Raman Spectroscopy of Liquid-Based Cervical Smear Samples as a Triage to Stratify Women Who Are HPV-Positive on Screening
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. ThinPrep Slide Preparation
2.3. Raman Spectroscopy
2.4. Data Preprocessing and Analysis
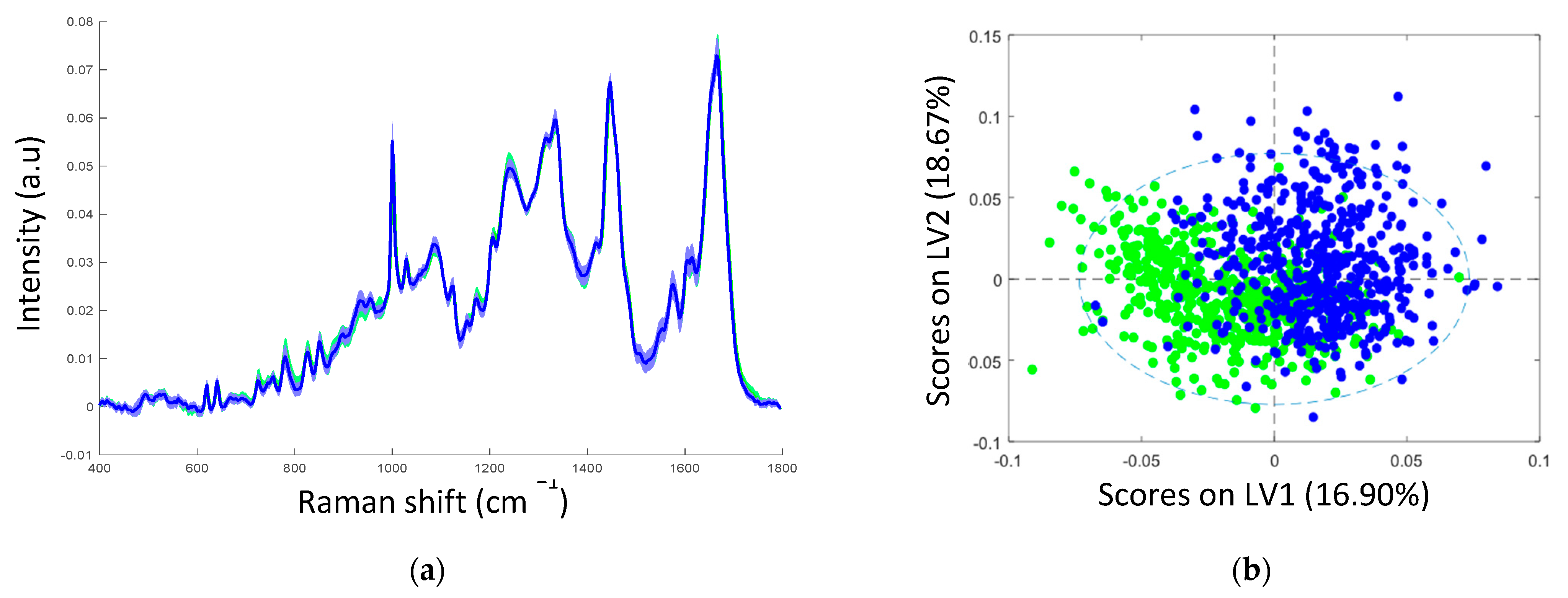
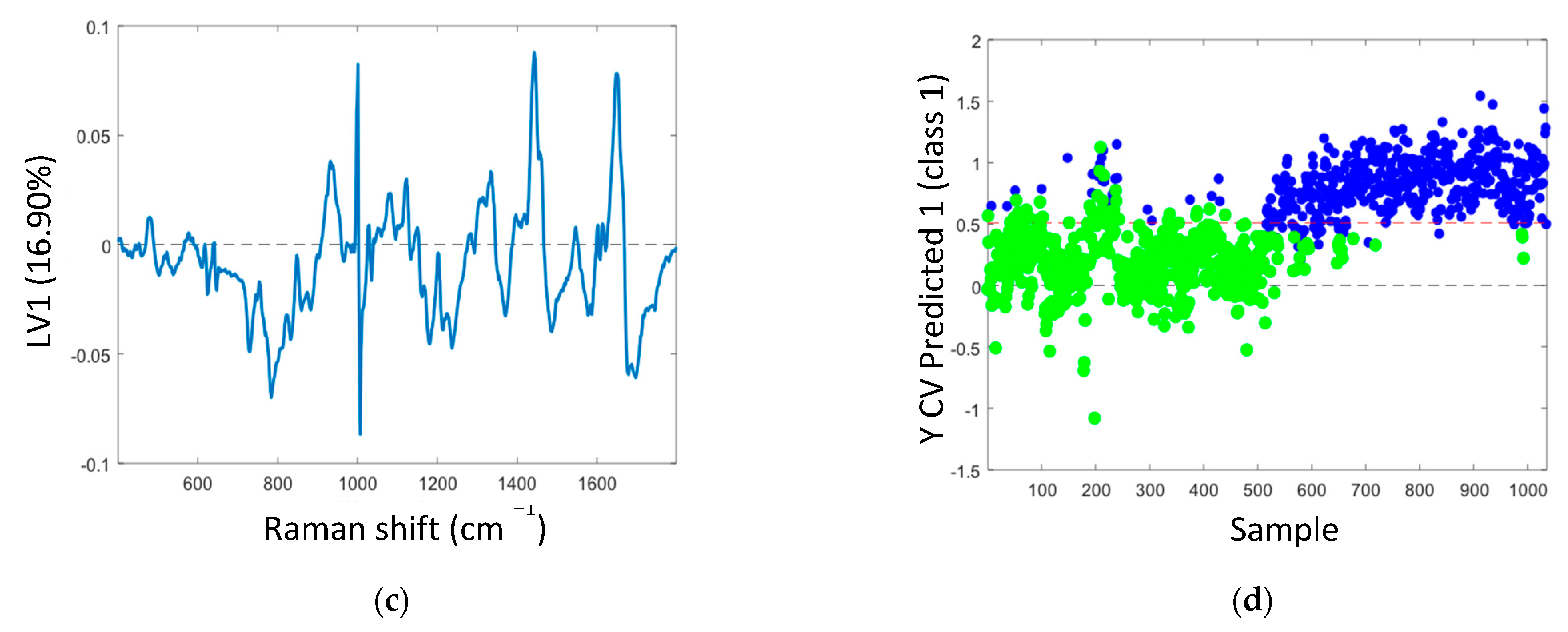
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Simms, K.T.; Steinberg, J.; Caruana, M.; Smith, M.A.; Lew, J.B.; Soerjomataram, I.; Castle, P.E.; Bray, F.; Canfell, K. Impact of Scaled up Human Papillomavirus Vaccination and Cervical Screening and the Potential for Global Elimination of Cervical Cancer in 181 Countries, 2020–2099: A Modelling Study. Lancet Oncol. 2019, 20, 394–407. [Google Scholar] [CrossRef]
- Canfell, K. Towards the Global Elimination of Cervical Cancer. Papillomavirus Res. 2019, 8, 100170. [Google Scholar] [CrossRef] [PubMed]
- Walboomers, J.M.M.; Jacobs, M.V.; Manos, M.M.; Bosch, F.X.; Kummer, J.A.; Shah, K.V.; Snijders, P.J.F.; Peto, J.; Meijer, C.J.L.M.; Muñoz, N. Human Papillomavirus Is a Necessary Cause of Invasive Cervical Cancer Worldwide. J. Pathol. 1999, 189, 12–19. [Google Scholar] [CrossRef]
- Paavonen, J.; Naud, P.; Salmerón, J.; Wheeler, C.M.; Chow, S.N.; Apter, D.; Kitchener, H.; Castellsague, X.; Teixeira, J.C.; Skinner, S.R.; et al. Efficacy of Human Papillomavirus (HPV)-16/18 AS04-Adjuvanted Vaccine against Cervical Infection and Precancer Caused by Oncogenic HPV Types (PATRICIA): Final Analysis of a Double-Blind, Randomised Study in Young Women. Lancet 2009, 374, 301–314. [Google Scholar] [CrossRef]
- De Sanjosé, S.; Brotons, M.; Pavón, M.A. The Natural History of Human Papillomavirus Infection. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 47, 2–13. [Google Scholar] [CrossRef]
- Tropé, A.; Sjøborg, K.; Eskild, A.; Cuschieri, K.; Eriksen, T.; Thoresen, S.; Steinbakk, M.; Laurak, V.; Jonassen, C.M.; Westerhagen, U.; et al. Performance of Human Papillomavirus DNA and MRNA Testing Strategies for Women with and without Cervical Neoplasia. J. Clin. Microbiol. 2009, 47, 2458–2464. [Google Scholar] [CrossRef]
- Tota, J.E.; Bentley, J.; Blake, J.; Coutlée, F.; Duggan, M.A.; Ferenczy, A.; Franco, E.L.; Fung-Kee-Fung, M.; Gotlieb, W.; Mayrand, M.H.; et al. Introduction of Molecular HPV Testing as the Primary Technology in Cervical Cancer Screening: Acting on Evidence to Change the Current Paradigm. Prev. Med. 2017, 98, 5–14. [Google Scholar] [CrossRef]
- Bonde, J.; Floore, A.; Ejegod, D.; Vink, F.J.; Hesselink, A.; Ven, P.M.; Valenčak, A.O.; Pedersen, H.; Doorn, S.; Quint, W.G.; et al. Methylation Markers FAM19A4 and miR124-2 as Triage Strategy for Primary Human Papillomavirus Screen Positive Women: A Large European Multicenter Study. Int. J. Cancer 2020, 148, 369–405. [Google Scholar] [CrossRef]
- Koliopoulos, G.; Nyaga, V.N.; Santesso, N.; Bryant, A.; Martin-Hirsch, P.P.L.; Mustafa, R.A.; Schünemann, H.; Paraskevaidis, E.; Arbyn, M. Cytology versus HPV Testing for Cervical Cancer Screening in the General Population. Cochrane Database Syst. Rev. 2017, 2017, CD008587. [Google Scholar] [CrossRef]
- Shiraz, A.; Crawford, R.; Egawa, N.; Griffin, H.; Doorbar, J. The Early Detection of Cervical Cancer. The Current and Changing Landscape of Cervical Disease Detection. Cytopathology 2020, 31, 258–270. [Google Scholar] [CrossRef] [PubMed]
- Santos, I.P.; Barroso, E.M.; Bakker Schut, T.C.; Caspers, P.J.; Van Lanschot, C.G.F.; Choi, D.H.; Van Der Kamp, M.F.; Smits, R.W.H.; Van Doorn, R.; Verdijk, R.M.; et al. Raman Spectroscopy for Cancer Detection and Cancer Surgery Guidance: Translation to the Clinics. Analyst 2017, 142, 3025–3047. [Google Scholar] [CrossRef] [PubMed]
- Upchurch, E.; Isabelle, M.; Lloyd, G.R.; Kendall, C.; Barr, H. An Update on the Use of Raman Spectroscopy in Molecular Cancer Diagnostics: Current Challenges and Further Prospects. Expert Rev. Mol. Diagn. 2018, 18, 245–258. [Google Scholar] [CrossRef]
- Kanter, E.M.; Vargis, E.; Majumder, S.; Keller, M.D.; Woeste, E.; Rao, G.G.; Mahadevan-Jansen, A. Application of Raman Spectroscopy for Cervical Dysplasia Diagnosis. J. Biophotonics 2009, 2, 81–90. [Google Scholar] [CrossRef]
- Vargis, E.; Kanter, E.M.; Majumder, S.K.; Keller, M.D.; Beaven, R.B.; Rao, G.G.; Mahadevan-Jansen, A. Effect of Normal Variations on Disease Classification of Raman Spectra from Cervical Tissue. Analyst 2011, 136, 2981–2987. [Google Scholar] [CrossRef]
- Vargis, E.; Tang, Y.-W.; Khabele, D.; Mahadevan-Jansen, A. Near-Infrared Raman Microspectroscopy Detects High-Risk Human Papillomaviruses. Transl. Oncol. 2012, 5, 172–179. [Google Scholar] [CrossRef]
- Kamemoto, L.E.; Misra, A.K.; Sharma, S.K.; Goodman, M.T.; Hugh, L.U.K.; Dykes, A.C.; Acosta, T. Near-Infrared Micro-Raman Spectroscopy for in Vitro Detection of Cervical Cancer. Appl. Spectrosc. 2010, 64, 255–261. [Google Scholar] [CrossRef]
- Tan, K.M.; Herrington, C.S.; Brown, C.T.A. Discrimination of Normal from Pre-Malignant Cervical Tissue by Raman Mapping of de-Paraffinized Histological Tissue Sections. J. Biophotonics 2011, 4, 40–48. [Google Scholar] [CrossRef]
- Rashid, N.; Nawaz, H.; Poon, K.W.C.; Bonnier, F.; Bakhiet, S.; Martin, C.; O’Leary, J.J.; Byrne, H.J.; Lyng, F.M. Raman Microspectroscopy for the Early Detection of Pre-Malignant Changes in Cervical Tissue. Exp. Mol. Pathol. 2014, 97. [Google Scholar] [CrossRef] [PubMed]
- Lyng, F.M.; Traynor, D.; Ramos, I.R.M.; Bonnier, F.; Byrne, H.J. Raman Spectroscopy for Screening and Diagnosis of Cervical Cancer. Anal. Bioanal. Chem. 2015, 407, 8279–8289. [Google Scholar] [CrossRef] [PubMed]
- Ramos, I.; Meade, A.D.; Ibrahim, O.; Byrne, H.; McMenamin, M.; McKenna, M.; Malkin, A.; Lyng, F. Raman Spectroscopy for Cytopathology of Exfoliated Cervical Cells. Faraday Discuss. 2015, 187, 187–198. [Google Scholar] [CrossRef]
- Duraipandian, S.; Zheng, W.; Ng, J.; Low, J.J.H.; Ilancheran, A.; Huang, Z. Near-Infrared-Excited Confocal Raman Spectroscopy Advances in Vivo Diagnosis of Cervical Precancer. J. Biomed. Opt. 2013, 18, 067007. [Google Scholar] [CrossRef][Green Version]
- Duraipandian, S.; Zheng, W.; Ng, J.; Low, J.J.H.; Ilancheran, A.; Huang, Z. Simultaneous Fingerprint and High-Wavenumber Confocal Raman Spectroscopy Enhances Early Detection of Cervical Precancer in Vivo. Anal. Chem. 2012, 84, 5913–5919. [Google Scholar] [CrossRef]
- Traynor, D.; Duraipandian, S.; Martin, C.M.; O’Leary, J.J.; Lyng, F.M. Improved Removal of Blood Contamination from ThinPrep Cervical Cytology Samples for Raman Spectroscopic Analysis. J. Biomed. Opt. 2018, 23, 1. [Google Scholar] [CrossRef] [PubMed]
- Kearney, P.; Traynor, D.; Bonnier, F.; Lyng, F.M.; O’Leary, J.J.; Martin, C.M. Raman Spectral Signatures of Cervical Exfoliated Cells from Liquid-Based Cytology Samples. J. Biomed. Opt. 2017, 22, 1. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Daniel, A.; Prakasarao, A.; Ganesan, S. Near-Infrared Raman Spectroscopy for Estimating Biochemical Changes Associated with Different Pathological Conditions of Cervix. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 190, 409–416. [Google Scholar] [CrossRef]
- Traynor, D.; Duraipandian, S.; Bhatia, R.; Cuschieri, K.; Martin, C.M.; O’Leary, J.J.; Lyng, F.M. The Potential of Biobanked Liquid Based Cytology Samples for Cervical Cancer Screening Using Raman Spectroscopy. J. Biophotonics 2019, 12, e201800377. [Google Scholar] [CrossRef] [PubMed]
- Bonnier, F.; Traynor, D.; Kearney, P.; Clarke, C.; Knief, P.; Martin, C.; O’Leary, J.J.; Byrne, H.J.; Lyng, F. Processing ThinPrep Cervical Cytological Samples for Raman Spectroscopic Analysis. Anal. Methods 2014, 6, 7831–7841. [Google Scholar] [CrossRef]
- Traynor, D.; Kearney, P.; Ramos, I.; Martin, C.M.; O’Leary, J.J.; Lyng, F.M. A Study of Hormonal Effects in Cervical Smear Samples Using Raman Spectroscopy. J. Biophotonics 2018, 11, e201700240. [Google Scholar] [CrossRef]
- Rubina, S.; Amita, M.; Kedar, K.D.; Bharat, R.; Krishna, C.M. Raman Spectroscopic Study on Classification of Cervical Cell Specimens. Vib. Spectrosc. 2013, 68, 115–121. [Google Scholar] [CrossRef]
- Jess, P.R.T.; Smith, D.D.W.; Mazilu, M.; Dholakia, K.; Riches, A.C.; Herrington, C.S. Early Detection of Cervical Neoplasia by Raman Spectroscopy. Int. J. Cancer 2007, 121, 2723–2728. [Google Scholar] [CrossRef]
- Ostrowska, K.M.; Malkin, A.; Meade, A.; O’Leary, J.; Martin, C.; Spillane, C.; Byrne, H.J.; Lyng, F.M. Investigation of the Influence of High-Risk Human Papillomavirus on the Biochemical Composition of Cervical Cancer Cells Using Vibrational Spectroscopy. Analyst 2010, 135, 3087–3093. [Google Scholar] [CrossRef] [PubMed]
- Movasaghi, Z.; Rehman, S.; Rehman, I.U. Raman Spectroscopy of Biological Tissues. Appl. Spectrosc. Rev. 2007, 42, 493–541. [Google Scholar] [CrossRef]
- Duraipandian, S.; Traynor, D.; Kearney, P.; Martin, C.; O’Leary, J.J.; Lyng, F.M. Raman Spectroscopic Detection of High-Grade Cervical Cytology: Using Morphologically Normal Appearing Cells. Sci. Rep. 2018, 8, 15048. [Google Scholar] [CrossRef] [PubMed]

| Scheme | Cytology | HPV DNA | HPV mRNA | Transcriptionally Active HPV Infection |
|---|---|---|---|---|
| 1–30 | HSIL | positive | negative | no |
| 31–60 | HSIL | positive | positive | yes |
| Raman Peak Position (cm−1) | Proteins | Lipids | Carbohydrates | Nucleic Acids |
|---|---|---|---|---|
| 482 | Glycogen | |||
| 577 | Glycogen | |||
| 622 | C–C twist Phe | |||
| 643 | C–C twist Tyr | |||
| 727 | CH2 def | C–C head | A | |
| 752 | Sym br. Trp | |||
| 781 | U, C, T ring br | |||
| 826 | Out of Plane ring br. Tyr | PO2 a.str | ||
| 851 | Ring br. Tyr, C–C str. Pro | |||
| 852 | Glycogen | |||
| 937 | Glycogen | |||
| 985 | C–C head | |||
| 1002 | Sym. Ring br. Phe | |||
| 1033 | C–H in plane Phe, C–C str | |||
| 1060 | C–N str | |||
| 1082 | C–N str | Chain C–C str | C–O str, Glycogen | |
| 1096 | Chain C–C str | C–C str | ||
| 1123 | C–N str | Chain C–C str | C–O str, Glycogen | |
| 1152 | C–N str | |||
| 1207 | C–C6H5 str. Phe, Trp | |||
| 1238 | C–N str, Amide III | |||
| 1334 | Glycogen | |||
| 1338 | Trp | G | ||
| 1366 | Sym. str. CH3 | |||
| 1381 | Glycogen | |||
| 1450 | CH2 def | CH2 def | ||
| 1485 | CH2 def | G, A | ||
| 1560 | Tyr, Trp | |||
| 1580 | A, G ring br | |||
| 1584 | C=C str, C=C bend. Trp, Phe | |||
| 1605 | C=C Phe, Tyr | |||
| 1642 | C=O str, C=C sym. str. | |||
| 1669 | C=O str. Amide I |
| Sample | Correct Classification | Individual Cellular Spectra | Mean Sample Spectra | ||
|---|---|---|---|---|---|
| Non-Transcriptionally Active | Transcriptionally Active | Non-Transcriptionally Active | Transcriptionally Active | ||
| 001 | Transcriptionally active | 0 | 16 | 0 | 1 |
| 002 | Transcriptionally active | 15 | 16 | 0 | 1 |
| 003 | Transcriptionally active | 1 | 12 | 0 | 1 |
| 004 | Transcriptionally active | 4 | 33 | 0 | 1 |
| 005 | Transcriptionally active | 7 | 25 | 0 | 1 |
| 006 | Transcriptionally active | 0 | 19 | 0 | 1 |
| 007 | Transcriptionally active | 2 | 17 | 0 | 1 |
| 008 | Transcriptionally active | 3 | 16 | 0 | 1 |
| 009 | Transcriptionally active | 4 | 44 | 0 | 1 |
| 010 | Transcriptionally active | 10 | 6 | 1 | 0 |
| 011 | Non-transcriptionally active | 29 | 0 | 1 | 0 |
| 012 | Non-transcriptionally active | 26 | 3 | 1 | 0 |
| 013 | Non-transcriptionally active | 21 | 3 | 1 | 0 |
| 014 | Non-transcriptionally active | 22 | 2 | 1 | 0 |
| Sample Number | Raman Classification | Cytology | Histology | HPV DNA | HPV mRNA |
|---|---|---|---|---|---|
| 001 | Transcriptionally active | HSIL | CIN2 | Positive | Positive |
| 002 | Transcriptionally active | HSIL | CIN3 | Positive | Positive |
| 003 | Transcriptionally active | HSIL | CIN3 | Positive | Positive |
| 004 | Transcriptionally active | HSIL | CIN2 | Positive | Positive |
| 005 | Transcriptionally active | HSIL | CIN1 | Positive | Positive |
| 006 | Transcriptionally active | HSIL | CIN2 | Positive | Positive |
| 007 | Transcriptionally active | HSIL | CIN2 | Positive | Positive |
| 008 | Transcriptionally active | HSIL | CIN3 | Positive | Positive |
| 009 | Transcriptionally active | HSIL | CIN2 | Positive | Positive |
| 010 | Non-transcriptionally active | LSIL | CIN1 | Positive | Positive |
| 011 | Non-transcriptionally active | LSIL | Negative | Positive | Negative |
| 012 | Non-transcriptionally active | Negative | Negative | Positive | Negative |
| 013 | Non-transcriptionally active | LSIL | Negative | Positive | Negative |
| 014 | Non-transcriptionally active | Negative | Negative | Positive | Negative |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Traynor, D.; Martin, C.M.; White, C.; Reynolds, S.; D’Arcy, T.; O’Leary, J.J.; Lyng, F.M. Raman Spectroscopy of Liquid-Based Cervical Smear Samples as a Triage to Stratify Women Who Are HPV-Positive on Screening. Cancers 2021, 13, 2008. https://doi.org/10.3390/cancers13092008
Traynor D, Martin CM, White C, Reynolds S, D’Arcy T, O’Leary JJ, Lyng FM. Raman Spectroscopy of Liquid-Based Cervical Smear Samples as a Triage to Stratify Women Who Are HPV-Positive on Screening. Cancers. 2021; 13(9):2008. https://doi.org/10.3390/cancers13092008
Chicago/Turabian StyleTraynor, Damien, Cara M. Martin, Christine White, Stephen Reynolds, Tom D’Arcy, John J. O’Leary, and Fiona M. Lyng. 2021. "Raman Spectroscopy of Liquid-Based Cervical Smear Samples as a Triage to Stratify Women Who Are HPV-Positive on Screening" Cancers 13, no. 9: 2008. https://doi.org/10.3390/cancers13092008
APA StyleTraynor, D., Martin, C. M., White, C., Reynolds, S., D’Arcy, T., O’Leary, J. J., & Lyng, F. M. (2021). Raman Spectroscopy of Liquid-Based Cervical Smear Samples as a Triage to Stratify Women Who Are HPV-Positive on Screening. Cancers, 13(9), 2008. https://doi.org/10.3390/cancers13092008







