivatives on the Development of Phage Infection in the Bacillus thuringiensis Culture. Mikrobiologiia 2000, 69, 266–269. [Google Scholar]
- Ly-Chatain, M.H.; Moussaoui, S.; Vera, A.; Rigobello, V.; Demarigny, Y. Antiviral effect of cationic compounds on bacteriophages. Front. Microbiol. 2013, 4, 46. [Google Scholar] [CrossRef]
- Amorim, J.H.; Del Cogliano, M.E.; Fernandez-Brando, R.J.; Bilen, M.F.; Jesus, M.R.; Luiz, W.B.; Palermo, M.S.; Ferreira, R.C.; Servat, E.G.; Ghiringhelli, P.D.; et al. Role of bacteriophages in STEC infections: New implications for the design of prophylactic and treatment approaches. F1000Research 2014, 3, 74. [Google Scholar] [CrossRef]
- Murata, A.; Oyadomari, R.; Ohashi, T.; Kitagawa, K. Mechanism of inactivation of bacteriophage deltaA containing single-stranded DNA by ascorbic acid. J. Nutr. Sci. Vitaminol. 1975, 21, 261–269. [Google Scholar] [CrossRef][Green Version]
- Kobayashi, S.; Ueda, K.; Morita, J.; Sakai, H.; Komano, T. DNA damage induced by ascorbate in the presence of Cu2+. Biochim. Biophys. Acta 1988, 949, 143–147. [Google Scholar] [CrossRef]
- Cloos, J.; Gille, J.J.; Steen, I.; Lafleur, M.V.; Retèl, J.; Snow, G.B.; Braakhuis, B.J. Influence of the antioxidant N-acetylcysteine and its metabolites on damage induced by bleomycin in PM2 bacteriophage DNA. Carcinogenesis 1996, 17, 327–331. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gião, M.S.; Borges, A.B.; Guedes, C.J.; Hogg, T.A.; Pintado, M.E.; Malcata, F.X. Determination of antioxidant capacity using the biological system bacteriophage P22/bacterium Salmonella typhimurium. J. Agric. Food Chem. 2009, 57, 22–25. [Google Scholar] [CrossRef]
- Kirtania, P.; Ghosh, S.; Bhawsinghka, N.; Chakladar, M.; Das Gupta, S.K. Vitamin C induced DevR-dependent synchronization of Mycobacterium smegmatis growth and its effect on the proliferation of mycobacteriophage D29. FEMS Microbiol. Lett. 2016, 363. [Google Scholar] [CrossRef]
- Harding, A.S.; Schwab, K.J. Using limes and synthetic psoralens to enhance solar disinfection of water (SODIS): A laboratory evaluation with norovirus, Escherichia coli, and MS2. Am. J. Trop. Med. Hyg. 2012, 86, 566–572. [Google Scholar] [CrossRef]
- Steiger, H.; Sinsheimer, R.L. Stimulation of phiX174 production in mitomycin C-treated Escherichia coli cells by caffeine. J. Virol. 1968, 2, 655. [Google Scholar] [CrossRef] [PubMed]
- Harris, S.M.; Yue, W.F.; Olsen, S.A.; Hu, J.; Means, W.J.; McCormick, R.J.; Du, M.; Zhu, M.J. Salt at concentrations relevant to meat processing enhances Shiga toxin 2 production in Escherichia coli O157:H7. Int. J. Food Microbiol. 2012, 159, 186–192. [Google Scholar] [CrossRef]
- Schons, M.; Inman, R.B. Caffeine-induced re-initiation of phage λ DNA replication. J. Mol. Biol. 1982, 159, 457–465. [Google Scholar] [CrossRef]
- Camire, M.E.; Kubow, S.; Donnelly, D.J. Potatoes and human health. Crit. Rev. Food Sci. Nutr. 2009, 49, 823–840. [Google Scholar] [CrossRef]
- Zaheer, K.; Akhtar, M.H. Potato Production, Usage, and Nutrition—A Review. Crit. Rev. Food Sci. Nutr. 2016, 56, 711–721. [Google Scholar] [CrossRef] [PubMed]
- Silva-Beltrán, N.P.; Chaidez-Quiroz, C.; Lpez-Cuevas, O.; Ruiz-Cruz, S.; Lpez-Mata, M.A.; Del-Toro-snchez, C.L.; Marquez-Rios, E.; Ornelas-Paz, J.D.J. Phenolic compounds of potato peel extracts: Their antioxidant activity and protection against human enteric viruses. J. Microbiol. Biotechnol. 2017, 27, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Quiñones, B.; Massey, S.; Friedman, M.; Swimley, M.S.; Teter, K. Novel cell-based method to detect Shiga toxin 2 from Escherichia coli O157:H7 and inhibitors of toxin activity. Appl. Environ. Microbiol. 2009, 75, 1410–1416. [Google Scholar] [CrossRef]
- Hadian, J.; Ebrahimi, S.N.; Mirjalili, M.H.; Azizi, A.; Ranjbar, H.; Friedt, W. Chemical and genetic diversity of zataria multifloraboiss. Accessions growing wild in Iran. Chem. Biodivers. 2011, 8, 176–188. [Google Scholar] [CrossRef]
- Chavez, J.H.; Leal, P.C.; Yunes, R.A.; Nunes, R.J.; Barardi, C.R.M.; Pinto, A.R.; Simes, C.M.O.; Zanetti, C.R. Evaluation of antiviral activity of phenolic compounds and derivatives against rabies virus. Vet. Microbiol. 2006, 116, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Gil, M.I.; Tomas-Barberan, F.A.; Hess-Pierce, B.; Holcroft, D.M.; Kader, A.A. Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing. J. Agric. Food Chem. 2000, 48, 4581–4589. [Google Scholar] [CrossRef] [PubMed]
- Haidari, M.; Ali, M. Pomegranate (Punica granatum) purified polyphenol extract inhibits influenza virus and has a synergistic effect with oseltamivir. Phytomedicine 2009, 16, 1127–1136. [Google Scholar] [CrossRef] [PubMed]
- Sichel, G.; Corsaro, C.; Scalia, M. In vitro scavenger activity of some flavonoids and melanins against O2- dot. Free Radic. Biol. Med. 1991, 11, 1–8. [Google Scholar] [CrossRef]
- Tyler, J.S.; Beeri, K.; Reynolds, J.L.; Alteri, C.J.; Skinner, K.G.; Friedman, J.H.; Eaton, K.A.; Friedman, D.I. Prophage induction is enhanced and required for renal disease and lethality in an EHEC mouse model. PLoS Pathog. 2013, 9, e1003236. [Google Scholar] [CrossRef]
- Sugita-Konishi, Y.; Hara-Kudo, Y.; Amano, F.; Okubo, T.; Aoi, N.; Iwaki, M.; Kumagai, S. Epigallocatechin gallate and gallocatechin gallate in green tea catechins inhibit extracellular release of Vero toxin from enterohemorrhagic Escherichia coli O157:H7. Biochim. Biophys. Acta 1999, 1472, 42–50. [Google Scholar] [CrossRef]
- Khan, N.S.; Ahmad, A.; Hadi, S.M. Anti-oxidant, pro-oxidant properties of tannic acid and its binding to DNA. Chem.-Biol. Interact. 2000, 125, 177–189. [Google Scholar] [CrossRef]
- Kamimoto, M.; Nakai, Y.; Tsuji, T.; Shimamoto, T.; Shimamoto, T. Antiviral effects of persimmon extract on human norovirus and its surrogate, bacteriophage MS2. J. Food Sci. 2014, 79, M941–M946. [Google Scholar] [CrossRef] [PubMed]
- Kameoka, S.; Leavitt, P.; Chang, C.; Kuo, S.M. Expression of antioxidant proteins in human intestinal Caco-2 cells treated with dietary flavonoids. Cancer Lett. 1999, 146, 161–167. [Google Scholar] [CrossRef]
- Tsafa, E.; Al-Bahrani, M.; Bentayebi, K.; Przystal, J.; Suwan, K.; Hajitou, A. The natural dietary genistein boosts bacteriophage-mediated cancer cell killing by improving phage-targeted tumor cell transduction. Oncotarget 2016, 7, 52135–52149. [Google Scholar] [CrossRef][Green Version]
- Nogueira, M.C.L.; Oyarzbal, O.A.; Gombas, D.E. Inactivation of Escherichia coli O157:H7, Listeria monocytogenes, and Salmonella in cranberry, lemon, and lime juice concentrates. J. Food Prot. 2003, 66, 1637–1641. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Horm, K.M.; Davidson, P.M.; Harte, F.M.; D’Souza, D.H. Survival and inactivation of human norovirus surrogates in blueberry juice by high-pressure homogenization. Foodborne Pathog. Dis. 2012, 9, 974–979. [Google Scholar] [CrossRef] [PubMed]
- Sheng, L.; Olsen, S.A.; Hu, J.; Yue, W.; Means, W.J.; Zhu, M.J. Inhibitory effects of grape seed extract on growth, quorum sensing, and virulence factors of CDC top-six non-O157 Shiga toxin producing E. coli. Int. J. Food Microbiol. 2016, 229, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Rasooly, R.; Do, P.M.; Levin, C.E.; Friedman, M. Inhibition of Shiga toxin 2 (Stx2) in apple juices and its resistance to pasteurization. J. Food Sci. 2010, 75, M296–M301. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Ruiz-Navajas, Y.; Fernndez-Lpez, J.; Prez-lvarez, J.A. Functional properties of honey, propolis, and royal jelly. J. Food Sci. 2008, 73, R117–R124. [Google Scholar] [CrossRef]
- Rufatto, L.C.; Luchtenberg, P.; Garcia, C.; Thomassigny, C.; Bouttier, S.; Henriques, J.A.P.; Roesch-Ely, M.; Dumas, F.; Moura, S. Brazilian red propolis: Chemical composition and antibacterial activity determined using bioguided fractionation. Microbiol. Res. 2018, 214, 74–82. [Google Scholar] [CrossRef]
- Iriti, M.; Varoni, E.M. Chitosan-induced antiviral activity and innate immunity in plants. Environ. Sci. Pollut. Res. 2015, 22, 2935–2944. [Google Scholar] [CrossRef]
- Su, X.; Zivanovic, S.; D’Souza, D.H. Effect of chitosan on the infectivity of murine norovirus, feline calicivirus, and bacteriophage MS2. J. Food Prot. 2009, 72, 2623–2628. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.; Zivanovic, S.; D’Souza, D.H.; Davidson, P.M. Effectiveness of chitosan on the inactivation of enteric viral surrogates. Food Microbiol. 2012, 32, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Chirkov, S.N. The antiviral activity of chitosan (review). Prikl. Biokhim. Mikrobiol. 2002, 38, 1–8. [Google Scholar]
- Liu, J.; Tian, S.; Meng, X.; Xu, Y. Effects of chitosan on control of postharvest diseases and physiological responses of tomato fruit. Postharvest Biol. Technol. 2007, 44, 300–306. [Google Scholar] [CrossRef]
- Dragsted, L.O.; Daneshvar, B.; Vogel, U.; Autrup, H.N.; Wallin, H.; Risom, L.; Møller, P.; Mølck, A.M.; Hansen, M.; Poulsen, H.E.; et al. A sucrose-rich diet induces mutations in the rat colon. Cancer Res. 2002, 62, 4339–4345. [Google Scholar] [PubMed]
- Duewel, H.S.; Daub, E.; Honek, J.F. Investigations of the interactions of saccharides with the lysozyme from bacteriophage lambda. Biochim. Biophys. Acta 1995, 1247, 149–158. [Google Scholar] [CrossRef]
- Taylor, A.; Gorazdowska, M. Conversion of murein to non-reducing fragments by enzymes from phage lambda and Vi II lysates. Biochim. Biophys. Acta 1974, 342, 133–136. [Google Scholar] [CrossRef]
- Aziz, M.; Karboune, S. Natural antimicrobial/antioxidant agents in meat and poultry products as well as fruits and vegetables: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 486–511. [Google Scholar] [CrossRef]
- Du, W.-X.; Olsen, C.W.; Avena-Bustillos, R.J.; McHugh, T.H.; Levin, C.E.; Friedman, M. Effects of allspice, cinnamon, and clove bud essential oils in edible apple films on physical properties and antimicrobial activities. J. Food Sci. 2009, 74, M372–M378. [Google Scholar] [CrossRef] [PubMed]
- Du, W.-X.; Olsen, C.W.; Avena-Bustillos, R.J.; McHugh, T.H.; Levin, C.E.; Mandrell, R.; Friedman, M. Antibacterial effects of allspice, garlic, and oregano essential oils in tomato films determined by overlay and vapor-phase methods. J. Food Sci. 2009, 74, M390–M397. [Google Scholar] [CrossRef] [PubMed]
- Nowicki, D.; Bloch, S.; Nejman-Faleczyk, B.; Szalewska-Paasz, A.; Wgrzyn, A.; Wgrzyn, G. Defects in RNA polyadenylation impair both lysogenization by and lytic development of Shiga toxin-converting bacteriophages. J. Gen. Virol. 2015, 96, 1957–1968. [Google Scholar] [CrossRef] [PubMed]
- Takemasa, N.; Ohnishi, S.; Tsuji, M.; Shikata, T.; Yokoigawa, K. Screening and analysis of spices with ability to suppress verocytotoxin production by Escherichia coli O157. J. Food Sci. 2009, 74, M461–M466. [Google Scholar] [CrossRef] [PubMed]
- Morgan, A.R.; Cone, R.L.; Elgert, T.M. The mechanism of DNA strand breakage by vitamin C and superoxide and the protective roles of catalase and superoxide dismutase. Nucleic Acids Res. 1976, 3, 1139–1149. [Google Scholar] [CrossRef] [PubMed]
- Morgan, W.A. DNA single-strand breakage in mammalian cells induced by redox cycling quinones in the absence of oxidative stress. J. Biochem. Toxicol. 1995, 10, 227–232. [Google Scholar] [CrossRef]
- Schoenfeld, J.D.; Sibenaller, Z.A.; Mapuskar, K.A.; Wagner, B.A.; Cramer-Morales, K.L.; Furqan, M.; Sandhu, S.; Carlisle, T.L.; Smith, M.C.; Hejleh, T.A.; et al. O2− and H2O2-mediated disruption of Fe metabolism causes the differential susceptibility of NSCLC and GBM cancer cells to pharmacological ascorbate. Cancer Cell 2017, 31, 487–500. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, N. Inactivation of T-group bacteriophages by ascorbic acid and some poly-phenol derivatives, and the catalytic effects of cupric ion and photo-excited riboflavin. Biochim. Biophys. Acta 1958, 27, 427–428. [Google Scholar] [CrossRef]
- Gorgus, E.; Lohr, C.; Raquet, N.; Guth, S.; Schrenk, D. Limettin and furocoumarins in beverages containing citrus juices or extracts. Food Chem. Toxicol. 2010, 48, 93–98. [Google Scholar] [CrossRef]
- Heckman, M.A.; Weil, J.; de Mejia, E.G. Caffeine (1, 3, 7-trimethylxanthine) in foods: A comprehensive review on consumption, functionality, safety, and regulatory matters. J. Food Sci. 2010, 75, R77–R87. [Google Scholar] [CrossRef]
- Cui, W.Q.; Wang, S.T.; Pan, D.; Chang, B.; Sang, L.X. Caffeine and its main targets of colorectal cancer. World J. Gastrointest. Oncol. 2020, 12, 149–172. [Google Scholar] [CrossRef]
- Moura, T.A.; Oliveira, L.; Rocha, M.S. Effects of caffeine on the structure and conformation of DNA: A force spectroscopy study. Int. J. Biol. Macromol. 2019, 130, 1018–1024. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.-W.; Dong, X.; Pan, P.; Chen, K.-W.; Fan, J.-X.; Cheng, S.-X.; Zhang, X.-Z. Phage-guided modulation of the gut microbiota of mouse models of colorectal cancer augments their responses to chemotherapy. Nat. Biomed. Eng. 2019, 3, 717–728. [Google Scholar] [CrossRef] [PubMed]
- Gelman, D.; Beyth, S.; Lerer, V.; Adler, K.; Poradosu-Cohen, R.; Coppenhagen-Glazer, S.; Hazan, R. Combined bacteriophages and antibiotics as an efficient therapy against VRE Enterococcus faecalis in a mouse model. Res. Microbiol. 2018, 169, 531–539. [Google Scholar] [CrossRef]
- Mai, V.; Ukhanova, M.; Reinhard, M.K.; Li, M.; Sulakvelidze, A. Bacteriophage administration significantly reduces Shigella colonization and shedding by Shigella-challenged mice without deleterious side effects and distortions in the gut microbiota. Bacteriophage 2015, 5, e1088124. [Google Scholar] [CrossRef] [PubMed]
- Mai, V.; Ukhanova, M.; Visone, L.; Abuladze, T.; Sulakvelidze, A. Bacteriophage administration reduces the concentration of listeria monocytogenes in the gastrointestinal tract and its translocation to spleen and liver in experimentally infected mice. Int. J. Microbi. 2010, 2010, 624234. [Google Scholar]
- Draper, L.A.; Ryan, F.J.; Dalmasso, M.; Casey, P.G.; McCann, A.; Velayudhan, V.; Ross, R.P.; Hill, C. Autochthonous faecal viral transfer (FVT) impacts the murine microbiome after antibiotic perturbation. BMC Biol. 2020, 18, 173. [Google Scholar] [CrossRef]
- Selle, K.; Fletcher, J.R.; Tuson, H.; Schmitt, D.S.; McMillan, L.; Vridhambal, G.S.; Rivera, A.J.; Montgomery, S.A.; Fortier, L.C.; Barrangou, R.; et al. In Vivo targeting of clostridioides difficile using phage-delivered CRISPR-Cas3 antimicrobials. mBio 2020, 11. [Google Scholar] [CrossRef]
- Cheng, M.; Liang, J.; Zhang, Y.; Hu, L.; Gong, P.; Cai, R.; Zhang, L.; Zhang, H.; Ge, J.; Ji, Y.; et al. The Bacteriophage EF-P29 Efficiently Protects against Lethal Vancomycin-Resistant Enterococcus faecalis and Alleviates Gut Microbiota Imbalance in a Murine Bacteremia Model. Front. Microbiol. 2017, 8, 837. [Google Scholar] [CrossRef]
- Galtier, M.; Sordi, L.D.; Sivignon, A.; De Vallée, A.; Maura, D.; Neut, C.; Rahmouni, O.; Wannerberger, K.; Darfeuille-Michaud, A.; Desreumaux, P.; et al. Bacteriophages Targeting adherent invasive escherichia coli strains as a promising new treatment for crohn’s disease. J. Crohn’s Colitis 2017, 11, 840–847. [Google Scholar] [CrossRef]
- Duan, Y.; Llorente, C.; Lang, S.; Brandl, K.; Chu, H.; Jiang, L.; White, R.C.; Clarke, T.H.; Nguyen, K.; Torralba, M.; et al. Bacteriophage targeting of gut bacterium attenuates alcoholic liver disease. Nature 2019, 575, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Febvre, H.P.; Rao, S.; Gindin, M.; Goodwin, N.D.M.; Finer, E.; Vivanco, J.S.; Lu, S.; Manter, D.K.; Wallace, T.C.; Weir, T.L. PHAGE Study: Effects of supplemental bacteriophage intake on inflammation and gut microbiota in healthy adults. Nutrients 2019, 11, 666. [Google Scholar] [CrossRef] [PubMed]
- Gindin, M.; Febvre, H.P.; Rao, S.; Wallace, T.C.; Weir, T.L. Bacteriophage for Gastrointestinal Health (PHAGE) study: Evaluating the safety and tolerability of supplemental bacteriophage consumption. J. Am. Coll. Nutr. 2019, 38, 68–75. [Google Scholar] [CrossRef]
- Grubb, D.S.; Wrigley, S.D.; Freedman, K.E.; Wei, Y.; Vazquez, A.R.; Trotter, R.E.; Wallace, T.C.; Johnson, S.A.; Weir, T.L. PHAGE-2 Study: Supplemental Bacteriophages Extend Bifidobacterium animalis subsp. lactis BL04 Benefits on Gut Health and Microbiota in Healthy Adults. Nutrients 2020, 12, 2474. [Google Scholar] [CrossRef] [PubMed]
- Cougnoux, A.; Dalmasso, G.; Martinez, R.; Buc, E.; Delmas, J.; Gibold, L.; Sauvanet, P.; Darcha, C.; Déchelotte, P.; Bonnet, M.; et al. Bacterial genotoxin colibactin promotes colon tumour growth by inducing a senescence-associated secretory phenotype. Gut 2014, 63, 1932–1942. [Google Scholar] [CrossRef] [PubMed]
- Ishaque, N.; Abba, M.L.; Hauser, C.; Patil, N.; Paramasivam, N.; Huebschmann, D.; Leupold, J.H.; Balasubramanian, G.P.; Kleinheinz, K.; Toprak, U.H.; et al. Whole genome sequencing puts forward hypotheses on metastasis evolution and therapy in colorectal cancer. Nat. Commun. 2018, 9, 4782. [Google Scholar] [CrossRef] [PubMed]
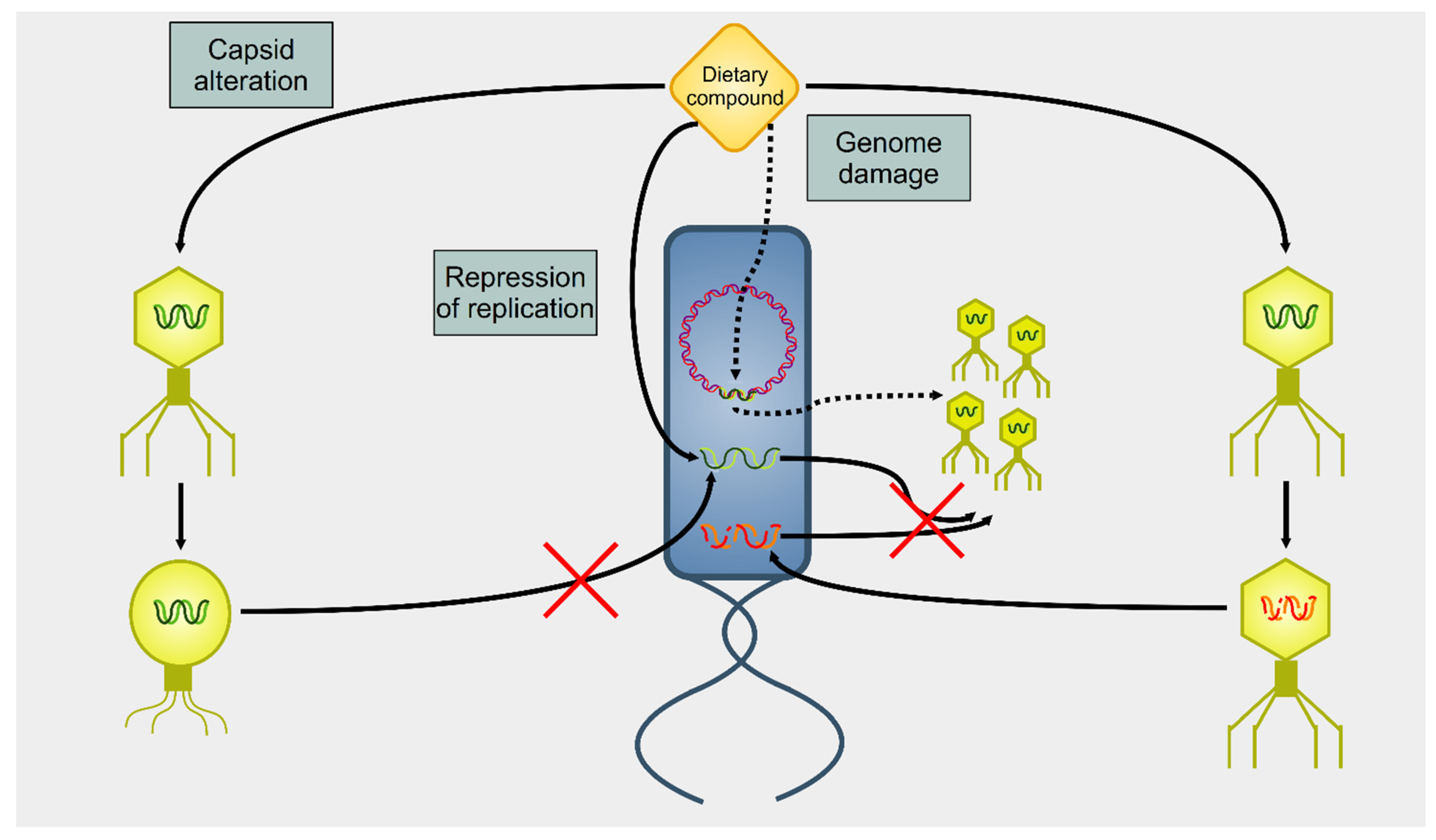
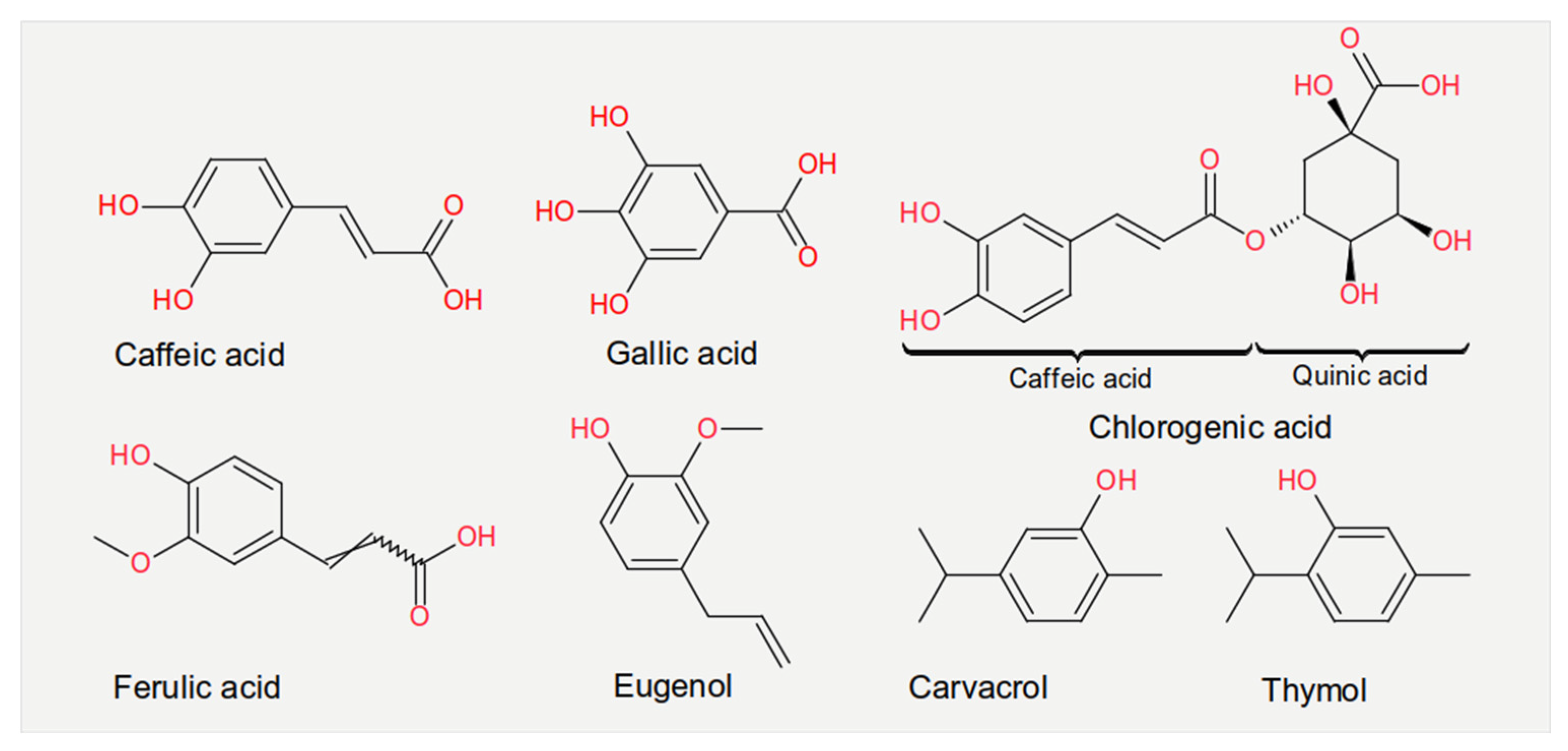
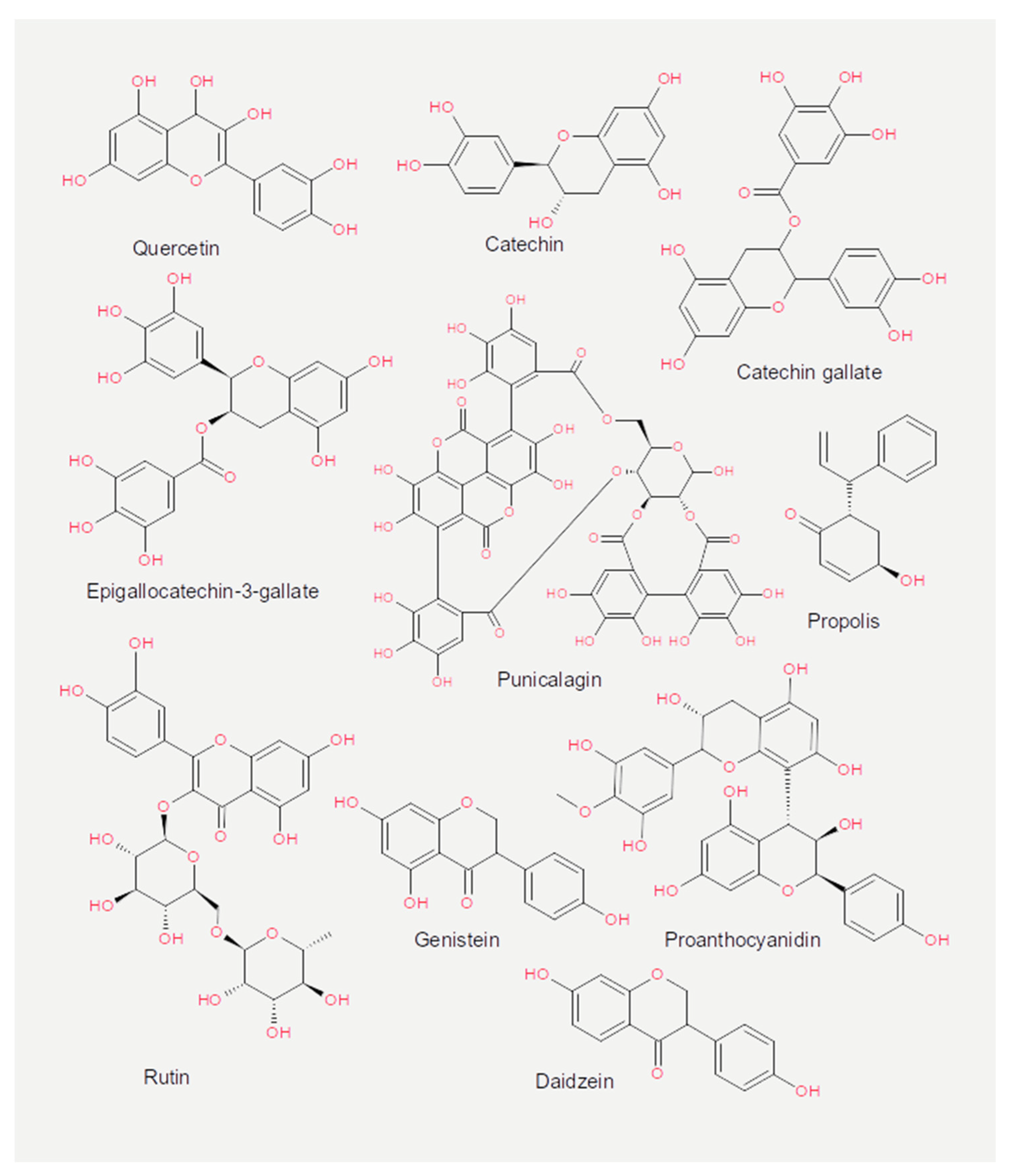
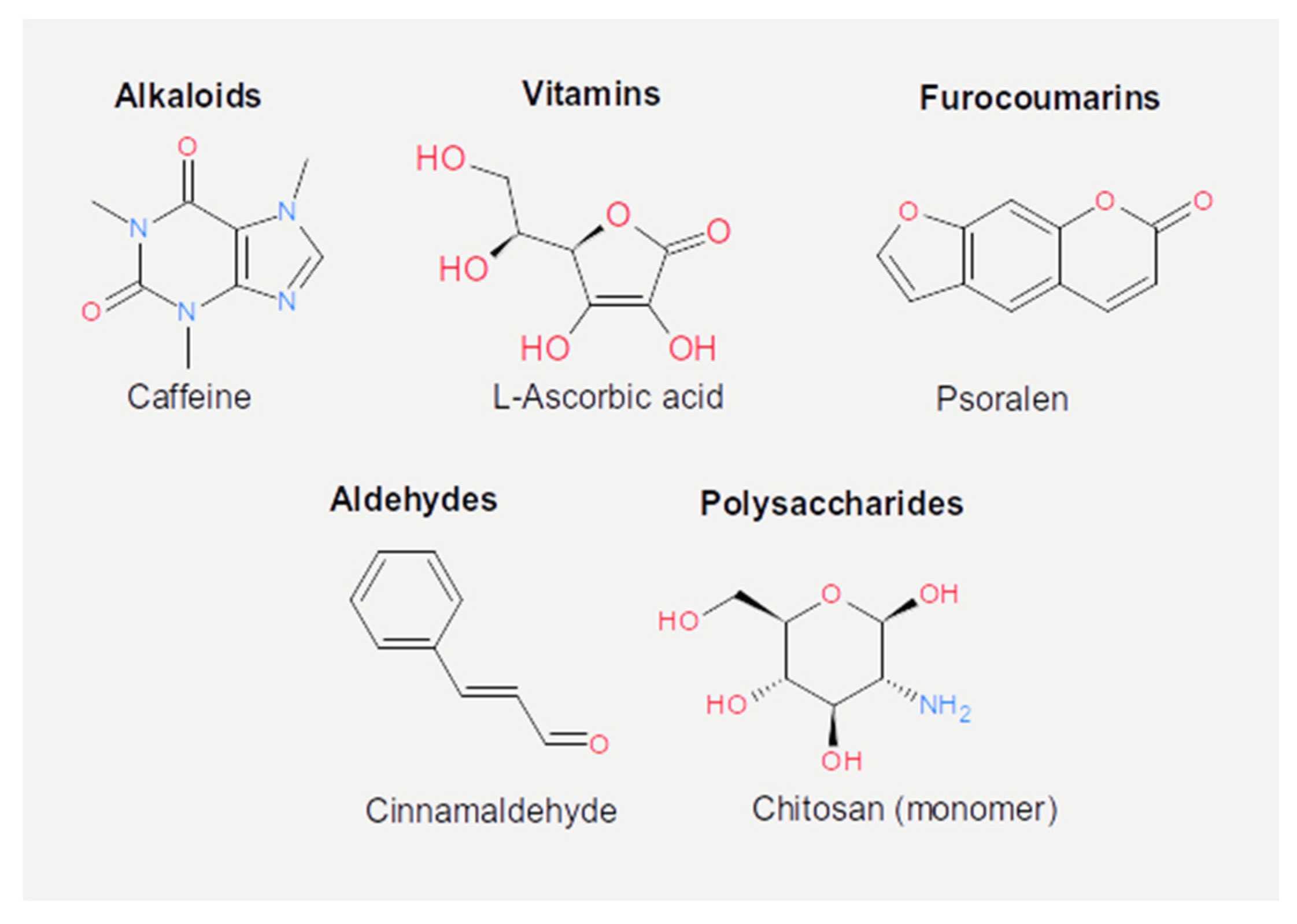
| Nutrient | Class | Virus | Effect | Mechanism | References |
|---|---|---|---|---|---|
| Caffeic acid (or carbonyls) | Phenolic acids (or hydrocarbons) | λ | Prophage induction | Stress response to DNA damage | [79] |
| Av-5, MS2 | Infectivity reduction | Inhibition of replication | [80] | ||
| Gallic acid, chlorogenic acid | Phenolic acids | Av-5, MS2 | Infectivity reduction | Inhibition of replication | [80] |
| Gallic acid | Phenolic acids | PL-1 | Infectivity reduction | Interference to infection | [81] |
| MS2 | No effect | Unreported | [82] | ||
| Carvacrol, thymol | Phenolic acids | 933 W | Prophage suppression | Unreported | [83] |
| Tea extracts | Phenolic acids or flavonoids | Felix 01 and P22 | Infectivity reduction | Unreported | [84] |
| Pomegranate juice (punicalagin) | Phenolic acids or flavonoids | MS2 | Infectivity reduction | Interference to infection (Capsid denaturation?) | [85] |
| Catechins | Flavonoids | T4 | Infectivity reduction | Unreported | [86] |
| EGCG | Flavonoids | 933 J | Prophage suppression | Repression of recA | [87] |
| GCG | Flavonoids | 933 W | Prophage induction | Stress response to ROS | [88] |
| Genistein, daidzein | Flavonoids | φX174 | Genome protection | Scavenging | [89] |
| Proanthocyanidin | Flavonoids | MS2, φX174 | Infectivity reduction | Capsid denaturation | [90,91] |
| PJE | Flavonoids | MS2 | Infectivity reduction | Capsid denaturation | [92] |
| GSE | Flavonoids | MS2 | Infectivity reduction | Interference to infection (Capsid denaturation?) | [93] |
| 933 W | Prophage suppression | Unreported | [94] | ||
| Cranberry juice | Flavonoids | T2, T4 | Infectivity reduction | Capsid denaturation | [95] |
| Propolis | Flavonoids | Unreported | Prophage induction or suppression | Unreported | [68] |
| Red propolis (formononetin) | Flavonoids | MS2, Av-08 | Infectivity reduction | Interference to infection (Capsid denaturation?) | [96] |
| Cinnamaldehyde (cinnamon) | Essential oil (aldehydes) | 933 W | Prophage suppression | Repression of recA | [94,97] |
| Oregano | Essential oil | Unreported | Prophage suppression | Unreported | [68] |
| Chamomile, lemongrass, cinnamon | Essential oils | T7, SA | Infectivity reduction | Unreported | [67] |
| Chitosan | Polysaccharide | MS2, φX174 | Infectivity reduction | Capsid denaturation | [98] |
| 1–97 A | Infectivity reduction | Capsid denaturation | [99] | ||
| c2 | Infectivity reduction | Capsid denaturation | [100] | ||
| 933 W | Infectivity reduction | Unreported | [101] | ||
| Ascorbic acid | Vitamin | δA, φX174, T7, P22, D29, PM2, MS2 | Infectivity reduction | Genome damage | [88,102,103,104,105,106] |
| Psoralen | Furocoumarins | MS2 | Infectivity reduction | Unreported | [107] |
| Caffeine | Alkaloids | φX174 | Prophage induction | Stress response to DNA damage | [108] |
| Sodium chloride | Salt | 933 W | Prophage induction | Unreported | [109] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marongiu, L.; Burkard, M.; Venturelli, S.; Allgayer, H. Dietary Modulation of Bacteriophages as an Additional Player in Inflammation and Cancer. Cancers 2021, 13, 2036. https://doi.org/10.3390/cancers13092036
Marongiu L, Burkard M, Venturelli S, Allgayer H. Dietary Modulation of Bacteriophages as an Additional Player in Inflammation and Cancer. Cancers. 2021; 13(9):2036. https://doi.org/10.3390/cancers13092036
Chicago/Turabian StyleMarongiu, Luigi, Markus Burkard, Sascha Venturelli, and Heike Allgayer. 2021. "Dietary Modulation of Bacteriophages as an Additional Player in Inflammation and Cancer" Cancers 13, no. 9: 2036. https://doi.org/10.3390/cancers13092036
APA StyleMarongiu, L., Burkard, M., Venturelli, S., & Allgayer, H. (2021). Dietary Modulation of Bacteriophages as an Additional Player in Inflammation and Cancer. Cancers, 13(9), 2036. https://doi.org/10.3390/cancers13092036









